Abstract
Although chemotaxis has been proposed to guide sperm to egg throughout the animal kingdom, sperm attractants released from mammalian eggs have not been identified. Since the G protein subunit α-gustducin is accepted as a marker of chemosensitive cells, attempts were made to explore whether α-gustducin is also expressed in spermatozoa of mammals. Immunohistochemical approaches using an anti-α-gustducin-specific antibody revealed the most intense immunoreactivity in differentiating spermatids. Further evidence for the α-gustducin expression was obtained analyzing testicular and sperm-derived tissue preparations in western blot analyses. To elucidate whether α-gustducin is retained in mature spermatozoa, epididymal mouse and rat sperm were subjected to immunocytochemistry as well as immunogold electron microscopy. A specific staining was obtained within the circumference of the midpiece-localized mitochondria, on the axoneme and the outer dense fibers surrounding the microtubules of this region, whereas no labeling was detectable in the end piece regions. The analysis of ejaculated bovine and human sperm revealed a comparable segmental distribution pattern for α-gustducin. Although a possible function for α-gustducin has yet to be determined, the axonemal-associated localization within the midpiece and principal piece of different mammalian spermatozoa raises the possibility that this G protein α-subunit may process intracellular signals controlling sperm motility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotaxis of sperm is well documented for marine invertebrates with external fertilization. Chemotaxis also is proposed to operate in sperm of mammals, including humans, to increase the likelihood of fertilization by chemically guiding spermatozoa to the mature oocyte within the oviduct (Eisenbach 2004). The search for sperm attracting activity has focused on the fluids secreted by the upper female genital tract, like the follicular fluid (Ralt et al. 1994; Oliveira et al. 1999; Fabro et al. 2002; Munuce et al. 2004) or secretions from the egg and its surrounding cumulus cells (Sun et al. 2005). However, in mammals, the molecular identities of physiological factors are still unknown. Although great progress has been made very recently in resolving the behavioural strategies (e.g., Ca2+ controlled swimming trajectories and flagellar waveforms) induced by a gradient of chemoattractants (Bohmer et al. 2005), the molecular transduction process(es) linking the detection of attracting factors to swimming responses are still ambiguous: in invertebrates, like sea urchin, resact, a chemoattractant peptide secreted by the egg, induces motor responses of the flagellum by a cGMP (cyclic guanosine 5′-monophosphate)-mediated transient increase in intracellular calcium (Kaupp et al. 2003). In contrast, in human spermatozoa, odorant-induced calcium influx as well as chemotaxis can be blocked by adenylyl cyclase inhibition (Spehr et al. 2004), thus suggesting that sperm chemotaxis in mammals may be mediated by a cAMP (3′, 5′ cyclic adenosine monophosphate)-signaling pathway resembling the one of the olfactory system (Breer 2003). This promising possibility is supported by the observation that olfactory receptor proteins (Parmentier et al. 1992; Vanderhaeghen et al. 1993, 1997; Walensky et al. 1995; Goto et al. 2001; Spehr et al. 2003; Fukuda et al. 2004), as well as key elements of the olfactory signaling cascade including adenylyl cyclase III (Defer et al. 1998; Gautier-Courteille et al. 1998; Livera et al. 2005) the cyclic nucleotide activated channel (Weyand et al. 1994; Wiesner et al. 1998) as well as the olfactory selective G protein α-subunit Gαolf (Fraser et al. 2003; Spehr et al. 2004) are also expressed in mammalian spermatozoa.
Especially for Gαolf in neurons of the nasal cavity, but also for transducins in rods and cones, a tissue-enriched expression profile has been described (Shepherd 1991; Milligan and Kostenis 2006). The same observation has been made for gustducin, a G protein α-subunit that has been demonstrated to play a key role in “bitter”, “sweet” and “umami” taste sensation (Wong et al. 1996; Ruiz-Avila et al. 2000; Caicedo et al. 2003; He et al. 2004). α-gustducin, which was originally discovered in a subset of taste cells on the tongue (McLaughlin et al. 1992), is also expressed in a variety of other sensory systems, like the gut (Hofer et al. 1996; Hofer and Drenckhahn 1998) and the vomeronasal organ (Zancanaro et al. 1999), as well as in solitary chemosensory cells (SCC) of the airway (Merigo et al. 2005), the nasal cavity (Finger et al. 2003; Gulbransen and Finger 2005) and the tongue (Sbarbati et al. 1999). To test the hypothesis that transduction mechanisms exist in mammalian sperm that are common to cells in different chemosensory systems, we looked for the expression of α-gustducin, a marker of chemosensitive cells (Sbarbati and Osculati 2003), using light and electron microscopy. We observed that α-gustducin is detectable in mature spermatozoa from mouse to humans and that the expression is notably enriched in the mitochondria-rich midpiece region as well as the proximal part of the principal region of the sperm tail.
Materials and methods
General reagents and antibodies
This study was conducted on male adult Balb/c mice and Wistar rats raised in the central departmental animal facility or purchased from Charles River (Sulzfeld, Germany). Testes and epididymides of mature bulls were obtained from a local slaughterhouse. Frozen bull semen samples were obtained from local veterinarians. Freshly ejaculated human semen samples were obtained from young healthy donors. A rabbit polyclonal anti-α-gustducin antibody generated against a peptide sequence of rat α-gustducin as well as an anti-Gβ3, -Gγ13 and anti-phospholipase C β2 (PLCβ2) antibody were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG was provided by BioRad (München, Germany) and fluorescein isothiocyanate (FITC)-conjugated secondary antibody against rabbit immunoglobulin was acquired from Sigma–Aldrich (Deisenhofen, Germany). Taq DNA polymerase, DNase I and dNTP mix were supplied by Fermentas (St. Leon-Rot, Germany). Primers were ordered from Metabion (Planegg-Martinsried, Germany). Unless specified otherwise, reagents were either purchased from Sigma–Aldrich (Deisenhofen, Germany) or Carl Roth (Karlsruhe, Germany).
Sperm preparation and capacitation
Sperm from adult mice, rats, and bulls were isolated as described previously (Wennemuth et al. 2000). Briefly, carefully dissected caudae epididymes were excised and washed in HS working solution (30 mM HEPES, 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM lactic acid, and 1 mM pyruvic acid), adjusted to pH 7.4 with NaOH. Subsequently, cleaned tissue was transferred to HS working solution supplemented with 0.5% BSA and 15 mM Na2CO3 (HS/BSA/Na2CO3), and incised several times to allow the sperm to exude into the medium. After a “swim out” period of 15 min at 37°C and 5% CO2, the medium was collected, the sperm were concentrated by centrifugation (5 min, 400g, RT), washed three times with HS working solution and used for immunofluorescence. To obtain spermatozoa of different capacitated status, motile cauda epididymal sperm were collected in Na2CO3/BSA supplemented HS working solution and subsequently incubated for different time periods (0, 60, 90, and 120 min) at 37°C in 5% CO2. At each experimental time point, two aliquots of cells were removed from the sperm suspension and carefully washed in PBS. One aliquot was smeared on slides, air-dried and used to either evaluate the acrosomal status by Commassie blue staining or directly deployed for immunocytochemistry. Cells of the second aliquot were used to control sperm motility.
Cryopreserved bovine semen samples and freshly ejaculated human semen were first washed two times with a ninefold volume of PBS to separate spermatozoa from seminal plasma. Subsequently, the sperm were concentrated by centrifugation (5 min, 500g, RT). The final bovine sperm pellet was resuspended in PBS (150 mM NaCl, 1.4 mM KH2PO4, 8 mM Na2HPO4, pH 7.4) and cells were directly used for immunocytochemical experiments. The human sperm pellet was carefully covered with prewarmed (37°C) PBS, incubated for 25 min at 37°C and 5% CO2, and subsequently, motile sperm in the supernatant were used for immunocytochemistry.
Total RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Tissue (pooled circumvallate papillae, testes and total epididymis not depleted of sperm) removed from adult male rats was frozen in liquid nitrogen and stored at −70°C. For RNA isolation the frozen tissue was mixed with Trifast reagent (Peqlab, Erlangen Germany), minced on ice and, subsequently total RNA was extracted according to the manufacturer’s instructions. After spectrophotometric determination of total RNA content, traces of genomic DNA were removed by treating 4 μg of RNA with RNase-free DNase I, and the corresponding cDNA was produced using the Revert Aid first-strand cDNA synthesis kit as described in the manufacturer’s specifications (Fermentas, St. Leon-Rot, Germany).
PCR reactions were performed in a volume of 25 μl, containing cDNA from different tissue (20–100 ng), 4 pmol of each primer, 0.4 mM dNTPs, 2 mM MgCl2, 2.5 U Taq DNA polymerase, and 2.5 μl 10× PCR buffer, pH 8.4. The PCR cycling profile for the amplification of α-gustducin was as follows: 5 min at 94°C, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 1 min and extension at 72°C for 1 min. The PCR program finished with a final annealing period at 72°C for 10 min. Primers used to amplify α-gustducin from cDNA derived from rat tissue were 5′-CAATCCGAGAAGTAGAGAGG-3′ and 5′-GCTGTTGAAGAGGTGAAGAC-3′ (sense position, nucleotide 401–420, antisense position, nucleotide 869–850, according to Genbank accession no. X65747). Based on the primer design, the expected size of the α-gustducin PCR product was 469 bp. To exclude an amplification of genomic DNA, the quality of the cDNA was monitored by using primer pairs spanning exon junctions of the ribosomal gene L8 of rat (data not shown). The expression of L8 was also used as an internal standard for different cDNA probes (Shi and Liang 1994). Following the PCR, 10 μl of the reaction products were analyzed on 1.5% agarose gels, subcloned into pGEM-T Easy (Promega, Mannheim, Germany), and subsequently subjected to sequencing (MWG Biotech, Ebersberg, Germany).
Immunocytochemistry and Confocal Microscopy
For immuncytochemical analyses, sperm from different species were prepared as described above, placed on glass slides and allowed to settle for 15 min. Adherent cells were subsequently washed with PBS, fixed for 2 min with ice-cold (−20°C) methanol and immediately transferred to PBS. All following steps were performed in a humidified chamber. To reduce non-specific binding of antibodies, samples were blocked for 30 min at RT with PBS supplemented with 10% FCS, and thereafter, incubated overnight at 4°C with the anti-α-gustducin antibody diluted in PBS containing 10% FCS. To check the specificity of antibody binding, control slides were incubated with PBS/10% FCS only. In addition, primary antibody was co-incubated with the corresponding synthetic peptide as specified by the manufacturer’s instructions. After removing the primary antibodies by three washes for 5–10 min in PBS, cells were incubated with a 1:750 dilution of a FITC-conjugated goat anti-rabbit IgG for 1 h at RT. Subsequently, slides were washed three times with PBS, and cell nuclei were counterstained with propidium iodide (Heydecke et al. 2006). After three additional washes in PBS, samples were coated with fluorescent mounting medium (DAKO Cytomation, Hamburg, Germany) and examined with a Zeiss LSM 510 Meta laser scanning confocal microscope (Zeiss, Jena, Germany).
Immunohistochemistry
Immunohistochemical experiments were performed as outlined previously (Aigner et al. 2002). Briefly, paraffin-embedded sections of adult mouse testis fixed in formalin were deparaffinized using xylene, and, subsequently, rehydrated with descending ethanol solutions (100, 96, 80, and 70%) and an incubation in 10 mM citrate buffer (pH 7.4) at 90°C for 10 min. Subsequently, sections were washed in PBS/0.1% Tween 20, and blocked for 2 h at RT with 10% normal goat serum in PBS. Control sections were treated in parallel but were incubated without the primary antibody. To verify the specificity of the labeling, the anti-α-gustducin antibody was co-incubated with the corresponding peptide as specified by the manufacturer’s instructions. After an overnight incubation at 4°C in a wet chamber, the sections were washed three times with PBS/0.1% Tween, and thereafter, samples were incubated with the FITC-conjugated secondary antibody. After 2 h incubation at RT, excess secondary antibody was removed by washes as described above, and the sections were coated with fluorescent mounting medium (DAKO) and coverslipped for microscopic analysis.
Immunoelectron microscopy
Immunoelectron microscopic analyses were essentially performed as described before (Neesen et al. 2002). Briefly, rat testes samples were immersion fixed for 2 h either in 0.625% paraformaldehyde, 1.25% glutaraldehyde, and 0.025% picric acid in 0.05 M sodium cacodylate buffer (pH 7.3) or in a fixative consisting of 1.25% paraformaldehyde and 0.5% glutaraldehyde in 0.05 M sodium cacodylate buffer (0.1 M, pH 7.3). Samples were washed with sodium cacodylate buffer, dehydrated in ethanol and propylene oxide as described (Neesen et al. 2002) and embedded in Epon. Ultrathin sections were cut on a Reichert ultramicrotome, equipped with a diamond knife, and mounted on 100 mesh gold grids. Grids were incubated with the anti α-gustducin antibody, diluted in TBS for 1 h at room temperature or 12 h at 4°C, washed with TBS three times for 15 min and, subsequently, incubated with a 1:100 dilution of a goat anti-rabbit IgG conjugated to colloidal 10 nm gold particles (BBInternational, Cardiff, UK). Then, sections were washed again with TBS, briefly treated with alkaline lead citrate and uranyl acetate solutions, and examined in an EM 10 electron microscope (EM 10). In control experiments, the primary antibody was replaced by TBS buffer used to dilute the primary antibody (negative control); under these conditions, no labeling could be detected (data not shown).
SDS/PAGE and western blot analysis
Dissected mouse and rat circumvallate papillae, testes and intact epididymis not depleted from sperm were homogenized in buffer containing 10 mM Tris, 2 mM EGTA, 3 mM MgCl2, 0.1 mM PMSF, 0.25% NP-40 and protease inhibitor cocktail (Set III, Calbiochem, Schwalbach am Taunus, Germany). To separate tissue debris, the homogenate was centrifuged for 10 min at 1,500g and the supernatant was collected. After a further centrifugation at 43,000 rpm for 30 min in a Beckman SW 50.1 rotor, the resulting membrane pellets were resuspended in homogenization buffer, and the protein concentration was determined according to Bradford (Bradford 1976).
For SDS-PAGE, protein samples of rodent tissue and isolated sperm were mixed with 5× sample buffer (625 mM Tris/HCl, pH 6.8, 50% glycerol, 5% SDS, 7.5 mM dithiothreitol, 0.05% bromphenol blue), heated at 94°C for 5 min and, subsequently, subjected to 10% polyacrylamide gel electrophoresis using the Laemmli buffer system (Laemmli 1970).
The separated proteins were transferred to nitrocellulose using a semidry blotting system. The blots were stained with Ponceau S and then, non-specific binding sites were blocked with 5% non-fat milk powder in 10 mM Tris/HCl, pH 8.0, 150 mM NaCl and 0.05% Tween 20 (TBST). The blots were then incubated overnight at 4°C with specific primary antibodies (0.2 μg/ml in TBST containing 3% non-fat milk powder) or antibodies together with their corresponding peptides (2 μg/ml in TBST containing 3% non-fat milk powder). After three washes with TBST, a horseradish-peroxidase-conjugated goat anti-rabbit IgG (1:7,500 in TBST containing 3% non-fat milk powder) was applied for 1 h at RT. Excess antibody was removed by three washes with TBST, and subsequently, the ECL-system (Amersham Biosciences Europe, Freiburg, Germany) was used to visualize bound antibodies.
Results
To address the question whether α-gustducin is expressed in testicular tissue, RT-PCR was performed analyzing testicular and tongue tissue samples from rat. Employing rat cDNA derived from isolated circumvallate papillae, testis and total epididymides together with primers specifically matching to rat α-gustducin yielded a PCR product with the predicted size (469 bp) in all examined cDNA samples (Fig. 1). Subsequent cloning and sequencing of the testicular PCR products confirmed sequence identity with the reported rat α-gustducin sequence. These results indicate the presence of α-gustducin mRNA in testicular tissue.
To determine whether the α-gustducin mRNA is actually translated, testicular tissue was assessed by means of western blot analysis (Fig. 2). Control immunoblots with membrane-enriched fractions of taste bud-containing circumvallate papillae of the mouse tongue revealed that the antibody recognized a band of about 40 kDa (Fig. 2a, left panel), a size, which corresponds well to the predicted molecular mass derived from the sequence data for α-gustducin (McLaughlin et al. 1992). In addition, a faint band with a molecular mass of about 60 kDa was detectable in taste tissue preparations, which recently has been described to represent α-gustducin within insoluble complexes (von Buchholtz et al. 2004). Analyzing testis- and epididymides-derived membrane fractions as well as isolated rat and mouse sperm, a strong 40 kDa α-gustducin-reactive band as well as the 60-kDa band were detectable (Fig. 2a), whereas kidney and heart tissue did not show α-gustducin staining (Fig. 2b). The immunoreactive bands from taste tissue preparations as well as from testis, epididymides and sperm fractions could be successfully eliminated by applying the immunogenic peptide (Fig. 2c), thus confirming the identity of α-gustducin.
Identification of α-gustducin in testicular tissue by western blot analyses. Analyzing different tissue preparations for α-gustducin immunoreactivity, a labelled band of the expected size (approximately 40 kDa) was detectable in membrane fractions of circumvallate papillae (mouse), testis (rat) and epididymidis (rat) as well as in total homogenates of isolated rat and mouse sperm (a), whereas in mouse kidney and heart preparations no staining was visible (b). A larger band at about 60 kDa might represent insoluble complexes containing α-gustducin. Note that immunoreactive signals were successfully blocked in control experiments employing the immunogen peptide (c). The positions of the molecular weight standards (MW) in kDa for each western blot are indicated on the left
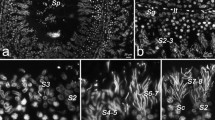
To define the testicular cell type in which α-gustducin is detectable at the protein level, immunohistochemical experiments were performed, incubating coronal sections of adult mouse testis with an anti-α-gustducin antibody. Initially, the specificity of the anti-α-gustducin antibody was verified by incubating coronal sections of circumvallate papillae with the anti-α-gustducin antibody, as well as with the primary antibody together with the antigenic peptide. As documented in Fig. 3, the anti-α-gustducin antibody shows an intense labeling of a subset of spindle-shaped taste bud cells and no staining in the surrounding lingual epithelium (Fig. 3c, d). Preincubation of the antibody with the immunogenic peptide eliminated the signal (Fig. 3b) thus confirming the specificity of the labeling. Incubating coronal testicular sections of adult male mouse with the anti-α-gustducin antibody revealed a bright immunofluorescence signal in the luminal part of the seminiferous epithelium and staining of some Leydig cells (Fig. 3g, arrowhead), whereas no staining was visible in the earliest precursors of spermatogonia, located at the outer region of the seminiferous tubule (Fig. 3g). At a higher magnification, it becomes obvious that the highest intensity of immunoreactivity marks the differentiating spermatid population next to and lining the lumen of the tubule (Fig. 3h). This α-gustducin-immunolabeling in late spermatids could be blocked by applying the antigenic peptide (Fig. 3f), whereas unspecific staining of Leydig cells, also detectable in sections incubated with secondary antibody only (Fig. 3e), was still visible (Fig. 3f).
Immunohistochemical localization of α-gustducin in mouse taste bud cells of circumvallate papillae and in late spermatids in mouse testis. An anti-α-gustducin-specific antibody labels a subset of spindle-shaped cells in the cleft of the taste bud whereas no signal was detectable in nonsensory epithelium and connective tissue (c, d). In coronal sections of testicular tissue, prominent α-gustducin-labeling was exclusively observed in differentiating spermatids covering the luminal surface of the seminiferous tubules (g, h). Note that the strong immunostaining in taste and testicular tissue was extinguished by the presence of the immunogenic peptide (b, f), whereas the weaker staining of Leydig cells, also observed in sections only incubated with the FITC-conjugated secondary antibody (e, arrowhead), was still visible. a, e Negative controls, in which the primary antibody was omitted. c, d, g and h Immunostaining of the anti-α-gustducin antibody. b, f Preabsorption of the primary antibody with the antigenic peptide. The superimposed box in (c) marks the higher magnification shown in (d). Pictures of the fluorescence channels (green) are overlaid with the corresponding transmitted-light channels
To verify whether α-gustducin is also expressed in mature spermatozoa that have moved to the epididymis, epididymal mouse and rat sperm were subjected to immunostaining using the anti-α-gustducin antibody and subsequently processed for immunofluorescence microscopy. To visualize the subcellular distribution of α-gustducin, fixed germ cells were counterstained with the DNA-intercalating dye propidium iodide (red). Immunocytochemical analysis of mouse sperm stained with the anti-α-gustducin antibody revealed an intense labeling of the sperm tail, but no detectable staining of the head (Fig. 4a, c). Interestingly, analyzing the labeling of the tail at a higher magnification, it becomes obvious that staining was not detectable along the entire length of the tail, but was greatly diminished in the distal part of the principal and end piece regions of the sperm tail (Fig. 4b, d). Remarkably, the staining pattern in the mitochondria-rich midpiece region and the proximal part of the principal piece of the flagellum varies between individual germ cells: whereas in some sperm, labeling was most abundant at the midpiece as well as the proximal part of the principal region (Fig. 4a, b), in other spermatozoa staining was more concentrated at the principal than at the midpiece region of the sperm tail (Fig. 4c, d). In order to examine the specificity of the staining, immunocytochemical experiments were repeated preincubating the primary antibody with an excess of the immunogenic peptide. Figure 4e and f shows that the corresponding peptide eliminated the staining in the midpiece, as well as in the principal piece of the flagellum, thus confirming the specificity of the α-gustducin expression in mouse spermatozoa.
Determination of subcellular localization of α-gustducin in mouse spermatozoa by indirect immunofluorescence. Isolated mouse sperm were fixed with ice-cold methanol and subsequently incubated with an anti-α-gustducin antibody. Bound primary antibody was visualized by a FITC-conjugated anti-rabbit-IgG. Note that staining pattern of the anti-α-gustducin antibody varies among individual epididymal mouse spermatozoa: Whereas in some sperm α-gustducin staining is visible in the midpiece and the proximal part of the principal region of the tail (a and b, arrowhead), other spermatozoa show a reduced labeling of the proximal principal part of the flagellum and the mid piece (c and d, arrowhead). After preabsorption of the primary antibody with an excess of the antigenic peptide, the signal was eliminated (e, f). Samples only incubated with the secondary antibody as a negative control were unstained (data not shown). The inserts in (a, c, e) indicate regions shown at higher magnification in (b, d, f). Confocal photomicrographs were created by the overlay of fluorescence channels (red channel for nuclear staining with propidium iodide; green channel for the FITC-conjugated secondary antibody) with the corresponding transmitted-light channels
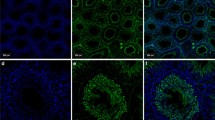
Analyzing the α-gustducin expression in epididymal rat sperm revealed a crescent-shaped staining of the acrosomal cap, although with varying intensities (Fig. 5a, c). In addition, the segmental distribution of α-gustducin at the flagellum differed from cell to cell, as observed for mouse spermatozoa: The fluorescence signal was either confined to the midpiece (Fig. 5a, b, arrowhead), or the midpiece and, especially, the proximal part of the principal piece of the sperm tail (Fig. 5c, d, arrowhead), whereas the end piece did not show apparent labeling. Specific neutralization of the primary antibody with the respective immunogenic peptide abolished staining (Fig. 5e, f), thus confirming the specificity of α-gustducin detection.
Confocal fluorescence images showing the subcellular localization of α-gustducin in rat spermatozoa. Subcellular localization of α-gustducin was demonstrated by indirect immunofluorescence using a FITC-conjugated secondary antibody. The overview presented in (a, c) reveal that (α-gustducin staining is detectable on the acrosomal cap of the sperm head as well as in the sperm tail. The arrowheads at higher magnification point to α-gustducin staining at the midpiece region (b) and the principal piece (d) of the tail. Primary antibody preincubated with the respective antigenic peptide gave no staining (e, f). In the absence of the primary antibody, no staining was observed (data not shown). The inserts in (a, c) indicate regions shown at higher magnification in (b, d). Confocal photomicrographs were created by the overlay of fluorescence channels with the corresponding transmitted-light channels
Since electron microscopy provides higher resolution and, thus, allows a more precise determination of the subcellular localization of α-gustducin expression within the sperm tail, immunoelectron microscopy was performed with ultrathin sections of adult rat testis tissue. Consistent with the immunofluorescence staining at the light microscopic level, few immunoreactive gold particles were detected in the acrosomal cap of elongated spermatids (Fig. 6a, arrowhead). However, the majority of α-gustducin gold particles were visible on the midpiece and proximal part of the principal piece of the sperm tail (Fig. 6b, c), whereas in the distal part of the principal piece (Fig. 6d), no gold labeling was detectable. Interestingly, α-gustducin gold particles were not localized at the surface of the flagellar axonema, but were restricted to the circumference of the spiral mitochondria, on the axonemal microtubules and most abundant at the outer dense fibers surrounding the microtubules (Fig. 6b, c, arrowheads). In control sections where incubation of the primary antibody was omitted, no gold labeling was detectable (data not shown).
Immunoelectron microscopic analysis of α-gustducin in adult rat testis. Rat testis samples were exposed to an anti-α-gustducin IgG followed by an incubation with a goat-anti-rabbit IgG conjugated to colloidal gold particles. The most intense staining was detectable at the outer dense fibers of the midpiece region (MP) of the tail (arrowheads in b and c) as well as in the circumference of the spiral mitochondria (b and c, arrowheads). In a longitudinal section of the acrosomal region of a sperm (a) a weak immunogold staining was detectable in the acrosomal cap (arrowhead). In a cross section of the sperm tail at the level of the distal part of the principal piece (PP)(d), no labeling was detectable. Arrowheads pinpoint to groups of gold particles. Results shown here are representative of those from two replicate experiments. ac Acrosomal cap, ax axonemal microtubules, fs fibrous sheath, ms mitochondrial sheath, nu nucleus, odf outer dense fibers, MP midpiece, PP principal piece
So far, the outlined results indicate that the G protein α-subunit gustducin is present in the midpiece and proximal principal region of the tail of rodent spermatozoa. To examine whether α-gustducin is also expressed in spermatozoa of other mammalian species, epididymal as well as ejaculated bovine sperm were subjected to immunostaining using the anti-α-gustducin antibody. Figure 7 documents that α-gustducin staining was different in epididymal bovine sperm compared to ejaculated spermatozoa: Although sperm of both stages of maturation are characterized by a weak labeling of the postacrosomal region (Fig. 7a, b, e, f), epididymal sperm show the strongest immunolabeling in the cytoplasmic droplet (Fig. 7a, b, arrowhead), a remnant of the germ cell cytoplasm that is shed off during spermatogenesis (Meggiolaro et al. 2003; Cooper 2005), whereas ejaculated bovine spermatozoa are characterized by the same tail-specific segmental distribution pattern observed for rodent spermatozoa (Fig. 7e, f, arrowhead).
Localization of α-gustducin in epididymidal and ejaculated bovine spermatozoa. Note that epididymal (a–d) as well as ejaculated bovine sperm (e–h) are characterized by a distinct labeling of the acrosomal region. However, in ejaculated spermatozoa, labeling is restricted to the midpiece (f, arrowhead) and proximal principal region of the tail, whereas in epididymal sperm, bright staining was only visible at the cytoplasmic droplet (cd), which is located either at the head–tail junction (a, arrow) or already migrated to the midpiece region (b, arrowhead). Inserts presented on the left (a, c, e, g) show higher magnification on the right (b, d, f, h). Green fluorescence indicates positive immunostaining revealed by an FITC-conjugated anti-rabbit IgG, red fluorescence indicates nuclear staining with propidium iodide and yellow fluorescence indicates overlapping of green and red fluorescence. a–h Overlay of corresponding fluorescence images. c, d, g, h Primary antibody preincubated with the antigenic peptide
To examine whether α-gustducin is also present in human spermatozoa, ejaculated human sperm were subjected to immunostaining for α-gustducin. In Fig. 8a and b, a typical labeling image is shown in which fluorescence is predominantly localized to the midpiece of the tail (Fig. 8, arrowhead), leaving the principal piece almost completely unstained. Competition experiments with an excess of immunogenic peptide revealed that the fluorescence signal was completely undetectable (Fig. 8c, d).
Immunolocalization of α-gustducin in human spermatozoa using confocal laser scanning microscopy. Indirect immunofluorescence staining with an anti-α-gustducin antibody and a goat anti-rabbit IgG-FITC-coupled secondary antibody. Note that immunolabeling was only visible in the midpiece region of the sperm tail (b, arrowhead), leaving the head completely unlabeled (a and b). The immunofluorescence pattern was completely abolished when the primary antibody was preincubated with the neutralizing peptide (c and d). Inserts presented on the left (a and c) show higher magnification on the right (b and d)
Discussion
The principle finding of this study is the identification of α-gustducin in spermatozoa of different mammalian species. Performing immunocytochemical experiments as well as immunogold electron microscopy, a segmental expression pattern of α-gustducin with an apparent accumulation within the mitochondrial-rich midpiece region and/or the proximal part of the principal piece of the sperm tail was observed.
The finding that sperm of different mammalian species are characterized by a segmental distribution of α-gustducin along the flagellum may suggest a common functional role for this G protein α-subunit in male germ cells. Remarkably, a strong expression level was detectable at the site of the axoneme (Fig. 6), a highly conserved and organized microtubule-based structure, that serves as general motility module but also as a sensory apparatus not only of sperm cells but also of trachea cells or inner-ear hair cells of mammals (Inaba 2003; Scholey 2003; Turner 2003). Thus, one may speculate that α-gustducin in mammalian spermatozoa plays a functional role either in signal transduction controlling sperm chemotaxis, or alternatively in the process of sperm motility.
Importantly, regarding the molecular transduction mechanisms of sperm chemotaxis, different models are currently discussed: In species with external fertilization, like the sea urchin, cGMP has clearly been demonstrated to control flagellar motor responses (Kaupp et al. 2003). However, in human spermatozoa, recent pharmacological approaches indicated that cAMP participates in the transduction of sperm chemotaxis in mammals (Spehr et al. 2004). Although it cannot be excluded that internally fertilizing species use a different signaling pathway than marine invertebrates, the two models may implicate that more than one transduction system is involved (Eisenbach 2004). In this context, it is important to mention that the detected segmental expression pattern of α-gustducin shows a striking co-localization with key elements of the olfactory cAMP signaling pathway (Vanderhaeghen et al. 1993; Walensky et al. 1995; Baxendale and Fraser 2003; Spehr et al. 2004) demonstrated to participate in sperm chemotactic signaling (Spehr et al. 2004; Spehr and Hatt 2005). Thus, it is reasonable to assume that the α-gustducin identified in sperm may represent a candidate component of an additional chemosensitive transduction cascade in sperm. In taste cells, where α-gustducin contributes to the transduction of bitter, sweet and umami, a Gβ3γ13-mediated stimulation of a PLCβ2 isoform has been described (Rössler et al. 1998, 2000; Huang et al. 1999; Zhang et al. 2003). However, using specific antibodies against these key molecules of taste signaling, we could not detect expression of Gβ3γ13 partners of α-gustducin, nor PLCβ2 in isolated spermatozoa (in preparation). Interestingly, taste cells respond to bitter tastants by a synchronous activation of the heterotrimeric G protein complex: a Gβ3γ13-mediated PLCβ2 stimulation and an α-gustducin controlled stimulation of PDEA1, a phosphodiesterase expressed in taste cells (Margolskee 2002). Thus, it may be possible that α-gustducin directly controls the activity of an yet unidentified effector enzyme of a chemosensitive signaling cascade in mammalian spermatozoa.
One key result of the present study is that localization of α-gustducin was not detectable at the plasma membrane of the tail axoneme, but was concentrated at cytoskeletal-associated subcellular compartments, such as mitochondria, the axonemal microtubules and the outer dense fibers, surrounding the axonema in the midpiece and principal piece of the sperm tail (Fig. 6). Although heterotrimeric G proteins were originally thought to be membrane-bound molecules, results from several laboratories currently also suggest that G proteins interact with intracellular compartments (Takesono et al. 1999). Interestingly, in a distinct taste cell population, there is also evidence for a cytoplasmic localization of α-gustducin (Sbarbati and Osculati 2003). In this context, it is important to mention that in retinal rods and cones, the cytoskeleton network affects translocation of transducin from the outer to the inner segment of the highly polarized photoreceptor cell (Peterson et al. 2005). Because we currently cannot exclude that under different experimental conditions, partial localization of α-gustducin may be detectable at the plasma membrane, it is possible that cytoskeletal elements in spermatozoa may also affect the distribution of α-gustducin to the membrane. Interestingly, a redistribution of α-gustducin was detectable during the transit of spermatozoa from seminiferous tubules through the epididymis (Fig. 7), but was obviously not altered during capacitation (data not shown). Since cytoskeletal proteins have been described as highly dynamic structures which actively participate in epididymal maturation (Howes et al. 2001), it will be interesting to examine whether the described cytoskeletal rearrangements during spermatozoal development may be accompanied by the observed redistribution of α-gustducin from the cytoplasmic droplet to the flagellum in ejaculated bovine sperm.
However, the observed cytoplasmic localization of α-gustducin could also implicate that this G protein α-subtype in sperm cells processes intracellular signals that do not directly involve cell surface receptors. Notably, G protein α-subunits and βγ-dimers are able to straightly interact with tubulin, a cytoskeletal protein that forms the microtubules within the axoneme, thus regulating microtubule polymerization and reorganization of cytoskeletal elements (for review see Rasenick et al. 2004). Since α-gustducin distribution was also found to be concentrated in the axonema, the flagellar motor module (Inaba 2003), it is intriguing to suggest that α-gustducin, or the associated Gβγ-subunits, which have not yet been identified, affect the dynamics of the microtubule cytoskeleton in spermatozoa. Thus, it will be crucially important for future work to identify Gβ- and Gγ-subtypes interacting with α-gustducin in mammalian spermatozoa. Furthermore, it will be challenging to examine whether α-gustducin in spermatozoa is involved in modulating cytoskeletal organization and function, and whether sperm of α-gustducin deficient mice (Wong et al. 1996) possess defects in motility, a phenotype, which may have escaped detection so far.
Abbreviations
- cAMP:
-
3′,5′-Cyclic adenosine monophosphate
- cGMP:
-
Cyclic guanosine 5′-monophosphate
- FITC:
-
Fluorescein isothiocyanate
- PDE:
-
Phosphodiesterase
- PLC:
-
Phospholipase C
- SCC:
-
Solitary chemosensory cells
References
Aigner A, Ray PE, Czubayko F, Wellstein A (2002) Immunolocalization of an FGF-binding protein reveals a widespread expression pattern during different stages of mouse embryo development. Histochem Cell Biol 117:1–11
Baxendale RW, Fraser LR (2003) Immunolocalization of multiple Galpha subunits in mammalian spermatozoa and additional evidence for Galphas. Mol Reprod Dev 65:104–113
Bohmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsumoto M, Hoshi M, Hildebrand E, Kaupp UB (2005) Ca2+ spikes in the flagellum control chemotactic behavior of sperm. Embo J 24:2741–2752
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breer H (2003) Sense of smell: recognition and transduction of olfactory signals. Biochem Soc Trans 31:113–116
Caicedo A, Pereira E, Margolskee RF, Roper SD (2003) Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci 23:9947–9952
Cooper TG (2005) Cytoplasmic droplets: the good, the bad or just confusing? Hum Reprod 20:9–11
Defer N, Marinx O, Poyard M, Lienard MO, Jegou B, Hanoune J (1998) The olfactory adenylyl cyclase type 3 is expressed in male germ cells. FEBS Lett 424:216–220
Eisenbach M (2004) Towards understanding the molecular mechanism of sperm chemotaxis. J Gen Physiol 124:105–108
Fabro G, Rovasio RA, Civalero S, Frenkel A, Caplan SR, Eisenbach M, Giojalas LC (2002) Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol Reprod 67:1565–1571
Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100:8981–8986
Fraser LR, Adeoya-Osiguwa SA, Baxendale RW (2003) First messenger regulation of capacitation via G protein-coupled mechanisms: a tale of serendipity and discovery. Mol Hum Reprod 9:739–748
Fukuda N, Yomogida K, Okabe M, Touhara K (2004) Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci 117:5835–5845
Gautier-Courteille C, Salanova M, Conti M (1998) The olfactory adenylyl cyclase III is expressed in rat germ cells during spermiogenesis. Endocrinology 139:2588–2599
Goto T, Salpekar A, Monk M (2001) Expression of a testis-specific member of the olfactory receptor gene family in human primordial germ cells. Mol Hum Reprod 7:553–558
Gulbransen BD, Finger TE (2005) Solitary chemoreceptor cell proliferation in adult nasal epithelium. J Neurocytol 34:117–122
He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S (2004) Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24:7674–7680
Heydecke D, Meyer D, Ackermann F, Wilhelm B, Gudermann T, Boekhoff I (2006) The multi PDZ domain protein MUPP1 as a putative scaffolding protein for organizing signaling complexes in the acrosome of mammalian spermatozoa. J Androl 27(3):390–404
Hofer D, Drenckhahn D (1998) Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 110:303–309
Hofer D, Puschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93:6631–6634
Howes EA, Hurst SM, Jones R (2001) Actin and actin-binding proteins in bovine spermatozoa: potential role in membrane remodeling and intracellular signaling during epididymal maturation and the acrosome reaction. J Androl 22:62–72
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2:1055–1062
Inaba K (2003) Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 20:1043–1056
Kaupp UB, Solzin J, Hildebrand E, Brown JE, Helbig A, Hagen V, Beyermann M, Pampaloni F, Weyand I (2003) The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat Cell Biol 5:109–117
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Livera G, Xie F, Garcia MA, Jaiswal B, Chen J, Law E, Storm DR, Conti M (2005) Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol 19:1277–1290
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277:1–4
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357:563–569
Meggiolaro D, Porcelli F, Scanziani E, Consiglio AL, Carnevali A, Ferrandi B (2003) ILA 147 immunoreactivity of the bull spermatozoa membrane during epididymal maturation. Acta Histochem 105:231–238
Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A (2005) alpha-Gustducin immunoreactivity in the airways. Cell Tissue Res 319:211–219
Milligan G, Kostenis E (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147(Suppl 1):S46–S55
Munuce MJ, Caille AM, Botti G, Berta CL (2004) Modulation of human sperm function by follicular fluid. Andrologia 36:395–401
Neesen J, Hartwich T, Brandhorst G, Aumuller G, Glaser B, Burfeind P, Mendoza-Lujambio I (2002) Tep22, a novel testicular expressed gene, is involved in the biogenesis of the acrosome and the midpiece of the sperm tail. Biochem Biophys Res Commun 297:737–748
Oliveira RG, Tomasi L, Rovasio RA, Giojalas LC (1999) Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J Reprod Fertil 115:23–27
Parmentier M, Libert F, Schurmans S, Schiffmann S, Lefort A, Eggerickx D, Ledent C, Mollereau C, Gerard C, Perret J et al (1992) Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 355:453–455
Peterson JJ, Orisme W, Fellows J, McDowell JH, Shelamer CL, Dugger DR, Smith WC (2005) A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci 46:3988–3998
Ralt D, Manor M, Cohen-Dayag A, Tur-Kaspa I, Ben-Shlomo I, Makler A, Yuli I, Dor J, Blumberg S, Mashiach S et al (1994) Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol Reprod 50:774–785
Rasenick MM, Donati RJ, Popova JS, Yu JZ (2004) Tubulin as a regulator of G-protein signaling. Methods Enzymol 390:389–403
Rössler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J (2000) G protein betagamma complexes in circumvallate taste cells involved in bitter transduction. Chem Senses 25:413–21
Rössler P, Kroner C, Freitag J, Noe J, Breer H (1998) Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol 77:253–261
Ruiz-Avila L, Ming D, Margolskee RF (2000) An In vitro assay useful to determine the potency of several bitter compounds. Chem Senses 25:361–368
Sbarbati A, Crescimanno C, Bernardi P, Osculati F (1999) Alpha-gustducin-immunoreactive solitary chemosensory cells in the developing chemoreceptorial epithelium of the rat vallate papilla. Chem Senses 24:469–472
Sbarbati A, Osculati F (2003) Solitary chemosensory cells in mammals? Cells Tissues Organs 175:51–55
Scholey JM (2003) Intraflagellar transport. Annu Rev Cell Dev Biol 19:423–443
Shepherd GM (1991) Sensory transduction: entering the mainstream of membrane signaling. Cell 67:845–851
Shi YB, Liang VC (1994) Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta 1217:227–228
Spehr M, Hatt H (2005) A potential role of odorant receptor agonists and antagonists in the treatment of infertility and contraception. Curr Opin Investig Drugs 6:364–368
Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H (2003) Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299:2054–2058
Spehr M, Schwane K, Riffell JA, Barbour J, Zimmer RK, Neuhaus EM, Hatt H (2004) Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J Biol Chem 279:40194–40203
Sun F, Bahat A, Gakamsky A, Girsh E, Katz N, Giojalas LC, Tur-Kaspa I, Eisenbach M (2005) Human sperm chemotaxis: both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum Reprod 20:761–767
Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S III, Duzic E, Lanier SM (1999) Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem 274:33202–33205
Turner RM (2003) Tales from the tail: what do we really know about sperm motility? J Androl 24:790–803
Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M (1993) Olfactory receptors are displayed on dog mature sperm cells. J Cell Biol 123:1441–1452
Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M (1997) Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics 39:239–246
von Buchholtz L, Elischer A, Tareilus E, Gouka R, Kaiser C, Breer H, Conzelmann S (2004) RGS21 is a novel regulator of G protein signalling selectively expressed in subpopulations of taste bud cells. Eur J Neurosci 19:1535–1544
Walensky LD, Roskams AJ, Lefkowitz RJ, Snyder SH, Ronnett GV (1995) Odorant receptors and desensitization proteins colocalize in mammalian sperm. Mol Med 1:130–141
Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF (2000) CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J Biol Chem 275:21210–21217
Weyand I, Godde M, Frings S, Weiner J, Muller F, Altenhofen W, Hatt H, Kaupp UB (1994) Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature 368:859–863
Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, Weyand I (1998) Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J Cell Biol 142:473–484
Wong GT, Gannon KS, Margolskee RF (1996) Transduction of bitter and sweet taste by gustducin. Nature 381:796–800
Zancanaro C, Caretta CM, Merigo F, Cavaggioni A, Osculati F (1999) alpha-gustducin expression in the vomeronasal organ of the mouse. Eur J Neurosci 11:4473–4475
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112:293–301
Acknowledgments
We thank Marga Losekamp for providing expert technical assistance, Tim Plant, Andreas Breit and Thomas Büch for critical reading of the manuscript, and Heinz Breer for helpful suggestions and support for some molecular and histological experiments. This work was supported in part by the P.E. Kempkes Stifung. The experiments comply with Principles of Animal Care, publication no. 85–23, revised 1985, of the National Institutes of Health and with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Johanna Fehr and Dorke Meyer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fehr, J., Meyer, D., Widmayer, P. et al. Expression of the G-protein α-subunit gustducin in mammalian spermatozoa. J Comp Physiol A 193, 21–34 (2007). https://doi.org/10.1007/s00359-006-0168-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0168-8