Abstract
Fecal microbial pollution is a major problem throughout the Danube River Basin, posing a threat to various types of water use, including drinking water production from river bank filtrates, water supply for agricultural and industrial use, and the role of the river as a recreational space. Fecal microbial pollution is introduced into the river by point sources, such as discharges of treated or untreated sewage from human sources or livestock, and by nonpoint sources, such as urban and agricultural runoff. In addition, fecal input from wildlife may be of importance in specific regions. Despite huge efforts to improve wastewater management in the past decade, in many sections, the river and its tributaries exhibit very high levels of fecal microbial pollution. To assess microbiological water quality, indicators of fecal pollution are used as surrogates for the potential presence of intestinal pathogens. However, the standard indicators cannot provide any reliable information regarding the origin of fecal pollution, nor can their concentration levels be directly related to human health risks for many types of exposure and situations.
The aim of this book chapter is to summarize the historical developments in microbiological water quality research and to reflect the most recent publicly available data on the fecal microbial pollution status of the Danube River. Moreover, the first results on fecal microbial source tracking by molecular biology methods are presented along with their applicability in river water quality monitoring, including the monitoring of riparian wells and alluvial groundwater resources. Finally, a discussion of the general state of water quality and public health is presented concerning (i) the current situation and potential limitations of the Water Framework Directive regarding the microbiological quality elements, (ii) further improvements regarding sampling and monitoring strategies, and (iii) the recently introduced concept of “integrated framework of fecal pollution monitoring and management” and expected further methodological developments in the context of the Danube watershed. Rapid progress in research and development is currently being made in the area of fecal microbial source tracking, pathogen detection, and health risk assessment, and these innovations are also likely to complement basic fecal pollution monitoring programs for river systems such as the Danube in the near future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fecal pollution
- Microbial source tracking
- Microbiological water quality
- Review
- Sustainable water management
1 Introduction
Microbes are fundamental in aquatic ecosystems and occupy – due to their dual role – a special position among biological quality elements. On the one hand, autochthonous microbes (including bacteria, viruses/phages, and protozoa) act as ecological components of system functioning and represent the most abundant group of organisms, being mainly responsible for the decomposition of organic matter, remineralization of inorganic nutrients, and energy and organic matter transfer to higher trophic levels (microbial loop [1]). In so doing, they predominantly contribute to the so-called “self-purification” process and thus characterize the saprobic status and ecological integrity of rivers. Moreover, if river water is used for drinking water production, the composition of organic matter and the capacity of the autochthonous microbes to degrade organic matter are crucial for the biostability of the end product. On the other hand, predominantly allochthonous microbes, which are introduced into rivers from external sources, can be important pollutants with relevance to human and animal health. Within the allochthonous microbes, those that are spread via the fecal-oral infection pathway are the most significant group. For the comprehensive characterization of river water quality, both components have to be considered, but to date only the aspect of pollution microbiology, due to its critical importance in public health, has been included in international regulations for water quality, such as the EU Bathing Water Directive [2] and the Drinking Water Directive [3]. The focus of this paper is restricted to the microbiological fecal pollution component, while the microbial ecological component is only discussed in the historical background section. Nevertheless, we want to reemphasize the importance of this topic, as understanding of microbial processes is the basis for understanding the whole system and the microbiological pollution patterns in rivers in particular. There is still a high demand for research in this area, and the debate on how microbial ecological methods can be integrated to define microbes as biological quality elements of rivers has not even started.
The Danube River has a total length of 2,870 km; its basin covers an area of 801,500 km2 with approximately 81 million inhabitants in 19 countries [4] contributing to a large extent to the water pollution of the Danube. In addition to chemical contamination, fecal microbial pollution is a major problem throughout the Danube River Basin, posing a threat to various types of water use [5], including drinking water production from river bank filtrates [6], the supply of water for agricultural and industrial use, and the role of the river as a recreational space. Approximately ten million people along the course of the Danube River receive treated drinking water from river bank filtration (www.iawd.at), and the International Association of Water Supply Companies in the Danube River (IAWD) issued a declaration as early as in 1992, “to improve and safeguard the water quality of the Danube and its tributaries and […] encouraging all measures and efforts aimed at avoiding and eliminating the pollution of, and threat to, the status of raw water in the interest of drinking water supply” [6]. Fecal microbial pollution is introduced into the river by point sources, such as discharges of treated or untreated sewage from human sources or livestock, and by nonpoint sources, such as urban and agricultural runoff. In addition, fecal input from wildlife may be important in regions where the main river is highly interconnected with floodplains. Despite huge efforts to improve wastewater management in the past decade, in many sections, the river and its tributaries still receive incompletely treated sewage, leading to serious deterioration of water quality [7].
Fecal microbial pollution of water sources relevant to human health is related to the occurrence of pathogenic microorganisms that are spread via the fecal-oral route. They may originate from infected humans (anthroponotic) or from animals (zoonotic) and are shed via their feces into the water source and subsequently taken up via ingestion. Because not all fecal-associated pathogens can be detected from a potentially contaminated water source, microbiological indicators of fecal pollution are used as surrogates for the potential presence of intestinal pathogens. Fecal indicator bacteria such as Escherichia coli (E. coli) and intestinal enterococci occur almost ubiquitously in high concentrations in human and animal fecal material and are valuable indicators for fecal pollution detection. These microbiological indicators are quantitatively determined via standard culture-based methods. Recovering quantitative information on fecal pollution is the basis for microbiological water quality monitoring. However, in the case of pollution problems, information on the origin of contamination is also needed for effective target-oriented management strategies. Furthermore, information on the expected health risk in relation to the respective type of usage (recreation, swimming, irrigation, drinking, aquaculture) is increasingly required for water safety management. However, the standard indicators, E. coli and intestinal enterococci, cannot provide any reliable information regarding the origin of fecal pollution (e.g., human vs. animal), nor can their concentration levels be directly related to human health risks for many exposition types and situations. Rapid progress in research and development is currently being made in the area of fecal microbial source tracking, pathogen detection, and health risk assessment, and these innovations are also likely to complement basic fecal pollution monitoring programs for river systems in the near future (see Sect. 5).
The aim of this book chapter is to summarize the historical developments in microbiological water quality research and to relay the most recent publicly available data on the fecal microbial pollution status of the Danube River. Recent data sets are mainly derived from two scientific reports and one publication in an international scientific journal and include data from two whole-river surveys (Joint Danube Survey 2001, 2007) and from the International Commission for the Protection of the Danube River (ICPDR) Transnational Monitoring Network. On a national basis, the Danube riparian states may have ample additional data sets on fecal microbial pollution concerning their section and its major tributaries. In addition, the IAWD supports microbiological monitoring activities at Danube sites where water is used for drinking water production. However, these data are not easily accessible because the data are not available in international scientific databases and could thus not be incorporated in the manuscript. As a further focus of this chapter, the first results on fecal microbial source tracking by molecular biological methods will be presented, and their applicability in river water quality monitoring – including the monitoring of riparian wells and alluvial groundwater resources – is discussed. The final Conclusion and Outlook section covers a discussion of the general state of microbial water quality and public health, the current situation and potential limitations of the Water Framework Directive regarding the microbiological quality elements, further improvements regarding sampling and monitoring strategies, and, finally, the recently introduced concept of “integrated framework of fecal pollution monitoring and management” and expected methodical developments in the context of the Danube watershed.
2 Historical Overview of Microbiological Research on the Danube River
This brief overview summarizes the historical development of the microbiological water quality research on the Danube River and important tributaries. This general overview is focused on relevant scientific publications and reports related to the field of pollution microbiology, primarily addressing allochthonous microorganisms such as intestinal indicator bacteria (e.g., E. coli, intestinal enterococci) and pathogenic microorganisms from external sources (e.g., sewage, runoff). Several publications in the field of microbial ecology, mainly concerning autochthonous microbial communities, referring to the assessment of water quality, are included as well. In addition, innovative studies involving first applications of molecular biological investigation techniques are cited. Transnational project studies in the Danube basin are emphasized. Early monitoring results from the Danube River were frequently published in German.
The first papers addressing microbiological water quality research of the Danube River were published around the turn of the nineteenth century. For example, Heider [8] already showed that the sewage from Vienna, after its discharge into the Danube River, flowed along the right bank of the stream, preserving its own bacterial characteristics and not mixing perfectly with the river water for more than 24 miles (44.5 km). Brezina [9] performed the first comparative investigations and found 1,900 culturable bacteria per ml in the Danube River upstream of Vienna and 110,000 culturable bacteria per ml downstream at the mouth of the Danube Canal in Vienna due to wastewater impact.
In the subsequent four decades, microbiological studies on the Danube River were scarce. Halophilic or salt-tolerant bacteria were investigated in the Danube Delta [10]. Joos [11] tested the presence of bacteria of the typhoid-paratyphoid group in Danube water and sewage. Investigations on the influence of pollution on bacterial nitrogen transformations were accomplished by Stundl [12].
The 1950s were the beginning of systematic investigation programs in the Danube River Basin [13]. Threats by microbiological pollution to human health via various types of water use were already observed [14].
In the 1960s, microbiological monitoring and research was extended to all riparian countries [13, 15–21]. In the book chapter “Die Mikrobiologie der Donau,” microbiological data from the riparian countries were summarized for the first time, e.g., total bacterial count, colony count, coliforms, E. coli, and enterococci (Mucha [22], in: Limnologie der Donau, edited by Liepolt 1967, in German with English summaries). The author noted the importance of the application of comparable and standardized methods and sent proposals to the national labs – with moderate success – as he commented himself. Microbiological research in the Danube River Basin received essential contributions from the International Association for Danube Research (IAD), an expert group in Microbiology/Hygienics and scientific platform for microbiologists working on Large River Ecosystems [22], which is still in operation today (www.iad.gs).
In the 1970s, research on pathogenic microorganisms (e.g., Salmonella spp.) was intensified (e.g., [16, 23–25]). A main finding was that Salmonella spp. occur frequently in wastewater and cannot be eliminated by biological sewage treatment plants. Therefore, Salmonella spp. can be easily isolated in polluted rivers downstream of wastewater discharges. Kohl [26] forced the bacteriological investigation of sediment and periphyton to improve the microbiological assessment of water bodies. An essential advance was the publication of a classification system for the heterotrophic plate count (colony count) and fecal indicator (fecal coliforms) parameters by Kohl [16].
In the 1980s, many investigations focused on the determination of the microbiological pollution of the Danube River (e.g., [27–31]). Several authors applied bacterial numbers and biomass as well as biochemical activity parameters, such as phosphatase activity, for the characterization of the microbiological water quality ([32–37]). In 1985 an international monitoring program was established based on the Bucharest Declaration, containing transboundary cross sections on the Danube River [7]. A highlight was the international Danube expedition in 1988, organized by the IAD, from Vienna resp. Bratislava to the Danube delta [38–43]. The data from the expedition indicated unacceptable fecal pollution levels in the Danube downstream of the cities Silistra, Nikopol, Vidin, Visegrad, Gabcikovo, and Bratislava because of sewage discharges [41]. Investigations of the German and Austrian Danube, due to technical reasons, could not be performed in this ambitious research program. Kasimir [42] investigated the total bacterial count, biomass, percentage of free living and attached bacterial cells, percentage of free dividing cells, and bacterial secondary production along the Danube River. Methodical comparisons of the direct count parameter from Austrian and Czechoslovakian labs demonstrated differences of approximately one order of magnitude, emphasizing again the requirements of standardized methods to obtain comparable results.
In the end of the last century, the focus was on microbiological long-term water quality alterations [44–48]. For example, in the Austrian section of the Danube River, bacteriological monitoring has been performed since 1957. The collected data suggested an improvement of bacteriological water quality between 1957 and 1997 [49]. Downstream of Vienna, the bacteriological data indicated the need for further action. The Transnational Monitoring Network (TNMN) was officially launched 1996 to support the implementation of the Danube River Protection Convention in the field of monitoring and assessment (www.icpdr.org). Popp et al. [50] published a classification scheme for the assessment of bacteriological water quality of running waters. Several papers also addressed natural microbial communities and their associated activities (microbial ecology), especially regarding water quality issues [51–57]. In 1998, the research boat MS Burgund traveled the great European waterway of the Rhine, Main, and Danube Rivers from Mainz on the Rhine to the Hungarian-Croatian-Serbian border, passing the German, Austrian, and Hungarian stretch of the Danube. The goal of this research trip was a joint evaluation and comparison of water quality, including microbiological parameters [58].
In the last decade (beginning in 2001), many innovative microbiological research activities have been undertaken in the Danube River Basin. Microbial community analysis, biotransformation processes, and enzymatic activities received special attention [59–62]. Farnleitner et al. [63, 64] presented enzymatic techniques for the rapid detection of E. coli in polluted river water. Schade et al. [65] discussed the wastewater UV disinfection method to improve water quality. Kolarevic et al. [66], Hosam et al. [67], and Ajeagah et al. [68] investigated the sanitary risks and aquatic ecosystem hazards in the Danube sections of Serbia, Hungary, and Romania. In its Annual Report for 2009/2010, the International Association of Water Supply Companies in the Danube River Catchment (IAWD) presented microbiological data from the current monitoring sites along the Danube River [6]. Monitoring focuses on the abstraction points for drinking water production at the respective Danube River bank filtration sites (bank filtrate).
During two whole-river surveys (Joint Danube Survey, JDS 2001, 2007), organized by the International Commission for the Protection of Danube River (ICPDR), samples were taken for the first time along the whole stretch of the Danube River from Germany to the Black Sea with uniform methods [5, 69, 70]. A third JDS was performed in 2013, but data from this survey was not integrated into the book chapter. The variation of fecal pollution in the longitudinal profile of the Danube and its main tributaries was determined by bacteriological standard parameters (details are presented in the next section). The first microbiological water quality map of the whole Danube River was created, illustrating the degree of fecal pollution at more than 75 Danube sampling stations and 21 tributaries [69]. A five-level classification system for microbiological fecal pollution to harmonize with other available classification systems according to the EU Water Framework Directive (EU-WFD; [2]) was developed by Kavka et al. [71] and applied in the Danube Survey 2007 [5, 70]. Longitudinal changes in the genetic and morphological population structure of the natural bacterial community of the Danube River, a fundamental part of ecosystem functioning and integrity, were studied during JDS1 (in 2001) and JDS2 (in 2007) [62, 72, 73]. The observed development of the bacterial compartment along the Danube River generally supported the river continuum concept [62]. Furthermore, DNA material recovered during the JDS 2001 and JDS 2007 water sampling activities was used to evaluate the applicability of DNA-based microbial source tracking approaches along the whole Danube River stretch to foster target-oriented management in the catchment [70, 74]. An overview on the applicability of microbial source tracking is presented in the next sections.
3 Fecal Microbial Pollution of the Danube River: A Snapshot Analysis
3.1 Methods Background
Cultivation-based methods are still the gold standard for the assessment of microbiological water quality. Within the current version of the EU Bathing Water Directive [2], which needed to be implemented by all European Union member states by 2008, the determination of E. coli and intestinal enterococci is compulsory. Previously, total coliforms, fecal coliforms, and fecal streptococci had to be investigated as fecal indicators, as well as salmonellae and enteroviruses as indicator pathogens [75]. Total coliforms were excluded in the new directive because several findings indicated that a significant portion of these bacterial species can multiply in the aquatic environment and are thus not suitable as fecal indicators ([76] and citations therein). E. coli is now used instead of fecal coliforms, as this species makes up the majority of fecal coliforms found in water and is a better indicator of fecal pollution. Similar arguments apply to the switch from fecal streptococci to intestinal enterococci. Finally, the mandatory investigation of salmonellae and enteroviruses has been omitted because (i) their detection is quite time consuming and (ii) the concentration of E. coli and intestinal enterococci shows a good correlation to epidemiological data of bathing water-associated diseases [77].
The data set presented in this article compiles the most recent available data from international scientific publications and public reports on microbiological water quality of the Danube River and its most important tributaries. This set includes information from two whole-river surveys (JDS1 and JDS2) and data from the ICPDR Transnational Monitoring Network (TNMN 2001–2005; http://www.icpdr.org/main/activities-projects/tnmn-transnational-monitoring-network) that were extracted from Kavka and Poetsch [69] and Kirschner et al. [5, 70].
Because the different data sets come from different years under different bathing water regulations, the assessment of microbiological water quality along the Danube River is based on a variety of parameters (fecal indicators) that were either assessed according to international standards [78–81] or other appropriate methods that were validated during the investigations with standard methods (Colilert 18, Idexx, Germany). Details can be found in the references mentioned above [5, 69, 70].
To translate concentrations of fecal indicators into levels of fecal microbial pollution, a five-level classification system [71] that integrates the guidelines for bathing water quality [2, 75] with the European Water Framework Directive (EU-WFD) [82] was applied. In this system, five classes of fecal pollution were defined such that classes I and II are below and quality classes III, IV, and V exceed the fecal pollution limit values for good bathing water quality (Table 1).
3.2 Joint Danube Surveys
During two whole-river surveys, samples from 96 (JDS 2007) and 98 (JDS 2001) sampling stations were taken, along a stretch of 2,600 km. In 2007, the selected sampling sites included 75 Danube River sites and 21 tributaries and branches; in 2001, they included 76 river sites and 22 tributaries/branches (Fig. 1). At all Danube and large tributary sampling stations, water samples were collected directly from the cruise ship in the middle of the river. Samples were taken with sterile 1 L glass flasks fixed to a sampling rod at a water depth of approximately 30 cm [81]. For smaller tributaries and branches, samples were taken in the same manner from small boats. All samples were immediately processed on board.
Sampling sites in the Danube River Basin selected during the Joint Danube Survey 2001 and 2007. Red circles indicate sampling stations common to JDS 2001 and JDS 2007, blue circles indicate sampling stations unique to JDS 2001, and yellow circles indicate sampling stations unique to JDS 2007 (Modified after [5]; with permission from Elsevier)
3.3 Summarized Data from the Joint Danube Surveys
Fecal pollution varies significantly along the course of the Danube and is to a large extent determined by the influence of the large urban areas of Vienna, Bratislava, Budapest, Belgrade, and Bucharest. The highest fecal pollution levels, however, are observed in specific tributaries and branches, related to the cities Györ (Mosoni Danube), Budapest (Rackeve-Soroksar Arm), Ruse (Rusenski Lom), and Arges (Bucharest).
According to levels of fecal indicator concentrations, six sections of fecal pollution along the Danube River can be delineated (Fig. 2). The first section has little to moderate pollution and ranges from the headwaters of the Danube in Germany to rkm (river kilometer) 1,942 (upstream of Vienna, Austria). Due to the influence of the urban areas of Vienna and Bratislava (Slovakia), the second section starts with a significant increase in fecal indicator concentrations to critical levels of fecal pollution, followed by a decreasing trend down to low levels until upstream of Budapest, Hungary (rkm 1,659). Because Budapest did not possess a state-of-the-art wastewater treatment plant until 2010, a dramatic increase of fecal indicator concentrations to strong pollution levels is observed at the beginning of section III. Critical levels of fecal pollution remain dominating in this section until downstream of Belgrade (Serbia). After the merging of Velika Morava (rkm 1,107), fecal pollution levels in the fourth section decreased markedly down to low levels until Orsova (Romania, rkm: 954) due to the absence of large cities and abundant agriculture in this section. Additionally, the deep Iron Gate reservoirs most likely enable sedimentation of particles and associated fecal indicators. The fifth section, ranging from rkm 954 to Cernavoda (Romania, rkm: 290), is characterized by a steady increase in fecal pollution from low to critical levels after merging with the excessively polluted water from the Arges (collecting untreated sewage via the Dambovita river from Romania’s capital Bucharest). In the last section, a slight decrease in fecal indicator concentrations to moderate levels is observed.
Longitudinal development of fecal microbiological pollution in the Danube River (small circles) and its major tributaries (large circles) during JDS 2001 (open symbols) and JDS 2007 (closed symbols). Colors were chosen according to the microbiology-based pollution classification system in Table 1: blue, little; green, moderate; yellow, critical; orange, strong; and red, excessive pollution. Upper panel, E. coli; mid panel, Enterococci; lower panel, total coliforms; tributaries/branches, 1; Inn, 2; Schwechat, 3; Morava, 4; Moson Danube, 5; Vah, 6; Hron, 7; Ipoly, 8; Rackeve-Soroksar Arm start, 9; Rackeve-Soroksar Arm end, 10; Sio, 11; Drava, 12; Tisza, 13; Sava, 14; Velika Morava, 15; Timok, 16; Iskar, 17; Olt, 18; Jantra, 19; Russenski Lom, 20; Arges, 21; Siret, 22; Prut (Taken from [5]; with permission from Elsevier)
The highest fecal pollution levels (strong-excessive) were observed in the Arges tributary and the Rusenski Lom (Bulgaria), Rackeve-Soroksar Arm, and Mosoni Danube (Slovakia, receiving wastewater from Györ, Hungary) branches. Other tributaries with pollution levels exceeding guideline values for bathing water quality are the Schwechat (Austria), Morava (Austria, Czech Republic, Slovakia), Vah (Slovakia), Ipoly (Hungary), Drava (Croatia, Hungary), Velika Morava (Serbia), Siret (Romania), and Prut (Romania, Moldova) Rivers. Other tributaries show moderate to low fecal pollution (Fig. 2).
3.4 Data from the Transnational Monitoring Network
Fifteen representative stations on the Danube River and one tributary (Arges River) that coincided with JDS sampling points and where continuous data sets from 2001 to 2005 for the middle of the river were available were chosen from the TNMN database. At each station, 20–120 measurements for each fecal indicator were available. Different methods were used in the different countries for the determination of fecal coliform (FC), enterococci, and total coliform concentrations. Figure 3 shows the variability of the fecal indicator concentrations over the 5-year period with concentrations ranging over two to four orders of magnitude. Despite the rather high variability of the TNMN data and despite the coarser spatial resolution, the data clearly reflect the pattern of fecal pollution developed from the two Joint Danube Surveys (Fig. 2). The average E. coli/FC concentrations of both surveys measured at the 16 common sampling points significantly correlated with the median TNMN concentrations (ρ = 0.624; p < 0.01). Because of the high number of zero values of enterococci measured during JDS 2007, no significant correlation to the TNMN data was obtained (Fig. 3). A weak but statistically insignificant correlation between the JDS and TNMN enterococci data was achieved when zero values were excluded from the data set (ρ = 0.453; p = 0.07). For total coliforms, a very high correspondence of the data sets was observed; only the data from the Arges tributary exceeded the maximal TNMN concentrations by approximately 1.5 orders of magnitude (Fig. 3). Median values from the two data sets were highly intercorrelated (ρ = 0.800; p < 0.001).
Box-Whisker plots of the TNMN sampling data recorded during 2001 and 2005. For each fecal indicator class, between 20 and 120 sampling points were measured at each sampling point. Different methods were used in the different countries for the determination of fecal coliform, enterococci, and total coliform concentrations. For comparison, the data obtained during the two Joint Danube Surveys were added as blue (JDS 2001) and yellow circles (JDS 2007). rkm river kilometer of the Danube (Modified after [5]; with permission from Elsevier)
3.5 Short Summary of Fecal Pollution Levels in the Danube River Sections
A clear picture of the longitudinal development of fecal microbial pollution could be drawn from the data set used in this study. Six sections of fecal pollution, which were mainly determined by the influence of the large capitals of the riparian countries, were delineated. Similarly, tributaries and branches receiving wastewater from large cities were hot spots of fecal pollution. In addition to the influence of municipal wastewater, the continuous increase of fecal indicator concentrations in section V (Romania, Bulgaria) likely indicates significant input from agricultural sources in this rural region. Of the identified sections, section I (Germany, Austria) and significant parts of section IV (Serbia, Romania) and V (Romania, Bulgaria) showed little to moderate fecal pollution.
4 Genetic Fecal Marker Detection and Fecal Microbial Source Tracking
4.1 Methods Background
Knowledge on the origin of fecal pollution in the Danube River and its tributaries is of high interest as it allows for targeted protection and for the evaluation of the effectiveness of environmental management practices. Furthermore it supports water safety assessment and health risk management regarding recreational activities, bathing, irrigation, and drinking water usage. As demonstrated above, the extent of fecal microbial pollution can be determined via standard bacterial fecal indicators. However, E. coli or enterococci do not easily allow fecal source differentiation as they occur – per definition – in humans and homoeothermic animals (i.e., giving a measure of the amount of total fecal pollution).
Microbial source tracking (MST) or the determination of fecal pollution sources using host-associated genetic fecal markers [83, 84] has become increasingly popular. One of the most frequently used methods is based on the quantitative polymerase chain reaction (qPCR) detection of host-associated Bacteroidetes populations [85]. Bacteroidetes are one of the dominating bacterial groups in human and animal fecal excreta representing up to 30% of the biomass in feces (in contrast E. coli or enterococci constitute less than 1‰). Furthermore some microbial cell lines of Bacteroidetes show remarkable host adaptations, i.e., they are strongly associated with a specific type of pollution source (human, ruminant, etc.). Hence, Bacteroidetes represent ideal candidate targets for MST. The detection method of choice is direct molecular detection as most of these Bacteroidetes populations cannot be detected by standard microbiological cultivation procedures (Fig. 4). MST is a very young and rapidly evolving discipline, and no standardized procedure – comparable to the detection of standard fecal bacteria – exists as of yet. Most detection approaches have also been developed based on the background conditions at the respective watersheds of interest and need to be evaluated for application to other regions.
Procedure for the quantification of genetic fecal markers (e.g., BacH) for library-independent microbial source tracking (MST). The working steps include sampling, filtration of the water samples via 0.2 μm polycarbonate membranes, extraction of DNA from microbial cells retained on the filter, and subsequent quantification of the respective genetic marker concentrations by quantitative polymerase chain reaction (qPCR) detection. DNA extracts can be stored at −80°C for several months until qPCR analysis is performed. A detailed description of the method can be found in [86, 87]
4.2 Testing Molecular MST at the Danube River Tributaries for Human Fecal Impact
Preserved DNA, recovered during the JDS 2001 and JDS 2007 water sampling activities, was used to evaluate the principle applicability of the Bacteroidetes-based MST approach along the whole Danube River stretch [70, 74]. Emphasis was placed on the tributaries as an exaggerated contamination range – from very low to excessive fecal pollution – was expected. The human-associated fecal marker, BacH, was detected in 82% of the investigated tributary samples for the JDS 2007. The marker equivalent concentrations (ME) ranged from 1.4 × 102 ME L−1 to 5.8 × 107 ME L−1 [70]. Statistical analysis revealed a high association between the human BacH marker and the E. coli enumerations (Fig. 5), strongly pointing to the importance of fecal pollution from sewage effluents at the investigated tributaries for the situation during the JDS 2007 [70]. Investigations regarding the JDS 2001 [74] revealed a very similar picture. Excluding the Timok River, a very high correlation between BacH and fecal coliforms for the rest of the investigated Danube River tributaries was evident (r = 0.93). The Timok River was heavily polluted with emissions from mining industries (e.g., high concentrations on heavy metals and organic pollutions), which might have strongly affected the performance characteristics of the investigated microbiological parameters [74].
Regression analysis between the logarithms of the E. coli (a measure for total fecal pollution) and the BacH marker (associated with human/sewage fecal pollution) concentrations from the Danube River tributaries during the JDS 2007 [70]. The statistical analysis revealed that 80% of the E. coli variations could be predicted by the human-associated BacH marker concentrations. This correlation is a strong indication that a dominant part of the fecal pollution, as measured by E. coli, could be traced back to sewage impact. The BacH marker was determined according to Reischer et al. [87]. Abbreviations: MPN most probable numbers, ME marker equivalents, a log10+1 transformation was applied
4.3 Testing the Genetic Fecal Marker Approach for Alluvial Well Water Resource Monitoring
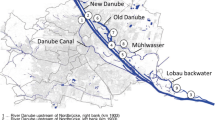
Very recently, the applicability of the genetic Bacteroidetes-based MST approach for water quality monitoring from alluvial groundwater fed by Danube River bank filtration was tested. Alluvial groundwater is of essential importance for the drinking water supply throughout the Danube River Basin (see Sect. 1). Thus, MST methods that are applicable at the river surface and corresponding alluvial groundwater locations would clearly add a significant benefit for the possibility of integrated water resource monitoring. From 2010 to 2012, five test wells at a Danube River backwater area southeast of Vienna (PGAW1 to PGAW5) were comparatively analyzed for cultivation-based standard fecal indicators, host-associated genetic fecal markers, genetic markers for total fecal pollution, and bacterial direct count numbers (Table 2, Fig. 6). Basic microbial characterization of the wells by bacterial direct counts indicated that wells generally had median cell counts below 100,000 cells per ml. However, PGAW4 and PGAW5 were less protected during periods of surface water influence compared to the others (as shown by the increased maximum bacterial count values). This observation corresponds with the proximity of these wells to the branches of the Danube River backwaters, the water levels of which can markedly increase during flood events at the Danube River. Analysis for fecal pollution vulnerability of the wells by standard indicators revealed no signs of fecal pollution, although the volume of investigation was 1 L. Except for one positive detection (enterococci), all samples (n = 91) were negative for E. coli, enterococci, and Clostridium perfringens spores (i.e., no colony forming units detectable per 1 L of analyzed well water, Table 2). Detection of human-associated (BacHum) and ruminant-associated (BacR) fecal markers revealed a similar picture compared to the standard fecal indicators (Table 2), although a somewhat higher positive detection rate was discernible. BacHum and the BacR were detected in 6 and in 1 case(s), respectively, of the ninety-one 1 L samples analyzed. Five of the seven positives were found at the more vulnerable wells PGAW4 and PGAW5. Furthermore, 50% of the human marker positives were from a single sampling date coinciding with a pronounced high water event at the Danube River (discharge peak approximately 7,000 m3). From this pilot study, it may be concluded that MST approaches also work in alluvial well water locations, and a combination with fecal standard parameters can provide very valuable information. The MST approach tended to show a higher sensitivity for fecal pollution detection in the wells compared to fecal standards and indicated the possibility of minute human fecal influence at the more vulnerable wells. However, the actual detection levels were extremely low, ranging from 5.3 × 101 ME L−1 to 6.1 × 102 ME L−1 (n = 7).
Results from a pilot study testing the applicability of Bacteroidetes-based genetic fecal marker detection in alluvial water wells (PGAW1–PGAW5) fed by Danube River bank filtration southeast of Vienna during 2010–2012 (lower panel, A). To increase detection sensitivity, 1 L of well water was investigated per parameter. The proposed genetic markers for total fecal pollution, AllBac (closed symbols) and BacUni (open symbols), were detected as described previously [86, 88–90]. To sensitively detect artificial contamination events potentially introduced during sampling or sample processing, rigorous controls were designed. Controls for sampling, filtrations, DNA extractions, PCR reagents, and PCR runs were performed. The threshold of marker detection was approximately 20 marker equivalents per filter. Standard fecal indicators were measured according to ISO standards (ISO 2000; ISO 2001, [78, 91]). For comparison, total bacterial direct count (upper panel) was performed as described in Kirschner and Velimirov [92]
In sharp contrast to the host-associated fecal markers, detection of genetic markers for total fecal pollution had no fecal indication value at all. The markers could be detected at all times at high concentrations, strongly suggesting their ubiquitous and natural occurrence (Fig. 6). This observation is in line with recent findings from other habitats that the proposed Bacteroidetes-based genetic markers for total fecal pollution are not specific for fecal pollution and also detect non-intestinal natural bacterial communities in water resources [90, 93].
5 Outlook and Conclusions
5.1 Public Health Aspects
Appropriate microbiological quality of water and water resources is considered of utmost importance for public health by the World Health Organization [94, 95]. Contrary to common public opinion, unacceptable health burdens due to unsafe water and sanitation are not restricted to developing countries – although massive epidemics are most easily recognized in these regions – and still occur in developed regions such as Europe. For example, Valent et al. [96] demonstrated that a large proportion of deaths and disability adjusted life years (DALYs) in European children are attributable to inadequate water and sanitation. Furthermore, the EFSA [97] reported 17 waterborne outbreaks involving 10,912 cases for eight member states of the European Union (Denmark, Finland, Ireland, the Netherlands, Poland, Slovakia, Spain, and Sweden) in 2007 alone. A study by Frost et al. [98] using surplus sera from Hungarian women revealed that those whose drinking water came from water supplies delivered from surface or surface-influenced water resources had significantly increased antigen titers from Cryptosporidium infections (a fecal-associated waterborne parasite) compared to women whose water was supplied from confined groundwater aquifers. Cryptosporidium and Giardia spp. parasites were regularly detected in the Danube River water at Budapest, although river bank filtration at these sites was found to be effective in removing these pathogens [99]. Although these investigations cannot be considered sufficient, the results indicate remarkably well that the impact of microbiologically polluted water can be measured and leaves its diagnosable footprint in the European population.
5.2 The Danube River: Fecal Microbiological Pollution Status and the Water Framework Directive
As seen in the present overview, several sections of the Danube River and its tributaries are currently critically affected by fecal microbiological pollution. Clearly, recreational activities such as swimming or drinking water production based on near-to-nature principles – as described in the Danube, Meuse, and Rhine Memorandum by the IAWR and the IAWD [6] –are unsafe at these locations. The data set used in this report does not contain data from the past 5 years (2008–2012), and does not reflect recent developments with respect to the implementation of the central wastewater treatment plant in Budapest (fully established in 2010) or the initiation of the wastewater treatment plant in Bucharest (established in 2011) as well as other measures and efforts taken to improve water quality. Nevertheless, data indicate the strong need to closely follow the status and the future developments on the microbial water quality alongside the Danube River.
One might assume that the good ecological health of aquatic systems would support the long-term health of surrounding human and animal populations. However, the current legal situation does not support this assumption. The Water Framework Directive (EU-WFD) currently defers to the EU Drinking Water Directive (EU-DWD) and EU Bathing Water Directive (EU-BWD) and does not directly define microbiological quality targets for aquatic systems [2]. Hence, general monitoring programs governed by the EU-WFD do not include any microbiological parameters, although they are considered a priority in the EU-DWD and EU-BWD. In contrast, chemical targets, including problematic micro-pollutants, are directly considered. The EU-WFD would benefit from including basic targets for fecal microbial pollution, which would promote equal treatment of chemical and microbiological hazards and support the harmonization of water quality monitoring programs in rivers.
5.3 Sampling Design and Methodological Aspects
In addition to these policy issues, current methodological constraints and future possibilities also have to be discussed. One important aspect concerns the sampling design of the monitoring programs. During both JDSs, samples were only taken from the middle of the river and not from its edges. Sampling location can have a highly significant influence. In contrast to impoundments of hydropower stations, where complete mixing may occur and river bottom clogging processes may have significant influence on the biota [100], in stretches devoid of meanders and hydropower plants, horizontal mixing of water masses from tributaries entering the Danube can be a very slow process, stretching out for dozens of kilometers [62]. Along such a stretch, however, intense vertical mixing may take place, and water of the boundary layers may be effectively cleaned by benthic filter feeders such as bivalves or amphipods, which represent the most important functional feeding groups at the bottom of the Danube River [62, 101]. By these processes, polluted water masses (tributaries or sewage inflows) may only partly enter the midstream if at all, and only taking midstream samples may not reflect the true pollution status of the Danube. Representative sampling at such a large river as the Danube should thus always include samples from the midstream and both boundary layers. Extrapolating the given results to the respective riverbank locations may thus result in a significant underestimation of the actual microbiological pollution.
The fecal indication capacity for bacterial standard indicators, especially for E. coli, has been increasingly questioned by many scientists recently. Naturalized E. coli populations, being not of immediate fecal origin, have been detected in the environment, such as in soil or algae mats (for an overview see [102]). This issue is far from being solved, and it is currently not clear in which environments and situations fecal monitoring programs might become methodologically limited. In contrast to this criticism, a recent detailed investigation on E. coli and enterococci in Austrian alpine water resources revealed excellent fecal indication capacity for both indicators [103].
The detection of microbiological fecal pollution by two biologically differing indicators, namely, E. coli and enterococci, yielded comparable fecal pollution patterns alongside the investigated sections of the Danube River for both JDS. Furthermore, a high correlation between E. coli and the human-associated genetic Bacteroidetes marker (BacH) was observed for the investigated Danube tributaries. Thus, a significant indication bias seems unlikely for the presented data set. However, basic research should be conducted into the fecal indication capacity of bacterial standard indicators for fecal microbial pollution monitoring at the Danube River watershed and its tributaries to provide a sound scientific basis for routine monitoring.
5.4 A Framework for Future Fecal Microbiological Pollution Analysis and Management
New methods and strategies for the analysis of microbiological water quality are emerging rapidly. This progress is mainly based on the continuously increasing importance of molecular biology diagnostics combined with improved data analysis and modeling. In Fig. 7 a framework for integrated fecal microbial pollution analysis and management in rivers is presented, visualizing the challenges but also the possibilities in the future [107]. The framework shows the different levels of data requirements, methods for its generation, and how it can be used for management activities. The basis of the approach is the detection of fecal pollution, as routinely performed using laboratory-based methods and monitoring programs [107]. At particularly sensitive points, such as at official bathing places or water abstraction sites for drinking water production, more rapid information on the actual microbiological water quality is required. Field monitoring methods will thus gain importance in the future. Near-real-time prediction of microbial water quality may be realized by modeling and/or measuring online surrogate variables or by automated and rapid detection of microbial parameters directly in the field. Both areas are currently a topic of intensive developments, and innovative technologies will likely be available in the future [107]. In cases where fecal pollution levels are above certain levels of acceptance, fecal hazard characterization or the determination of the sources of pollution becomes important [107]. Knowing the actual source(s) of pollution allows for target-oriented management and the evaluation of options available to solve the problem. Source apportionment, transport, and load modeling, based on sound hydrological principles and information [108], are required to support adequate predictions in the future. Thus, interdisciplinary collaboration between microbiologists and hydrologists is needed. Molecular fecal source tracking (MST) will largely support fecal microbial source characterization (see also chapter 4). MST is a very young discipline and far from being standardized. However, in the near future, it can be expected, that robust methods will be available, allowing the simultaneous quantification and host-specific discrimination of fecal pollution sources. Very intensive research activities throughout the world reflect the need and the progress that is made regarding this area [83]. Information about the expected health risk in relation to the extent of fecal microbial pollution and the respective type of usage (recreation, swimming, irrigation, drinking, aquaculture) is required for water safety management [107]. For example, to achieve the required safety levels for consumers of drinking water, a clear understanding of the extent of water treatment and disinfection is required regarding the actual level of microbiological pollution in the river water. Knowing the source of pollution helps to search for the representative fecal-associated pathogens (reference pathogens) and to foster the selection of adequate infection and disease risk models [107]. State-of-the-art primary and secondary biological wastewater treatment is a first fundamental barrier to significantly reduce fecal microbial pollution loads. However, wastewater treatment plants (WWTP) are not designed to quantitatively remove pathogens, and effluents of WWTP have to be considered infectious sources [109]. Quantitative microbial risk assessment (QMRA) can help to evaluate whether a further disinfection step (tertiary treatment) can help to reach the water quality targets at specific locations (e.g., WWTP upstream of a bathing area). As for the area of MST, QMRA is a young discipline as well and similarly requires the close cooperation between microbiologists, hydrologists, and modelers. Health risk assessment is considered an essential element in sustainable water and environmental management of the future, although data availability may limit the level of precision achievable. The suggested framework for integrated fecal microbial pollution analysis and management in rivers provides a basis where traditional pollution monitoring and further novel investigation targets can be integrated to use the strength of each individual approach in a sustainable way.
The conceptual framework for fecal pollution analysis of water resources adapted for river systems (Modified after [104–106]). Three interacting levels characterize the backbone of the concept with relevance to the following issues: (i) is there any problem with fecal pollution, (ii) if yes, who is responsible for it, and (iii) what is the actual health risk in relation to the fecal source(s) contributing to the observed pollution? Note that various methods are available at each level. The suggested framework was also referred to as a “bottom-up approach” because it starts at the most general level (i.e., level of general fecal pollution monitoring) and becomes more specific as it proceeds to the right end of the diagram
References
Pomeroy LR, Williams PJLB, Azam F, Hobbie JE (2007) The microbial loop. Oceanography 20:28–33
European Parliament & Council (2006) Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off J L064:37–51
European Council (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J L330:32–54
Schmedtje U, Bachmann J, Behrendt H, Birk S, Biza P, D’Eugenio J, v Gils J, Grath J, Hamchevici C, Hansen W, Interwies E, Kampa E, Lindinger H, Liska I, Popescu L, Popovici M, Pottgiesser T, Sigmund G, Sommerhäuser M, Speck S, Subauer I, Vogel B, Weller P, Winkelmann-Oei G, Zinke A (2005) Danube basin analysis (WFD roof report 2004). The Danube River Basin district - river basin characteristics, impacts of human activities and economic analysis required under article 5, annex II and annex III, and inventory of protected areas required under article 6, annex IV of the EU Water Framework Directive (2000/60/EC) – part A – basin-wide overview. ICPDR, Vienna, 191 pp
Kirschner AKT, Kavka GG, Velimirov B, Mach RL, Sommer R, Farnleitner AH (2009) Microbiological water quality along the Danube River: integrating data from two whole-river surveys and a transnational monitoring network. Water Res 43:3673–3684
International Association of Water Supply Companies in the Danube River Catchment Area (IAWD) (2011) Annual report 2009/2010. IAWD c/o Vienna Waterworks, Vienna, Austria (www.iawd.at)
Liska I, Wagner, F, Slobodnik, J (eds) (2008) Joint Danube Survey 2, final scientific report. ICPDR, Vienna (Austria), 242 pp. http://www.icpdr.org/main/activities-projects/joint-danube-survey-2
Heider A (1893) Untersuchungen über die Verunreinigungen der Donau durch Abwasser der Stadt Wien. Das Österreichische Sanitätswesen 6: 53 [In: Prescott SG, Winslow CEA (eds) Water bacteriology with special reference to sanitary water analysis, 6th edn. Wiley/Chapman & Hall, New York/London (1946)]
Brezina E (1906) Die Donau vom Leopoldsberge bis Pressburg, die Abwässer der Stadt Wien und deren Schicksal nach ihrer Einmündung in den Strom. Zeitschrift für Hygiene 53: 369 [In: Prescott SG, Winslow CEA (eds) Water bacteriology with special reference to sanitary water analysis, 6th edn. Wiley/Chapman & Hall, New York/London (1946)]
Lacroix H (1926) Wissenschaftliche Forschungsergebnisse aus dem Gebiete der unteren Donau und des Schwarzen Meeres. IV. Chemische und bakteriologische Untersuchung eines Wassers aus dem Donaudelta. Arch Hydrobiol 16(4):644–648
Joos I (1935) Untersuchungen von Donauwasser und Kanälen auf Typhus-Paratyphus Bazillen. Zentralbl Bakt 1 Abt Orig 135(4/5):266–269
Stundl K (1943) Untersuchungen über den Einfluss des Wassercharakters auf bakterielle Stickstoffumsetzungen. Zeitschr Fisch u Hilfsw 41(1):11–21
Mucha V, Daubner I (1965) Hydromikrobiologie im Rahmen der limnologischen Erforschung der Donau in der CSSR. Arch Hydrobiol Suppl 30:1–23
Megay K (1957) Die Güte des Donauwassers im Linzer Stadtgebiet und die Voraussetzungen für ein Strombad. Naturkundliches Jahrbuch der Stadt Linz 3:51–77
Weber J (1963) Ergebnisse der Enterokokken-Untersuchungen im Donaustrom. 7. Konferenz der Int. Arbeitsgemeinschaft Donauforschung (IAD) der SIL in Smolenice, CSSR. Referate. Hrsg: IAD, Wien, Österreich
Kohl W (1975) Über die Bedeutung bakteriologischer Untersuchungen für die Beurteilung von Fließgewässern, dargestellt am Beispiel der österreichischen Donau. Arch Hydrobiol Suppl 44:392–461
Deufel J (1968) Die Häufigkeit der Enterobakterien, Enterokokken und anaeroben sporenbildenden Bakterien im Oberlauf der Donau bis Ulm. Arch Hydrobiol Suppl 34:74–87
Mateeva E, Simon L (1963) Die physikalisch-chemischen und mikrobiologischen Ergebnisse der in der Donau im bulgarischen Sektor von km 845 bis 375 durchgeführten Untersuchungen 1959 bis 1962. In: 8. Konferenz der Internationalen Arbeitsgemeinschaft Donauforschung, Bukarest 1963. Referate. Hrsg: IAD, Wien, Österreich
Szemes G, Bozzay E (1964) The chemical and microbiological quality of the Danube water under ice cover in the extremely cold winter of 1962/63 as related to the supply of Budapest. Ann Sci Bp Biol 7:201 (cited by Mucha 1967: Die Mikrobiologie der Donau 3: 132–157)
Ristic O (1965) Mikrobiologische Untersuchungen der jugoslawischen Donaustrecke. Technologische Fakultät Novi Sad – Petrovaradin, unpubl. (cited by Mucha 1967: Die Mikrobiologie der Donau 3: 132–157)
Zamfir G, Balteanu EC, Nastase V, Finichiu M, Schreiner M (1968) Mikrobiologische und physikalisch-chemische Charakterisierung des Donauwassers im Abschnitt Braila-Tulcea. 10. Jubiläumstagung der Arbeitsgemeinschaft Donauforschung (IAD), Sofia. Limnologische Berichte. Hrsg: IAD, Wien, Österreich, pp 137–140
Mucha V (1967) Die Mikrobiologie der Donau 3: 132–157 [In: Liepolt R (Hrsg): Limnologie der Donau. Eine monographische Darstellung. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart 1967]
Kadlecova O, Odler J, Enterova K (1970) Die Salmonellen-Verunreinigung der Donau durch Abwässer in Bratislava. Arch Hydrobiol Suppl 36:255–262
Mucha V, Daubner I (1971) Über die hydromikrobiologischen Erforschung der Donau. Aquat Sci Res Across Boundaries 33:252–268
Michailenko LE, Ftomov AS (1979) Vergleichende sanitäts-mikrobiologische Auswertung der Mündungsgebiete von Dnepr, Dnestr und Donau. 21. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung der SIL in Novi Sad, Sept 1981. Wissenschaftliche Kurzreferate. Hrsg: IAD, Wien, Österreich, pp 562–567
Kohl W (1973) Salmonellen im Schlamm von Donaustauräumen. Kurzreferate der 16. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung (IAD), Bratislava, CSSR. Hrsg: IAD, Wien, Österreich
Kisselinov C (1981) Mikrobiologische Untersuchung der bakteriellen Belastung der Donau im bulgarischen Abschnitt. Referate - III. Hydromikrobiologisches Symposium, Smolenice, CSSR, 3.-6. Juni 1980, Verlag der Slowakischen Akademie der Wissenschaften, Bratislava, pp 175–186
Miklosovicova L (1982) Der gegenwärtige Stand der Wassergüte der Donau im Bereich des Wasserkraftwerkes Gabcikovo-Nagymaros vom mikrobiologischen Standpunkt. 23. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung in Wien, Sept 1982. Wissenschaftliche Kurzreferate. Hrsg: IAD, Wien, Österreich, pp 56–58
Zamfir G, Raileanu L (1982) Hygienisch-sanitäre Eigenheiten des Donaustromes hinsichtlich seiner Verwendung als Trinkwasserquelle. 23. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung (IAD) der SIL in Wien, Sept. 1982. Wissenschaftliche Kurzreferate. Hrsg: IAD, Wien, Österreich, pp 72–74
Kavka GG (1985) Salmonellen in Stauräumen des österreichischen Donauabschnittes. Wissenschaftliche Kurzreferate – 25. Arbeitstagung der Internationalen ARGE Donauforschung der SIL in Bratislava, pp 159–163
Kavka GG (1987) Die bakteriologische Wasserbeschaffenheit der österreichischen Donau. Wasser und Abwasser 31:305–344
Nicolescu D (1982) Daten zum Bakterioplankton der Donau im rumänischen Abschnitt im Jahre 1981. 23. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung der SIL in Wien, Sept 1982. Wissenschaftliche Kurzreferate. Hrsg: IAD, Wien, Österreich, pp 53–55
Gajin S (1982) Die Bewertung der Wasserqualität der jugoslawischen Donaustrecke aufgrund einiger mikrobiologischer Parameter. 23. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung (IAD) der SIL in Wien, 13–17 Sept 1982. Wissenschaftliche Kurzreferate. Hrsg: Österreichisches Nationalkomitee der IAD, Wien, Österreich, pp 62–64
Matavulj M, Petrovic O, Gajin S, Gantar M, Bokorov M, Erbenzik M (1984) Korrelation zwischen Phosphatase-Aktivität und mikrobiologischen Kennziffern der Qualität des Donauwassers. 24. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung in Szentendre, Sept 1984. Wissenschaftliche Kurzreferate I. Ed. Dr. A Berczik, Göd, Ungarn, pp 57–60
Matavulj M, Bokorov M, Gajin S, Gantar M, Stoilkovic S, Flint KP (1990) Phosphatase activity of water as a monitoring parameter. Water Sci Technol 22:63–68
Kasimir GD, Kavka GG (1988) Untersuchung der zählbaren und züchtbaren Bakterien in einem Laufstauökosystem (Donaustau Altenwörth). Wasser und Abwasser 32:57–88
Baschmakova IC (1985) Strukturelle und funktionelle Charakteristik des Bakterioplanktons im Kilia-Delta. 25. Arbeitstagung der Internationalen Arbeitsgemeinschaft Donauforschung (IAD), Bratislava, CSSR, 17–21 Sept 1985. Wissenschaftliche Kurzreferate. Hrsg: IAD, Wien, Österreich, pp 151–154
Albinger O (1990) Bakteriologische Wasser- und Sedimentuntersuchungen der Donau von Str.-km 16 bis Str.-km 1868 im März 1988. Ergebnisse der Internationalen Donauexpedition 1988. Hrsg: Internationale Arbeitsgemeinschaft Donauforschung (IAD) der Societas Internationalis Limnologiae (SIL), Wien, Österreich, Eigenverlag, pp 249–256
Trzilova B, Miklosovicova L (1990) Mikrobiologische Ergebnisse der Donauexpedition 1988 (Strom-km 20 bis Strom.-km 1868). Ergebnisse der Internationalen Donauexpedition 1988 Hrsg.: Internationale Arbeitsgemeinschaft Donauforschung (IAD) der Societas Internationalis Limnologiae (SIL), Wien, Österreich, Eigenverlag, pp 293–300
Stilinovic B (1990) Bakteriologische Eigenschaften des Donauwassers von Bratislava bis Vilkovo im März 1988. Ergebnisse der Internationalen Donauexpedition 1988. Hrsg.: Internationale Arbeitsgemeinschaft Donauforschung (IAD) der Societas Internationalis Limnologiae (SIL), Wien, Österreich, Eigenverlag, pp 281–292
Beij TW, Schmargun LM (1990) Hygienisch-bakteriologische Charakteristik des Donauwassers im März 1988. Ergebnisse der Internationalen Donauexpedition 1988. Hrsg: Internationale Arbeitsgemeinschaft Donauforschung (IAD) der Societas Internationalis Limnologiae (SIL), Wien, Österreich, Eigenverlag, pp 257–262
Kasimir GD (1990) Bacterial density, bacterial biomass and production in the river Danube along a longitudinal profile (in German). Ergebnisse der Internationalen Donauexpedition 1988. Internationale ARGE Donauforschung (IAD) der SIL, Eigenverlag, pp 263–273
Michajlenko LJ, Baschmakova IC, Jakuschin WM (1990) Die funktionelle Struktur des Bakterienplanktons der Donau. Ergebnisse der Internationalen Donauexpedition 1988. Hrsg: Internationale Arbeitsgemeinschaft Donauforschung (IAD) der Societas Internationalis Limnologiae, Wien, Österreich, Eigenverlag, pp 273–280
Kavka GG, Ludwig C, Ranner H, Humpesch UH, Kohl W (1990) Long-term quality alteration of the Danube near Vienna (1934–1902 km from the river mouth) (in German, English summary). Österreichische Wasserwirtschaft 42:26–33
Gajin S, Gantar M, Matavuly M, Petrovicy O (1990) The long term investigation of the River Danube water quality in the Yugoslav section according to microbial parameters. Water Sci Technol 22:39–44
Daubner I, Trzilova B (1991) Mikrobiologische Langzeituntersuchungen (1954–1989) der Donau aus limnologischer und hygienischer Sicht. Wasser und Abwasser, Wien 35: 31–51
Kavka GG, Berger B, Hoch BM, Herndl G (1996) Assessment of microbiological water quality in the Austrian section of the River Danube. Arch Hydrobiol Suppl Large Rivers 113(10):79–86
Kavka GG (1997) Bacteriological water quality in the Austrian section of the River Danube in consideration of EEC-Directives (in German with English summary). In: Güteentwicklung der Donau - Rückblick und Perspektiven. Schriftenreihe des Bundesamtes für Wasserwirtschaft, vol 4. Wien, Österreich, pp 52–69
Kavka GG (2000) Mikrobiologischer Zustand des österreichischen Donauabschnittes. In: Kavka GG und Kreitner P (Hrsg): Wasserbeschaffenheit und Güte der österreichischen Donau unter besonderer Berücksichtigung der langzeitlichen Entwicklung. Schriftenreihe des Bundesamtes für Wasserwirtschaft, vol 10. Wien, Österreich, pp 224–259
Popp W, Baumann M, Moller de Vargas D (1993) Bewertungsschema zur bakteriologisch-hygienischen Beurteilung der Wasserqualitat von Fließgewässern anhand von Fäkalindikatorbakterien als Ergänzung zur biologischen Gewassergütebeurteilung. Münchner Beiträge zur Abwasser-, Fischerei- und Flussbiologie, vol 47, pp 63–86
Baschmakova IC (1990) Estimation of the readily oxidizable organic matter reserve and its effect on the intensity of organic matter destruction by bacteria in the Danube. Water Sci Technol 22:31–33
Trzilova B, Miklosovicova L (1991) Das Vorkommen physiologischer Mikroben im Donauwasser. der 29.Tagung der Internationale Arbeitsgemeinschaft Donauforschung (IAD) in Kiew. Limnologische Berichte. Hrsg.: IAD, Wien, Österreich, pp 34–36
Berger B, Hoch BM, Kavka G, Herndl GJ (1995) The bacterial community of the Danube near Vienna: microbial-ecological parameters as compared with bacteriological water quality parameters and their influencing factors (in German). Wasser Abfallwirtsch 47:282–288
Hoch B, Berger B, Kavka G, Herndl GJ (1996) Influence of waste water treatment on the ecology of a large temperate river system – the Danube River. Hydrobiologia 321:205–218
Berger B, Hoch BM, Kavka G, Herndl GJ (1996) Bacterial colonization of suspended solids in the River Danube. Aquat Microb Ecol 10:37–44
Berger B, Hoch BM, Kavka G, Herndl GJ (1996) Bacterial metabolism in the Danube River: parameters controlling bacterial production. Freshw Biol 34:601–616
Farnleitner AH, Kasimir DG (1996) Bacterial activities in newly deposited sediments of the River Danube in Lower Austria. Arch Hydrobiol Suppl Large Rivers 113:397–403
Krauss-Kalweit I (2000) Vom Rhein zur ungarischen Donau. Messfahrt der MS Burgund auf Main, Main-Donau-Kanal und Donau vom 11. Mai bis 20. Juni 1998. Bd. I und II (Untersuchungsergebnisse). Hrsg: Ministerium für Umwelt und Forst, Rheinland-Pfalz, Germany (in German with English summary), 62 pp
Nemes K, Matavulj M, Simeunović J, Bugarski R, Gajin S, Lozanov-Crvenković Z (2007) Development of bacterioplankton biotransformation processes in the Danube River. In: Proceedings of the 11th international eco-conference 2007, Novi Sad, 26–29 Sept 2007, pp 89–96
Peduzzi P, Luef B (2008) Viruses, bacteria and suspended particles in a backwater and main channel site of the Danube (Austria). Aquat Sci 70:186–194
Nemes K, Matavuly M, Gajin S, Bugarski R, Simeunović J, Lozanov-Crvenković Z, Dalmacija B (2008) Phosphatase activity index (PAI): a reliable parameter for monitoring the point sources of pollutants and sustainable development. In: Proceedings - Balwois 2008 – Ohrid, Republic of Macedonia – 27–31 May 2008, 8 pp
Velimirov B, Milosevic N, Kavka GG, Farnleitner AH, Kirschner AKT (2011) Development of the bacterial compartment along the Danube River: a continuum despite local influences. Microb Ecol 61:955–967
Farnleitner AH, Hocke L, Beiwl C, Kavka GG, Zechmeister T, Kirschner AKT, Mach LR (2001) Rapid enzymatic detection of Escherichia coli contamination in polluted river water. Lett Appl Microbiol 33:246–250
Farnleitner AH, Hocke L, Beiwl C, Kavka GG, Mach RL (2002) Hydrolysis of 4- methylumbelliferyl-b-D-glucuronide in differing sample fractions of river waters and its implication for the detection of fecal pollution. Water Res 36:975–981
Schade M, Kopf W, Reischer GH, Farnleitner AH (2010) Wastewater disinfection at River Ilz to improve bacteriological water quality: effects and constraints. In: 38th conference international association for Danube research (IAD), Dresden 2010. Abstract book. IAD Germany, German Federal Institute of Hydrology (BfG) & IAD General Secretary, Wilhering, p 116
Kolarević S, Knežević-Vukčević J, Paunović M, Tomović J, Gačić Z, Vuković-Gačić B (2011) The anthropogenic impact on water quality of the River Danube in Serbia: microbiological analysis and genotoxicity monitoring. Arch Biol Sci Belgrade 63:1209–1217
Hosam EAF, Hamuda B, Patko I (2012) Ecological monitoring of Danube water quality in the Budapest region. Am J Environ Sci 8:202–211
Ajeagah G, Cioroi M, Praisler M, Constantin O, Palela M, Bahrim G (2012) Bacteriological and environmental characterisation of the water quality in the Danube River Basin in the Galati area of Romania. Afr J Microbiol Res 6:292–301
Kavka GG, Poetsch E (2002) Microbiology. In: Literáthy P, Koller-Kreimel V, Liska I (eds) Joint Danube Survey, technical report, ICPDR Vienna (Austria), vol 261. pp 138–150. http://www.icpdr.org/main/activities-projects/joint-danube-survey-1
Kirschner AKT, Kavka GG, Velimirov B, Reischer GH, Mach RL, Farnleitner AH (2008) Microbiological water quality and DNA based quantitative microbial source tracking. In: Liska I, Wagner F, Slobodnik J (eds.) Joint Danube Survey 2, final scientific report. ICPDR, Vienna, pp 86–95. http://www.icpdr.org/main/activities-projects/joint-danube-survey-2
Kavka GG, Kasimir GD, Farnleitner AH (2006) Microbiological water quality of the River Danube (km 2581 - km 15): longitudinal variation of pollution as determined by standard parameters. In: Proceedings of the 36th international conference of IAD, Austrian Committee Danube Research, Vienna, pp 415–421
Farnleitner AH, Reischer G, Winter C, Hein T, Mach RL, Kavka GG (2006) Progress in aquatic microbiology: introducing molecular biological approaches on a whole River Danube scale from Germany to the Black Sea. Danube news, vol 50. International Association of Danube Research, Wilhering, pp 8–12
Winter C, Hein T, Kavka GG, Mach RL, Farnleitner AH (2007) Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Appl Environ Microbiol 73:421–431
Reischer GH, Kavka GG, Kasper DC, Winter C, Mach RL, Farnleitner AH (2008) Applicability of DNA based quantitative microbial source tracking (QMST) on a large scale in the Danube River and its important tributaries. Fundam Appl Limnol Suppl 162:117–125
European Council (1976) Council Directive 76/160/EEC of 8 December 1975 concerning the quality of bathing water. Off J L031:1–7
Evanson M, Ambrose RF (2006) Sources and growth dynamics of fecal indicator bacteria in a coastal wetland system and potential impacts to adjacent waters. Water Res 40:475–486
Wade TJ, Pai N, Eisenberg JNS, Colford JM (2003) Do US environmental protection agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect 111:1102–1109
International Standard Organisation (ISO 7899-1) (1998) Water quality – detection and enumeration of intestinal enterococci in surface and waste water - part 1: miniaturized method (most probable number) for surface and waste water. International Organization of Standardization, Geneva, 21 pp
International Standard Organisation (ISO 7899-2) (2000) Water quality – detection and enumeration of intestinal enterococci – part 2: membrane filtration method. International Organization of Standardization, Geneva, 7 pp
International Standard Organisation (ISO 9308-1) (2000) Water quality – detection and enumeration of Escherichia coli and coliform bacteria – part 1: membrane filtration method. International Organization of Standardization, Geneva, p 10
International Standard Organisation (ISO 19458) (2006) Water quality – sampling for microbiological analysis. International Organisation for Standardisation, Geneva, Switzerland, 27 pp
European Parliament & Council (2000) Directive 2000/60/EC of the European Parliament and the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official J L327:1–72
Hagedorn C, Haarwood J, Blanch A (2011) Microbial source tracking: methods, applications and case studies. Springer, New York, 642 pp
Domingo JWS, Bambic DG, Edge TA, Wuertz S (2007) Quo vadis source tracking? Towards a strategic framework for environmental monitoring of fecal pollution. Water Res 41:3539–3552
Wuertz S, Wang D, Reischer G, Farnleitner AH (2011) Library independent bacterial methods. In: Hagedorn C, Haarwood J, Blanch A (eds) Microbial source tracking: methods, applications and case studies. Springer, New York, pp 61–112
Reischer GH, Kasper DC, Steinborn R, Mach RL, Farnleitner AH (2006) Quantitative PCR method for sensitive detection of ruminant faecal pollution in freshwater and evaluation of this method in alpine karstic regions. Appl Environ Microbiol 72:5610–5614
Reischer GH, Kasper DC, Steinborn R, Farnleitner AH, Mach RL (2007) A quantitative real-time PCR assay for the highly sensitive and specific detection of human faecal influence in spring water from a large alpine catchment. Lett Appl Microbiol 44:351–356
Kildare BJ, Leutenegger CM, McSwain SM, Bambic DG, Rajal VB, Wuertz S (2007) 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: a Bayesian approach. Water Res 41:3701–3715
Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G (2006) Development of bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224
Vierheilig J, Farnleitner AH, Kollanur D, Blöschl G, Reischer GH (2012) High abundance of genetic Bacteroidetes markers for total fecal pollution in pristine alpine soils suggests lack in specificity for feces. J Microbiol Methods 88:433–435
International Standard Organisation (ISO⁄CD 6461-2) (2002) Water quality – detection and enumeration of Clostridium perfringens – part 2: method by membrane filtration. International Organization of Standardization, Geneva
Kirschner A, Velimirov B (1997) Seasonal study of bacterial community succession in a temperate backwater system indicated by variation in morphotype numbers, biomass and secondary production. Microb Ecol 34:27–38
Van der Wielen PWJ, Medema G (2010) Unsuitability of quantitative Bacteroidales 16S rRNA gene assays for discerning fecal contamination of drinking water. Appl Environ Microbiol 76:4876–4881
World Health Organisation (2003) Guidelines for safe recreational water environments - volume 1: coastal and freshwaters. World Health Organisation, Geneva, 219 pp
World Health Organisation (2011) Guidelines for drinking-water quality, 4th edn. World Health Organisation, Geneva, 541 pp
Valent F, Little DA, Bertolli R, Nemer LE, Barbone F, Tamburlini G (2004) Burden of disease attributable to selected environmental factors and injury among children and adolescent in Europe. Lancet 363:2032–2039
European Food Safety Authority (2009) Food-borne outbreaks in the European Union in 2007. EFSA J 271:128 pp. ISBN: 978-92-9199-133-4
Frost F, Muller T, Craun G, Mihály K, György B, Calderon B (2005) Serological response to Cryptosporidium antigens among women using riverbank-filtered water, conventionally filtered surface water and ground water in Hungary. J Water Health 3:77–82
Putzer J, Takó MH, Márialigeti K, Törökné A, Karanis A (2007) First investigation into the prevalence of Cryptosporidium and Giardia spp. in Hungarian drinking water. J Water Health 5:573–584
Blaschke AP, Steiner K-H, Schmalfuß R, Gutknecht D, Sengschmitt D (2003) Clogging processes in hyporheic interstices of an impounded river, the Danube at Vienna, Austria. Int Rev Hydrobiol 88:397–413
Literáthy P, Koller-Kreimel V, Liska I (eds) (2002) Joint Danube Survey, technical report, ICPDR Vienna (Austria), 261 pp. http://www.icpdr.org/main/activities-projects/joint-danube-survey-1
Ishii S, Sadowsky M (2008) Escherichia coli in the environment: implications for water quality and human health. Microbes Environ 23:101–108
Farnleitner AH, Ryzinska-Paier G, Reischer GH, Burtscher MM, Knetsch S, Kirschner AKT, Dirnböck T, Kuschnig G, Mach RL, Sommer R (2010) Escherichia coli and enterococci are sensitive and reliable indicators for human, livestock, and wild life faecal pollution in alpine mountainous water resources. J Appl Microbiol 109:1599–1608
Farnleitner AH, Stadler H, Reischer GH, Sommer R, Kirschner AKT, Burtscher MM, Ryzinska G, Kuschnig G, Mach RL, Zerobin W (2008) Methods and strategies for alpine karstic water resource management: opening pollution microbiology’s “black box”. World Water Conference and Exhibition, 8.-12.09.2008 Vienna, Int. Water Association (IWA)
Farnleitner AH, Reischer GH, Savio DF, Frick C, Schuster N, Schilling K, Mach RL, Derx J, Kirschner A, Blaschke AP, Sommer R (2014) Diagnostik mikrobiologischer Fðkalkontaminationen in Wasser und Gewðssern: Status Quo und gegenwðrtige Entwicklungen. Wiener Mitteilungen 230:157–184, 978-3-85234-124-8
Farnleitner AH, Reischer GH, Stadler H, Kollanur D, Sommer R, Zerobin W, Barrella KM, Truesdale JA, Casarez EA, DiGiovanni GD (2011) Agricultural and rural watersheds. In: Hagedorn C et al (eds) Microbial source tracking: methods, applications and case studies. Springer, New York
Stalder G, Sommer R, Walzer C, Mach RL, Beiglbck C, Blaschke AP, Farnleitner AH (2011) Gefðhrdungs- und risikobasierende Konzepte zur Bewertung der mikrobiologischen Wasserqualitðt - Teil 1. Vet Med Austria 98:9–24
Derx J, Blaschke AP, Blöschl G (2010) Three-dimensional flow patterns at the river-interface – a case study at the Danube. Adv Water Resour 33:1375–1387
Farnleitner AH, Mach RL, Reischer G, Kavka GG (2007) Mikrobiologisch – hygienische Risiken trotz Klðranlagen am Stand der Technik? Wiener Mitteilungen 201:209–242 (in German)
Farnleitner AH, Winter C, Hein T, Mach RL, Kavka G (2006) Longitudinal changes in the bacterial community of the Danube by 16S rDNA profiling: a whole river approach. In: Proceedings of the 36th international conference of the IAD, Austrian Committee Danube Research/International Association of Danube Research, Vienna, pp 326–331
Kohl W (1969) Vorkommen und Nachweis von Salmonellen in Oberflächengewässern Österreichs. Wien Tierarztl Monatsschr 56:379–381
Stadler H, Skritek P, Sommer R, Mach RL, Zerobin W, Farnleitner AH (2008) Microbiological monitoring and automated event sampling at karst springs using LEO-satellites. Water Sci Technol 58:899–909
Acknowledgments
This paper was supported by the ICPDR (Joint Danube Survey 2001, 2007), the Austrian Science Fund (FWF) projects # P22309-B20 granted to A.H.F. and # W1219-N22 (Vienna Doctoral Programme on Water Resource Systems) as well as by the Vienna Water Works (project “Groundwater Resource Systems Vienna”), as part of the “(New) Danube – Untere Lobau Network Project” (Gewässervernetzung (Neue) Donau–Untere Lobau (National Park Donau-Auen)), funded by the Government of Austria (Federal Ministry of Agriculture, Forestry, Environment and Water Management), the Government of Vienna, and the European Agricultural Fund for Rural Development (project LE 07-13).
This is a joint publication of the Interuniversity Cooperation Center Water and Health (www.waterandhealth.at).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kirschner, A.K.T. et al. (2014). Microbiological Water Quality of the Danube River: Status Quo and Future Perspectives. In: Liska, I. (eds) The Danube River Basin. The Handbook of Environmental Chemistry, vol 39. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2014_307
Download citation
DOI: https://doi.org/10.1007/698_2014_307
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47738-0
Online ISBN: 978-3-662-47739-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)