Abstract
Multiple sclerosis (MS) is a disease with a resilient inflammatory component caused by accumulation into the CNS of inflammatory infiltrates and macrophage/microglia contributing to severe demyelination and neurodegeneration. While the causes are still in part unclear, key pathogenic mechanisms are the direct loss of myelin-producing cells and/or their impairment caused by the immune system. Proposed etiology includes genetic and environmental factors triggered by viral infections. Although several diagnostic methods and new treatments are under development, there is no curative but only palliative care against the relapsing-remitting or progressive forms of MS. In recent times, there has been a boost of awareness on the role of histamine signaling in physiological and pathological functions of the nervous system. Particularly in MS, evidence is raising that histamine might be directly implicated in the disease by acting at different cellular and molecular levels. For instance, constitutively active histamine regulates the differentiation of oligodendrocyte precursors, thus playing a central role in the remyelination process; histamine reduces the ability of myelin-autoreactive T cells to adhere to inflamed brain vessels, a crucial step in the development of MS; histamine levels are found increased in the cerebrospinal fluid of MS patients. The aim of the present work is to present further proofs about the alliance of histamine with MS and to introduce the most recent and innovative histamine paradigms for therapy. We will report on how a long-standing molecule with previously recognized immunomodulatory and neuroprotective functions, histamine, might still provide a renewed and far-reaching role in MS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Clinical trials
- Demyelination
- Drug therapy
- Experimental autoimmune encephalomyelitis
- Histamine
- Multiple sclerosis
1 The Histamine System
Heterogeneous genetic and molecular mechanisms contribute to multiple sclerosis (MS), a disabling central nervous system (CNS) disease causing permanent deterioration of axons. These gradually lose their myelin ensheathment because of an autoimmune reaction to myelin, caused by T lymphocytes entering the brain after blood-brain-barrier (BBB) injury. Signs and symptoms of MS are very variable and depend on the extent and exact location of axonal damage. While there is no cure for MS, pharmacological treatments can modulate disease progression, manage the symptoms, and accelerate recovery (Derdelinckx et al. 2021; McGinley et al. 2021).
The potent physiological actions of the hydrophilic vasoactive histamine have been identified a while ago by Sir Henry Dale and Patrick Laidlaw (Dale and Laidlaw 1910). Over the years, histamine has proven to be a master molecule in pharmacology and immunology, with two Nobel Prizes awarded for the identification of anti-H1R and anti-H2R antagonists. In addition to their use for allergic, gastric, and immune disorders, histamine drugs have entered clinical testing for obesity, neurological disorders, and memory [for reviews (Hu and Chen 2017; Ghamari et al. 2019; Volonté et al. 2019; Provensi et al. 2020a, b)].
Histamine is obtained exclusively from decarboxylation of the amino acid histidine, a reaction catalyzed by l-histidine decarboxylase (HDC, encoded in humans by HDC gene, generating an active homodimer of 54 kDa per unit), which is localized in the intracellular compartment of specific cell phenotypes. Major histamine producing cells are: (1) mast cells (resident cells of connective tissue that contain many histamine and heparin granules, a sort of granulocytes derived from myeloid stem cells and part of the immune and neuroimmune systems); (2) basophil (the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells); (3) enterochromaffin-like cells (a type of neuroendocrine cell found beneath the epithelium of gastric mucosa gland cells and contributing to the production of gastric acid via the release of histamine); (4) and histaminergic neurons (histamine releasing neurons present exclusively in the tuberomammillary nucleus of the posterior hypothalamus, and involved in the control of arousal, learning, memory, sleep, and energy balance). Minor histamine producing cells are also dendritic, T cells, macrophages/microglia, neutrophils, monocytes, platelets, and epithelial cells (Huang et al. 2018; Thangam et al. 2018).
Once synthetized intracellularly, histamine can be stored in intracellular granules/vesicles, released extracellularly, and/or rapidly metabolized by its primary degradative enzymes. The vesicular monoamine transporter-2 is responsible for loading monoamines, among them also histamine, into secretory vesicles (Schafer et al. 2013). Histamine degrading enzymes are histamine-N-methyltransferase (HNMT, encoded by HNMT gene in humans, generating a 33 kDa protein) catalyzing the methylation of histamine, and diamine oxidase (DAO, encoded in humans by AOC1 gene, generating a homodimer of theoretical 73 kDa per unit) catalyzing oxidative deamination, an enzyme whose shortage in the human body causes allergy or histamine intolerance (Comas-Basté et al. 2020). The HNMT enzyme resides in the cytosol, whereas DAO metabolizes extracellular free histamine. Synthesis, storage in granules/vesicles, degradation, and release of histamine are highly regulated mechanisms, under the control of a plethora of different extracellular and intracellular signals among them trophic factors, hormones, transmitters, and various stressors, thus rendering the histaminergic system a complex sensor and effector of cellular and environmental modifications (Haas et al. 2008).
Active extracellular release of histamine from granules/vesicles occurs mainly by wide diffusion through a concentration gradient and slow transmission mechanisms, being mediated: (1) by IgE/antigen crosslinking, complement activation, or the presence of allergens in mast cells and basophils (Borriello et al. 2017); (2) by somatostatin- or gastrin-dependent activation in enterochromaffin-like cells (Barocelli and Ballabeni 2003); (3) by activation of N-methyl-D-aspartate, U opioid, D2 dopamine, or serotonin receptors in histaminergic neurons (Haas et al. 2008). The actions of released histamine are terminated not only by DAO (see above), but also by the cellular reuptake system through specific monoamine transporters such as serotonin, dopamine, and norepinephrine transporters, i.e. Na+- and Cl−-dependent high affinity transporters, defined as uptake-1 system, and through Na+- and Cl−-independent low affinity, high-capacity uptake-2 system transporters (Slamet Soetanto et al. 2019).
In the extracellular space, histamine exerts its effects by primarily binding to the 7-transmembrane rhodopsin-like family of G protein-coupled receptors classified as H1R, H2R, H3R, and H4R, respectively, encoded in humans by HRH1, HRH2, HRH3, and HRH4 genes (Haas et al. 2008). Recently, histamine was also shown to activate ligand-gated chloride channels in the brain and intestinal epithelium (Panula et al. 2015).
In eukaryotic cells, H1R is found in smooth and cardiac muscles, vascular endothelial cells, and in the CNS. The downstream pathways activated after binding of histamine to H1R are Gq protein, phospholipase C leading to inositol triphosphate-dependent release of calcium from intracellular stores, and diacylglycerol formation with modulation of voltage-dependent calcium channels (Leurs et al. 2002). As a key regulator of inflammatory processes, NF-κB expression and downstream pathways are tightly controlled by activation or constitutive activity of H1R in target cells, to the point that H1R antagonists were shown to mitigate inflammation through NF-κB modulation (Apolloni et al. 2016). The class of molecules commonly known as H1R antihistamines and generally used to treat allergies can function as either receptor antagonists or inverse agonists at H1R, although only limited H1 antihistamines act as inverse agonists (H1 receptor. IUPHAR/BPS Guide to Pharmacology, http://www.guidetopharmacology.org). In the CNS, H1R activation induces excitatory stimulation, moreover controls nutritional state and wake–sleep cycles, and also regulates neuroinflammatory processes (Fukui et al. 2017).
H2R is present in vascular smooth muscles, where it controls muscle relaxation and vasodilation; in neutrophils, it prevents activation and chemotaxis; in T and B cells, it modulates proliferation and antigen-specific responses as antibody synthesis and cytokine production; in gastric gland cells, it stimulates gastric acid secretion (Thangam et al. 2018); not last, in mast cells enriched in histamine granules. In the CNS, the receptor is found in cerebral cortex, caudate-putamen, hippocampus, and dentate nucleus of cerebellum, playing a role in neuronal plasticity, synaptic transmission, and cognitive performance (Haas et al. 2008). H2R is positively coupled to adenylate cyclase through activation of Gs protein that induces cyclic adenosine monophosphate production, protein kinase A activation, and phosphorylation of target proteins.
H3R is mainly expressed in cortical and subcortical areas of the CNS (being involved in cognitive processes, wakefulness, and eating behaviors) and, to a lesser extent, in the peripheral nervous system (Nieto-Alamilla et al. 2016), other than in the heart, lung, and gastrointestinal tract and endothelial cells. H3R shows very little sequence homology with H1R and H2R. Differently from the other histamine receptors, H3R has the peculiarity to act as autoreceptor in presynaptic histaminergic neurons and to feedback regulate the turnover of histamine by inhibiting its synthesis and release. This inhibitory activity is exerted also on presynaptic dopamine, gamma-aminobutyric acid, glutamate, noradrenaline, serotonin, and acetylcholine receptors, thus H3R also behaves as inhibitory heteroreceptor (Panula et al. 2015). Consequent to histamine binding to H3R, the first downstream effector to become activated is Gi protein, causing inhibition of cyclic AMP production. Through inhibition of N-type voltage-gated Ca2+ channels mediated by β and γ subunits of G proteins, H3R also inhibits Ca2+ uptake mediated by action potentials, thus further reducing neurotransmitter release.
In humans, H4R is a receptor subtype mainly present peripherally in oral epithelium, bone marrow, and leukocytes, where it regulates neutrophils release from bone marrow, eosinophil shape change, and mast cells chemotaxis, through modulation of actin polymerization and cytoskeleton stability. It operates through Gαi-dependent inhibition of adenylate cyclase and Gβγ-dependent stimulation of phospholipase C, leading to inositol triphosphate and diacylglycerol formation, Ca2+ mobilization from intracellular stores, and activation of protein kinase C (Thurmond 2015).
Histamine is an important pleiotropic factor actively participating in multiple physiological functions such as neurotransmission, circadian rhythms, sleep–wake cycle, mood, learning, appetite, and eating behavior. Moreover, histamine levels are modulated in the CNS as a function of age, sex, and disease insurgence and progression. Histamine deficiency is related to narcolepsy, food intake and sleep disorders, and to neuropsychiatric conditions comprising schizophrenia and several different neurodegenerative/neuroinflammatory diseases (Cacabelos et al. 2016a, b). Several studies have described the histaminergic system as directly involved in various pathological conditions of the CNS, among them ischemia, traumatic brain and spinal cord injury, Alzheimer’s, Huntington’s, Parkinson’s diseases, Wernicke’s encephalopathy, Tourette syndrome and, of course, MS. The pathophysiological relevance of central histamine signaling has thus accelerated several attempts to pharmacologically manipulate brain histamine concentrations for the treatment of various neurological disorders (Naganuma et al. 2017). We believe that further research will certainly stimulate a deeper comprehension of disease-related histaminergic mechanisms, with potential identification of histamine-dependent therapeutic opportunities.
2 From Central and Peripheral Inflammation to Myelination Defects in MS
MS is a chronic autoimmune, inflammatory, and neurodegenerative disease that affects both white and gray matter of the CNS, although historically identified as a predominantly affecting white matter disease. MS occurs within various stages and evolves as a continuum from a clinically isolated acute syndrome to a secondary-progressive disease through relapsing-remitting phases. MS is very heterogeneous indeed: in approximately 85% of patients the disease exhibits a relapsing-remitting course characterized by acute attacks followed by partial or complete recovery. Over time, many relapsing-remitting patients switch to the secondary-progressive phase, where neurological lesions and disabilities gradually accumulate even without further relapses. On the other hand, about 10–15% of patients have a progressive primary course, characterized by a continuous accumulation of neurological lesions and disabilities that already start at the insurgence of the disease (Milo and Kahana 2010).
Pathological studies described the presence of cerebral and cerebellar cortical demyelination in MS patients and led to the identification of three types of lesions: subpial, intracortical, and leukocortical (Bö et al. 2006). Cortical demyelination also present in early MS phases is topographically associated with conspicuous meningeal inflammation and may precede white matter plaques formation in MS patients, and be associated with irreversible disability and cognitive impairment (Popescu and Lucchinetti 2012). The pathogenic events that characterize MS are various and include lymphocytes infiltration through the BBB, inflammation, microglia activation, and astrocyte proliferation with consequent nerve conduction impairment, demyelination, axonal transection, and neuronal injury. These causally related events lead to plaques formation (Ciccarelli et al. 2014).
Although MS can be considered as a primary autoimmune disease [“outside-in” hypothesis (Lucchinetti et al. 2011; Malpass 2012; Baecher-Allan et al. 2018)], many scientists now doubt that inflammation and/or autoimmunity are really the unique promoters of the disease and have proposed that MS originates as a neurodegenerative disease [the “inside-out” hypothesis (Stys et al. 2012; Duffy et al. 2014)]. Although the exact etiology remains unknown, the debate on whether the immune or the nervous system initiates the disease is certainly open, and now scientists tend to consider MS a neuroimmune system disease, initiated when multiple biochemical signals triggered by neurotransmitters, neurohormones, cytokines, chemokines, and growth factors in neurons, glia and immune cells lose their homeostasis. With no doubt, MS is a disease with critically altered communication between the nervous and immune systems.
Clear evidence now suggests that the contribution of the immune system is less in the progressive than in the initial acute forms of the disease. In the progressive form, CNS resident cells as microglia and astrocytes indeed sustain a low-grade inflammation that leads to oligodendrocyte damage and neurodegeneration (Correale and Farez 2015; das Neves et al. 2020; Prinz et al. 2021).
In particular, the astrocytes highly contribute to inflammation and play a dual role in MS, characterized by both pathogenic alterations and beneficial repair, depending on the stage of the disease, the type and microenvironment of the lesion, the interaction with other cell phenotypes and several exogenous factors (Williams et al. 2007; Nair et al. 2008; Correale and Farez 2015; Amadio et al. 2017; Rao et al. 2019; das Neves et al. 2020). This dual function is documented by means of RNA sequencing, electron microscopy, immunohistochemistry, and imaging techniques that have recognized also high degrees of astrocyte heterogeneity. Indeed, astrocytes are a diversified population of cells possessing specific properties and functions according to their localization and pathophysiological state (Khakh and Deneen 2019; Linnerbauer et al. 2020; Escartin et al. 2021; Schirmer et al. 2021; Werkman et al. 2021).
In addition, the brain-resident immune cells, microglia, exhibit high heterogeneity in MS, contributing to both damage and repair events (Tsouki and Williams 2021). Not surprisingly, one of the pathological hallmarks of MS is the infiltration of microglia into CNS lesions, where they become the first responders and remain within the lesions until they heal the damaged tissue, or until the damage becomes irreversible and the lesion inactive. As for astrocytes, microglia are conditioned by the microenvironment, the anatomical location of the lesion, and the presence or absence of remyelination during the different stages of MS (Guerrero and Sicotte 2020; Pons and Rivest 2020; Zia et al. 2020).
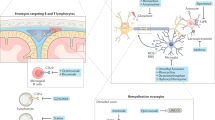
Also mast cells generally associated with allergic reactions are crucial players of the innate immune system and involved in autoimmune diseases and particularly MS. First of all, brain mast cells are located in the perivascular space where they can secrete various pro-inflammatory and vasoactive molecules able to further weaken an already damaged BBB during MS. Second, several neural factors including substance P, myelin basic protein, and corticotropin-releasing hormone can induce mast cells to release inflammatory mediators during MS. Finally, mast cells can directly participate to inflammation and demyelination in MS by presenting myelin antigens to T cells and permitting inflammatory cells and cytokines to enter through the BBB. Not surprisingly, compounds blocking mast cells can reduce T cell stimulation and EAE (Theoharides et al. 2008; Conti and Kempuraj 2016; Elieh-Ali-Komi and Cao 2017).
3 An Overview of Histamine Preclinical Studies
Different features of MS among them inflammation, demyelination, remyelination, and neurodegeneration have been studied using different animal models, none of which, however, covers the full spectrum of clinical, pathological, or immunological characteristics of the disease. So, the right model for MS research needs to be selected at each time, depending on the specific aspects to be addressed (Lassmann and Bradl 2017). The experimental autoimmune encephalomyelitis (EAE) mouse model and the cuprizone/rapamycin toxic demyelination (useful to investigate only mechanisms of protection and repair, but not inflammation) are among the most frequently used models to study MS. However, EAE can be induced in all vertebrates with different degrees of efficacy, frequently using mice, rats, and primates. Because the EAE models share several histopathological and immunological features with MS, their use has allowed to dissect the pathogenic mechanisms of relapsing-remitting and progressive forms of the disease, proving to be excellent systems for preclinical experimentation as well (Schreiner et al. 2009).
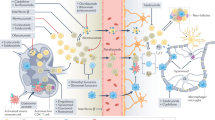
As described above, histamine is a ubiquitous inflammatory mediator involved in the pathogenesis of various allergic, autoimmune, inflammatory, and neurodegenerative diseases (Hu and Chen 2017; Branco et al. 2018). For this reason, numerous studies were conducted also on the involvement of histamine in EAE, overall demonstrating that the histaminergic system indeed plays a significant role in MS pathogenesis (Panula and Nuutinen 2013). However, the interaction of histamine with its cell surface receptors can induce either detrimental or beneficial actions in the context of EAE, often with multiple and contrasting effects depending on the specifically activated receptors and target tissues (Passani and Ballerini 2012). For instance, histamine can modify the BBB permeability and increase the number of infiltrated cells in the CNS, therefore inducing a process of deleterious neuroinflammation. On the other hand, histamine can sustain a protective role in MS and EAE by reducing demyelination and improving remyelination (Jadidi-Niaragh and Mirshafiey 2010). Through the years, thanks to the use of genetically modified mice deprived of histaminergic enzymes or receptors, and the availability of relatively selective agonists and antagonists, scientists have identified and characterized the direct involvement of histamine and its receptors in EAE/MS (Fig. 1).
The dual effect of histamine in improving or worsening EAE pathological features is due to several properties of four histamine receptors. Genetic or pharmacological inhibition (HR-), as well as presence or activation (HR+) of H1-4R receptors can induce different courses of the disease. H3R is the only receptor playing an overall antipathogenic role
3.1 Targeting H1 Receptor
A key mechanism in the development of EAE and MS is the breakdown of the BBB. Sensitization of the endothelium by environmental factors such as Bordetella pertussis or biogenic amines such as histamine is believed to lead to increased permeability of the BBB. Pertussis-induced histamine sensitization is an intermediate phenotype of EAE controlled by the histamine receptor H1R. Ma and collaborators have shown that susceptibility to pertussis-induced histamine sensitization and EAE needs expression of Hrh1, the gene encoding H1R. Indeed, the authors observed a decreased EAE susceptibility in H1R-KO mice (Ma et al. 2002), while the expression of H1R in T cells is instead disease promoting (Noubade et al. 2007).
To highlight the cell-specific effects of the Hrh1 in the pathogenesis of EAE and to optimize any cell phenotype-specific therapeutic intervention, Saligrama and collaborators re-expressed H1R in CD11b+ cells of H1R-KO mice, in order to test the hypothesis that H1R signaling in CD11b+ monocytes, macrophages/microglia, and natural killer cells might contribute to EAE susceptibility. Unpredictably, the re-expression of H1R exclusively in CD11b+ cells did not restore EAE severity and affect T cell responses in H1R-KO mice (Saligrama et al. 2012b). The propathogenic role of H1R was also confirmed in additional work in which drug treatment with the H1R antagonist hydroxyzine (known to block mast cells) or pyrilamine reduced clinical severity and pathology in EAE rats and mice, respectively (Dimitriadou et al. 2000; Pedotti et al. 2003).
In contrast, the selective transgenic overexpression of H1R in endothelial cells of Hrh1-KO mice demonstrated that these mice were resistant to Bordetella pertussis-induced histamine sensitization, also having reduced permeability of the BBB and greater protection from EAE than H1R-KO mice. This suggested that endothelial H1R may be important for sustaining cerebrovascular integrity (Lu et al. 2010).
3.2 Targeting H2 Receptor
Gene targeting studies established that also H2R plays significant roles in EAE/MS pathogenesis and vulnerability. Similarly to H1R, H2R appears to have a propathogenic role, but in addition H2R induces a beneficial restraint of the autoimmune response, thus assuming a concomitant antipathogenic action. In particular, in H2R-KO mice the attenuation of Th1 effector cells with decreased susceptibility to acute early-phase EAE compared to wild-type mice is due to dysregulation of cytokine production by antigen presenting cells (Teuscher et al. 2004). By breeding transgenic mice expressing H2R exclusively in T cells, Saligrama and collaborators (Saligrama et al. 2014) have extended the previous study determining that T-cell intrinsic H2R signaling is necessary and sufficient to re-establish EAE susceptibility as in wild-type control mice, as previously observed also with H1R (Noubade et al. 2007). Furthermore, the results demonstrated that EAE severity and neuropathology in H2R-KO mice expressing H2R exclusively in T cells become the same as in wild-type mice, only when adjuvant pertussis toxin modeling environmental factors and susceptibility to disease is used to induce EAE (Saligrama et al. 2014). This proves that unlike H1R, the H2R is also linked to inhibition of inflammatory states, thus possessing also an antipathogenic role. H2R was previously found on monocytes and associated with the suppression of superoxide formation via inhibition of NADPH oxidase (Burde et al. 1990), an enzyme highly involved in the development of EAE (van der Veen et al. 2000). A second possible mechanism for the antipathogenic role of H2R is the inhibition of the production of pro-inflammatory cytokines involved in EAE insurgence. Activation of H2R decreases TNF-α production by inflammatory cells and suppresses IL-12 expression (Vannier et al. 1991; Azuma et al. 2001). For these reasons, treatment with the H2R agonist dimaprit reduces clinical severity and pathology associated with EAE in both C57BL/6 and iNOS deficient EAE mice (Emerson et al. 2002).
3.3 Targeting H3 Receptor
The H3R, unlike the other histaminergic receptors, is not present on hematopoietic cells, but mainly in the CNS (Passani et al. 2011). In 1983, Arrang and collaborators identified the H3R as an autoreceptor that controls the activities of histaminergic neurons such as histamine production, release and electrophysiological response (Arrang et al. 1983). In addition, behaving as a presynaptic heteroreceptor, H3R regulates the release of a variety of other neurotransmitters (Passani and Blandina 2011), thus rendering this receptor a fundamental player at the crossover of central neurotransmission.
Teuscher and co-workers established the antipathogenic role of H3R in EAE pathology (Teuscher et al. 2007). H3R-KO mice develop more severe EAE and neuroinflammation. This result is associated with dysregulation of BBB permeability and increased expression of chemokines/chemokine receptors on peripheral T cells facilitating their entrance into the CNS. H3R effects on EAE seemed to be related both to neurogenic control of cerebrovascular tone and to alterations of immune cells that however do not express H3R. The authors suggested that the lack of presynaptic inhibition in H3R-KO mice leads to increased release of neurotransmitters and augmented postsynaptic activity that performs a neurogenic control of BBB permeability and T cell chemokine profile (Teuscher et al. 2007). Consequently, activation of H3R may be a potential strategy to treat MS/EAE. Indeed, further studies proved that a strong and highly selective histamine H3R agonist, immethridine, could alleviate the severity of EAE when used in EAE mouse model (Shi et al. 2017). EAE mice treated with immethridine showed lower clinical scores and reduced pathology with respect to control EAE mice. Fewer inflammatory infiltrates and decreased demyelination in spinal cord were also reported. In addition, reduced levels of inflammatory cytokines such as TNFα, IFN-γ, and IL-17A were observed in splenocytes isolated from EAE mice treated with immethridine, thus suggesting a widespread action of this agonist in improving the severity of EAE. Later studies reported that in immethridine-treated EAE mice compared to control EAE the percentage of Th1 and Th17 cells were decreased, and surface molecules such as CD40, CD86, and MHCII were downregulated on dendritic cells thus inhibiting their function (Shi et al. 2017).
Furthermore, an antipathogenic role of H3R has been confirmed in a very recent work in which two new soluble piperidine derivatives acting as histamine H3R antagonists/inverse agonists reduce the lymphocyte numbers and diminish disease symptoms in EAE (Imeri et al. 2021).
3.4 Targeting H4 Receptor
Expression of H4R is mostly restricted to T and B cells, monocytes, eosinophils, dendritic and natural killer cells, therefore playing an important role in the modulation of the immune system. Not surprisingly, this selective localization suggested therapeutic use in inflammatory disorders and autoimmune diseases (Zampeli and Tiligada 2009). However, evidence also demonstrated the topological and functional localization of H4R in human and rodent CNS (Strakhova et al. 2009).
In light of these findings, del Rio and collaborators investigated the potential role of H4R in MS, by inducing EAE in H4R-KO mice and demonstrating that the presence of H4R elicits an antipathogenic role and modulates EAE severity. In addition, H4R signaling exerts control over the abundance of regulatory T cells in secondary lymphoid tissues, regulates their chemotaxis and suppressive ability. H4R-KO mice exhibit augmented neuroinflammation, increased BBB permeability, and more severe EAE compared with wild-type mice. Consistent with this, H4R deficiency leads to lower infiltration of regulatory T cells into the CNS during the acute phase of the disease, causing impairment of anti-inflammatory responses in association with increased encephalitogenic Th17 cells (del Rio et al. 2012).
These data were corroborated by Ballerini and co-authors, which demonstrated that the H4R antagonist JNJ7777120 administrated to EAE mice caused increased inflammation and demyelination in spinal cord, augmented expression of IFN-γ and suppression of IL-4 and IL-10 in lymph nodes, with a general worsening of disease symptoms, thus suggesting a protective role of this receptor in the context of EAE (Ballerini et al. 2013). Despite this antipathogenic action, conflicting results were reported on its propatogenic/antipathogenic role during immune and allergic responses, with a growing interest in the therapeutic anti-inflammatory potential of H4R antagonists in the immune system, where the H4R is ubiquitous. In light of these results, there is urgent need to further investigate this receptor to anticipate potential clinical benefits and/or predict possible deleterious effects (Passani and Ballerini 2012).
3.5 Targeting HDC
Due to the overlapping but often opposite functions played by the different histamine receptors in the presence of endogenous histamine, single receptor-blocking strategies cannot always attain complete elimination of histamine signaling in vitro; similarly, it is difficult to achieve complete and long-lasting inhibition of histamine receptors using pharmacological or genetic approaches in vivo (Ohtsu et al. 2001). Therefore, HDC-deficient mice were generated to provide a more exhaustive model in which to ablate endogenous histamine synthesis and study biological responses in the CNS.
In particular, Musio and co-workers using HDC-KO mice investigated the effect of endogenous histamine removal in the insurgence and progression of EAE. They established that EAE pathology is significantly more severe in histamine-deficient mice, showing diffuse inflammatory infiltrates with a prevalent granulocytic component in the brain and cerebellum. In particular, splenocytes from HDC-KO mice do not produce histamine in response to myelin antigen immunization, but secrete increased amounts of pro-inflammatory cytokines, such as IFN-γ, TNF, and leptin. Therefore, endogenous histamine notably restrains the harmful autoimmune response against myelin and immune impairment in the CNS (Musio et al. 2006).
These results were confirmed by Saligrama and collaborators, who studied the function of endogenous histamine on EAE susceptibility in H1-4R-KO and HDC-KO mice, both deficient in histamine signaling. Surprisingly, H1-4R-KO mice were found to be significantly resistant to EAE, whereas HDC-KO mice were highly susceptible. H1-4R-KO mice develop less severe neuropathological conditions and EAE symptoms than wild-type and HDC-KO mice. Furthermore, splenocytes from immunized H1-4R-KO mice produce lower amounts of Th1/Th17 effector cytokines. Overall, these findings suggest that histamine can mediate increased resistance to EAE also acting through mechanisms independent from its known receptors (Saligrama et al. 2013).
3.6 Combinatorial Histamine Receptor Actions
The previous results, in addition to the pleiotropism of histamine, created some difficulties in the comprehension of the exact pathophysiological roles of histamine in EAE, further complicated by the simultaneous recruitment on the same cell of different histamine receptors that sometimes play different, or even opposite, functions. While the selective combinatorial expression of different receptors subtypes on a given cell can contribute to explain diverse pathological conditions (Volonté et al. 2006, 2008), a similar mechanism can also apply to EAE/MS. In trying to explain the accurate and peculiar alliance between histamine and MS, a study indeed suggested that combinatorial targeting of histamine receptors may be an effective disease-modifying therapy in MS. H3H4R-KO EAE mice developed a significantly more severe clinical disease course than control or H1H2R-KO EAE mice. Furthermore, histopathological analysis demonstrated increased inflammation and pathology in the brain, but not spinal cord, of H3H4R-KO mice that moreover exhibited augmented BBB permeability during the acute early phase of the disease, compared to H1H2R-KO mice. These data indicate that the joined effect of deleting H1R and H2R signaling becomes antipathogenic in EAE, whereas the combined H3R and H4R ablation is propathogenic. It’s important to notice that EAE severity and pathology in H1H2R-KO and H3H4R-KO mirrors that of the single HR-KO mice, where EAE is less severe in H1R-KO and H2R-KO mice, but more pronounced in H3R-KO and H4R-KO mice. This might occur because of a compensatory upregulation of residual HRs in single HR-KO, H1H2R-KO, and H3H4R-KO mice. As a consequence, simultaneous treatment with H1R and H2R antagonists may be protective in the CNS, perhaps due to the upregulation of the antipathogenic H3R and H4R. On the other hand, the absence of H3R or H4R signaling has a negative effect on EAE susceptibility and encephalitogenic T-cell activity, suggesting that H3R and H4R agonists might have a beneficial impact in the treatment of CNS diseases by intervening on histamine signaling through the propathogenic H1R and H2R subtypes (Saligrama et al. 2012a).
So far, we have reported that genetically modified mice lacking single or different combinations of histaminergic enzymes or receptors, together with fairly selective histamine receptor agonists and antagonists, have greatly contributed to identify and in part characterize the involvement of histaminergic signaling in EAE/MS. To allow more successful preclinical development of therapeutic strategies against MS, it is mandatory to have access to more extensive data on the disease progression in animal models, to broader understanding of disease pathology, and to more robust outcome measures that can be used to assess treatment efficacy. We are confident that renewed experimental interest and research on histaminergic axis and mechanisms in EAE/MS will contribute to fill this gap.
4 Histamine Markers in Biological Fluids and CNS Tissues from MS Patients
Discovering reliable and early biomarkers is of fundamental importance in neurological disorders where the diagnosis often occurs after the onset of symptoms, mainly due to lack of specific markers that allow discriminative diagnosis for different pathologies. Significant variations in the peripheral and central levels of histamine have been detected in patients with neurological diseases (Cacabelos et al. 2016a) and histamine levels have been extensively investigated in biological fluids of MS patients as well.
The first study aimed to evaluate histamine-related changes in MS reported that in the cerebrospinal fluid (CSF) of a small cohort of patients with remitting and progressive forms of the disease, histamine levels were about 60% higher than in controls, and the patients showed a concomitant decrease in histamine-degrading enzyme HNMT, indicating an altered histamine metabolism in the CNS during MS (Tuomisto et al. 1983). Furthermore, a significant increase of the mast cell-specific proteolytic enzyme tryptase (an enzyme released with histamine when mast cells are activated as part of immune responses or allergic hypersensitivity) was found in the CSF of MS patients with respect to control subjects or patients affected by other neurological diseases, suggesting that the activation of mast cell was a pathological feature of MS (Rozniecki et al. 1995). In accordance with these observations, in a recent trial enrolling 36 MS patients and 19 age- and gender-matched healthy volunteers, histamine content was found significantly higher in the CSF of MS patients. Remarkably, the authors demonstrated that histamine levels further increased with age in the CSF of patients (Kallweit et al. 2013). Finally, gene-microarray analysis has shown that H1R expression is upregulated in acute MS lesions (Lock et al. 2002). Independently from the relative up or down values of expression, the overall modulated levels of central histamine found in MS patients could be correlated with enhanced inflammatory response known to contribute to the onset and progression of the disease.
Interestingly, in the serum of MS patients histamine shows an opposite trend with respect to the CNS (Cacabelos et al. 2016b). In particular, the levels of both histamine and the enzyme responsible for its degradation, DAO, have been found decreased in the serum of relapsing-remitting MS patients compared to healthy individuals (Rafiee Zadeh et al. 2018). Moreover, a recent study has shown that the levels of histamine precursor histidine are lower in the serum of MS women with disabling and persistently perceived fatigue, suggesting a strong involvement of histamine also in MS-associated symptoms (Loy et al. 2019).
Finally, by analyzing the expression of HRH1, HRH2, and HRH4 genes in peripheral blood mononuclear cells derived from patients with different forms of MS, i.e. relapsing-remitting, primary-progressive, and secondary-progressive, Costanza and co-authors demonstrated that H1R transcript was significantly decreased in secondary-progressive-MS patients compared to healthy individuals and, conversely, H4R was increased in secondary-progressive-MS compared to controls and relapsing-remitting-MS, indicating a distinct involvement of histamine receptors in the different forms of the disease (Costanza et al. 2014). A synoptic view on the modulation of histamine-related markers in biospecimen from MS patients is reported in Table 1.
5 Toward a Histamine-Based Pharmacology in MS Patients
As discussed above, MS is a complex disease requiring different approaches for investigation such as prevention strategies, disease-modifying drugs, and symptomatic treatments. Recently, histamine-based pharmacology has been confirmed as a new avenue in the field of neurodegenerative diseases, especially those associated with a strong neuroinflammatory component. Particularly in MS, histamine-related molecules are getting to the root causes of the disease and are emerging as potential therapeutic strategies.
Actually, transdermal application of histamine was adopted more than 20 years ago to treat the symptoms of both relapsing-remitting and progressive MS, demonstrating efficacy in improving extremity strength, balance, fatigue in daily activities and cognitive abilities. The authors linked these effects to a rise of histamine in the CNS, leading to improved cerebral blood flow, decreased autoimmune responses, and augmented remyelination of demyelinating fibers (Gillson et al. 1999, 2000). Accordingly, as measured by the Modified Fatigue Impact Scale, the feeling of fatigue was moreover reduced in a small cohort of MS patients compared to placebo group in a 12-week trial with the therapeutic mixture Prokarin, a blend of histamine and caffeine (Gillson et al. 2002).
Regarding H1R, the antagonist hydroxyzine has shown efficacy in reducing mood symptoms in a pilot open-label clinical trial with relapsing-remitting or relapsing-progressive MS patients (Logothetis et al. 2005). Moreover, the compound AVN-101, a BBB-permeable 5-HT7 receptor antagonist with anxiolytic and anti-depressive efficacy in animal models of CNS diseases, also exhibiting high affinity for H1R, demonstrated good tolerability in a phase I MS study, thus suggesting its potential use in alleviating mood symptoms in MS (Ivachtchenko et al. 2016).
Finally, an epidemiological study aimed to associate the risk of developing MS to environmental, lifestyle factors and former pharmacological treatments demonstrated a reduced incidence of MS in patients exposed to sedating H1R antagonists, thus confirming the involvement of H1R in MS and suggesting the use of these drugs as potential targets to prevent MS (Yong et al. 2018).
H2R as well was investigated for its potential involvement in MS. In particular, H2R drugs used to treat gastric disorders were suggested to affect the activation state of the immune system (Atabati et al. 2021). In particular, activation of H2R could serve as anti-inflammatory strategy and, conversely, inhibition of H2R by antagonists commonly adopted as standard therapy for gastritis could become pro-inflammatory by over-stimulating the immune system. In line with this hypothesis, studies on MS animal models demonstrated that H2R blockers often used as antacids for treating corticosteroid-dependent dyspeptic pain in MS patients have damaging effects in accelerating the disease (Biswas et al. 2012). In addition, H2R antagonists and additional antacid drugs as proton pump inhibitors could also modify the intestinal microbiota, which may in turn activate immune responses. Confirming this hypothesis, an interesting issue concerns the activation of intestinal H2R as one of the mechanisms proposed for the beneficial effects of probiotics on the immune system during several multisystemic inflammatory diseases among them MS (Liu et al. 2018).
The finding that an exonic single nucleotide polymorphism in the HRH3 gene was linked to higher susceptibility to develop MS (Chen et al. 2017) demonstrated the strategy of directly targeting H3R as promising for improving remyelination. In particular, high expression of H3R was detected in oligodendrocytes present in demyelinated lesions of MS patients, and H3R antagonists/inverse agonists were soon identified by high-content screening assays as compounds able to stimulate the differentiation of oligodendrocyte precursor cells (OPC). Interestingly, the authors demonstrated that the expression of H3R was first upregulated and then downregulated during OPC differentiation. Remarkably, while the knockdown of HRH3 gene in OPC augmented the expression of differentiation markers and the number of mature oligodendrocytes, its overexpression exerted opposite effects, by decreasing both differentiation markers and the number of mature oligodendrocytes (Chen et al. 2017).
Furthermore, the BBB-permeable H3R inverse agonist GSK247246 reduced intracellular cyclic AMP and cAMP response element-binding protein phosphorylation in vitro, leading to improved remyelination and axonal integrity in a mouse model of demyelination induced by cuprizone/rapamycin. This result strengthens the role of H3R in promoting remyelination during MS (Chen et al. 2017). Finally, the high H3R expression in oligodendroglial cells from patients with MS presenting demyelinating lesions has validated a genetic association between an exonic single nucleotide polymorphism in HRH3 and the susceptibility to MS (Chen et al. 2017). Following this evidence, the efficacy, safety, and pharmacokinetics of another potent and brain penetrant H3R inverse agonist, GSK239512, were evaluated in patients with relapsing-remitting MS in a phase II, randomized, parallel-group, placebo-controlled, double-blind, multicenter study (NCT01772199). As measured by the magnetization transfer ratio, i.e. by magnetic resonance imaging for myelination markers, the once-daily oral dose of GSK239512, along with interferon-β1a or glatiramer acetate, demonstrated a small but positive effect on lesion remyelinating activity, with an incidence of adverse events in patients very similar to that found in the placebo group (Schwartzbach et al. 2017).
6 Concluding Remarks
Ever since the achievement of the human genome sequence, clinicians and scientists through molecular and phenotypic analysis characterizing genetic keystones of many common and rare diseases and introducing transformative-targeted therapies have obtained more refined diagnoses, rational treatments, and prevention of diseases. We are just beginning to see the fruits of these efforts also in MS. Thanks to the use of genetically modified mouse models deprived of histaminergic enzymes or receptors, and the availability of relatively selective agonists and antagonists, scientists have now recognized and started to dissect the role of histamine and its receptors in EAE/MS, as we have described in this work. However, successful clinical translation depends on the quality of preclinical findings and on the predictive value of the experimental models used in the initial drug development. Further research on the pleiotropic actions and functional validation of histaminergic signaling in the various EAE models of MS will certainly help to shed further light on the disease. Ultimately, we have also presented a bulk of information that highlights the use of histamine biomarkers to trace MS pathology and the pitfalls of successfully moving a histaminergic therapeutic strategy to the clinic.
In our quest in understanding MS, providing essential evidence for innovative treatments, and designing successful clinical trials, we trust that the data we described about the histamine alliance in MS will become an invaluable source for inspiring further research and approaches to modify the disease course.
Abbreviations
- BBB:
-
Blood brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DAO:
-
Diamine oxidase
- EAE:
-
Experimental autoimmune encephalomyelitis
- HDC:
-
l-Histidine decarboxylase
- HNMT:
-
Histamine-N-methyltransferase
- MS:
-
Multiple sclerosis
- OPC:
-
Oligodendrocyte precursor cells
References
Amadio S, Parisi C, Piras E, Fabbrizio P, Apolloni S, Montilli C et al (2017) Modulation of P2X7 receptor during inflammation in multiple sclerosis. Front Immunol 8:1529. https://doi.org/10.3389/fimmu.2017.01529
Apolloni S, Fabbrizio P, Amadio S, Volonté C (2016) Actions of the antihistaminergic clemastine on presymptomatic SOD1-G93A mice ameliorate ALS disease progression. J Neuroinflammation 13(1):191. https://doi.org/10.1186/s12974-016-0658-8
Arrang JM, Garbarg M, Schwartz JC (1983) Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 302(5911):832–837. https://doi.org/10.1038/302832a0
Atabati H, Yazdanpanah E, Mortazavi H, Bajestani SG, Raoofi A, Esmaeili SA et al (2021) Immunoregulatory effects of tolerogenic probiotics in multiple sclerosis. Adv Exp Med Biol 1286:87–105. https://doi.org/10.1007/978-3-030-55035-6_6
Azuma Y, Shinohara M, Wang PL, Hidaka A, Ohura K (2001) Histamine inhibits chemotaxis, phagocytosis, superoxide anion production, and the production of TNFalpha and IL-12 by macrophages via H2-receptors. Int Immunopharmacol 1(9-10):1867–1875. https://doi.org/10.1016/s1567-5769(01)00112-6
Baecher-Allan C, Kaskow BJ, Weiner HL (2018) Multiple sclerosis: mechanisms and immunotherapy. Neuron 97(4):742–768. https://doi.org/10.1016/j.neuron.2018.01.021
Ballerini C, Aldinucci A, Luccarini I, Galante A, Manuelli C, Blandina P et al (2013) Antagonism of histamine H4 receptors exacerbates clinical and pathological signs of experimental autoimmune encephalomyelitis. Br J Pharmacol 170(1):67–77. https://doi.org/10.1111/bph.12263
Barocelli E, Ballabeni V (2003) Histamine in the control of gastric acid secretion: a topic review. Pharmacol Res 47(4):299–304. https://doi.org/10.1016/s1043-6618(03)00009-4
Biswas S, Benedict SH, Lynch SG, LeVine SM (2012) Potential immunological consequences of pharmacological suppression of gastric acid production in patients with multiple sclerosis. BMC Med 10:57. https://doi.org/10.1186/1741-7015-10-57
Bö L, Geurts JJ, Mörk SJ, van der Valk P (2006) Grey matter pathology in multiple sclerosis. Acta Neurol Scand Suppl 183:48–50. https://doi.org/10.1111/j.1600-0404.2006.00615.x
Borriello F, Iannone R, Marone G (2017) Histamine release from mast cells and basophils. Handb Exp Pharmacol 241:121–139. https://doi.org/10.1007/164_2017_18
Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN (2018) Role of histamine in modulating the immune response and inflammation. Mediat Inflamm 2018:9524075. https://doi.org/10.1155/2018/9524075
Burde R, Buschauer A, Seifert R (1990) Characterization of histamine H2-receptors in human neutrophils with a series of guanidine analogues of impromidine. Are cell type-specific H2-receptors involved in the regulation of NADPH oxidase? Naunyn Schmiedeberg’s Arch Pharmacol 341(5):455–461. https://doi.org/10.1007/BF00176340
Cacabelos R, Torrellas C, Fernández-Novoa L, Aliev G (2016a) Neuroimmune crosstalk in CNS disorders: the histamine connection. Curr Pharm Des 22(7):819–848. https://doi.org/10.2174/1381612822666151209150954
Cacabelos R, Torrellas C, Fernández-Novoa L, López-Muñoz F (2016b) Histamine and immune biomarkers in CNS disorders. Mediat Inflamm 2016:1924603. https://doi.org/10.1155/2016/1924603
Chen Y, Zhen W, Guo T, Zhao Y, Liu A, Rubio JP et al (2017) Histamine receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS One 12(12):e0189380. https://doi.org/10.1371/journal.pone.0189380
Ciccarelli O, Barkhof F, Bodini B, De Stefano N, Golay X, Nicolay K et al (2014) Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol 13(8):807–822. https://doi.org/10.1016/S1474-4422(14)70101-2
Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, Latorre-Moratalla M, Vidal-Carou MDC (2020) Histamine intolerance: the current state of the art. Biomol Ther 10(8):1181. https://doi.org/10.3390/biom10081181
Conti P, Kempuraj D (2016) Important role of mast cells in multiple sclerosis. Mult Scler Relat Disord 5:77–80. https://doi.org/10.1016/j.msard.2015.11.005
Correale J, Farez MF (2015) The role of astrocytes in multiple sclerosis progression. Front Neurol 6:180. https://doi.org/10.3389/fneur.2015.00180
Costanza M, Di Dario M, Steinman L, Farina C, Pedotti R (2014) Gene expression analysis of histamine receptors in peripheral blood mononuclear cells from individuals with clinically-isolated syndrome and different stages of multiple sclerosis. J Neuroimmunol 277(1-2):186–188. https://doi.org/10.1016/j.jneuroim.2014.09.018
Dale HH, Laidlaw PP (1910) The physiological action of beta-iminazolylethylamine. J Physiol 41(5):318–344. https://doi.org/10.1113/jphysiol.1910.sp001406
das Neves SP, Sousa JC, Sousa N, Cerqueira JJ, Marques F (2020) Altered astrocytic function in experimental neuroinflammation and multiple sclerosis. Glia. https://doi.org/10.1002/glia.23940
del Rio R, Noubade R, Saligrama N, Wall EH, Krementsov DN, Poynter ME et al (2012) Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol 188(2):541–547. https://doi.org/10.4049/jimmunol.1101498
Derdelinckx J, Cras P, Berneman ZN, Cools N (2021) Antigen-specific treatment modalities in MS: the past, the present, and the future. Front Immunol 12:624685. https://doi.org/10.3389/fimmu.2021.624685
Dimitriadou V, Pang X, Theoharides TC (2000) Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. Int J Immunopharmacol 22(9):673–684. https://doi.org/10.1016/s0192-0561(00)00029-1
Duffy SS, Lees JG, Moalem-Taylor G (2014) The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int 2014:285245. https://doi.org/10.1155/2014/285245
Elieh-Ali-Komi D, Cao Y (2017) Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol 52(3):436–445. https://doi.org/10.1007/s12016-016-8595-y
Emerson MR, Orentas DM, Lynch SG, LeVine SM (2002) Activation of histamine H2 receptors ameliorates experimental allergic encephalomyelitis. Neuroreport 13(11):1407–1410. https://doi.org/10.1097/00001756-200208070-00012
Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Fukui H, Mizuguchi H, Nemoto H, Kitamura Y, Kashiwada Y, Takeda N (2017) Histamine H. Handb Exp Pharmacol 241:161–169. https://doi.org/10.1007/164_2016_14
Ghamari N, Zarei O, Arias-Montaño JA, Reiner D, Dastmalchi S, Stark H et al (2019) Histamine H. Pharmacol Ther 200:69–84. https://doi.org/10.1016/j.pharmthera.2019.04.007
Gillson G, Wright JV, DeLack E, Ballasiotes G (1999) Transdermal histamine in multiple sclerosis: part one -- clinical experience. Altern Med Rev 4(6):424–428
Gillson G, Wright JV, DeLack E, Ballasiotes G (2000) Transdermal histamine in multiple sclerosis, part two: a proposed theoretical basis for its use. Altern Med Rev 5(3):224–248
Gillson G, Richard TL, Smith RB, Wright JV (2002) A double-blind pilot study of the effect of Prokarin on fatigue in multiple sclerosis. Mult Scler 8(1):30–35. https://doi.org/10.1191/1352458502ms777oa
Guerrero BL, Sicotte NL (2020) Microglia in multiple sclerosis: friend or foe? Front Immunol 11:374. https://doi.org/10.3389/fimmu.2020.00374
Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88(3):1183–1241. https://doi.org/10.1152/physrev.00043.2007
Hu W, Chen Z (2017) The roles of histamine and its receptor ligands in central nervous system disorders: an update. Pharmacol Ther 175:116–132. https://doi.org/10.1016/j.pharmthera.2017.02.039
Huang H, Li Y, Liang J, Finkelman FD (2018) Molecular regulation of histamine synthesis. Front Immunol 9:1392. https://doi.org/10.3389/fimmu.2018.01392
Imeri F, Stepanovska Tanturovska B, Zivkovic A, Enzmann G, Schwalm S, Pfeilschifter J et al (2021) Novel compounds with dual S1P receptor agonist and histamine H. Neuropharmacology 186:108464. https://doi.org/10.1016/j.neuropharm.2021.108464
Ivachtchenko AV, Lavrovsky Y, Okun I (2016) AVN-101: a multi-target drug candidate for the treatment of CNS disorders. J Alzheimers Dis 53(2):583–620. https://doi.org/10.3233/JAD-151146
Jadidi-Niaragh F, Mirshafiey A (2010) Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology 59(3):180–189. https://doi.org/10.1016/j.neuropharm.2010.05.005
Kallweit U, Aritake K, Bassetti CL, Blumenthal S, Hayaishi O, Linnebank M et al (2013) Elevated CSF histamine levels in multiple sclerosis patients. Fluids Barriers CNS 10:19. https://doi.org/10.1186/2045-8118-10-19
Khakh BS, Deneen B (2019) The emerging nature of astrocyte diversity. Annu Rev Neurosci 42:187–207. https://doi.org/10.1146/annurev-neuro-070918-050443
Lassmann H, Bradl M (2017) Multiple sclerosis: experimental models and reality. Acta Neuropathol 133(2):223–244. https://doi.org/10.1007/s00401-016-1631-4
Leurs R, Church MK, Taglialatela M (2002) H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy 32(4):489–498. https://doi.org/10.1046/j.0954-7894.2002.01314.x
Linnerbauer M, Wheeler MA, Quintana FJ (2020) Astrocyte crosstalk in CNS inflammation. Neuron 108(4):608–622. https://doi.org/10.1016/j.neuron.2020.08.012
Liu Y, Alookaran JJ, Rhoads JM (2018) Probiotics in autoimmune and inflammatory disorders. Nutrients 10(10). https://doi.org/10.3390/nu10101537
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H et al (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508. https://doi.org/10.1038/nm0502-500
Logothetis L, Mylonas IA, Baloyannis S, Pashalidou M, Orologas A, Zafeiropoulos A et al (2005) A pilot, open label, clinical trial using hydroxyzine in multiple sclerosis. Int J Immunopathol Pharmacol 18(4):771–778. https://doi.org/10.1177/039463200501800421
Loy BD, Fling BW, Sage KM, Spain RI, Horak FB (2019) Serum histidine is lower in fatigued women with multiple sclerosis. Fatigue 7(2):69–80. https://doi.org/10.1080/21641846.2019.1611786
Lu C, Diehl SA, Noubade R, Ledoux J, Nelson MT, Spach K et al (2010) Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 107(44):18967–18972. https://doi.org/10.1073/pnas.1008816107
Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H et al (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365(23):2188–2197. https://doi.org/10.1056/NEJMoa1100648
Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T et al (2002) Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science 297(5581):620–623. https://doi.org/10.1126/science.1072810
Malpass K (2012) Multiple sclerosis: ‘Outside-in’ demyelination in MS. Nat Rev Neurol 8(2):61. https://doi.org/10.1038/nrneurol.2011.217
McGinley MP, Goldschmidt CH, Rae-Grant AD (2021) Diagnosis and treatment of multiple sclerosis: a review. JAMA 325(8):765–779. https://doi.org/10.1001/jama.2020.26858
Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 9(5):A387–A394. https://doi.org/10.1016/j.autrev.2009.11.010
Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G et al (2006) A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol 176(1):17–26. https://doi.org/10.4049/jimmunol.176.1.17
Naganuma F, Nakamura T, Yoshikawa T, Iida T, Miura Y, Kárpáti A et al (2017) Histamine N-methyltransferase regulates aggression and the sleep-wake cycle. Sci Rep 7(1):15899. https://doi.org/10.1038/s41598-017-16019-8
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65(17):2702–2720. https://doi.org/10.1007/s00018-008-8059-5
Nieto-Alamilla G, Márquez-Gómez R, García-Gálvez AM, Morales-Figueroa GE, Arias-Montaño JA (2016) The histamine H3 receptor: structure, pharmacology, and function. Mol Pharmacol 90(5):649–673. https://doi.org/10.1124/mol.116.104752
Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M et al (2007) Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest 117(11):3507–3518. https://doi.org/10.1172/JCI32792
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G et al (2001) Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett 502(1-2):53–56. https://doi.org/10.1016/s0014-5793(01)02663-1
Panula P, Nuutinen S (2013) The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14(7):472–487. https://doi.org/10.1038/nrn3526
Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL et al (2015) International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67(3):601–655. https://doi.org/10.1124/pr.114.010249
Passani MB, Ballerini C (2012) Histamine and neuroinflammation: insights from murine experimental autoimmune encephalomyelitis. Front Syst Neurosci 6:32. https://doi.org/10.3389/fnsys.2012.00032
Passani MB, Blandina P (2011) Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol Sci 32(4):242–249. https://doi.org/10.1016/j.tips.2011.01.003
Passani MB, Blandina P, Torrealba F (2011) The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther 336(1):24–29. https://doi.org/10.1124/jpet.110.171306
Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R et al (2003) Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci U S A 100(4):1867–1872. https://doi.org/10.1073/pnas.252777399
Pons V, Rivest S (2020) Beneficial roles of microglia and growth factors in MS, a brief review. Front Cell Neurosci 14:284. https://doi.org/10.3389/fncel.2020.00284
Popescu BF, Lucchinetti CF (2012) Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol 12:11. https://doi.org/10.1186/1471-2377-12-11
Prinz M, Masuda T, Wheeler MA, Quintana FJ (2021) Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol 39:251–277. https://doi.org/10.1146/annurev-immunol-093019-110159
Provensi G, Costa A, Izquierdo I, Blandina P, Passani MB (2020a) Brain histamine modulates recognition memory: possible implications in major cognitive disorders. Br J Pharmacol 177(3):539–556. https://doi.org/10.1111/bph.14478
Provensi G, Passani MB, Costa A, Izquierdo I, Blandina P (2020b) Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol 177(3):557–569. https://doi.org/10.1111/bph.14476
Rafiee Zadeh A, Falahatian M, Alsahebfosoul F (2018) Serum levels of histamine and diamine oxidase in multiple sclerosis. Am J Clin Exp Immunol 7(6):100–105
Rao VTS, Fuh SC, Karamchandani JR, Woulfe JMJ, Munoz DG, Ellezam B et al (2019) Astrocytes in the pathogenesis of multiple sclerosis: an in situ MicroRNA study. J Neuropathol Exp Neurol 78(12):1130–1146. https://doi.org/10.1093/jnen/nlz098
Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC (1995) Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol 37(1):63–66. https://doi.org/10.1002/ana.410370112
Saligrama N, Noubade R, Case LK, del Rio R, Teuscher C (2012a) Combinatorial roles for histamine H1-H2 and H3-H4 receptors in autoimmune inflammatory disease of the central nervous system. Eur J Immunol 42(6):1536–1546. https://doi.org/10.1002/eji.201141859
Saligrama N, Noubade R, Case LK, Poynter ME, Teuscher C (2012b) H(1)R expression by CD11B(+) cells is not required for susceptibility to experimental allergic encephalomyelitis. Cell Immunol 278(1-2):27–34. https://doi.org/10.1016/j.cellimm.2012.06.012
Saligrama N, Case LK, del Rio R, Noubade R, Teuscher C (2013) Systemic lack of canonical histamine receptor signaling results in increased resistance to autoimmune encephalomyelitis. J Immunol 191(2):614–622. https://doi.org/10.4049/jimmunol.1203137
Saligrama N, Case LK, Krementsov DN, Teuscher C (2014) Histamine H2 receptor signaling × environment interactions determine susceptibility to experimental allergic encephalomyelitis. FASEB J 28(4):1898–1909. https://doi.org/10.1096/fj.13-239939
Schafer MK, Weihe E, Eiden LE (2013) Localization and expression of VMAT2 aross mammalian species: a translational guide for its visualization and targeting in health and disease. Adv Pharmacol 68:319–334. https://doi.org/10.1016/B978-0-12-411512-5.00015-4
Schirmer L, Schafer DP, Bartels T, Rowitch DH, Calabresi PA (2021) Diversity and function of glial cell types in multiple sclerosis. Trends Immunol 42(3):228–247. https://doi.org/10.1016/j.it.2021.01.005
Schreiner B, Heppner FL, Becher B (2009) Modeling multiple sclerosis in laboratory animals. Semin Immunopathol 31(4):479–495. https://doi.org/10.1007/s00281-009-0181-4
Schwartzbach CJ, Grove RA, Brown R, Tompson D, Then Bergh F, Arnold DL (2017) Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. J Neurol 264(2):304–315. https://doi.org/10.1007/s00415-016-8341-7
Shi Y, Li Z, Chen R, Zhang J, Hu X, He C et al (2017) Immethridine, histamine H. Oncotarget 8(43):75038–75049. https://doi.org/10.18632/oncotarget.20500
Slamet Soetanto T, Liu S, Sahid MNA, Toyama K, Maeyama K, Mogi M (2019) Histamine uptake mediated by plasma membrane monoamine transporter and organic cation transporters in rat mast cell lines. Eur J Pharmacol 849:75–83. https://doi.org/10.1016/j.ejphar.2019.01.050
Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD et al (2009) Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res 1250:41–48. https://doi.org/10.1016/j.brainres.2008.11.018
Stys PK, Zamponi GW, van Minnen J, Geurts JJ (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 13(7):507–514. https://doi.org/10.1038/nrn3275
Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD et al (2004) Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen-presenting cells. Am J Pathol 164(3):883–892. https://doi.org/10.1016/S0002-9440(10)63176-8
Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF et al (2007) Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc Natl Acad Sci U S A 104(24):10146–10151. https://doi.org/10.1073/pnas.0702291104
Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB et al (2018) The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 9:1873. https://doi.org/10.3389/fimmu.2018.01873
Theoharides TC, Kempuraj D, Kourelis T, Manola A (2008) Human mast cells stimulate activated T cells: implications for multiple sclerosis. Ann N Y Acad Sci 1144:74–82. https://doi.org/10.1196/annals.1418.029
Thurmond RL (2015) The histamine H4 receptor: from orphan to the clinic. Front Pharmacol 6:65. https://doi.org/10.3389/fphar.2015.00065
Tsouki F, Williams A (2021) Multifaceted involvement of microglia in gray matter pathology in multiple sclerosis. Stem Cells. https://doi.org/10.1002/stem.3374
Tuomisto L, Kilpeläinen H, Riekkinen P (1983) Histamine and histamine-N-methyltransferase in the CSF of patients with multiple sclerosis. Agents Actions 13(2-3):255–257. https://doi.org/10.1007/BF01967346
van der Veen RC, Dietlin TA, Hofman FM, Pen L, Segal BH, Holland SM (2000) Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J Immunol 164(10):5177–5183. https://doi.org/10.4049/jimmunol.164.10.5177
Vannier E, Miller LC, Dinarello CA (1991) Histamine suppresses gene expression and synthesis of tumor necrosis factor alpha via histamine H2 receptors. J Exp Med 174(1):281–284. https://doi.org/10.1084/jem.174.1.281
Volonté C, Amadio S, D'Ambrosi N, Colpi M, Burnstock G (2006) P2 receptor web: complexity and fine-tuning. Pharmacol Ther 112(1):264–280. https://doi.org/10.1016/j.pharmthera.2005.04.012
Volonté C, Amadio S, D'Ambrosi N (2008) Receptor webs: can the chunking theory tell us more about it? Brain Res Rev 59(1):1–8. https://doi.org/10.1016/j.brainresrev.2008.04.004
Volonté C, Apolloni S, Sabatelli M (2019) Histamine beyond its effects on allergy: potential therapeutic benefits for the treatment of amyotrophic lateral sclerosis (ALS). Pharmacol Ther 202:120–131. https://doi.org/10.1016/j.pharmthera.2019.06.006
Werkman IL, Lentferink DH, Baron W (2021) Macroglial diversity: white and grey areas and relevance to remyelination. Cell Mol Life Sci 78(1):143–171. https://doi.org/10.1007/s00018-020-03586-9
Williams A, Piaton G, Lubetzki C (2007) Astrocytes--friends or foes in multiple sclerosis? Glia 55(13):1300–1312. https://doi.org/10.1002/glia.20546
Yong HY, McKay KA, Daley CGJ, Tremlett H (2018) Drug exposure and the risk of multiple sclerosis: a systematic review. Pharmacoepidemiol Drug Saf 27(2):133–139. https://doi.org/10.1002/pds.4357
Zampeli E, Tiligada E (2009) The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol 157(1):24–33. https://doi.org/10.1111/j.1476-5381.2009.00151.x
Zia S, Rawji KS, Michaels NJ, Burr M, Kerr BJ, Healy LM et al (2020) Microglia diversity in health and multiple sclerosis. Front Immunol 11:588021. https://doi.org/10.3389/fimmu.2020.588021
Acknowledgments
This work was supported by Italian Ministry of Health through Fondazione Santa Lucia IRCCS Ricerca Corrente.
Author Contributions
Conceptualization, CV; writing original draft, review and editing, CV, SAp, SAm; graphical representation, SAm, Sap, CV. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Volonté, C., Apolloni, S., Amadio, S. (2021). The Histamine and Multiple Sclerosis Alliance: Pleiotropic Actions and Functional Validation. In: Yanai, K., Passani, M.B. (eds) The Functional Roles of Histamine Receptors. Current Topics in Behavioral Neurosciences, vol 59. Springer, Cham. https://doi.org/10.1007/7854_2021_240
Download citation
DOI: https://doi.org/10.1007/7854_2021_240
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16996-0
Online ISBN: 978-3-031-16997-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)