Abstract
The thymus is an organ that generates and educates T cells, supplying T subsets that ensure adaptive immunity and self-tolerance. Intrathymic positive selection of T cells with further post-thymic maturation and signaling provided by peripheral antigen-presenting cells (APCs) sustains a functional and regulated T cell pool. Intrathymic negative selection and generation of natural regulatory T cells (Tregs) in the thymus are essential to contain T cell autoimmunity. Humanized mouse models provide a unique opportunity to investigate these processes and their underlying mechanisms. Humanized mice can be generated by injecting human hematopoietic stem cells, with or without cotransplantation of fetal thymic tissue, to immunodeficient mice. Human thymopoiesis and T cell development in the former and latter models occur in the recipient mouse thymus and human thymic grafts, respectively. In this chapter, we summarize information on human T cell development, post-thymic interactions between T cells and APCs, as well as mechanisms maintaining tolerance that has been learned from these humanized mouse models.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Humanized mouse models
- T cell development
- Negative selection
- Positive selection
- Post-thymic maturation
- Regulatory T cells
- Thymic transplantation
- Mixed hematopoietic chimerism
- Tolerance
- Xenograft
- Xenotransplantation
1 Overview of T Cell Selection and Maturation
The immune system of mammals is needed to fight infections while maintaining tolerance to the self. T cells play a central role in these processes. The development of T cells in the thymus endows them with the ability to recognize and mount immune responses to millions of pathogen-derived nonself-antigens while still being tolerant to “self” antigens. Mouse studies demonstrate that the thymus can perform three critical functions for development of a functional, self-tolerant T cell repertoire, namely positive selection , negative selection and generation of CD4+CD25+Foxp3+ regulatory T cells (Tregs). T cell progenitors migrate to the thymus, where they develop into T cells. Absence of thymus, such as in nude mice and human patients lacking a functional FOXN1 gene, results in the absence of normal functional T cells [1]. Newly generated T cells undergo further post-thymic maturation to become fully functional in the periphery [2]. Intrathymic positive selection of T cells results in a repertoire that most efficiently recognizes exogenous peptide antigens presented by the same “self” MHC molecules. Engagement by T cell receptor (TCR) of naïve T cells of the same MHC/self-peptide complexes expressed on peripheral antigen-presenting cells (APCs), as those on which they are positively selected in the thymus, provides a critical survival signal [3]. These processes sustain a functional T cell pool. Negative selection and generation of Tregs ensure that the majority of strongly autoreactive T cells are purged and those that escape negative selection are kept in check by Tregs in the periphery [4]. Positive and negative selection of developing thymocytes is achieved through differential affinities of the TCR on the thymocytes with MHC-peptide complexes on the thymic epithelial cells and intrathymic APCs. Thymocytes expressing TCR with low and intermediate affinities for MHC-peptide complexes expressed on thymic epithelium are positively selected, enabling effective recognition of peptides presented by autologous MHC molecules in the periphery. Positively selected thymocytes expressing TCR with high affinities for self peptides presented by autologous MHC molecules undergo apoptosis during negative selection [5] . This process, also known as central tolerance, ensures the elimination of the strongly self-reactive T cells and is one of the key mechanisms for establishing self-tolerance.
2 Humanized Mouse Models Involving Human T Cell Development
Models allowing investigation of human T cell development, such as humanized mouse models, provide a unique opportunity to investigate the human immune system and T cell development . Initially, humanized mice were generated by injecting human mature immune cells, such as human peripheral blood mononuclear cells (PBMCs) into severe combined immunodeficiency (SCID) mice [6]. Later, humanized mice were established by injecting human hematopoietic stem cells (HSCs) into irradiated neonatal or adult immunodeficient mice [7, 8]. Engraftment of human HSCs gives rise to human immune cells, including T cells, B cells, monocytes, etc. However, both types of humanized mouse models have limitations for the study of human T cell development. Grafting of human PBMCs includes mature T cells and thus does not allow the investigation of human T cell development. In addition, mature human T cells that are reactive to mouse xenoantigens can dominate and preclude good functional immunity [9, 10]. T cells in humanized mice generated by transplanting human HSCs develop in the mouse thymus rather than the human thymus. Thymopoiesis occurs at only a low level and the small number of T cells generated show functional defects [11, 12] (discussed in detail below). Therefore, these models are not optimal for the investigation of human T cell development .
Early work by McCune et al. demonstrated that implantation of human fetal thymic tissue under the kidney capsule of unconditioned adult SCID mice together with intravenous or intrathymic injection of fetal liver cells led to robust thymopoiesis [13]. However, very few human T cells populated the periphery and these were only detected transiently, from week 4 to 10 postimplantation. Implantation of fetal thymic tissue together with fetal liver led to more persistent T cells in peripheral blood [14]. Despite the presence of human IgG and human HLA Class I+ cells in peripheral blood in some animals, human hematopoietic reconstitution of non-T cells seemed to be largely confined to the human graft microenvironment [13]. The failure to achieve systemic human hematopoiesis in this model may reflect the susceptibility of human hematopoietic cells to rapid destruction by mouse macrophages in CB.17-SCID mice [15] . Subsequently, we combined i.v. injection of human HSCs and implantation of human thymic tissue under the kidney capsule in nonobese diabetic SCID (NOD/SCID) mice and achieved much more robust human T cell and APC reconstitution. It is likely that the reduced phagocytosis of human cells in mice on the NOD background, reflecting the compatibility of the NOD SIRPα allele with human CD47 [16], explains the improved human hematopoietic reconstitution in our model over the original model described by McCune et al. In our model, humanized mice are generated by transplanting human fetal thymic/liver tissue under the kidney capsule and coinjecting fetal liver-derived CD34+ HSCs from the same donors to irradiated adult NOD/SCID mice [17–20]. The model has been replicated and termed the “BLT” mouse [21]. The transplantation of autologous thymic tissue is an important improvement to the injection of human HSCs. In this model, not only major immune cell populations, such as B cells, dendritic cells and monocytes, but also large numbers of T cells appear in peripheral lymphoid tissues, which develop significant structure by 12 weeks post-transplant [17, 19]. Because human T cells in these mice develop in autologous thymic tissues, this model is highly relevant and valuable for the study of human T cell development and offers advantages compared to other models. First, in sharp contrast to humanized mice generated by grafting human HSCs only [7, 8, 11] , thymopoiesis occurs at a high level, with thymocyte numbers (> 108) that are similar to or even greater than those in a normal, immunocompetent mouse thymus [20]. Large numbers of T cells migrate to the peripheral lymphoid tissues [17–19]. Importantly, robust immune functions are seen, such as spontaneous rejection of xenogeneic skin grafts and class-switched antibody responses following immunization [17, 18, 22]. Secondly, this model not only allows the investigation of human T cell development in physiological conditions, but also enables characterization of human T cell generation in pathological conditions, such as viral infection [21, 23, 24]. Thirdly, as this model allows the de novo development of human T cells in human thymic tissue, it can be utilized to investigate immune abnormalities arising from HSC-intrinsic factors .
We have modified this model by transplanting adult bone marrow-derived CD34+ HSCs and partially HLA-matched, T-cell depleted allogeneic fetal thymic tissue to NOD/SCID/ Common γ-chain-deficient (NSG) mice [25]. These humanized mice, similar to those generated by grafting autologous fetal thymic tissue and fetal liver-derived CD34+ cells, demonstrate polyclonal human T cell reconstitution and robust T cell function [25]. This humanized mouse model allows the study of T cell development and function in any individual human and is thus termed the “Personalized Immune” (PI) mouse model. With this PI mouse model, we are characterizing the development and function of T cells in type I diabetic patients, addressing the hypothesis that T cell abnormalities in these patients are intrinsically determined in their HSCs. In this chapter, we will summarize knowledge on human T cell development learned in humanized mouse models generated by transplantation of human fetal thymic tissue and fetal or adult CD34+ HSCs .
3 Positive Selection
Interactions of T cell progenitors entering the thymus with MHC/peptide complexes expressed by thymic stromal cells, including thymic epithelial cells (TECs), trigger positive selection [26, 27] . Studies using transgenic mice exclusively expressing MHC I [28] or II [29] molecules on cortical TECs demonstrated that these cells are required for positive selection of CD8 or CD4 T cells, respectively. During positive selection, thymocytes expressing a TCR that is able to recognize an autologous MHC/self-peptide complex expressed on cortical TECs with at least low to medium affinity survive this process [5, 30]. This low level of self-reactivity is critical for the homeostasis [3] and immune functions of T cells in the periphery [30, 31].
In humanized mice generated by transplanting only human HSCs to immunodeficient mice, T cells develop in the mouse thymus, where human thymocytes are positively selected by mouse MHC molecules expressed on mouse TECs. The ability of murine MHC to positively select human T cells is not unexpected in view of previous work. Ample evidence from our porcine thymus transplant models shows that xenogeneic MHC molecules are able to mediate positive selection. A series of early studies demonstrated that mouse T cells [32–35] or human T cells [36, 37] developed robustly, with diverse repertoires, in porcine thymus. Importantly, porcine MHC molecules were shown to positively select mouse T cells with no contribution from the mouse MHC [35]. Nevertheless, mouse T cells selected by porcine MHC molecules could still respond to cognate antigen stimulation and were able to control infection in vivo, apparently due to cross-reactivity of the diverse repertoire combined with post-thymic selection for T cells that cross-reacted with mouse MHC [32]. The ability of xenogeneic MHC molecules to mediate positive selection is presumably due to the broad MHC cross-reactivity for TCR that is conserved between species .
Although the native thymi of these mice receiving human HSCs alone demonstrate relatively normal histologic structure due to colonization by developing human T cells, the number of thymocytes is low (about 1 million/thymus) compared to the much larger numbers in human thymic grafts (of the order of 100 fold more), and this difference is reflected in lymphoid tissues [11]. Multiple factors may contribute to the low level of thymopoiesis in this model. Failed thymic structural development, as TECs depend on interactions with thymocytes for their own development [38, 39], is partly circumvented by introducing human HSCs close to the time of birth [8]. Incompatibility of adhesion molecules and cytokines between mouse and human may lead to decreased homing of human thymocyte progenitors to and decreased survival of developing thymocytes in the mouse thymus, respectively. Indeed, supplementation of human cytokines, such as IL-7 [40] and IL-15 [41], increased human thymopoiesis. In addition, increased thymopoiesis (but still only of few a million thymocytes) was found in recipient mice expressing a single human HLA Class II molecule (HLA-DR4), showing that reduced ability of mouse MHC molecules to positively select human thymocytes plays a role [11]. However, these issues arising from xenoincompatibility cannot be generalized to all species, as robust human thymopoiesis occurs in pig thymic grafts (again, about 100-fold the level in the native mouse thymus). In fact, humanized mice grafted with fetal pig thymic tissues and human HSCs demonstrate thymopoiesis as robust as that seen in recipients of human thymic tissues and HSCs from the same donor [36, 37]. Thus, the mouse thymus is not an optimal microenvironment for the development of human T cells in humanized mice generated by grafting human HSCs alone. In contrast, in humanized mice generated by transplantation of autologous fetal thymic tissues and fetal liver-derived CD34+ HSCs, T cell development occurs in autologous thymic tissue and the problems described above are thereby circumvented, allowing robust thymopoiesis. Large numbers of thymocytes (> 1 × 108/thymic graft) [20] and splenic T cells (10–20 million/spleen) can be recovered 10–12 weeks post transplantation. The developing thymocytes show a normal ratio of CD4+, CD8+, CD4+CD8+ and CD4−CD8− subsets [17, 19].
4 Post-thymic Interactions Between T Cells and APCs
T cells that have survived positive and negative selection in the thymus mature and are exported to the periphery, where their interactions with APCs are critical for their survival, further maturation, function, and homeostasis. TCRs interact most efficiently in the periphery with the MHC/peptide complexes on which they are positively selected in the thymus [3, 42], providing critical signals for naïve T cell survival in the periphery [3]. In addition, self peptide-MHC complexes promote the responsiveness of peripheral T cells to their cognate antigens [31, 43] by acting as co-agonists to enhance their functional sensitivity [30, 44].
In mouse models, proliferation of T cells occurs when they are transferred to lymphopenic hosts, including rapid and slow proliferation known as lymphopenia-induced proliferation (LIP) [3, 45]. Recognition of commensal microorganisms causes the rapid proliferation [45], whereas the slower LIP is dependent on interactions between TCR and self MHC/peptide complexes and γc cytokines, such as IL-7 and IL-15 [3, 45]. Although insights into homeostatic and lymphopenia-driven proliferation of T cells have been obtained in mouse models, it is more difficult to investigate these events in humans, underscoring the importance of a suitable humanized mouse model to study homeostatic proliferation of human T cells. We have addressed this issue using humanized mice generated by grafting human fetal thymic tissue and HSCs [19]. Transfer of CD45RO− naïve T cells isolated from humanized mice, generated with human thymic tissue and intravenously-administered HSCs, to T cell-deficient humanized mice reconstituted with only the HSCs from the same donor, led to two forms of LIP, similar to results in RAG−/− mice receiving naïve mouse T cells [45]. Like mouse T cells, human naïve T cells undergoing rapid proliferation acquired a memory phenotype and production of IFN-γ, while T cells undergoing slow proliferation retained a naïve phenotype and did not produce IFN-γ. Importantly, the recovery of transferred T cells that had undergone LIP was correlated with the level of human APC chimerism in the secondary recipients and no cells were recovered from adoptive recipients lacking any human hematopoiesis. This result suggests that the LIP of transferred T cells was dependent on interactions with autologous APCs in the periphery, probably via interactions between TCR and autologous HLA molecules [19]. Thus, the survival of human T cells, like that of mouse T cells, depends on interactions with autologous MHC in the periphery. This requirement might in part explain the absence of T cells in the peripheral lymphoid tissues in the humanized mouse model established by McCune et al. [13]. Because no human APCs were found in the peripheral lymphoid tissues in that model, T cells egressing the thymus were unable to interact with peripheral APCs to receive a survival signal, resulting in a lack of accumulation of T cells. Consistently, the addition of intravenous CD34 cells to human fetal thymus and liver grafts in NOD/SCID mice led not only to the presence of human APCs in the periphery, but also to markedly increased T cell reconstitution [17, 18]. Moreover, only the T cells in animals that received CD34 cells intravenously were sufficiently functional to reject xenografts spontaneously [17], consistent with the need for tonic interactions with positive selecting MHC/peptide complexes in the periphery to maintain T cell function [30, 31, 43, 44].
Mouse studies demonstrate that T cells termed recent thymic emigrants, which have recently completed intrathymic development and been exported to the periphery, undergo further post-thymic maturation to gain full immune function. This process requires their entry into secondary lymphoid organs to interact with APCs, but may be independent of TCR engagement with self MHC-peptide complexes [2, 46]. Due to the difficulty in investigating this process, it is unclear whether similar phenomena prevail in humans. However, humanized mice generated with autologous thymic tissue and i.v. injection of HSCs can be a useful tool to unravel this process.
Acquisition of MHC preference for peptide antigen recognition occurs through positive selection . This preference is of critical importance in achieving efficient immune responses to antigens. One example is the interactions between CD4 T helper cells and B cells in the production of antigen-specific antibodies. Because human CD4 T cells developing in humanized mice grafted only with human HSCs are selected by and thus preferentially recognize antigens presented by mouse MHC, they are not able to provide efficient help to antigen-specific human B cells via the interactions of T cell receptor and HLA molecules on B cells. This may contribute to the failure to induce antigen-specific IgG production following immunization. Consistent with this notion, transgenic expression of a human HLA class II molecule in recipient mice enhances IgG responses [11]. In contrast, humanized mice generated by grafting autologous thymic tissue and HSCs should have effective interactions between CD4 T cells and B cells, since T cells develop in the thymic tissue from the same donor and are thus positively selected on their own HLA molecules. Consistently, immunization of these mice leads to production of antigen-specific IgG [22]. Another demonstration of the importance of having the positive selecting MHC also present in the periphery is provided by human T cells developing in pig thymic tissue. In humanized mice grafted with pig thymic tissue and human HSCs, robust thymopoiesis and T cell reconstitution is seen. However, T cell responses to tetanus toxoid following immunization were markedly reduced compared to those in mice with an autologous human thymus graft [47]. Presumably T cells developing in the pig thymic tissue and thus positively selected on pig MHC molecules were not able to efficiently recognize antigen presented by human APCs in the periphery.
5 Negative Selection
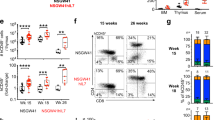
Mouse studies demonstrate that negative selection is critical for the induction of tolerance to self. The majority of autoreactive T cells are purged by this process, resulting in a T cell pool that is tolerant to self antigens [4, 48]. The negative selection process has been demonstrated in several conditions in humanized mice receiving fetal thymic tissue and HSCs. In some mice receiving fresh human thymic tissues, human anti-mouse xenogeneic GVHD can be seen about 12–16 weeks post-transplantation, as reflected by hunched posture, hair loss, and dermatitis. Target organs show typical GVHD histopathologies [49]. In contrast, the incidence of xenogeneic GVHD is clearly reduced by the use of freeze/thawed thymic tissue and administration of T cell-depleting antibody following transplantation, both of which deplete pre-existing thymocytes, before and after they migrate to the periphery respectively (Fig. 11.1, reference 25 and unpublished data). T cells from humanized mice that are grafted with freeze/thawed autologous thymic tissue and HSCs do not mount responses to mouse xenoantigens in in-vitro mixed lymphocyte reaction (MLR) cultures, demonstrating tolerance of human T cells to mouse xenoantigens [47]. Pre-existing thymocytes within human fetal thymic grafts can cause xenogeneic GVHD in these humanized mice because they have not undergone negative selection in the presence of mouse APCs. Mouse APCs populate the freeze/thawed human thymic tissue (Fig. 11.2) when grafted to NSG mice receiving T cell depleting mAb [25], resulting in negative selection in the human thymic graft during de novo human T cell development.
Cryopreservation depletes thymocytes in human fetal graft. Fresh and cryopreserved thymus (~ 0.002 g of a single piece of tissue) from the same donor were dissociated and stained for live cells and thymocytes markers CD4 and CD8. 1 × 105 total events were collected and subgated on live (DAPI−) thymocytes. FCM plots are shown on the left and total cell number for CD4CD8 double negative, single positive and double positive populations are shown in the graft at the right. Cryopreservation decreased single positive CD4 and CD8 cells 510 fold and 454 fold respectively after adjusting for tissue weight difference before freezing (SPCD4 fresh 1.9 × 105 vs. cryopreserved 425 cells; SPCD8 fresh 1.1 × 105 vs. cryopreserved 275 cells; from Kalscheuer et al. [25]. Reprinted with permission from AAAS)
Antigen-presenting cells from the recipient mouse in human thymic graft of PI mouse. The thymic graft from a PI mouse reconstituted with adult CD34+ cells (center), a thymus from a normal C57BL/6 mouse (left) and a thymus from a human (right) were sectioned and stained with anti-mouse pan-MHC class II mAb to reveal the presence of mouse-derived MHC class II positive cells. In the normal mouse thymus, m denotes the medullary and c denotes the cortical region. When tested for cross-reactivity, the anti-mouse MHCII antibody did not bind to human thymus tissue (right). (From Kalscheuer et al. [25]. Reprinted with permission from AAAS)
We have investigated the induction of tolerance to pig xenoantigens by thymic transplantation . Our early studies demonstrated that transplantation of pig thymic tissue to thymectomized and T and NK cell-depleted mice led to generation of a mouse T cell repertoire that was specifically tolerant to the recipient mouse and donor pig, suggesting that thymic transplantation could be a potential approach to inducing tolerance to pig xenoantigens in humans [34, 50]. Negative selection of mouse developing T cells by the pig thymus was found to be responsible for much of the tolerance in this model [33], but a role for Tregs was also suggested [51]. Later studies showed that cotransplantation of pig thymus prevented rejection of pig kidney grafts in baboons, further indicating the potential of this approach to induce tolerance to pig xenoantigens [52]. Humanized mice have been used in our recent studies to investigate this approach for human T cell tolerance. Transplantation of pig thymic tissue with human HSCs leads to generation of a human T cell repertoire that is specifically tolerant to the donor pig xenoantigens [36, 53]. T cells from spleens of humanized mice transplanted with SLAd/d pig thymic tissues and human HSCs showed strong responses to human alloantigens and to third party SLAc/c pig xenoantigens, with specific unresponsiveness to the pig donor and mouse recipient [47]. Alternatively, induction of pig/human mixed hematopoietic chimerism in humanized mice generated by grafting human fetal thymic tissue and autologous human HSCs with pig bone marrow cells also led to specific unresponsiveness of human T cells to donor pig xenoantigens [18]. Pig MHC Class II+ cells were found in the human thymic grafts in the pig/human mixed chimeric humanized mice, suggesting that negative selection of human T cells by these pig MHC Class II+ APCs led to the deletion of pig-reactive human T cells [18]. These data suggest that human T cells become tolerant to pig xenoantigens by undergoing negative selection in pig thymus or in human thymus containing pig bone marrow-derived APCs. Pig thymus transplantation and induction of mixed porcine hematopoietic chimerism thus are two potential solutions for inducing human T cell tolerance to pig xenoantigens. Induction of tolerance is likely to be essential for successful pig-to-human xenogeneic organ transplantation.
6 Treg Generation and Maturation
Ample studies demonstrate that CD4+CD25+Foxp3+ naturally occurring Tregs play a critical role in maintenance of self tolerance [54, 55] . Tregs are a promising candidate for therapy and prophylaxis against multiple diseases [56]. Mouse “natural” Tregs are generated in the thymus [54] in processes involving both positive and negative selection [5, 57]. The process by which Tregs are generated, including the location and molecular signals, has been under active investigation [5, 57, 58]. Naturally occurring Tregs exist in humans [59, 60], but little is known about their development. Thus, humanized mouse models that enable the investigation of human Treg development are needed. Although human Tregs with in vitro suppressive activities are present in humanized mice generated by transplantation of human HSCs alone, these Tregs develop in the mouse thymus and the developmental process may not fully recapitulate that of normal human Tregs [61]. We therefore investigated human Treg development in humanized mice grafted with fetal thymic tissue and HSCs [20]. CD25+CD127low thymocytes were present at similar percentages among CD4 single positive thymocytes in thymic grafts of the humanized mice and in human fetal thymic tissues. This subset of T cells expresses both Foxp3 and Helios, which are typical markers for human natural Tregs. Like the Tregs in fetal thymus, thymus-derived Tregs from humanized mice express HLA-DR and are mainly CD45RA− and CD45RO+ [20]. These data demonstrate that human Tregs develop normally in the thymic grafts of the humanized mice. Tregs were also detected in multiple tissues of humanized mice, including peripheral blood, spleen and lymph nodes. Despite the similarity of Tregs in the thymus grafts, there are differences between Tregs in the PBMCs of humanized mice and those in adult human PBMCs. A greater percentage of CD4+CD25+Foxp3+Tregs showed a naïve phenotype (CD45RA+CD45RO−) in the PBMCs of humanized mice compared to human PBMCs and the percentage of HLA-DR+ Tregs was lower in the PBMCs of humanized mice. However, a subset of peripheral Tregs in humanized mice expressed CD45RO and HLA-DR, suggesting that post-thymic encounter with self MHC-peptide complexes on human APCs had taken place. The lower percentage of these “activated” Tregs in humanized mice compared to adult PBMCs might be explained simply by the difference in age of the human immune system in each type of host and/or by the greater exposure of humans to microorganisms compared to the mice. Functional assays demonstrated that Tregs isolated from spleens of humanized mice are as potent on a “per cell” basis as those from human peripheral blood in suppressing anti-CD3-induced proliferation of CD4+CD25− conventional T cells [20].
7 Concluding Remarks
In summary, insights into human T cell development have been obtained using humanized mice generated by transplantation of autologous fetal thymic tissue and HSCs. These studies collectively indicate that thymocyte development, including positive selection , negative selection and generation of Tregs occur in human thymus grafts and that humanized mice generated by transplantation of autologous fetal thymic tissue and HSCs (Fig. 11.3) are of considerable utility for the study of human T cell development in both physiological and pathological conditions. In addition to allowing the detailed investigation of normal human T cell development, this model has enabled studies of human T cell homeostasis and provides a model for testing therapeutic strategies to induce tolerance for the treatment of human diseases, such as thymus transplantation to induce tolerance to pig xenoantigens for xenogeneic organ transplantation. Moreover, this model can be further optimized by using genetic manipulation. For example, transplantation of thymic tissue and TCR gene-transduced autologous CD34+HSCs enables the study of the development of antigen-specific T cell clones. While humanized mice generated by grafting of fetal tissues allows the investigation of human T cell development in general, the “PI” humanized mouse model makes it possible to explore the roles of abnormalities of T cell development in the pathogenesis of immune-mediated diseases, such as autoimmune diseases. The “PI” humanized mice thus provide a novel and powerful tool to shed light on human diseases and in which to test immunotherapies in a personalized fashion.
Positive and negative selection of T cells and development of CD4 + CD25 + Foxp3 natural Tregs in humanized mice generated by grafting autologous human fetal thymic tissue and fetal liver-derived CD34 + cells. NSG mice are conditioned by 2 Gy total body irradiation (TBI) followed by implantation of human fetal thymic tissue under the kidney capsule and injection of autologous fetal liver-derived CD34 + cells. Human lymphoid progenitors home to the autologous thymic tissue and undergo selection and maturation, resulting in robust thymopoiesis. Positive selection, which is mediated by autologous cortical thymic epithelial cells expressing HLA molecules, allow conventional αβ T cells to optimally recognize peptides presented by autologous HLA molecules expressed on peripheral antigen-presenting cells (APCs), permitting T cells to respond to antigen, provide help to B cells and undergo homeostatic proliferation. Negative selection in the thymus is mediated by autologous human medullary thymic epithelial cells, autologous human APCs in the thymic graft and mouse bone marrow-derived APCs. This negative selection results in a human T cell repertoire that is tolerant to both human tissue donor and the recipient mouse. CD4 + CD25 + Foxp3 natural Tregs are generated in human thymic tissue. Their interactions with autologous APCs in the periphery may lead to further maturation
References
Mecklenburg L, Tychsen B, Paus R. Learning from nudity: lessons from the nude phenotype. Exp Dermatol. 2005;14(11):797–810.
Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11(8):544–9.
Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–62.
Joller N, Peters A, Anderson AC, Kuchroo VK. Immune checkpoints in central nervous system autoimmunity. Immunol Rev. 2012;248(1):122–39.
Moran AE, Hogquist KA. T-cell receptor affinity in thymic development. Immunology. 2012;135(4):261–7.
Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–9.
Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood. 2005;106(5):1565–73.
Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti J-C, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7.
Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180(5):1817–27.
Tary-Lehmann M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992;175(2):503–16.
Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu T-D, et al. Expression of HLA class II molecules in humanized NOD. Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS ONE. 2011;6(5):e19826.
Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γcnull (NOG) mice (hu-HSC NOG mice). Int Immunol. 2009;21(7):843–58.
McCune J, Namikawa R, Kaneshima H, Shultz L, Lieberman M, Weissman I. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–9.
Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172(4):1055–63.
Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109(2):836–42.
Takenaka K, Prasolava TK, Wang JCY, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–23.
Lan P, Tonomura N, Shimizu A, Wang S, Yang Y-G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–92.
Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964–9.
Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang Y-G, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184(12):6756–65.
Onoe T, Kalscheuer H, Danzl N, Chittenden M, Zhao G, Yang Y-G, et al. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J Immunol. 2011;187(7):3895–903.
Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–22.
Tonomura N, Habiro K, Shimizu A, Sykes M, Yang Y-G. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111(8):4293–6.
Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83(14):7305–21.
Hongo D, Hadidi S, Damrauer S, Garrigue V, Kraft D, Sachs DH, et al. Porcine thymic grafts protect human thymocytes from HIV-1-induced destruction. J Infect Dis. 2007;196(6):900–10.
Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, et al. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4(125):125ra30.
Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1(1):31–40.
Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21(1):139–76.
Capone M, Romagnoli P, Beermann F, MacDonald HR, van Meerwijk JPM. Dissociation of thymic positive and negative selection in transgenic mice expressing major histocompatibility complex class I molecules exclusively on thymic cortical epithelial cells. Blood. 2001;97(5):1336–42.
Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383(6595):81–5.
Lo W-L, Allen P. Self-peptides in TCR repertoire selection and peripheral T cell function. Curr Top Microbiol Immunol. 2014;373:49–67.
Krogsgaard M, Juang J, Davis MM. A role for “self” in T-cell activation. Semin Immunol. 2007;19(4):236–44.
Zhao Y, Fishman JA, Sergio JJ, Oliveros JL, Pearson DA, Szot GL, et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J Immunol. 1997;158(4):1641–9.
Zhao Y, Sergio JJ, Swenson K, Arn JS, Sachs DH, Sykes M. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol. 1997;159(5):2100–7.
Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2(11):1211–6.
Zhao Y, Swenson K, Sergio JJ, Sykes M. Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. J Immunol. 1998;161(3):1320–6.
Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162(6):3402–7.
Shimizu I, Fudaba Y, Shimizu A, Yang Y-G, Sykes M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation. 2008;86(4):601–10.
Alves NL, Huntington ND, Rodewald H-R, Di Santo JP. Thymic epithelial cells: the multi-tasking framework of the T cell “cradle”. Trends Immunol. 2009;30(10):468–74.
van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15(5):214–7.
van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183(12):7645–55.
Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Plet A, et al. Autonomous and extrinsic regulation of thymopoiesis inhuman immune system (HIS) mice. Eur J Immunol. 2011;41(10):2883–93.
Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192(4):F9–14.
Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–34.
Lo W-L, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10(11):1155–61.
Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang H-Q, et al. Cutting edge: Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174(6):3158–63.
Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol. 2013;31(1):31–50.
Kalscheuer H, Onoe T, Dahmani A, Li H-W, Hölzl M, Yamada K, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192(7):3442–50.
Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209(1):290–6.
Greenblatt MB, Vbranac V, Tivey T, Tsang K, Tager AM, Aliprantis AO. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS ONE. 2012;7(9):e44664.
Lee LA, Gritsch HA, Sergio JJ, Arn JS, Glaser RM, Sablinski T, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A. 1994;91(23):10864–7.
Rodriguez-Barbosa JI, Zhao Y, Barth R, Zhao G, Arn JS, Sachs DH, et al. Enhanced CD4 reconstitution by grafting neonatal porcine tissue in alternative locations is associated with donor-specific tolerance and suppression of preexisting xenoreactive T cells. Transplantation. 2001;72(7):1223–31.
Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of [alpha]1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–4.
Habiro K, Sykes M, Yang YG. Induction of human T-cell tolerance to pig xenoantigens via thymus transplantation in mice with an established human immune system. Am J Transplant. 2009;9(6):1324–9.
Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18(1):423–49.
Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45.
Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–65.
Bettini ML, Vignali DAA. Development of thymically derived natural regulatory T cells. Ann N Y Acad Sci. 2010;1183(1):1–12.
Yuan X, Malek TR. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum Immunol. 2012;73(8):773–82.
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–53.
Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+ CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31(4):1247–54.
Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+ FoxP3+ regulatory T cells in human stem cell factor–, granulocyte-macrophage colony-stimulating factor–, and interleukin-3–expressing NOD-SCID IL2Rγnull humanized mice. Blood. 2011;117(11):3076–86.
Acknowledgments
We thank Shavree Washington for assistance with the manuscript. The work of the authors discussed in this chapter was supported by the following NIH grants: RO1AI084074 (to Sykes), RC1HL100117 and R01 AI064569 (to Yang) and P01AI045897 (to Sykes and Yang) and JDRF grant 1-2007-1057 (to Sykes).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Li, H., Yang, YG., Sykes, M. (2014). Thymic Education of Human T Cells and Regulatory T Cell Development in Humanized Mice. In: Poluektova, L., Garcia, J., Koyanagi, Y., Manz, M., Tager, A. (eds) Humanized Mice for HIV Research. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1655-9_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1655-9_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1654-2
Online ISBN: 978-1-4939-1655-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)