Abstract
Sensing protein breakdown products in the luminal content is of particular importance for the regulation of digestive activities in the stomach which are mainly governed by gastric hormones. The molecular basis for tuning the release of hormones according to the protein content is still elusive. In this study we have analysed the murine stomach for candidate nutrient receptors. As a promising candidate we have concentrated on the broadly tuned amino acid receptor GPRC6A. Expression of GPRC6A could be demonstrated in different regions of the murine stomach; especially in the gastric antrum. Using immunohistochemical approaches, a large cell population of GPRC6A-positive cells was visualized in the basal half of the antral gastric mucosa. Molecular phenotyping of GPRC6A-immunoreactive cells revealed that most of them contained the peptide hormone gastrin. A small population turned out to be immunoreactive for somatostatin. In search for additional amino acid receptors in antral gastric mucosa, we obtained evidence for expression of the gustatory amino acid receptor subunit T1R3 and the calcium-sensing receptor CaSR. Many CaSR-cells were found in the gastric antrum and most of them also contained gastrin; very similar to GPRC6A-cells. In contrast, T1R3 was found only in a small population of gastrin-negative cells. The finding that GPRC6A-and CaSR-receptors are both expressed in many if not all gastrin cells strongly suggests that both receptor types are co-expressed in the same cells, where they could form heterodimers providing a unique response spectrum of these cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gastrointestinal tract is sensing the luminal content to adapt the motility, as well as secretory activities according to the content and to the region of the gut. For the stomach, sensing of proteins seems to be of particular importance to regulate the secretion of gastrin, the central hormone for controlling gastric activities (Dockray et al. 2001). The 17-aa carboxy-amidated peptide gastrin activates chief cells to release pepsinogen and stimulates parietal cells to secrete hydrochloric acid either directly or via ECL cells releasing histamine (Gregory 1968; Mulholland and Debas 1988; Dockray 1999). Gastrin is generated in G-cells which are almost exclusively located in the antral gastric mucosa and is released in response to food in the stomach (Dockray et al. 2005). Proteins or its breakdown products are considered as most potent food constituents for initiating gastrin release; in fact, amino acids have been found as major stimulus (McArthur et al. 1983; Zeng et al. 1995). The mechanisms underlying the responsiveness of G-cells to food constituents in the stomach lumen remain unknown. G-cells could either be activated via paracrine signals from nearby chemosensors in the mucosa or they could be able to interact directly with nutrients in the gastric chyme. The notion that G-cells have contact to the lumen (Fujita and Kobayashi 1977; Mortensen 1980) would favour the concept of direct sensing, which requires molecular entities for recognizing the appropriate food constituents, i.e. receptors which detect protein breakdown products. Very little is known about the mechanisms how gastrin-cells perceive luminal constituents, although a very recent study suggests a role for the calcium-sensing receptor (CaSR; Feng et al. 2010). In search for molecular entities which enable gastric mucosa cells to recognize and respond to protein breakdown products, in this study we set out to investigate which type of candidate amino acid receptors are expressed in the gastric mucosa and especially in G-cells. In particular, we focused on the family C receptor GPRC6A which responds to basic amino acids, including arginine, lysine, ornithine and various aliphatic amino acids (Wellendorph et al. 2005; Pi et al. 2005). Previous studies have described the expression of this receptor in different tissues and cells e.g. soft palate, liver, blood vessels and taste cells (Kuang et al. 2005; Wellendorph et al. 2007; Harno et al. 2008; Bystrova et al. 2010). As all identified endogenous agonists for family C receptors are nutrient-like molecules reaching from ions and sugars to amino acids (Quiocho and Ledvina 1996; Wellendorph et al. 2009a; Geraedts et al. 2010), the broadly tuned L-amino-acid receptor GPRC6A presents a plausible candidate for being a nutrient sensor of emerging physiological significance (Kuang et al. 2005; Wellendorph et al. 2007; Wellendorph et al. 2009a). Therefore this promiscuous nutrient sensing receptor (Wellendorph et al. 2010) GPRC6A could be a suitable element to sense important nutrients in the luminal content of the stomach and thereby influence gastric secretion.
Materials and methods
Mice
Analyses were performed with wild type mouse strains C57/BL6 J from Charles River (Sulzfeld, Germany). Animals were fed with standard laboratory chow ad libitum and had free access to water. All experiments comply with the Principles of animal care, publication no. 85–23, revised 1985, of the National Institutes of Health and with the current laws of Germany.
RNA isolation and cDNA synthesis
Total RNA was isolated from dissected tissue preparations of soft palate and the stomach compartments with a NucleoSpin RNA kit (Macherey–Nagel, Düren, Germany). First-strand cDNA was prepared with an oligo(dT) primer and Superscript III reverse transcriptase (Invitrogen, Carlsbad, Calif., USA).
Reverse transcriptase polymerase chain reaction (RT-PCR)
RT-PCR amplification was conducted by using normalized cDNA from different tissues as described above. PCR amplifications were performed with the following primer combinations: GPRC6A forward, 5′-GCT CCA AGG AAT GCA GCC CTG GTC-3′; GPRC6A reverse, 5′-CAC GCA AAG TCT TGT GGT TCT CC-3′; rpl8 forward, 5′-GTG CCT ACC ACA AGT ACA AGG C-3′; rpl8 reverse, 5′-CAG TTT TGG TTC CAC GCA GCC G-3′; T1R3 forward, 5′-GCT GCC TTG CAC AAC AAC CAA TGG C-3′; T1R3 reverse, 5′-GAT AGC ACC CAT CTG CAC AGC TGG C-3′; CaSR forward, 5′-GGA GGG AGA GGT TAC CCG GTT G-3′; CaSR reverse, 5′-GTT ACA GAG ACA GCT CGT TGG G-3′.
RT-PCR was carried out using High Fidelity PCR Enzyme Mix (Fermentas, St. Leon-Rot, Germany) and a Peltier PTC-200 thermo cycler (MJ Research). For exponential amplification of GPRC6A the following PCR cycling profile was used. One cycle: 3 min at 94°C; 20 cycles: 30 s at 94°C, 40 s at 65°C with −0.5°C per cycle, 40 s at 72°C; 20–31 cycles: 30 s at 94°C, 20 s at 55°C, 40 s at 72°C; and one cycle: 1 min at 72°C.
For amplification of CaSR and T1R3 the same PCR cycling profile was used with adjusted annealing temperatures (CaSR 62–52°C; T1R3 69–59°C) and optimized numbers of cycles to achieve exponential amplification (25 cycles). PCR products were run on a 1.5% agarose gel containing EtdBr. Amplification of a 205 bp fragment from mouse housekeeping control gene ribosomal protein l8 (rpl8) was used as control to confirm equal quality and quantity of the cDNA preparations. PCR products were subsequently cloned into pGem-T (Promega, Madison, Wis., USA) and subjected to sequence analysis in an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif., USA).
Tissue preparation
For immunoreactivity to GPRC6A, mice were gassed with CO2 and perfused via the left heart ventricle with 1× PBS (0.85% NaCl, 1.4 mM KH2PO4, 8 mM Na2HPO4, pH 7.4) followed by 4% ice-cold paraformaldehyde (in 150 mm phosphate buffer, pH 7.4). The stomach was removed and rinsed with 1× PBS. After fixation in the same fixative for 10 min the tissue was cryoprotected by incubation in 25% sucrose overnight at 4°C. Finally, the tissue was embedded in Tissue Freezing Medium and quickly frozen on dry ice or liquid nitrogen. Cryosections (8 μm) were generated using a CM3050S cryostat (Leica Microsystems, Bensheim, Germany) and adhered to Superfrost Plus microscope slides (Menzel Gläser, Braunschweig, Germany).
For immunoreactivity to calcium-sensing receptor (CaSR), removed stomachs were rinsed in 1× PBS and immersed in 4% formalin solution (Roti-Histofix, Roth, Karlsruhe, Germany) for 1 h. After fixation the tissue was cryoprotected by incubation in 25% sucrose overnight at 4°C. Finally, the tissue was embedded in Tissue Freezing Medium and quickly frozen on dry ice or liquid nitrogen. Cryosections (8 μm) were generated using a CM3050S cryostat (Leica Microsystems, Bensheim, Germany) and adhered to Superfrost Plus microscope slides (Menzel Gläser, Braunschweig, Germany).
Immunohistochemistry
Cryosections (8 μm) were air-dried, rinsed in 1× PBS for 10 min at room temperature and blocked in 0.3% Triton X-100 in 1× PBS containing either 10% normal goat serum (NGS; Dianova, Hamburg, Germany) or 10% normal donkey serum (NDS; Dianova, Hamburg, Germany) for 30 min at room temperature.
For immunoreactivity to CaSR, cryosections underwent citrate-antigen-retrieval. Therefore, frozen sections were incubated in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for 45 min at 4°C. Afterwards sections were immersed in the same sodium citrate buffer for 10 min at 100°C. After three rinses for 5 min in 1× PBS, cryosections were blocked in 0.3% Triton X-100 in 1× PBS containing either 10% normal goat serum (NGS) or 10% normal donkey serum (NDS) for 30 min at room temperature.
For single- and double-labeling experiments, primary antibodies were diluted in 0.3% Triton X-100 in 1× PBS containing either 10% NGS (for single-labelings including rabbit anti-GPRC6A, as well as mouse anti-calcium sensing receptor and double-labelings including rabbit anti-GPRC6A/guinea pig anti-gastrin, mouse anti-calcium sensing receptor/guinea pig anti-gastrin and rabbit anti-T1R3/guinea pig anti-gastrin) or 10% NDS (for double-labelings including rabbit anti-GPRC6A/goat anti-somatostatin and mouse anti-calcium sensing receptor/goat anti-somatostatin). Antibodies were used in the following dilutions: rabbit anti-GPRC6A (SP4276P, Acris Antibodies, Herford, Germany) 1:50; guinea pig anti-gastrin (BP5046, Acris Antibodies, Herford, Germany) 1:1,000; goat anti-somatostatin (sc-7819, Santa Cruz Biotechnology, Santa Cruz, Calif., USA) 1:2,000; rabbit anti-T1R3 (ab-74732; Abcam, Cambridge, UK; Hass et al. 2010) 1:50; mouse anti-calcium sensing receptor (MA1-934, ABR Affinity BioReagents, Golden, USA) 1:100. Blocked sections were incubated with the diluted primary antibodies overnight at 4°C. After washing in 1× PBS, the bound primary antibodies were visualized using appropriate secondary antibodies conjugated to Alexa 488, Alexa 568 or Cy3 (Invitrogen, Karlsruhe, Germany, 1:400) diluted in 1× PBS with 0.3% Triton X-100 containing either 10% NGS or 10% NDS for 2 h at room temperature. After three rinses for 5 min in 1× PBS, the sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; 1 μg/mL, Sigma Aldrich, Schnelldorf, Germany) for 3 min at room temperature, rinsed with bidest, and finally mounted in MOWIOL (10% polyvinylalcohol 4–88 (Sigma), 20% glycerol in 1× PBS). No immunoreactivity could be observed when the primary antibodies were omitted.
Microscopy and photography
Immunohistochemical staining was documented by using a Zeiss Axiophot microscope (Carl Zeiss MicroImaging, Jena, Germany). Images were captured using a Zeiss Axiocam for transmitted light and a ‘Sensi-Cam’ CCD-camera (PCOimaging, Kelheim, Germany) for fluorescent images. Brightness and contrast were adjusted for the whole images.
Cell quantification
Putative chemosensory cells were counted manually on consecutive sections from antral glandular regions of one animal that were stained with the anti-GPRC6A, the anti-gastrin antibody and DAPI to visualize cell nuclei, as well as from one animal that were stained with the anti-GPRC6A, the anti-gastrin antibody and DAPI. For quantification the numbers of single-labeled cells, as well as of double-labeled cells were counted per three hundred nucleated epithelial cells in mucosal areas where strongly immunoreactive cells resided. The average cell count of immunopositive cells per field was determined and the proportion (%) of GPRC6A-positive cells which were gastrin-positive, as well as the proportion of gastrin-positive cells which were GPRC6A-positive was determined. In addition, also the numbers for GPRC6A-cells and somatostatin-cells were determined.
Results
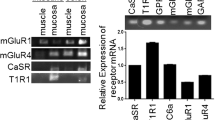
The molecular basis for chemosensory cells in the stomach mucosa to sense nutrients, especially protein breakdown products and amino acids, is still elusive. Therefore, in this study we have focused on the family C receptor GPRC6A which has been characterized as a promiscuous, seven transmembrane receptor for amino acids and is therefore considered as a candidate receptor for nutrients (Kuang et al. 2005; Conigrave and Hampson 2006; Wellendorph et al. 2007; Wellendorph et al. 2009a). As initial experiments, semi-quantitative RT-PCR analyses were performed using primers specific for GPRC6A on normalized cDNA from the murine stomach as well as from soft palate (Fig. 1). Amplicons of the expected size were obtained for tissue samples from the corpus and antrum region. The stronger amplification product for the antrum sample (Fig. 1a) indicates that the antral tissue contained more mRNA for GPRC6A than the corpus region. For comparison, we have analyzed cDNA from soft palate which is known to have gustatory function (Ikeda et al. 2002) and has previously been described as an example of GPRC6A expressing tissue (Wellendorph et al. 2007). Interestingly, the band amplified from antral tissue was even stronger than the amplicon obtained from soft palate (Fig. 1b). Subsequent cloning and sequencing of the amplicons revealed that they indeed encoded GPRC6A.
Identification of mRNA for the receptor GPRC6A and the ribosomal protein l8 (rpl8). Semi-quantitative RT-PCR experiments performed with primer pairs specific for GPRC6A (414 bp) or rpl8 (202 bp). a On using normalized cDNA from gastric tissue from the corpus and antrum region, amplicons of the expected sizes were obtained. While experiments with cDNA from corpus mucosa yielded a comparatively weak band for GPRC6A, cDNA from gastric antral mucosa yielded a stronger band. No bands were observed in water controls lacking template. b As positive control and for comparison cDNA from soft palate was analysed. Amplicons of the expected sizes were obtained, however, the band for GPRC6A was weaker than the one obtained from antral tissue. This result indicates a relatively high level of GPRC6A mRNA in the antral tissue. No amplicons were observed in reactions lacking template (water control)
In order to visualize, localize and identify those cells in the stomach mucosa which express the receptor GPRC6A, immunohistochemical experiments were performed using a GPRC6A specific antibody. Tissue sections from the pyloric antral region depicted in Fig. 2 show the stomach wall and mucosa visualized by DAPI staining; the gastric mucosa appears in a light blue, whereas the submucosa is more darkly stained. Dependent on the section plane, different views of the antral mucosa were obtained; longitudinal sections resulted in a typical view with the gastric invaginations forming the antral glands (Fig. 2c); diagonal sections resulted in a typical view of the ring-shaped gastric antral glands forming the densely arranged annuli (Fig. 2d). An assessment of tissue sections through the antral mucosa by means of a GPRC6A-specific antibody led to a visualization of numerous labeled cells at the basal region of the gastric invaginations (Fig. 3). Higher magnification revealed that GPRC6A-positive cells are arranged as part of the typical annuli (Fig. 3a). Within one annulus, two to three labeled cells were observed (Fig. 3b, c). The majority of these immunoreactive cells appear to be part of the luminal epithelium of the basal annuli and therefore contact the central lumen. No staining was observed when the primary antibody was omitted (data not shown). Since most of the GPRC6A expressing cells were found at the base of antral glands, it was of immediate interest to investigate if this receptor may be expressed in G-cells which are located at the base of gastric glands (Mortensen 1980). To approach this question, double-labeling experiments were performed using antibodies for GPRC6A and gastrin, respectively. It was found that the majority of GPRC6A-positive cells also displayed intense gastrin immunoreactivity (Fig. 4a–c). Yet, a small number of GPRC6A-immunoreactive cells did not show any labeling for gastrin (arrowheads Fig. 4c). Counting the number of immunolabeled cells revealed that 81.3% of the GPRC6A-expressing cells were gastrin-positive and that 98.8% of the gastrin-cells expressed the receptor GPRC6A. As the gastrin-negative GPRC6A-cells were located in close vicinity to gastrin-positive cells, D-cells, which are described to be closely located to G-cells and release somatostatin (Gugger and Reubi 2006; Corleto 2010), were considered as candidates. Therefore, tissue sections were assessed with specific antibodies for GPRC6A and somatostatin. This approach revealed that a few of the GPRC6A-positive cells coexpressed the peptide hormone somatostatin (Fig. 5a–c). A rough quantitative analysis revealed that about 27% of the GPRC6A-expressing cells contained the hormone somatostatin and that 70% of somatostatin-positive cells expressed the receptor GPRC6A. A subpopulation of somatostatin-positive cells did not express GPRC6A. These findings indicate that two distinct cell populations in the antral glands express the GPRC6A-receptor; both are endocrine cells responding to nutrients. Therefore, it is conceivable that the GPRC6A receptor may in fact function as a nutrient sensor in endocrine cells of the gastric mucosa. Some recent studies have shown that other candidate nutrient receptors, such as CaSR and T1R3 are also expressed in the stomach mucosa (Cheng et al. 1999; Geibel and Hebert 2009; Hass et al. 2010). Therefore, we have tried to explore, if these receptors are also expressed in the pyloric antral region and if they are expressed in different cells than GPRC6A. As a first step, expression of CaSR and T1R3 in the stomach compartments corpus and antrum was assessed by semi-quantitative RT-PCR analyses. Amplicons for CaSR and T1R3 were obtained with cDNA from corpus as well as from antrum tissue (Fig. 6). Interestingly, the amplification product for CaSR from antrum was stronger compared to corpus (Fig. 6a) which is reminiscent of the results obtained for GPRC6A (Fig. 1). For T1R3 amplicons of similar intensity were obtained from corpus and antrum tissue (Fig. 6b). To follow up the stronger expression levels for CaSR in the antrum, attempts were made to visualize the CaSR-expressing cells. Immunohistochemical experiments with a CaSR-specific antibody resulted in a labeling of numerous cells in annuli of the antral mucosa (Fig. 7a). In longitudinal tissue section it became evident that the CaSR-positive cells were located mainly at the base of antral glands (Fig. 7b). For some of the cells a long process could be visualized which seems to contact the gastric lumen (Fig. 7c). The number and spatial distribution of CaSR-positive cells resembled that of GPRC6A cells in the antrum region. The notion that the two receptors may be coexpressed in the same cells could not be assessed directly via double-immunohistochemical experiments due to the technical limitation that each of the antibodies needed a quite different fixation of the tissue. Based on the finding that most of the G-cells express the receptor GPRC6A (Fig. 4) it was tried to explore, if CaSR is also expressed in G-cells. Double-labeling experiments combining the CaSR antibody with the gastrin antibody clearly demonstrated that the vast majority of CaSR-positive cells also expressed the hormone gastrin (Fig. 8a–c). The extent of co-expression for CaSR and gastrin clearly resembled the overlap of GPRC6A and gastrin depicted in Fig. 4. Therefore, these results strongly suggest a coexistence of CaSR- and GPRC6A-receptors in antral G-cells. In order to explore if the receptor subunit T1R3 is also expressed in G-cells, double-immunohistochemical analysis with a T1R3 antibody and the gastrin antibody were performed. The results are depicted in Fig. 9. Only relatively few T1R3-immunoreactive cells could be visualized at the base of antral glands (Fig. 9a) compared to the large population of gastrin-positive cells (Fig. 9b) and for none of the T1R3-expressing cells a coexpression with gastrin was observed (Fig. 9c).
Structural features of the stomach wall and mucosa in the pyloric antral region. a Digital photograph showing a view onto the mouse stomach. The dotted line indicates the section plane through the gastric antrum. Cryosectioning along this line resulted in an accordant view shown in b. b Exemplary view onto a section along the white dotted line (a); assembly of multiple photographs. In this proximal antrum region, lumina of antrum and corpus are connected. However, there exists an mucosal upfolding between these two compartments. Due to the used DAPI staining, the gastric mucosa appears in a light blue color shape, whereas the submucosa exhibits a more dark blue staining. c Exemplary demonstration of a longitudinal section through the antral mucosa. Mucosal invaginations are longitudinally truncated. Diagonal sectioning along the white dotted line results in a view shown in d. d Diagonal sectioning reveals the unique morphology of the antral mucosa with the characteristic, densely arranged annuli. Sections are stained with DAPI (blue). Scale bars: a = 1 mm; b = 200 μm; c, d = 50 μm. (Color figure online)
GPRC6A-positive cells in the gastric antrum region. Horizontal tissue section through the basal portion of the antral mucosa were assessed with an anti-GPRC6A antibody (red) and counterstained with DAPI (blue). a The anti-GPRC6A antibody labels numerous cells located in basal annuli. b Higher magnification reveals that one annulus contained three GPRC6A-positve cells, which appear to be part of the luminal epithelium of the basal annuli; thus GPRC6A-positive cells seem to have contact to the stomach lumen. c Other annuli comprise only two GPRC6A-immunoreactive cells. Scale bars: a = 20 μm; b, c = 5 μm. (Color figure online)
Most of GPRC6A-positive cells contain gastrin. Assessment of horizontal tissue section through the basal portion of the antral mucosa for GPRC6A (red) and gastrin (green). a GPRC6A-immunoreactive cells in the gastric antrum. b Gastrin-positive cells are located in the same basal region of the antral mucosa. c Overlay of a and b clearly demonstrates that the majority of GPRC6A-positive cells are also gastrin positive. A relatively small number of GPRC6A-positive cells show no labeling for gastrin (arrowheads). Sections are counterstained with DAPI (blue). Scale bars: a–c = 20 μm. (Color figure online)
Few of the GPRC6A-positive cells contain somatostatin (Sst). Assessment of horizontal tissue section through the basal portion of the antral mucosa for GPRC6A (red) and somatostatin (green). a GPRC6A-immunoreactive cells in basal antral annuli. b Few somatostatin-positive cells are located in the same basal regions. c Overlay of a and b reveals the coexpression of GPRC6A and somatostatin in few cells. Sections are counterstained with DAPI (blue). Scale bars: a–c = 20 μm. (Color figure online)
Identification of mRNA for the receptors CaSR and T1R3. Semi-quantitative RT-PCR experiments were performed with primer pairs specific for CaSR (680 bp), T1R3 (500 bp) and rpl8 (202 bp), respectively. a PCR products for CaSR were amplified from cDNAs of both corpus and antrum tissue; with cDNA from antral mucosa the band was stronger than from corpus mucosa. No bands were observed in water controls lacking template. b For T1R3 amplicons of a similar strength were obtained for the corpus and the antrum tissue. No bands were observed in water controls lacking template. (Color figure online)
CaSR-positive cells in the gastric antrum region. a Immunohistochemistry using an anti-CaSR antibody (green) on a horizontal tissue section through the antral region labels a large cell population in the basal annuli of the antral mucosa. b In a longitudinal section plane, CaSR-positive cells are located at the base of pyloric glands. c At higher magnification it became apparent that for some of the cells a long process could be visualized which seems to extend onto the gastric lumen (arrowhead). Sections are counterstained with DAPI (blue). Scale bars: a, b = 20 μm; c = 5 μm. (Color figure online)
The majority of CaSR-expressing cells contain the hormone gastrin. Assessment of horizontal tissue section through the basal portion of the antral mucosa for CaSR (green) and gastrin (red). a CaSR-immunoreactive cells in basal annuli of the antrum region. b Numerous gastrin-positive cells are located in the same basal region. c Overlay of a and b; the majority of CaSR-positive cells are also gastrin positive. Sections are counterstained with DAPI (blue). Scale bars: a–c = 20 μm. (Color figure online)
Localization of T1R3-expressing cells in relation to gastrin-positive cells in the gastric antrum. Assessment of horizontal tissue section through the basal portion of the antral mucosa for T1R3 (green) and gastrin (red). a Few T1R3-positive cells were detected in the basal portion of the antral stomach mucosa. b Gastrin-positive cells are located in the same region of the antrum. c Overlay of a and b; T1R3-positive cells show no immunoreactivity for gastrin. Sections are counterstained with DAPI (blue). Scale bars: a–c = 20 μm. (Color figure online)
Discussion
Sensing of protein breakdown fragments is an essential prerequisite for regulating activities of the stomach, including the secretion of hydrochloric acid and pepsinogen which are governed by the central gastric hormone gastrin. In search for molecular entities which enable gastric mucosa cells to sense luminal constituents that result from proteolysis, the finding of this study that the promiscuous amino acid receptor GPRC6A is expressed in the gastric antral mucosa of the stomach appears to be of high relevance. The observation that apparently all G-cells which release gastrin, the key hormone for regulating gastric activities, express GPRC6A is of particular importance in view of the unsolved question how these cells may sense the luminal content and thereby initiate and regulate the release of gastrin according to the amount of ingested protein. So far, the calcium-sensing receptor (CaSR) has been proposed as a candidate sensor for the regulation of acid secretion and gastrin release (Feng et al. 2010). However, the CaSR is considered as major sensor and regulator of extracellular Ca2+-concentration, which is allosterically affected by amino acids, small peptides and pH (Conigrave and Hampson 2010; Ohsu et al. 2010). In fact, CaSR is supposed to respond primarily to calcium and organic compounds such as polyamines and amino acids alter the EC50 of the CaSR for Ca2+-ions, whereas their direct agonist effects are rather weak (Geibel and Hebert 2009). The finding of this study that both GPRC6A and CaSR coexist in gastrin-cells may be of particular physiological relevance for governing the gastrin release. Interestingly, the response spectrum of both receptors to various proteinogenic L-α-amino acids seems to be quite complementary; whereas the receptor GPRC6A responds to basic and small neutral amino acids (Wellendorph et al. 2005; Kuang et al. 2005; Christiansen et al. 2007), the CaSR responds primarily to aromatic amino acids (Geibel and Hebert 2009). So it is conceivable that the presence of both receptor types provides the endocrine cells with the capacity to recognize a broad spectrum of protein breakdown products. Furthermore, coexistence of both GPRC6A and CaSR receptors in G-cells offers the possibility for an active interplay of both receptor types which could lead to additive or synergistic effects; this could be due to a cross-talk at the downstream signaling pathways. For the class C GPCRs it is well established that they form obligatory dimers and it appears that dimerisation is mandatory for the function of these receptors. In fact, a dimeric receptor complex is essential for the unique mode of activation for class C GPCRs and dimers are indeed the signaling entities (Kniazeff et al. 2011). Although both receptor types appear to be functionally active as homodimers in heterologous systems, it is conceivable that the two receptor types may form heterodimers. Heterodimerization of GPRC6A and CaSR could lead to the formation of a “new” receptor entity. In view of this concept, it is interesting to note, that closely related class C GPCRs, e.g. T1Rs and GABAB receptors, form heterodimers (Conigrave and Hampson 2010); in fact, the closest receptor homologue of GPRC6A is the CaSR (Wellendorph and Bräuner-Osborne 2004). Furthermore, the heterologous expression of GPRC6A in mammalian cell lines has proven very difficult (Kuang et al. 2005; Wellendorph et al. 2005) and has been proposed that GPRC6A may form heterodimers with unidentified partners (Conigrave and Hampson 2010). In addition, analyses of knock-out mice revealed that exon-2 null mice which lack the ligand binding domain, showed complex metabolic disturbance (Pi et al. 2008), whereas the exon-6 null mice which lack the signaling domain of the receptor showed no phenotypic disturbance (Wellendorph et al. 2009b). The results suggest that the recognition domain for amino acids and/or divalent cations is critical for sensing nutrients but that the signaling function of exon-6 is redundant and could be mediated by a signaling partner in a heterodimeric complex. Transcomplementation experiments will be necessary to evaluate if a functional collaboration between the two class C receptors GPRC6A and CaSR is possible as shown for other class C GPCRs including the taste receptors T1R2/T1R3 and T1R1/T1R3 (Chandrashekar et al. 2006), but also between metabotropic glutamate receptors and the calcium-sensing receptor (Chang et al. 2007).
Abbreviations
- CaSR:
-
Calcium-sensing receptor
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- GPCR:
-
G-protein coupled receptor
- GPRC6A:
-
G-protein coupled receptor class C member 6A
- T1R:
-
Taste receptor family-1
References
Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS (2010) Functional expression of the extracellular-Ca2+–sensing receptor in mouse taste cells. J Cell Sci 123:972–982
Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444:288–294
Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, Bettler B, Margeta M, Jan LY, Shoback D (2007) Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem 282:25030–25040
Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, Cima RR, Rogers KV, Hebert SC, Geibel JP, Brown EM, Soybel DI (1999) Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology 116:118–126
Christiansen B, Hansen KB, Wellendorph P, Bräuner-Osborne H (2007) Pharmacological characterization of mouse GPRC6A, an L-alpha-amino-acid receptor modulated by divalent cations. Br J Pharmacol 150:798–807
Conigrave AD, Hampson DR (2006) Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab 17:398–407
Conigrave AD, Hampson DR (2010) Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther 127:252–260
Corleto VD (2010) Somatostatin and the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 17:63–68
Dockray GJ (1999) Topical review. Gastrin and gastric epithelial physiology. J Physiol 518:315–324
Dockray GJ, Varro A, Dimaline R, Wang T (2001) The gastrins: their production and biological activities. Annu Rev Physiol 63:119–139
Dockray G, Dimaline R, Varro A (2005) Gastrin: old hormone, new functions. Pflugers Arch 449:344–355
Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA (2010) Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci 107:17791–17796
Fujita T, Kobayashi S (1977) Structure and function of gut endocrine cells. Int Rev Cytol Suppl 6:187–233
Geibel JP, Hebert SC (2009) The functions and roles of the extracellular Ca2 +–sensing receptor along the gastrointestinal tract. Annu Rev Physiol 71:205–217
Geraedts MC, Troost FJ, Saris WH (2010) Gastrointestinal targets to modulate satiety and food intake. Obes Rev. doi:10.1111/j1467-789X
Gregory RA (1968) Recent advances in the physiology of gastrin. Proc Roy Soc B 170:81–88
Gugger M, Reubi JC (2006) Somatostatin. In: Kastin AJ (ed) Handbook of biologically active peptides. Elsevier, London, pp 1123–1130
Harno E, Edwards G, Geraghty AR, Ward DT, Dodd RH, Dauban P, Faure H, Ruat M, Weston AH (2008) Evidence for the presence of GPRC6A receptors in rat mesenteric arteries. Cell Calcium 44:210–219
Hass N, Schwarzenbacher K, Breer H (2010) T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339:493–504
Ikeda M, Ikui A, Tomita H (2002) Gustatory function of the soft palate. Acta Otolaryngol Suppl 546:69–73
Kniazeff J, Prézeau L, Rondard P, Pin JP, Goudet C (2011) Dimers and beyond: the functional puzzles of class C GPCRs. Pharmacol Ther 130:9–25
Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR (2005) Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem 93:383–391
McArthur KE, Isenberg JI, Hogan DL, Dreier SJ (1983) Intravenous infusion of L-isomers of phenylalanine and tryptophan stimulate gastric acid secretion at physiologic plasma concentrations in normal subjects and after parietal cell vagotomy. J Clin Invest 71:1254–1262
Mortensen NJ (1980) The anatomy of the gastrin cell. Ann R Coll Surg Engl 62:462–469
Mulholland MW, Debas HT (1988) Physiology and pathophysiology of gastrin: a review. Surgery 103:135–147
Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y (2010) Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem 285:1016–1022
Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209
Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J et al (2008) GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3:e3858
Quiocho FA, Ledvina PS (1996) Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol 20:17–25
Wellendorph P, Bräuner-Osborne H (2004) Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335:37–46
Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H (2005) Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol 67:589–597
Wellendorph P, Burhenne N, Christiansen B, Walter B, Schmale H, Bräuner-Osborne H (2007) The rat GPRC6A: Cloning and characterization. Gene 396:257–267
Wellendorph P, Johansen LD, Bräuner-Osborne H (2009a) Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol 76:453–465
Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P et al (2009b) No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42:215–223
Wellendorph P, Johansen L, Bräuner-Osborne H (2010) The emerging role of promiscuous 7TM receptors as chemosensors for food intake. Vitam Horm 84:151–184
Zeng N, Zeng TK, Walsh JH, Scott D, Sachs G (1995) Specific amino acids activate calcium signals in isolated G cells of rat antrum. Gastroenterology 108:A1020
Acknowledgment
The authors would like to thank Kerstin Bach for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, BR 712/25-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haid, D., Widmayer, P. & Breer, H. Nutrient sensing receptors in gastric endocrine cells. J Mol Hist 42, 355–364 (2011). https://doi.org/10.1007/s10735-011-9339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-011-9339-1