Abstract
Metals, metalloids and nonmetals concentrations along the Suquía River basin have been monitored in sediment and surface water, during the wet and dry season, at different points and by different authors since 1997 until 2014. The potential ecological risk (PER) in surface sediments along some studied stations is presented on the basis of measured data.
In general, metal/loids concentrations were highest in sediments and lower in water, being sediments the major sink for metal/loids pollution in this river. The concentrations of metal/loids from the Suquía River pristine areas (upper catchment) were, as expected, the lowest measured. It was also demonstrated how the environmental impact of Córdoba City (e.g. WWTP discharge) becomes evident in the Suquía River basin, which is not only marked by the presence of metals at a sampling station located few kilometres downstream the WWTP but also by the influence of agricultural and small industrial activities downstream from Córdoba City.
According to ecological risk indexes of metal/loids in the pseudo-total fraction of sediments, the best scenario was found in La Calera (LC), upstream from Córdoba City. Results indicate that this site presented low to moderate ecological risk. On the other hand, the worse situation is observed in Corazón de María (CM), ca. 16 km downstream the WWTP, where the ecological risk ranges from moderate to severe.
The use of Generalised Procrustes analysis (GPA) shows that the different ecological compartments studied (water and sediment) are closely related and that the interaction between them determines the characteristics of each site.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent decades, studies have been conducted to evaluate the pollutants that are discharged into different water sources. Many of these contaminants are not detected in the water column, and, in order to know their fate and effect on the environment, numerous studies have been carried out worldwide in the last decade to assess concentrations of contaminants not only in waterbodies but also in sediments, suspended material, etc. Sediment has been considered a sink of contaminants, and a record of anthropogenic pollution, since the input of diverse contaminants in the water column is many times stored in the sediment (settling) or transported (adsorbed–absorbed) associated with particulate matter [1]. However, available metals in the sediment could be also reintroduced into the water or be uptaken by plants and benthic organisms [2].
Metals are among the main pollutants, since they are easily transported and accumulated in the environment. They are considered serious pollutants due to their persistence in the environment, bioaccumulation and high toxicity [3]. These compounds may be biomagnified through the food chain, resulting in sublethal concentrations affecting the biota, or even reaching concentrations that are lethal to local populations [4].
The study of metal/loids in river waters and sediments is a contribution to the provision of information on the environmental character of these rivers and also to the diagnosis of each of their catchment areas, facilitating the decision making, especially at the government level. Toxic metal/loids are a major environmental concern because of their toxicity to both humans and animals as in the case of fish impact. Investigating the presence of toxic metal/loids in certain water reservoirs can improve the knowledge about the routes of contaminants and their interaction with other substances and organisms in the water.
The presence of toxic metal/loids in waters and sediments of rivers also causes a serious health problem to the inhabitants of populations served by these rivers, which implies an increased spending on medical treatments, a reduction in the productive capacity of residents and, of course, a negative economic impact.
The origin or presence of metal/loids in coastal sediments can be originated from physical and chemical weathering of parent rocks, wastewater discharge and atmospheric deposition [5]. Metal/loids discharged into aquatic systems are distributed between the aqueous phase and sediments during their transport. Due to adsorption, hydrolysis and co-precipitation of soluble ions, a large quantity of these metal/loids are deposited in the sediment, while only a small portion of free ions stay dissolved in the water column. The accumulation and mobility of elements in sediments is controlled by various factors, such as the nature of the sediment, properties of adsorbed compounds, metal/loid characteristics, redox reactions and biodegradation of sorptive substances under specific conditions [6,7,8,9,10]. Hence, sediments are enumerated as a major source of metal/loids in the environment, playing a key role in their transmission and deposition. Accumulated metal/loids in sediments can be chemically altered by aquatic organisms and converted into organic complexes, some of which may be more hazardous to animal and human life, via the food chain.
When environmental conditions change (pH, cationic exchange capacity, nutrient status, redox potential, etc.), some of the sediment-bound elements may be remobilised and released back into the water, where they can have adverse effects on living organisms [11]. In fact, the mobility of metal/loids in the environment strongly depends on their chemical forms or types of binding of the elements [12]. Numerous analytical techniques have been used to identify the key factors that control distribution and speciation of metal/loids in coastal and estuarine sediments in order to understand their mobility and potential ecological risks [13].
Sediments from various water environments reveal the differences in hydrodynamic regime, redox potential, sorting process, mineral and chemical components. These differences are reflected by geochemical properties of sediments [14]. River sediments usually derive from ambient soils and road deposits [15]. These sediments undergo the effect of one-way water flow and exhibit a relatively high proportion of coarse matter [16].
The sediment contamination by inorganic elements is traditionally evaluated in terms of total concentrations or pseudo-totals of each element; however, it is shown that the danger that toxic elements pose to living organisms is determined more by their availability to living organisms than by their total concentration [17]. For the extraction of the pseudo-total fraction of sediments, a mix of HCl and HNO3 at different proportions is commonly used, being the extraction performed during long times at high temperatures. Conversely, the available fraction is extracted using various reagents and different extraction methods. Among the methods reported in the literature, the use of diluted hydrochloric acid (0.5 M) is a low-cost and widely used procedure to extract the available fraction [18]. In connection with this last method, the use of 0.5 M HCl [19] satisfies the minimum requirements for the extraction of metal/loids that are part of the exchangeable fraction, with minimum disturbance of the silicate matrix [17, 18, 20]. Thus, metal/loids extracted by this method can be interpreted as the mobilisable fraction of metal/loids in soil, mainly because diluted HCl releases the metal/loid carbonates associated with Fe and Mn oxides [20]. Studies of metal/loids in the sediment of the Suquía River basin include both total and bioavailable fractions; so, from now on, the discussion will explain to which sediment fraction the metal/loid belongs.
2 Metals, Metalloids and Nonmetals
“Heavy metal” is a somewhat imprecise term commonly used to refer to certain metals and some of their related compounds, to which certain environmental pollution, toxicity and ecotoxicity effects are attributed.
According to the International Union of Pure and Applied Chemistry (IUPAC), the term “heavy metal” may be a “meaningless term”, because there is no standardised definition for a heavy metal. In fact, some light metals or metalloids are toxic, while some high-density metals are not. For a given metal/loid, the toxicity varies widely depending on its allotrope or oxidation state. For instance, hexavalent chromium is deadly; while trivalent chromium is nutritionally significant in many organisms, including humans. Today, a new classification is being used:
-
Metals are generally shiny, malleable and hard. Metals are also good conductors of electricity. Examples of metals are gold, silver, iron, uranium and zinc.
-
Nonmetals do not conduct heat or electricity very well. Nonmetals are typically brittle and are not easily moulded into shapes. Examples of nonmetal elements are selenium and phosphorous.
-
Metalloids share characteristics of both metals and nonmetals and are also called semimetals. Metalloids are typically semiconductors, meaning that they both insulate and conduct electricity. This semiconducting property makes metalloids very useful as a computer chip material. Examples of metalloid elements are arsenic and boron.
So, metals, metalloids and nonmetals are naturally present in the soil, at concentration levels called background levels or simply “background”, whose origin is not external. Background levels come from the original parent rocks. Often found as cations, they strongly interact with the soil matrix, which sometimes means that even at high concentrations they can be found in harmless concentrations or as chemically inert forms. However, these elements can move and change their shape due to chemical changes in response to different environmental conditions [21].
For the exposed general characteristics, it is necessary to identify the source of these elements in benthic sediments of waterbodies. There are different sources of metals, metalloids and nonmetals in the environment. These sources can be either of natural or anthropogenic origin [5, 22].
The weathering of rocks and soils, directly exposed to the action of water, is the major contribution from natural sources. On the other hand, human activities such as agriculture, industry and urban waste are of great importance to the contribution of these inorganic compounds in the sediment of natural water courses [5].
2.1 Anthropogenic Sources of Inorganic Compounds
Metals, metalloids and Se are released into the environment by many human activities. They are also used in a large variety of industrial products, which in the long term have to be deposited as waste. They are released into the environment at the beginning of the production chain, whenever ores are mined, or during the use of products containing them, and also at the end of the production chain (trash, etc.). Here, we present an overview on anthropogenic sources and uses of these inorganic compounds, through which they can be introduced into the environment. The natural sources are dominated by parent rocks and metallic minerals, while the main anthropogenic sources are agricultural activities, where fertilisers, animal manures and pesticides containing metal/loids are widely used. Also, metallurgical activities, including mining, smelting, metal finishing among others, in addition to energy production and transportation, microelectronic products and waste disposal, contribute as anthropic sources of metal/loids. Furthermore, metals, metalloids and nonmetals can be released into the environment in gaseous, particulate, aqueous or solid form, emanating from both diffuse and point sources [5].
-
As: Used as additive to animal feed, wood preservative (copper chrome arsenate), special glasses, ceramics, pesticides, insecticides, herbicides, fungicides, rodenticides, algaecides, sheep dip, electronic components (gallium arsenate semiconductors, integrated circuits, diodes, infrared detectors, laser technology), nonferrous smelters, metallurgy, coal-fired and geothermal electrical generation, textile and tanning, pigments and anti-fouling paints, light filters, fireworks, veterinary medicine
-
Be: Used in alloys (with Cu), electrical insulators in power transistors, moderator of neutron deflectors in nuclear reactors
-
Cd: Used in Ni/Cd batteries, pigments, anticorrosive metal coatings, plastic stabilisers, alloys, coal combustion, neutron absorbers in nuclear reactors
-
Co: Used in metallurgy (superalloys), ceramics, glasses, paints
-
Cr: Manufacturing of iron alloys (special steels), plating, pigments, textiles and leather tanning, passivation of corrosion of cooling circuits, wood treatment and audio, video and data storage
-
Cu: Good conductor of heat and electricity, water pipes, roofing, kitchenware, chemicals and pharmaceutical equipment, pigments, alloys
-
Fe: Cast iron, wrought iron, steel, alloys, construction, transportation, machine manufacturing
-
Hg: Extracting of metals by amalgamation, mobile cathode in the chloride–alkali cell for the production of NaC1 and Cl2 from brine, electrical and measuring apparatus, fungicides, catalysts, pharmaceuticals, dental fillings, scientific instruments, rectifiers, oscillators, electrodes, mercury vapour lamps, X-Ray tubes, solders
-
Mn: Production of ferromanganese steels, electrolytic manganese dioxide for use in batteries, alloys, catalysts, fungicides, antiknock agents, pigments, dryers, wood preservatives, coating welding rods
-
Mo: Alloying element in steel, cast irons, nonferrous metals, catalysts, dyes, lubricants, corrosion inhibitors, flame retardants, electroplating
-
Ni: Alloying element in the steel industry, electroplating, Ni/Cd batteries, arc-welding, rods, pigments for paints and ceramics, surgical and dental prosthesis, moulds for ceramic and glass containers, computer components, catalysts
-
Pb: Antiknock agents, tetramethyllead, lead–acid batteries, pigments, glassware, ceramics, plastic, in alloys, sheets, cable sheathings, solder, ordinance, pipes or tubing
-
Sb: Type-metal alloy (with lead to prevent corrosion), in electrical applications, Britannia metal, pewter, Queen’s metal, in primers and tracer cells in munition manufacture, semiconductors, flameproof pigments and glass, medicines for parasitic diseases, as an expectorant, combustion of fossil fuels
-
Se: In the glass industry, semiconductors, thermoelements, photoelectric and photo cells, and xerographic materials, inorganic pigments, rubber production, stainless steel, lubricants, dandruff treatment
-
Sn: Tin-plated steel, brasses, bronzes, pewter, dental amalgam, stabilisers, catalysts, pesticides
-
Ti: For white pigments (TiO2), as UV-filtering agents (sun cream), nucleation Agent for glass ceramics, as Ti alloy in aeronautics
-
Tl: Used for alloys (with Pb, Ag or Au) with special properties, in the electronics industry, for infrared optical systems, as a catalyst, deep temperature thermometers, low melting glasses, semiconductors, supra conductors
-
V: Steel production, in alloys, catalyst
-
Zn: Zinc alloys (bronze, brass), anticorrosion coating, batteries, cans, PVC stabilisers, precipitating Au from cyanide solution, in medicines and chemicals, rubber industry, paints, soldering and welding fluxes
3 Metals, Metalloids and Se in Water and Sediment from the Suquía River Basin: Studies Over the Years
The Suquía River basin has been monitored since 1991. The first study on metals (Mn, Fe, Zn, Pb, Cu and Ni) in the available fraction of Suquía River sediments was reported by Gaiero et al. [23].
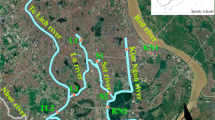
In this study, river sediments were sampled in two seasons. Samples were collected in June 1991 (autumn), after the rainy season, and in October 1991 (spring), after the dry winter period, coinciding with the initial phase of the rainy season. Eight sampling stations (S1 to S6, and LI–L2) were established along the main course. Two of these stations (L1 and L2) were located in the mixing zone of the Mar Chiquita Lake, where the Suquía Rivers discharges its water into the lake. The active upper catchment was also sampled in eight additional stations: IC1–IC2, Y1–Y2, SFI–SF2 and LM 1–LM2 (Fig. 1).
Stations IC1, Y1, LM1 and SF1 were representative of the conditions dominating in the upper catchments of the main tributaries.
These stations, located in the Punilla Valley, were mainly placed on modern sedimentary terrain and were subjected to various degrees of environmental impact (Table 1). The city of Córdoba is Argentina’s second largest urban and industrial centre. To show its impact on the river, stations S1 and S2 were located upstream and downstream from the city. Stations S3, S4 and S5 were distributed along the lower 100 km section, upstream from the river mouth in the Mar Chiquita Lake. Small towns, with less than 6,000 inhabitants, justified the location of S3 and S4. Stations S5, S6, Ll and L2 were influenced by extensive farming activities.
Table 1 lists Mn, Fe, Zn, Pb, Cu and Ni concentrations in sediment measured during the wet and dry season at the different sampling sites from the Suquía River basin.
During this first study, the uppermost area exhibited a low population impact; thus, it was considered a nearly pristine basin in terms of potential man-made sources of metals.
The concentrations of these metals in sediment at sites located in the upper basin showed many similarities; this could be attributed to the similarity in the geochemical conditions in these places (Table 1). The concentrations of some metals were slightly affected by the different hydrological conditions (dry or wet/rainy seasons).
As expected, concentrations of some metals like Pb and Ni exhibited lower values along the entire basin, probably due to the generation of hydrous oxides by weathering reactions, while Fe and Mn exhibited a highly relative abundance in the sediment fraction.
In the upper basin, areas considered as representative between the transition of low and moderate population were stations LM1, LM2, IC2 and Y2.
The increase in the concentrations of some elements (e.g. Pb, Cu, Zn) in these transition zones is clearly related to the increase in urban settlements with respect to the pristine areas. In contrast, Fe and Mn showed no significant changes with values recorded in the upper pristine basin.
Sampling stations SF1 and SF2 correspond to the San Francisco River (Fig. 1a). This river drains through a valley (Punilla Valley), where urban activities release different wastes, with little or no treatment, into the riverbed. In these sampling sites, increased levels of Pb, Cu and Zn during the rainy season were observed, in agreement with increased levels of organic matter and, to a lesser extent, precipitated carbonates [23]. In the dry season, a drop in Pb, Cu and Zn concentrations was observed [23]. On the other hand, concentrations of Fe and Mn in these sites were among the lowest throughout the entire basin; a possible explanation for this might be associated with reducing conditions in this area. Ni concentrations showed minimal temporal and spatial changes.
During the wet season, an increase in the concentrations of metals downstream from sampling sites SF1 and SF2 was observed, in addition to an increase in the content of organic matter and carbonates.
The upper basin supplies the water stored at the San Roque reservoir (Fig. 1). This dam is considered the limit between the upper and the medium drainage basin, where the city of Córdoba is located. The river crosses the city, receiving industrial and municipal effluents as well as urban runoff inputs.
The concentrations of most of the measured elements (with the sole exception of Mn) were higher downstream from Córdoba City, while a subsequent decrease in the downstream direction was also observed (Table 1). Metal concentrations, measured at the sampling point S2, were higher during the wet season compared to the dry one, in opposition to values recorded in both the upstream and downstream sections. This increase was approximately 40% above base values during the dry season, and it can be attributed to metals washed out from the city via urban runoffs.
Downstream from the city of Córdoba, in S2 monitoring station, a marked reducing environment determines low concentrations of Mn and high concentrations of Fe. Such reducing environment is likely to be caused by the discharge of the wastewater treatment plant (WWTP), which causes a severe oxygen drop downstream, leading to such reducing conditions. Under these conditions, Fe, along with other metals, probably precipitates as sulphide, given the presence of bioavailable organic matter, sulphates and other oxidising compounds – such as Fe+3 [24].
A reasonable explanation for the observed decrease of most heavy metals, further downstream from S2, is the dilution by “native sediments”, relatively free from heavy metals, introduced into the main stream by bank erosion from the surrounding area. This river section does not present major point sources of metals, although minor fluctuations can be attributed to the presence of small towns located along the riverbank.
Finally, stations L1 and L2 represented the transition zone between the freshwater river mouth (L1, conductivity: 23,754 μS) and the Mar Chiquita saline lake (L2, conductivity: 27,000 μS). Settling of small particles determined the increase observed with most metal concentrations (Fig. 1).
As in some estuaries (e.g. [25]), the concentrations of Fe appeared to be higher in the low-salinity river mouth than in the high-salinity sector. Probably, Fe (along with A1 and Ti) remained associated with fine colloidal particles in offshore waters [26]. An increase of organic matter and carbonates in bottom sediments (L2) from the saline river mouth was also observed [23].
Some years later, Contardo-Jara et al. [27] also reported the amounts of the available metal fraction, extracted from field-sampled sediments and surface water during the spring of 2007. In this case, four sampling sites were monitored, covering a pollution range from quasi-pristine to heavily polluted areas. The monitoring station at Río Yuspe corresponds to the Y2 station in Gaiero et al. [23]. A second station, El Diquecito, located 30 km upstream from Córdoba City, is slightly polluted as a consequence of less treated sewage and urban runoff from smaller cities further upstream from the eutrophic San Roque reservoir [28], where the Suquía River is born (Fig. 1). A third monitoring station was Isla de los Patos, located close to Córdoba City downtown, where the river is flanked on both sides by frequently used highways. Further reasons for the pollution at Isla de los Patos are in connection with urban drainage (runoff), where illegal garbage and domestic sewage is sometimes introduced. The most polluted site reported by Contardo-Jara et al. [27] was Corazón de María, located ca. 16 km downstream the WWTP (Fig. 1). It is worth noting that only 0.7 out of 1.2 million inhabitants of Córdoba City are connected to the municipal sewage, with the rest discharging home-treated sewage (septic tanks) into cess pools, which then infiltrate the ground and pollute the groundwater. This last site (Corazón de María) corresponds to the S2 sampling site in the work of Gaiero et al. [23].
Contardo-Jara et al. [27] showed that metals tend to concentrate in the sediment, where they reach concentrations of several magnitudes higher than in the overlaying water.
Conversely, iron showed the highest concentration in sediments of the Yuspe River (514 μg g−1), which could be a consequence of the geological composition of the surrounding soil (metamorphic granite with gneiss ducts). This result cannot be compared with previous studies [23], since Fe was not reported during spring monitoring in this previous work.
Iron content in surface water in Yuspe River (24.1 μg L−1) was in the same magnitude as the most polluted site Corazón de María (33.5 μg L−1). At Isla de los Patos, even higher amounts were detected (55.2 μg L−1), while at El Diquecito values were below detection limit. In some cases, metal content in sediments did not show a clear increasing or decreasing trend throughout the studied basin section (e.g. Co, K, Mn, Na). Others metals are strongly associated with human activities or sewage, showing their highest levels at Corazón de María compared to the other studied basin sections (Cr, 1.36 μg g−1; Cu, 17.45 μg g−1; Mg, 913 μg g−1; Ni, 7,08 μg g−1; Pb, 11.8 μg g−1; and Zn, 160 μg g−1)
Changes in copper concentration in basin sediments seem to be associated with urban activities, changing by almost sixfold from Río Yuspe (1.52 μg g−1) to El Diquecito (8.64 μg g−1) and Isla de los Patos (8.64 μg g−1), with a further increase by more than tenfold at Corazón de María (17.45 μg g−1) with respect to Yuspe River. This trend was also reported by [23] some years before during the spring time (Table 1).
Concentrations of Fe, Cu, Ni and Pb in sediment collected during spring time at both Y1 and S2 stations in Gaiero et al. [23] were higher in all cases than concentrations reported by Contardo-Jara et al. [27] in the same site, with the exception of Mn and Zn in S2 (Corazón de María) station, where in both papers they showed similar concentrations.
Nickel amounts in surface water of Yuspe River (17.8 μg L−1) are strikingly high, being sixfold higher than in Corazón de María (2.6 μg L−1), which can be explained by the geochemical background of the surrounding soils [27].
Two years later, Monferrán et al. [29] reported concentrations of Ag, Cr, Cu, Mn, Ni, Pb, Fe and Zn in the available fraction of sediments and surface water throughout five stations studied for the period 2008–2009 at the Suquía River basin, during the dry and rainy season.
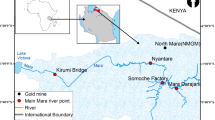
Sampling areas used by Monferrán et al. [29] were selected, considering previous reports on pollution sources and water quality of the Suquía river basin [23, 27, 30, 31]. All of these reports point out to Córdoba City as the main responsible area for the pollution of the Suquía River. So far, a reference area located upstream from the city (La Calera,LC; Fig. 1) was established. The four sampling areas located downstream from Córdoba City, Corazón de María (CM), Capilla de los Remedios (CR), Río Primero (R1) and Santa Rosa de Río Primero (SR) are primarily affected by the input of pollutants from the city sewage [30, 31]. Closer to the WWTP, downstream from Córdoba City, the basin could receive agricultural runoffs or additional domestic wastes [23].
The mean values, determined in both water and sediment by Monferrán et al. [29], are given in Table 2. Clearly, sediments show the negative impact of the city, with increased amounts of Pb, Cu, Cr and, particularly, Zn. Considering previous reports [23, 27, 31], it is likely to think that these metals arise from the city WWTP, though this point cannot be definitively concluded because the sewage exit was not analysed during these works. On the other hand, Ni remained roughly constant in sediments throughout the studied area; this trend was also reported by [23] some years before (Table 1). It is worth to mention that the amount of Fe is drastically reduced in sediments downstream from Córdoba City but proportionally increased in the water. Thus, in agreement with reports by [23], it is demonstrated that the tendency remained unchanged over the time (>10 years).
Additionally, higher values of dissolved Cr, Cu, Mn, Ni and Pb are observed during the wet (rainy) season, probably due to the increased amount of these metals coming from the urban runoffs at the beginning of the rainy season.
The uppermost area (La Calera) exhibits low population impact, and it is considered quasi-pristine in terms of potential man-made source of toxic metal/loids. Thus, current results show that the riverbed sediment is projecting a clear image of the impact produced by diverse activities, but it is mainly affected by the city sewage.
In some cases, as previously reported by others authors, the levels of soluble metals show the impact of the WWTP discharge, followed by a drop downstream from this point (i.e. Cr and Mn at Corazón de María – CM – and further downstream, Table 2). However, other metals like Cu showed the highest values at R1 during the wet season (Table 2), which is less influenced by the sewage discharge. In this case, high concentrations of soluble Cu could be the consequence of agricultural runoffs (CuSO4 is used as a common fungicide in this area) or any other point source pollution.
Considering the studied metals in stream sediments by Monferrán et al. [29], it can be seen that concentrations of Cu, Zn and Pb were lowest at the reference site (LC). The environmental impact caused by Córdoba City (e.g. WWTP) became evident in the Suquía River system because of some toxic metals (Zn, Cu and Pb) at CM, with moderate or less drop further downstream (Table 2). Thus, the impact of sewage point source pollution is reflected downstream in river sediments, though values in water tend to decrease (Table 2). This trend was also observed in previous years [23, 27].
Many pollutants measured during the work of Monferrán et al. [29] are well above levels considered as hazardous for aquatic life, exceeding the levels of the Argentinean Environmental Water Quality Guidelines [32]. For instance, values observed for Cr at LC, CM, CR and SR during the wet season (Table 2) clearly exceed the threshold-regulated value of 2.5 μg L−1. A similar situation is observed with Pb, which exceeds the threshold value (1.6 μg L−1) throughout the entire basin during the wet season and at CM during the dry season. In the sediment, some metals exceed the risk levels defined by the Management of Aquatic Sediment Quality ([33]; Argentinean regulations do not stipulate guideline values for sediments). Concentrations of Cu (17.5, 20.1 and 22.1 μg g−1 DW at CM, CR and R1, respectively; Table 2) were in excess up to 1.4-fold (threshold value, 16 μg g−1 DW), while loadings of Ni (ca. 17 μg g−1 DW at CM, CR and R1; Table 2) also exceeded levels for the protection of the aquatic biota established in Canada (16 μg g−1 DW).
These results complement the previous measurements of metal levels in available fraction in sediments of the Suquía River basin [23, 27]. Thus, current Cu, Ni and Pb concentrations in sediments are similar to those previously reported by Gaiero et al. [23] at similar monitoring places. Current concentrations of Zn present lower values upstream from Córdoba City but higher values downstream. It is worth to mention that Fe in sediments presents much higher values during this work in comparison to previous reports by Gaiero et al. [23].
Later on, Monferrán et al. [34, 35] reported concentrations of metals, metalloids and Se (Li, B, Be, Al, V, Cr, Mn, FMo, Ag, Cd, Ce, Hg, Tl, e, Co, Ni, Cu, Zn, As, Se, Rb, Sr, Pb, Bi, U, Pd, Sn, Sb, Pt and Au) in the pseudo-total fraction of sediments and water throughout five studied stations at the Suquía River basin: La Calera (LC) was established as the reference area located upstream from the city and four sampling areas downstream from Córdoba City, Corazón de María (CM), Rio Primero (R1), Santa Rosa de Rio Primero (SR) and La Para (LP) (Fig. 1).
Higher values of dissolved Al, V, Mn, Co, Ba and Ce were observed during the wet season; this could be attributed to runoffs of the basin area during rainfall (Table 1); higher values of dissolved elements were observed in the dry season in comparison to the wet season. In some cases, the levels of soluble metal/loids showed the impact of the WWTP discharge, followed by a drop downstream from this point source (i.e. Cr, Mn, Hg, Ni, Cu, Zn, Pb and Sn at CM and further downstream) (Table 3). These results agree with those reported by Contardo-Jara et al. [27] and Monferrán et al. [29].
It can be seen that levels of As in the water increase as the river flows towards the Mar Chiquita lake (1.8 to 14.6 μg g−1 from west to east). The Chaco–Pampas plain in Argentina is considered the largest region in the world (one million km2) affected by the presence of arsenic in groundwater. Within this region, the eastern part of the Province of Cordoba is one of the most affected areas. Levels of As reported by different authors in surface waters from this area are generally lower than those reported in groundwater. In rivers and lakes, the average concentration of As reported in the literature is generally less than 0.8 μg L−1. However, downstream from Córdoba City, the Suquía River flows through an area with intensive agriculture and stockbreeding, where there is a frequent extraction of groundwater for irrigation purposes and the provision of drinking water to cattle. Thus, As contained in this groundwater can reach the river in this area, increasing levels of this metalloid in surface waters [36].
Finally, Harguinteguy et al. [37] also reported levels of some metals (Co, Cu, Fe, Mn, Ni, Pb and Zn) in surface water and sediment samples of the Suquía River. In this case, sampling was carried out in July 2006 and February 2009, during the dry and wet seasons. To evaluate the spatial variation, they selected seven sampling sites:
-
Site 1 (31°21′60″ S, 64°30′52″ W, 766 m), established as the reference, was located on Los Chorrillos brook before the San Roque reservoir (Fig. 1).
-
Site 2 (31°20′36″ S, 64°21′18″ W, 539 m) was located 18 km upstream from Córdoba City, before La Calera town.
-
Site 3 (31°17′54″ S, 64°19′53″ W; 594 m) was located 15 km upstream from Córdoba City, before the Saldán brook.
-
Site 4 (31°19′16″ S, 64°18′58″ W, 516 m) was located on the Saldán brook, before the mouth of the Suquía river.
-
Site 5 (31°20′46″ S, 64°16′58″ W; 463 m) was located 12 km upstream from Córdoba City, after Villa Rivera Indarte, upstream from Córdoba downtown.
-
Site 6 (31°24′19″ S, 64°05′29″ W, 397 m) was located 1 km downstream the WWTP.
-
Site 7 (31°25′48″ S, 64°01′22″ W, 360 m) was located 9 km downstream from Córdoba City, after the discharge of a channel containing industrial effluents (automotive, metallurgical and metal–mechanical industries) in the southeast of Córdoba City.
Metal concentrations in surface waters found by Harguinteguy et al. [37] in 2006 and 2009 revealed significant differences between the sampling sites. In general, metal concentrations were higher downstream from Córdoba City (Sites 6 and 7) in both sampling campaigns, which was probably related to the contribution of pollutants from effluent discharges from anthropogenic sources (WWTP and the industrial channel). The mean concentrations of all metals in river water, except for Cu and Pb, were well above the levels considered hazardous for aquatic life, exceeding the levels established by the Argentinean Environmental Water Quality Guidelines [32].
It should be mentioned that metals in sediment in this work resulted in concentration values much higher than those observed in previous studies conducted in the same river and in the same sampling stations [23, 27, 29]. This could be due to methodological differences as Harguinteguy et al. [37] measured the pseudo-total fraction in sediment, while previous works reported the labile fraction [23, 27, 29]. The evaluation of pseudo-total concentrations involves a more exhaustive extraction than the one performed to determine the bioavailable or labile fraction. However, results by Harguinteguy et al. [37] can be compared to those reported by Monferrán et al. [34, 35].
Reports by Harguinteguy et al. [37] and Monferrán et al. [34, 35] show the negative impact of Córdoba City, particularly through the WWTP and industrial channel discharges. Thus, downstream from the city, increased amounts of Pb, Cu, Cr, Zn, Cd, Ni, Hg, Bi, Sn and Pt were observed. On the other hand, Be, Co, V, Rb, Tl and Pd remained roughly constant in sediments throughout the studied area.
It is worth to mention that Harguinteguy et al. [37] reported that levels of Fe in sediments were higher in 2009 than in 2006 (5,842 and 7,892 μg g−1, respectively), with the maximum concentrations of this metal being registered in 2009 in areas where large amounts of organic matter were deposited (site 6 and 7, corresponding to the site CM in Monferrán et al. [34, 35] work). In this regard, Charzeddine et al. [38] noted that the external supply of Fe in the rainy season was able to form colloidal dispersions of amorphous iron hydroxide, Fe(OH)3 and goethite, α-FeO(OH), which were retained by the organic matter in sediments. Similarly, Wedepohl [39] indicated that this element is found in large proportions in the upper crust, and consequently, its concentrations in aquatic environments tend to increase considerably due to the drag action exerted by rainfall, surface runoff and/or leaching. This increase in iron concentration in sediments during the wet season, compared to the dry season, is not as marked in Monferrán et al.’s [34, 35] work, probably because of methodological differences, as Monferrán et al. [34, 35] monitored dry and wet season within the same year (2012), while Harguinteguy et al. [37] reported results from the dry season of 2006 and the rainy season of 2009. So far, consideration of the hydrological issues and the analytical method used is necessary to compare results by different authors, taken in different years, under different weather conditions. A normalisation of data should be attempted considering the total load of metals and metalloids transported by the river. Unfortunately, the lack of hydrological stations coincident with monitoring sites precludes such data normalisation.
3.1 Metals and Metalloids Concentrations in Water and Sediment of the San Roque Reservoir
Seventeen elements (Mn, Fe, Zn, Cu, Cd, Cr, Ni, Ag, Mo, Nd, Al, Ce, As, Sr, Pb, Pt and Hg) were sampled from water and sediment on the San Roque reservoir (Fig. 1) during both wet and dry seasons throughout 2012 [35]. In this case, the available fraction of sediments was analysed.
The mean values, determined in both water and sediment, are given in Table 3. In general, the highest concentrations for measured metal/loids in water were detected during the dry season (P < 0.05) (Table 3). This could be the result of low water volumes supplied by tributaries during the dry season, resulting in a concentration of studied elements in the reservoir because of the lower water amount. Conversely, higher flows observed during the wet season could dilute elements in the reservoir (Table 3). These results are in agreement with the previously detected trend, measuring several physical and chemical parameters in the lake [31]. Some of the measured elements exceed the limit considered dangerous to aquatic wildlife, established by the Argentinean Environmental Water Quality Guidelines [32]. For instance, values observed for Al, Cu, Cr, Fe, Ni and Zn, during the dry season (Table 3), clearly exceed the threshold regulated (100, 2.87, 2.5, 1.37*, 4.2, 4.54 μg L−1, respectively, with the exception of Fe, where values are expressed as mg L−1). A similar situation is observed with Cu and Zn during the wet season, exceeding the threshold value (2.87 and 4.54 μg L−1, respectively).
Water pollution has also affected the upper layer of sediment (0–15 cm). The highest concentrations for most measured metals in reservoir sediments were detected during the dry season (P < 0.05) (Table 3). Sediment samples presented different textures along the studied period, varying from low to high silt sludge. It is noticeable that the deposition of suspended material, due to the slow water flow (larger residence time) during the dry season, determined a high metal concentration in sediments, in contrast with more sandy sediments typical of the rainy season (Table 1). These results complement the few previous measurements of metal/loids in sediments of the San Roque reservoir. Thus, current Cr, Cu, Ni and Fe concentrations in sediments are lower than those previously reported by Monferrán et al. [29], in sediments of the Suquía River, close to the San Roque dam (La Calera) (Fig. 1) during both wet and dry seasons (Table 2). Although Zn concentration presents higher values in the San Roque reservoir than those previously found in La Calera, both concentrations do not exceed the risk levels defined by the Canadian Guidelines for the Protection and Management of Aquatic Sediment Quality ([33], Argentinean regulations do not stipulate guideline values for sediments).
4 Ecological Risk Assessment
Potential ecological risk was calculated using Håkanson [8] methodology in which the sensitivity of the aquatic system depends on its productivity. The potential ecological risk index (RI) was introduced to assess the degree of heavy metal pollution in sediments, according to the toxicity of metal and metalloids pollution and the response of the environment:
where RI is calculated as the sum of all risk factors for metals studied in sediment, E ir is the monomial potential ecological risk factor, T ir is the toxic-response factor for a given substance, which accounts for the toxic and sensitivity requirements. C if is the contamination factor, C io is the concentration of metals in sediment and C in is a reference value for metals (Table 3).
The risk factor RI proposed by Håkanson [8] was based on eight parameters (PCB, Hg, Cd, As, Pb, Cu, Cr and Zn) measured in total or pseudo-total fraction of sediments. To calculate the ecological risk assessment, the data presented in Table 4 [34, 35] was used, since this study contains much elements measured in pseudo-total fraction, excluding PCB that was not measured during this work. Using Eq. (1) and the parameters listed in Table 4, the potential ecological risk indexes and R1 for each sampling site were calculated. T ir is the toxicity coefficient, which represents the toxic-response factor for a given metal/loid. The value of T ir for Hg, Cd, As, Cu, Pb, Cr and Zn was 40, 30, 10, 5, 5, 2 and 1, respectively [40]. C if is the contamination factor, C io is the concentration of metal in the sediment of the Suquía River and C in is the background value of the heavy metal in coastal sediments [41].
Based on Eqs. (1)–(3), ecological risk indexes of metal/loids in the five monitoring stations, considering dry and wet seasons, were calculated and are listed in Table 4. The results indicated that there was a relatively low degree of ecological risk associated with toxic metal/loids in LC, R1, SR and LP during the wet season. Conversely, moderate ecological risk was found in LC, R1, SR and LP in the dry season and in CM in the wet season. It is worth noting that severe ecological risk was determined for CM during the dry season. The potential ecological risk index of a single-element showed that Hg exhibited the most severe risk for potential pollution risk out of seven studied metal/loids in the sediments of the Suquía River basin, mainly due to the highest toxicity coefficient of Hg.
5 Multivariate Statistical Analysis
Looking for evidence on the correspondence between the two studied matrixes (water and sediment), we decided to apply the Generalised Procrustes analysis (GPA). Specifically, GPA constructs the consensus configuration of a group of datasets by applying transforms in an attempt to superimpose them. Therefore, GPA theory and algorithms can be applied to match abiotic parameters (metals and metalloids in this case), measured in different matrixes, namely, water and sediment in this case. Additionally, GPA produces a configuration corresponding to different studied sites that reflect the consensus among the two matrixes (metal/loids in water and sediment from different sites). The result is a consensus alignment that uses all the variables from both datasets.
Variables used are those from Tables 5 and 6, since these datasets contain the highest number of measured elements. So far, all the variables showed in Tables 5 and 6 were used as descriptors for grouping water and sediments. In Fig. 2a, b, the consensus configuration projected onto the plane defined by its first and second principal axis is shown, explaining 68.4% of variability between samples during the wet season and 73.7% during the dry season.
We can observe that the five monitoring sites considered are well separated in terms of levels of metals and metalloids measured in both water and sediment. This last result gives further indication of the connection between both studied matrixes.
Through this multivariate statistical technique (GPA), we can presume that the different ecological compartments (water and sediment) studied along the Suquía River basin are closely related and that the interaction between them determines the characteristics of each site. These results allow us to highlight the importance of integrating studies from different compartments to determine the quality of water resources by means of a pollution gradient as the one observed along the Suquía River basin from upper to lower sections.
6 Conclusions
According to studies conducted by different authors over 17 years, it can be concluded that, although not all mentioned reports sampled at the same monitoring sites, or at the same time, when analysing them all together, a wide overview of metal/loids concentrations in water and sediment along the Rio Suquía basin is observed, showing differences between the dry and wet season throughout the studied years. A brief summary of all these studies allow the following conclusions:
The concentrations of metal/loids in stream available sediments from the pristine areas were similar in both sampling seasons.
Some metal/loids values (e.g. Pb and Ni) in the upper catchment were, as expected, the lowest, considering the entire drainage basin. Conversely, Cu and Zn exhibited moderate concentrations, especially in LM1 and LM2 (Table 1) sites when compared to levels for the protection of the aquatic biota established in Canada (16 μg g−1 DW).
The environmental impact of Córdoba City (mainly from the WWTP) became evident in the Suquía River system with the increase of toxic metal/loids at the sampling stations located downstream the WWTP, with a greater impact closer to the sewage exit.
A reduced number of point sources of pollutants further downstream the WWTP and the industrial effluents determine a decreasing metal/loids concentration trend downstream from these points. Other processes, such as dilution by relatively metal-free sediment supplied by bank erosion, may also support the observed decreasing concentration trend.
The increase in As concentration observed between Córdoba City and the river mouth at the Mar Chiquita lake could be explained by nonpoint sources, arising from runoffs from surrounding fields dedicated to both agriculture and stock breeding, which use groundwater for irrigation and provision of water to cattle.
The concentrations of some elements in river waters are also characterised by a seasonal dependence. Namely, higher concentrations are observed during the wet/rainy season for some elements, probably due to increased urban runoffs at the beginning of the rainy season, while other elements present higher values during the dry season, probably as a consequence of a lower amount of water, causing a concentration effect when an almost constant charge is released into the water.
Ecological risk indexes of metals in sediments indicate that sediments located few kilometres downstream from the WWTP have moderate to severe ecological risk. Therefore, the downstream area close to the WWTP can be considered as the most polluted site.
Using multivariate statistical analysis (GPA), it can be demonstrated that the different ecological compartments studied (water and sediment) are closely related and that the interaction between them determines the quality of the aquatic environment at each studied site.
References
El-Said GF, Youssef DH (2013) Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediments from Red Sea, Egypt. Environ Monit Assess 185:393–404
DeWolf H, Rashid R (2008) Heavy metal accumulation in Littoraria scabra along polluted and pristine mangrove areas of Tanzania. Environ Pollut 152:636–643
Chen Y, Siyue L, Yulong Z, Quanfa Z (2011) Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater 191:366–372
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159(10):2575–2585
Bradl HB (2005) Sources and origins of heavy metals. In: Bradl HB (ed) Heavy metals in the environment. Elsevier, Amsterdam, pp 1–27
Bastami KD, Bagheri H, Haghparast S, Soltani F, Hamzehpoor A, Bastami MD (2012) Geochemical and geo-statistical assessment of selected heavy metals in the surface sediments of the Gorgan Bay, Iran. Mar Pollut Bull 64:2877–2884
ElNemr AH, El Sikaily A, Khaled A (2007) Total and leachable heavy metals in muddy and sandy sediments of Egyptian coast along Mediterranean Sea. Environ Monit Assess 129:151–168
Håkanson L (1980) An ecological risk index for aquatic pollution control of sediment ecological approach. Water Res 14:975–1000
Maher WA, Aislabie J (1992) Polycyclic aromatic hydrocarbons in near shore marine sediments of Australia. Sci Total Environ 112:143–164
Rivail Da Silva M, Lamotte M, Donard OFX, Soriano-Sierra EJ, Robert M (1996) Metal contamination in surface sediments of mangroves, lagoons and southern Bay in Florianopolis Island. J Environ Technol 17:1035–1046
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Yu RL, Hu GY, Wang LJ (2010) Speciation and ecological risk of heavy metals in intertidal sediments of Quanzhou Bay, China. Environ Monit Assess 163:241–252
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21:1335–1346
Wu YH, Hou XH, Cheng XY, Yao SC, Xia WL, Wang SM (2007) Combining geochemical and statistical methods to distinguish anthropogenic source of metals in lacustrine sediment: a case study in Dongjiu Lake, Taihu Lake catchment. China Environ Geol 52:1467–1474
Taylor KG, Owens PN (2009) Sediments in urban river basins: a review of sediment-contaminant dynamics in an environmental system conditioned by human activities. J Soils Sediments 9:281–303
Pettine M, Camusso M, Martinotti W, Marchetti R, Passino R, Queirazza G (1994) Soluble and particulate metals in the Po River: factors affecting concentrations and partitioning. Sci Total Environ 145:243–265
Sutherland RA, Tack FMG, Ziegler AD, Bussen JO (2004) Metal extraction from road-deposited sediments using nine partial decomposition procedures. Appl Geochem 19:947–955
Sutherland RA (2002) Comparison between non-residual Al, Co, Cu, Fe, Mn, Ni, Pb and Zn released by a three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment. Appl Geochem 17:353–365
Agemian H, Chau A (1977) A study of different analytical extraction methods for nondetrital heavy metals in aquatic sediments. Arch Environ Contam Toxicol 6:69–82
Andrews S, Sutherland RA (2004) Cu, Pb and Zn contamination in Nuuanu watershed, ahu, Hawaii. Sci Total Environ 324:173–182
Yuan X, Zhang L, Li J, Wang C, Ji J (2014) Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain, China. Catena 119:52–60
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer, Berlin
Gaiero D, Ross GR, Depetris P, Kempe S (1997) Spatial and temporal variability of total non-residual heavy metals content in stream sediments from the Suquia River system, Cordoba, Argentina. Water Air Soil Pollut 93:303–319
Marnette ECL, Van Breemen N, Hordjijk KA, Cappemberg TE (1993) Pyrite formation in two freshwater systems in the Netherlands. Geochim Cosmochim Acta 57:4165–4177
Eaton A (1979) Leachable trace elements in San Francisco Bay sediments: indicators of sources and estuarine processes. Environ Geol 2:333–339
Yan L, Stallard RF, Key RM, Crerar DA (1991) Trace metals and dissolved organic carbon in estuaries and offshore waters of New Jersey, USA. Geochim Cosmochim Acta 55:3647–3656
Contardo-Jara V, Galanti LN, Amé MV, Monferrán MV, Wunderlin DA, Wiegand C (2009) Biotransformation and antioxidant enzymes of Limnoperna fortunei detect site impact in watercourses of Córdoba, Argentina. Ecotoxicol Environ Saf 72:1871–1880
Amé MV, Díaz MD, Wunderlin DA (2003) Occurrence of toxic cyanobacterial blooms in San Roque Reservoir (Córdoba, Argentina): a field and chemometric study. Environ Toxicol 18:192–201
Monferrán MV, Galanti LN, Bonansea RI, Amé MV, Wunderlin DA (2011) Integrated survey of water pollution in the Suquía River basin (Córdoba, Argentina). J Environ Monit 13:398–409
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Cordoba City (Argentina) on Suquia River. Water Res 34:2915–2926
Wunderlin DA, Diaz MP, Amé MV, Pesce SF, Hued AC, Bistoni MA (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquía River basin (Córdoba-Argentina). Water Res 12:2881–2894
Argentinean Environmental Water Quality Guidelines (AEWQGNiveles Guı’a Nacionales de Calidad de Agua Ambiente) (2003) Subsecretaria de Recursos Hídricos de la Nación, República Argentina. www.hidricosargentina.gov.ar/NivelCalidad1.html. Accessed 27 July 2012
Canadian Council of Ministers of the Environment (2001) Canadian sediment quality guidelines for the protection of aquatic life: introduction. Updated. In: Canadian environmental quality guidelines, 1999. Canadian Council of Ministers of the Environment, Winnipeg, Canada
Monferrán MV, Garnero P, Bistoni MA, Ambar AD, Gordon GW, Wunderlin DA (2012) Acumulación de metales pesados y metaloides en diferentes matrices del lago San Roque (Córdoba, Argentina). Implicancias asociadas al consumo de peces. IV Congreso Argentino de la Sociedad de toxicología y química ambiental SETAC-Argentina. Buenos Aires, Argentina
Monferrán MV, Pignata ML, Wunderlin DA (2012) Bioaccumulation of heavy metal and trace elements by Potamogeton pusillus as bioindicator of aquatic pollution. VI SETAC Word Congress, SETAC Europe 22nd Annual meeting. Berlin, Germany
Pérez-Carrera A, Fernández A (2013) Cirelli1,2. http://revistas.unlp.edu.ar/index.php/domus/issue/current/showToc
Harguinteguy CA, Fernández Cirelli A, Pignata ML (2014) Heavy metal accumulation in leaves of aquatic plant Stuckenia filiformis and its relationship with sediment and water in the Suquía river (Argentina). Microchem J 114:111–118
Charzeddine L, Andrade J, Martins C, Gharseddine S, Pérez M (2002) Variación estacional demetales pesados en Americonuphismagna (Annelida: Polychaeta) y en sedimentos de la región nororiental de Venezuela, Saber 14
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Hilton J, Davison W, Ochsenbein U (1985) A mathematical model for analysis of sediment coke data. Chem Geol 48:281–291
Li J, Zheng C (1988) Handbook of environmental background values. China Environmental Science Press, Beijing
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Monferrán, M.V. (2015). Metals and Metalloids in Water and Sediment of the Suquía River Basin: Spatial and Temporal Changes. In: Wunderlin, D.A. (eds) The Suquía River Basin (Córdoba, Argentina). The Handbook of Environmental Chemistry, vol 62. Springer, Cham. https://doi.org/10.1007/698_2015_444
Download citation
DOI: https://doi.org/10.1007/698_2015_444
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67755-2
Online ISBN: 978-3-319-67757-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)