Abstract
Colorectal cancer is the third most common form of cancer worldwide leading to escalating mortality rates and mainly includes hereditary, sporadic and colitis-associated cancer development. The escalated mortality rates is due to the limited treatment options as this form of cancer is usually not easy to diagnose in its early stages and are highly invasive leading to rapid metastasis of the malignant cells to the neighbouring tissue. In order to combat this limitation several chemotherapeutic regimens are now being combined with targeted therapies after the knowledge acquired on the inevitable effects of the tumor microenvironment on the colon cancer growth and progress. The colon tumor niche mainly consists of a large mass of tumor cells along with various immune cells, inflammatory cells, tumor macrophages and fibroblasts that infiltrate the tumor as it is a site of predominant inflammation. Among cells of the microenvironment, mesenchymal stem cells (MSCs) exhibiting ability to evolve into cancer associated fibroblasts (CAFs) have recently generated a major interest in the field. The physiological state of the tumor microenvironment is closely connected to discrete steps of tumorigenesis. The colon cancer cells elicit various factors with their direct interaction with MSCs or via paracrine fashion, which modulate these cells to promote cancer instead of performing their innate function of abating cancer progression. This review intends to highlight the necessity to exploit the cellular landscape of tumor microenvironment of colon cancer and a detailed understanding of the interactions between tumor epithelial cells and their stromal/inflammatory elements will aid in future perspectives for designing therapeutic regimens targeting tumor microenvironment to improve the clinical outcome of colon cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Colon cancer (CC) initiates as a benign or a metastatic mass of cells at any given point in the lining of the colon or rectum which mainly includes hereditary, sporadic and colitis-associated cancer development. Adenocarcinomas accounting for 95% of the colorectal tumors usually begin as benign polyps also known as adenomas that have the potential to develop into cancerous outgrowths as a result of accumulated mutations having a profound impact on the signalling pathways involved in maintenance of cell proliferation and tumor suppression (Colangelo et al. 2017). It is the third most common form of cancer worldwide leading to escalating mortality rates. The aberrated cell signalling pathways controlling the cell proliferation, stem cell maintenance and tumor suppression ultimately lead to the invasive forms of CC that metastasizes to distant organs wherein the tumor cells detach from the primary tumors, intravasate the vascular networks and reach the neighbouring tissues leading to rapid deaths. The late diagnosis of this form of cancer often leads to reduced treatment options as the tumor cells tend to metastasize at faster rates. The predominant cause for the progression of CC is the development of therapy refractory metastatic disease. Complex surgical resections of the tumors mostly do not completely cure the patient of cancer as there usually occurs a relapse of CC in a more invasive metastatic form as most of the tumor cells tend to metastasize even prior to the surgical removal of the tumor. Prolonged chemotherapy has also been observed to have evident effects on the patients however it did not prove to be of great use in the metastatic disease and rather lead to predominant drug resistance and tumor development (Tauriello and Batlle 2016). Several upcoming treatment options involve chemotherapy in combination with targeted therapy that targets not only the tumor cells but also its unique tumor niche that can lead to a more effective treatment of the metastatic forms of CC. Multifold studies have now highlighted that the escalation and recurrence of tumors are governed by the genetic alterations in the tumor microenvironment factors as well apart from the aberrated cancer cells (Quail and Joyce 2013). Tumor microenvironment (TME) plays a dominant role in influencing the tumor cells for their development and progression of CC. A coordinated network of interface cell types mainly include pericytes, adipocytes, immune cells, endothelial cells, fibroblasts, and mesenchymal stem cells through the extracellular matrix and soluble factors such as cytokines, chemokines, growth factors and various metabolites enhancing the tumorigenesis. Tumor assorted macrophage and myeloid suppressive cells characterize tumor-promoting immune cells enclave with their derived cytokines such as interleukin (IL-6, IL-1β, IL-23) and tumor necrosis factor (TNF- α). VEGF stimulates vascular endothelial cells for the formation of blood capillaries and is enhanced in the perivascular environment of tumor blood vessels exhibiting an abnormal physiology, due to deviant pericytes and permeable endothelial layers leads to the development of hypoxia and metastasis. It has been reported that the existence of MSCs can enhance the metastatic ability of various cancer including colon cancer.
Cancer-associated fibroblasts (CAFs) in the TME from the produce an excess of growth factors, cytokines, chemokines, structural protein components, and metabolites which are resultant of diverse precursors like mesenchymal stem cells (MSC) or endothelial cells so as to further endorse oncogenesis. An abnormal tumor-associated MSC can gain distinct functions such as secretion of TGF-ß to be a factor of epithelial-to-mesenchymal transition (EMT) and immune-suppressive activities following their interactions with tumor cells. Further, deviant MSC are known to secrete CXCL12 (SDF-1) and also liberate VEGF to sustain tumor cell growth and survival. MSC have the potential to either impede or elevate tumor progression within the TME by their distinctive kind of cellular interactions. MSCs can be recognized as RANTES (Regulated on Activation, Normal T cell Expressed and Secreted) which are expelled by the CC-chemokines ligand 5 (CCL-5) and further act together with suitable cytokine receptors like CCR1, CCR3 and CCR5. The adaptation of tumor growth and propagation is influenced by the extracellular matrix (ECM).
The precise contribution of the TME in promoting cancer in highlighted when only a confined success rate in met when the cancer cells are only targeted. The tumor microenvironment has therefore been considered to play an additional critical role in cancer progression as targeting only the cancer cells has led limited success rates in most of the CC patients (Ribatti et al. 2006). This review will briefly summarize the current understanding of the role of various cellular compartments of the tumor microenvironment in which CCs proliferate and metastasize and thereafter focus the discussion on the therapeutic aspect of targeting the major paracrine factors that govern the fate of colon cancer progression and metastasis.
2 Tumor Microenvironment (TME)
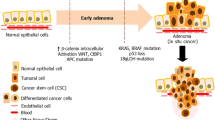
The escalating significance towards ecological therapy for various forms of cancer has given rise to extensive analysis of the cellular and non-cellular compartments of tumors which are commonly referred to as the tumor microenvironment (Wang et al. 2017). These solid tumors are predominantly a mass of several cell types providing a tumor specific niche having a profound effect on the immune status, neovascularization and establishment of an ECM that promotes the interactions among the various cells present in the TME. Tumor niche has been found to play a crucial role in the tumor growth and metastasis that was well explained by the “seed and soil” hypothesis put forward by Stephen Paget years back in 1889 (Ribatti et al. 2006). According to him the cancer cells that are represented by the “seed” can be maintained well only in its specific environment that was represented by the “soil” thereby, highlighting the importance of the TME in the maintenance and progression of cancer (O’Malley et al. 2016). The tumor microenvironment or niche is mainly composed of the tumor associated cells, extracellular matrix, inflammatory cytokines and matrix associated molecules that play a crucial role in the tumor cells maintenance, progression and metastasis. The tumor associated cells mainly include, immune cells such as T-cells, tumor associated macrophages, monocytes, neutrophils, natural killer cells, dendritic cells along with endothelial cells, platelets, cancer-associated fibroblasts and mesenchymal stromal cells (Peddareddigari et al. 2010). Due to excessive infiltration of the TME by the immune cells a chronic inflammatory response is elicited that plays a critical role in the neoplastic process. The essential role of inflammation in cancer progression was first described by Rudolf Virchow in 1863 (David 1988). The neoplastic process has several signaling pathways similar to those observed during an inflammatory response such as apoptosis, angiogenesis and escalated proliferation rates. The inflammatory process involved in normal tissue repair is resolved post tissue regeneration and pathogen elimination and homeostasis is maintained. However, chronic inflammation of the tissues in some cases has been observed to give rise to malignant transformation of the normal stromal cells and inhibit the restoration of normal homeostasis thereby leading to carcinogenesis (Landskron et al. 2014). The TME enables the malignant cells to evade clearance by the immune system and promote vascularization of the tumor by the release of various cytokines and matrix molecules which ablate the efficacy of several therapeutic regimens (O’Malley et al. 2016). The chronic inflammation and release of chemokines by the tumor cells as well as the stromal cells of TME are the key factors governing the tumor niche (Melzer et al. 2016). The complex mechanisms of tumor development and progression can therefore be analysed clearly by observing the interactions between the several factors and cells residing in the tumor specific niche (Melzer et al. 2016) (Fig. 1).
2.1 Structural Scaffold for the Tumor Stroma
Fibrous protein in the tumor stroma includes elastin, collagen, fibronectin, proteoglycans like chondroitin sulphate and hyaluronic acid which are subsisted in the ECM and is also mainly enriched with collagen. Soluble factors such as growth factors, angiogenic factors, cytokines and chemokines are abundantly present in tumor stroma. Collagen involves the deposition and tight organization of matrix proteins like elastin, laminins and also the altering enzymes such as lysyl oxidase, leads to a more inflexible phenotype of complete tumor. Fibronectin is associated in tumor invasion and metastasis (Chen and Huang 2014).
2.2 Cellular Components of the TME in Colon Cancer
Tumor niche consists of a class of non-malignant cell types such as immune cells, endothelial cells, fibroblast and MSC which expand the tumor-assisted functions along with the soluble factors and ECM components interconnects with the cancer cells to disseminate tumorigenesis. The tumor microenvironment in CC is comprised of multiple components such as vasculature, tumor-infiltrating cells, extracellular matrix (ECM), and other matrix-associated molecules. A detailed characterisation of the cellular landscape and their role in cancer progression has been explained in the below sections.
2.2.1 Immune Cells
Immune cells play a prominent role as gate-keepers and protectors of the body from various infections and cancers. Cancer immune-surveillance involves the role of both the innate and the adaptive immune system to eradicate the tumor from the body. However, the immune-surveillance tends to be affected due to the interactions of the immune cells with other cellular components, cytokines and ECM molecules present in the TME. Due to varied response of the immune system during cancer progression the concept of immune-editing has been introduced which includes three stages namely, elimination, equilibrium and escape. These cancer cells are either eliminated by the immune cells else they acquire adaptations making them resistant to the immune clearance and thereby maintain their pool resulting in cancer progression (Colangelo et al. 2017). The first stage mainly involves the complete clearance of the tumor cells effectively by the immune cells and various other critical signaling molecules involved in the immune clearance process. Post tumor clearance these immune cells and active molecules result in immune-editing wherein the immune cells in the equilibrium state are unable to completely eliminate the tumor from the body as the cancer cells have adapted to the immune-surveillance by establishing phenotypic alterations such as epithelial to mesenchymal transition. However, at this stage even though the immune cells are unable to completely eliminate the tumor cells they are capable to limit the tumor growth to a certain extent. Therefore, the immune microenvironment have been found to play a role in the retaining a pool of dominant cancer cells that can repopulate the tumor and maintain tumor growth. In the escape stage, the immune cells assist the tumor cells in effectively escaping from the body’s immune clearance and promote cancer invasion by releasing anti-cancer proteins and cytokines. Therefore targeting these interactions of the cellular components of the tumor niche will be proving to be a more potent cancer therapeutic regimen (David 1988).
2.2.2 Tumor Associated Macrophages (TAM’s)
Macrophage plasticity has been observed to massively impact the tumor growth and invasion potential. TAM’s play a critical role in the regulation of the tumor niche in colon cancer. Macrophages have always been recognized as critical effector cells for the clearance of the tumors. However, recent studies have highlighted the role of macrophages as TAM’s in assisting the cancer cells in altering their phenotypes as well as promoting tumor invasion. TAM’s have been observed to crowd at the tumor edges and induce apoptosis of the cancer cells however; those populated near the tumor invasion sites have been found to possess reduced potential in tumor ablation (Wang et al. 2017). TAM’s are main contributors in promoting tumor angiogenesis (Balkwill et al. 2012). This dual role of the macrophages can be explained by their ability to alter their phenotypes and their potent plasticity that can be attained by altering their polarization according to the current conditions. Macrophages are mainly classified into two distinct types based on their polarization states such as M1 and M2 macrophages. The M1 macrophages are the normally activated macrophages that release type I pro-inflammatory cytokines and elicit an anti-tumorigenic response. However, the M2 macrophages that are alternatively activated release type II cytokines that elicit an anti-inflammatory response and thereby promote tumor growth and invasion. These TAM’s have been observed to enhance tumor invasiveness by intricate paracrine interactions between the cancer and the macrophages mainly including factors such as tumor-derived CSF-1 and macrophage-derived EGF. Targeting this switch of macrophage toward tumor enhancing phenotype and characteristics can be useful to block tumor increase (Quail and Joyce 2013).
2.2.3 Mesenchymal Stem Cells (MSCs)
MSCs are distinctive multipotent cells which possess distinct characteristics such as self-renewal, anchorage- dependent and their differentiation potential that exist in perivascular environment of the human tissues and organs such as bone marrow, adipose tissue and fetal tissues comprising of placenta, amniotic membranes and umbilical cord. MSCs have an excellent potential to drift to the site of inflammation and to sustain tissue repair, angiogenesis, stem cell homeostasis, immune inflection and thus elevate tumor progression by liberating various endocrine and paracrine signals. Exosomes are extracellular vesicles which aid in the intercellular contact with the neighbouring tumor cells and mesenchymal stroma cells. Movement of MSCs to the site of inflammation or tumor microenvironment is facilitated by the exosomes to secrete chemokines such as CXCL1, CCL2, IL-6 and growth factors like TGF-β1,VEGF and PDGF-BB (Rhee et al. 2015). Thus, the focal point of the extracellular vesicles to the tumor cells helps in their migration and paves the way to tumor malignancy. Exosomes arising from MSCs receive their stimulation from matrix metalloproteinase-2 (MMP-2) and ecto-5′-nucleotidase activity along with miRNA facilitates cancer progression.
2.2.3.1 Migration of MSC’s to the TME and Their Potential Interface
The MSCs can be attracted and recruited into damaged tissues by releasing enormous amounts of inflammatory cytokines and chemokines. The malignant tissues are well known for the release of several chemokines during the tumor development. The chemokines such as CCL2, CCL15, CCL20, CCL25, CXCL1 and CXCL8 have been identified to greatly impact the homing of the MSC’s to the tumor niche. The possible paracrine interactions between MSCs and CC cells in the TME during CC progression have been summarized in Fig. 2. These active factors can be secreted to implement profound effects on the cells via paracrine signaling or by releasing exosomes that are extracellular vesicles containing substantial amounts of the secreted factors. The recruitment of MSC’s therefore activates these stem cells and results in spontaneous release of a set of other inflammatory cytokines that promote tumor development and maintenance of tumor niche (Ali et al. 2015a). These MSC’s upon activation in the TME transform into malignant cells commonly known as the cancer associated fibroblasts (CAFs). These CAF’s promote tumor progression and metastasis. These CAF’s are mainly characterized by expression of α-smooth muscle actin, PDGF receptor-β. These malignant fibroblast have also been observed to induce epithelial to mesenchymal transitions in the cancer cells by the expression of proteins such as MMP, TWIST, WnT5A and TGF-β type-I receptors that in turn give rise to aggressive forms of tumors (Peddareddigari et al. 2010). Based on recent research for the underlying mechanism of interaction between MSCs and CC cells in tumor niche, some significant factors has emerged which upon targeting might lead to suppression of cancer progression (Fig. 3).
2.2.4 Cancer Associated Fibroblasts (CAF’s)
The stroma is the most essential component that maintains the tissue architecture that provides a base for the residing cells. Fibroblasts are the most common cells present in almost all the tissues of the body. These spindle-shaped cells predominantly found the colon in the normal colonic mucosa are situated adjacent to the colon mucosal epithelium. They are known to play a role in the synthesis, deposition and turnover of the basement membrane components. The crosstalk between these fibroblasts and the epithelium via paracrine signaling results in the maintenance of tissue integrity (Wang et al. 2017). Therefore these fibroblasts are also found to infiltrate the tumors and play a major role in maintaining the tumor growth and TME. These fibroblasts get activated in the TME and give rise to cancer associated fibroblasts (CAF). The CAF’s have been found to promote cancer cell proliferation and metastatic potential. These cells are mainly characterized by the altered expression of α-SMA, vimentin, fibroblast activating protein (FAP), platelet-derived growth factor receptors α and β, fibroblast specific protein-1 (FSP1), the chondroitin sulphate proteoglycan neuron-glial antigen-2 (NG2), and prolyl-4-hydroxylase (Colangelo et al. 2017). These cells secrete considerable amounts of stromal-cell derived factor 1 that specifically binds to the CXCR-4 expressed by the tumor cells thereby promoting tumor invasion and angiogenesis (Chen and Huang 2014). The CAF’s formed from the modified fibroblasts present in the tumor niche have been observed to secret multiple factors which influences the invasive potential of the tumor, its angiogenesis as well as tumor progression. These factors mainly include miRNAs 200b and 155, angiogenesis factor VEGF, chemokines such as SDF1 also known as CXCL-12, hepatocyte growth factor (HGF), epidermal growth factor (EGF), macrophage migration inhibitory factor (MIF), and various other interleukins as well (Wang et al. 2017; Bahrami et al. 2018). TGF-β released by the fibroblasts has been known to induce epithelial to mesenchymal transition of the cancer cells thereby contributing to the immune-suppressive tumor niche. Studies have indicated that elimination of these FAP- positive cells resulted in spontaneous tumor necrosis mediated by the factors IFN-γ and TNF-α (Quail and Joyce 2013).
2.2.5 Vascular Endothelial Cells and Pericytes
Tumor cells are well known to promote the infiltration of endothelial cells into the tumor compartment in order to promote neovascularization. Several soluble factors such as VEGF, FGF, PDGF and various other chemokines play important roles in promoting tumor growth by promoting tumor vasculature. When a blood vessel ruptures in the tumor vicinity the angiogenic signals are released from the malignant cells stimulating formation of new blood vessels. The formation of these new aberrant and uneven blood vessels results in the leakiness which is one of the major reasons of excessive metastasis of the malignant cells (Chen and Huang 2014). Pericytes are cells of mesenchymal origin that form a support system for blood vessel formation and function. These mainly provide structural support and therefore depletion in their pool results in the formation of excessive aberrant and leaky blood vessels which results in a higher rate of tumor metastasis (Wang et al. 2017).
2.2.6 Myeloid Derived Suppressor Cells (MDSC)
MDSC or immature myeloid precursor cells are of immense interest as they are implicated in cancer immune suppression as well as immune suppressive states of colon cancer. These cells are broadly characterized as CD11b+, CD33+ and LIN−, HLA-DR−, cells in humans. MDSC consists of a heterogeneous set of precursor myeloid cells capable of suppressing the adaptive immune response. MDSCs proliferation is influenced by pro-myelopoetic factors produced by colon cancer cells which include M-CSF, IL-6, PGs, GM-CSF, VEGF and stem cell factor (SCF). As the MDSC proliferate, they induce a negative feedback inhibition on the T cell and NK cells activity thereby arresting the immune response against the colon cancer progression (Wang et al. 2017).
3 Non-cellular Components of the TME of Colon Cancer
The colon cancer TME is not only composed of several cell populations but also includes multifold active molecules and ECM components that equally contribute in maintaining the malignant microenvironment as well as promote the tumor growth and metastasis.
3.1 miRNA in Colon TME
These small non-coding RNA’s have been known to play a crucial role in several physiological processes and also have conspicuous role in various pathological conditions including cancers. Interestingly this microRNA’s have been observed to not only regulate the cancer cells but also have an impact on the tumor stroma which in turn gives rise to drastic progression of the cancer (Jiang et al. 2017). The alteration in the function and the stages of different tumors has been found to be associated with altered miRNA expression. The miRNA expression in colorectal cancer has been well elucidated. The miRNA’s with escalated expression levels during CRC include miR-21, miR17, miR-155, miR-146, miR-221, miR-31, miR-25 and miR-196 (Strubberg and Madison 2017). Around 35 microRNAs have been observed to be either escalated or abated in CC. A detailed chart of the highly up and down regulated miRNAs, their target genes and possible signaling pathways involved are depicted in Table 1 (Inoue et al. 2012; Sansom et al. 2010; Ibrahim et al. 2011; Wang et al. 2015; Zhang et al. 2010, 2018; Xu et al. 2014; Schee et al. 2010; Mohammadi et al. 2016). These variations in the miRNA expression are found to be result of repeated chromosomal aberrations. Both normal as well as malignant cells have been found to release miRNA’s into the peripheral blood that are protected from RNase degradation by protective vesicles known as exosomes. The crosstalk between the other cells of the tumor niche and the cancer cells via autocrine or paracrine signalling is crucial for the progression of cancer. These interactions are brought about by these exosomal miRNA that are released by the cancer cells in order to promote the neighbouring cells to maintain the required tumor niche for enhanced cancer progression and metastasis. The released exosomal miRNA’s have also been observed to provide a premetastatic environment that enhances permeability and vascularization of the tumor that in turn promotes the metastasis of the malignant cells to the neighbouring organs (Strubberg and Madison 2017). This exosomal microRNA’s can be easily detected in the plasma levels thereby coming forward as an effective target for diagnosis as well as CC treatment. The microRNA’s miR-17-3p and miR-92a have been observed to be drastically abated in CC patient’s post-surgery thereby indicating their excessive expression during cancer progression. MiRNA-29a has been observed to help differentiate between advanced stages of CC from other bowel disorders. Colon cancer progression can be effectively regulated by directly targeting these miRNA expressions by directly blocking/inhibiting these miRNA’s or by downregulating the expression of these miRNA’s by antisense oligonucleotides or promoter methylation (Inoue et al. 2012). Multifold tumor suppressor miRNA’s have also contributed widely to the regulation of CC progression. These beneficial miRNA’s are mostly transferred by viral vectors that release these RNA’s at the tumor site to achieve maximal tumor abatement. Several tumor suppressive miRNA’s are now being delivered not only to the malignant cells but also the tumor stromal cells present in the immediate tumor microenvironment. The delivery of these miRNA’s have been found to modulate the tumor associated dendritic cells from an immunosuppressive to an immune-stimulatory state resulting in enhanced reduction of the tumor as they are subjected to the body’s immune system.
3.2 miRNA from TAMs in Colon Cancer TME
Recent studies have highlighted the role of the immune cells to also play a vital role in the initiation, progression and metastasis of various cancers. The macrophages in the tumor niche have been observed to be polarized from an anti-tumorigenic (M1) to a pro-tumorigenic (M2) state by alterations in their metabolic pathways. This has been analysed due to the predominant dysregulation of the miRNA expression profiles of the normal macrophages after the onset of cancer. Therefore, several approaches have been made to target these dysregulated miRNA profiles to revere the polarization of the TAM’s to normal macrophages and bring about effective anti-tumorigenic effects. The miR-511-3p that encodes for the macrophage-mannose receptor has been found to possess altered expression in the MRC1+ TAM’s by escalating the expression of this miRNA in the TAM’s resulted in enhanced suppression of the pro-tumoral genes thereby inhibiting tumor growth and alterations in the blood supply to the tumors. The escalated release of cytokines from this TAM’s resulted in aberrated cancer progression. Excessive expression of miRNA-155 has been found to attenuate the release of multifold cytokines such as IL-6, IL-10 and TNF-alpha that play a vital role in promoting malignancies. The escalated expression of miRNA-155 resulted in evident reversal of the TAM’s to normal anti-tumorigenic macrophages (Sansom et al. 2010).
3.3 Extracellular Matrix (ECM)
Almost all the mammalian cells remain in close contact with their surrounding stromal matrix whose components and composition varies in different organs, cell types and medical conditions such as cancer. The extra cellular matrix is mainly composed of 5 essential components namely collagens, laminins, fibronectins, proteoglycans and hyaluronans. These ECM components bind to the integrins present on the cell surface and provide the required mechanical and physicochemical support to the cells. The ECM is also critically involved in the cell migration and bears several growth factors that play a major role in maintaining the cell pool. The stromal cells and epithelial cells in contact with each other coordinate and produce the basement membrane (BM) that has a significant role in cancer progression. The loss of the BM components or their inappropriate synthesis alters the cell physiology thereby being a key player in the onset of the disease. For instance the loss of the ECM component laminin-5 is major contributor in colon cancer progression. An altered ECM composition has been found to give rise to a switch in the role of the integrin α6β4 that is also known as the tumor antigen. Enhanced expression of this integrin has been found to give rise to escalated aggressiveness and poor prognosis of the developing tumors. In colon carcinoma this integrin promotes cell migration on laminins-1 thereby contributing to the metastatic potential of the malignant cells. The metabolism of the ECM molecules is an essential aspect involved during tissue homeostasis and the response of cells towards acute and chronic stresses. Several types of proteinases participate in ECM turnover, however matrix metalloproteinases (MMPs) are known to be the major ECM degrading enzymes along with urokinase-type plasminogen activator (Rhee et al. 2015). The ECM degradation being a common phenomenon during cancer development, these enzymes involve the interactions of the stromal and the cancer cells upon ECM loss thereby inducing a profound effect on the stromal cells via direct contact or paracrine signaling. These interactions therefore promote the metastasis of the malignant cells (Ali et al. 2015a).
3.4 Matrix Metalloproteinases
The MMP’s are a group of zinc-dependant endopeptidases that play a crucial role in the hematogenous metastasis process by carrying out the degradation of the ECM. Many matrix metalloproteinases have been linked to the colon cancer progression and metastasis (Ibrahim et al. 2011) MMP-9 is a common 92-kDa proenzyme which has the potential to degrade type IV collagen post activation. MMP-2 another important matrix metalloproteinase plays a crucial role in CC progression and invasion. Elevated expression of MMP-2 has been observed in bladder cancer, lung carcinoma, colorectal cancer gastric and breast cancer. Studies working on the silencing of MMP-2 in colon cancer cell lines have indicated significant ablation of the cell proliferation, invasion and colony forming capacity (Wang et al. 2015; Zhang et al. 2010). Particularly, MMP-7 overexpression has been observed to occur during the intiation of the carcinogenic cascade post transformation of the normal mucosa into carcinogenic adenomas. Data obtained from in vitro studies have shown that MMP-7 expression is related to the invasiveness of the primary tumor cells (Xu et al. 2014) in colon cancer and overexpression of MMP-7 has also been reported in pancreatic (Schee et al. 2010) and breast (Zhang et al. 2018) carcinomas, respectively. Recent studies reported that MMP-7 appears to be an early event in the adenoma-to-carcinoma pathway (Mohammadi et al. 2016), hence multiple experiments are being carried out by utilizing MMP inhibitors for the prevention or treatment of colorectal cancer.
3.5 Cytokines
The colon cancer onset is mainly observed in patients initially afflicted by inflammatory bowel disease that occurs due to a noticeable loss of balance between the pro-inflammatory and regulatory cytokines. The malignant cells possess the ability to modulate the stroma and the immediate microenvironment by secreting multifold factors that recruit inflammatory cells and activate stroma cells in the TME. These cells in turn release several factors such as cytokines, chemokines, growth factors and proteases that promote tumor progression and metastasis. Along with these factors these cells also release several relative oxygen and nitrogen species which induce genetic alterations thereby promoting cancer. Chemokines can be directly secreted into to the extracellular space or via vesicles. Multifold cytokines such as TNFα, IL-8, IL-6 and VEGF, have been noticed to be elevated in colorectal carcinoma (CRC) patients (Kuninty et al. 2016). Escalated levels of TNF-α and IL-6 have been observed to result in the activation of NF-κB and STAT3 pathways. Interleukin 1β a potent pro-inflammatory cytokine secreted in voluminous amounts by the macrophages in the tumor niche is known to promote the secretion of other inflammatory cytokines and chemokines such as TNFα, IL-6, IL8, IL-17, COX-2 and PGE2 that promote the colon cancer cell growth and progression. The colon cancer cells stimulate the macrophages present in the TME to secrete multifold amounts of IL-6 that is known to activate STAT3 in the malignant cells. This cytokine plays a crucial role in maintaining the growth of the tumor cells as the inhibition of this chemokine resulted in evident decrease in the growth potential of the malignant cells. This cytokine is also secreted in substantial quantities by various other stimulated cells residing in the tumor vicinity mainly including fibroblasts, monocytes, endothelial cells and immune cells namely the B and T-lymphocytes. Another critical cytokine in the TME is IL-10, this cytokine has predominant inhibitory effects on the pro-inflammatory cytokines such as IL-1β, TNFα and IL-6and is mainly secreted by Th2 cells, B cells, tumor cells and macrophages. Abated levels of this cytokine result in drastic progression of the tumors. Several chemokines released by the cancer cells such as CC15, CCL-2, CCL-20, CXCL-8 that promote the attraction of the MSC’s towards them. The tropism of the MSC’s is also affected by the release of certain active factors such as VEGF, HGF and TGFβ. The MSC’s upon activation by the colon cancer cells secrete detectable amounts of CXCL-1, CXCL-2, CXCL-12 and IL-6 (Zucker and Vacirca 2004). These pro-inflammatory cytokines and its associated pathways have emerged as potential targets for effective and alternative cancer therapy.
4 Future Perspectives and Therapy
4.1 Effective Molecular Interaction of MSCs with Tumor Cells
Cellular and molecular mechanisms involved when mesenchymal stem cells are co-cultured along with distinctive cancer cell types such as colon cancer, lung cancer, breast cancer, ovarian cancer cell lines exemplify an intercellular contact with tumor microenvironment evolving the expression of MSC surface markers such as CD90, CD105, and CD73 on the cell surface both in vitro by appropriate differentiating conditions (Melzer et al. 2016). For instance, when co-culturing adipose derived MSCs with colon cancer cell lines such as HCT116 cells, LoVo cells, SW480, LS174T, and CCD-18 Co, it has been recognized to explicit epithelial- mesenchymal transition (EMT) correlated genes like ZEB1, ZEB2, Slug, Snail, Twist and also stemness genes such as Oct4, Sox2, Nanog, Bmi1 which are known to be intensified in co-cultured system along with other associated cancer genes namely MMP1, IL10, TGF-(α, β), COL1A1, IFN-γ, VEGFA etc. (Dong et al. 2011). Thus, it is proven that the MSCs enhance the tumor propagation and malignancies in conjunction with knocking down or up-regulating certain genes along with other growth factors and signaling pathways in their tumor niche (Melzer et al. 2016). In tumor malignancy, it has been identified as tumor cells along with MSCs imply certain gene transcripts and other growth factors trigger various signaling pathways. They are instigated that according to their diverse cancer cell types in the tumor niche, it is comprised of notch, Hedgehog, Wnt, PI3K, NF-κB, and STAT pathways which are specifically activated or inhibited depending on the paracrine signals in their tumor stroma. Therefore, paracrine signals are proven to enhance the MSCs residing in the tumor niche of colon cancer more precisely (Illemann et al. 2006; Gout and Huot 2008). In contrast to the tumor-enhancing ability of MSCs, different studies have shown that MSCs inhibit tumor progression and metastasis by inhibiting angiogenesis, suppressing immune responses, suppressing Wnt and Akt signaling, and inducing apoptosis or cell cycle arrest in the G0-G1 phase of the cancer (Lazennec and Lam 2016). Due to their tropism to the tumor niche, mesenchymal stem cells are considered to be a promising vector for the delivery of antitumor agents.
4.2 Targeting of TME by miRNAs
A number of malignancies have been associated with characteristic miRNA signatures (Chen et al. 2015; Lu et al. 2005; Bullock et al. 2013). Compared to normal tissues, miRNAs have been found to be dysregulated in cancers. Recent studies have highlighted the inevitable role of miRNAs in the progression of CRC. The miRNAs of the cancer cells via the non-cell autonomous mechanism have been found to elicit an impact on the TME by altering the miRNA profiles of the non aberrated neighbouring cells and resulting into their conversion into cells possessing cancer promotive effects. Many studies have highlighted the role of CAF in the initiation and progression of cancer. Changing of phenotype to CAFs is responsible for trans-differentiation properties as well as CAF associated tumorigenic actions of stromal cells in TME (Sansom et al. 2010). One of the most common miRNAs that has been reported to be predominantly present in the tumor cells as well as the CAFs of pancreatic and colorectal tumors is miRNA-21 (Lu et al. 2005; Bullock et al. 2013; Ali et al. 2015b). Hence studies that utilized antagomiR for the inhibition of miR-21 resulted in decreased migration/invasion potential of the CAFs (Chen et al. 2015; Lu et al. 2005). The CAF phenotype can be induced by both altered escalated as well as abated expression of certain miRNAs (Bullock et al. 2013; Ali et al. 2015b). Abrupt expansion of the roles of miRNAs in tumor microenvironments has come forward. For the use of miRNAs in therapeutics, it is vital to deliver miRNA mimics cells or antagomiRs directly into the target cells. However, the polyanionic nature, hydrophilicity, and high molecular weight of naked miRNAs make it impossible of them to pass through cell membranes. Rapid removal by urine has been observed when the chemically modified anti-miR oligonucleotides are administered in the absence of a carrier as they exhibit limited tissue distribution. Viral and non-viral encapsulation strategies and nanoencapsulation of miRNAs to protect miRNAs from degradation by nucleases, along with improved circulating half-life had been reported earlier (Sansom et al. 2010).
5 Concluding Remarks
The progression and metastatic potential of the colon cancer cells is predominantly supported by the immediate tumor microenvironment that consist of several cellular and non-cellular components that promote the sustenance and spread of these malignant cells to other organs of the afflicted patient. The TME mainly comprising of the immune cells such as T-cells, B-cells, Tumor associated Macrophages, fibroblasts, CAF’s, and acellular components mainly comprising of the ECM, cytokines and chemokines that have predominant effects on the signalling pathways involved in colon cancer progression and metastasis. Therapeutic agents that can alter the colon cancer ecosystem may be effective in preventing or treating the metastatic diseases. Colon cancer cells rely on stromal factors to proliferate and migrate (Rhee et al. 2015; Heslin et al. 2001). Therapeutic targeting of stromal cellular components, including inflammatory cells such as CAFs, TAMs, immune cells, endothelial cells and the vasculature, ECM, and matrix-associated molecules, must therefore be considered eventually. Therapies targeting the tumor niche mainly the small molecule inhibitors, antibodies blocking the interactions between the tumor cells and the other cells of the TME will give rise to a more effective alternative therapy as the cancer progression is blocked at the molecular level by targeting the cytokines and the exosomal interactions and thereby abating the activation of signalling pathways involved in the progression and metastasis of colon cancer cells. In order to develop multifold strategies for the in-vivo delivery of miRNA the mechanisms of miRNAs have been extensively analysed. However, there are a few setbacks that are being worked on. One of the major issues is the poor cancer tissue permeability in miRNA based therapy. Heterogeneous tumor perfusion and interstitial fibrosis has resulted in the inefficiency of the penetrating miRNA-containing delivery vesicles with or without targeting moieties in the tumor microenvironment. In order to promote alternative and more effective cancer therapies the complicated nature of the cancer cell–host cell interactions and cell–ECM interactions in the tumor are to be understood.
Abbreviations
- CAFs:
-
Cancer Associated, Fibroblasts
- CC:
-
Colon Cancer
- CRC:
-
Colo Rectal Carcinoma
- ECM:
-
Extracellular Matrix
- EGF:
-
Epidermal Growth Factor
- EMT:
-
Epithelial to Mesenchymal Transition
- FAP:
-
Fibroblast Activating Protein
- HGF:
-
Hepatocyte Growth Factor
- MDSC:
-
Myeloid Derived Suppressor Cells
- MIF:
-
Migration Inhibitory Factor.
- MMP:
-
Matrix Metalo Proteinases
- MSCs:
-
Mesenchymal Stem Cells
- RANTES:
-
Regulated on activation, Normal T-cell Expressed and Secreted
- SCF:
-
Stem Cell Factor
- TAMs:
-
Tumour Associated Macrophages
- TME:
-
Tumour Microenvironment
- TNF:
-
Tumour Necrosis Factor
- VEGF:
-
Vascular Endothelial Growth Factor
References
Ali S, Suresh R, Banerjee S et al (2015a) Contribution of microRNAs in understanding the pancreatic tumor microenvironment involving cancer associated stellate and fibroblast cells. Am J Cancer Res 5(3):1251
Ali S, Dubaybo H, Brand RE, Sarkar FH (2015b) Differential expression of microRNAs in tissues and plasma co-exists as a biomarker for pancreatic cancer. J Cancer Sci Ther 7(11):336
Bahrami A, Khazaei M, Hassanian SM et al (2018) Targeting the tumor microenvironment as a potential therapeutic approach in colorectal cancer: rational and progress. J Cell Physiol 233(4):2928–2936
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23):5591–5596
Bullock MD, Pickard KM, Nielsen BS et al (2013) Pleiotropic actions of miR-21 highlights the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis 4(6):e684
Chen S, Huang EH (2014) The colon cancer stem cell microenvironment holds keys to future cancer therapy. J Gastrointest Surg 18(5):1040–1048
Chen J, Li C, Chen L (2015) The role of microvesicles derived from mesenchymal stem cells in lung diseases. Biomed Res Int 2015:985814
Colangelo T, Polcaro G, Muccillo L et al (2017) Friend or foe?: The tumour microenvironment dilemma in colorectal cancer. Biochim Biophys Acta Rev Cancer 1867(1):1–8
David H (1988) Rudolf Virchow and modern aspects of tumor pathology. Pathol Res Pract 183(3):356–364
Dong W, Li H, Zhang Y, Yang H, Guo M, Li L, Liu T (2011) Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim Biophys Sin 43(11):840–848
Gout S, Huot J (2008) Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron 1(1):69–83
Heslin MJ, Yan J, Johnson MR, Weiss H, Diasio RB, Urist MM (2001) Role of matrix metalloproteinases in colorectal carcinogenesis. Ann Surg 233(6):786
Ibrahim AF, Weirauch U, Thomas M, Grünweller A, Hartmann RK, Aigner A (2011) MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res 71(15):5214–5224. https://doi.org/10.1158/0008-5472.CAN-10-4645
Illemann M, Bird N, Majeed A et al (2006) MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res 4(5):293–302
Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R (2012) Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep 27(6):1759–1764
Jiang X, Hu S, Liu Q, Qian C, Liu Z, Luo D (2017) Exosomal microRNA remodels the tumor microenvironment. PeerJ 5:e4196
Kuninty PR, Schnittert J, Storm G, Prakash J (2016) MicroRNA targeting to modulate tumor microenvironment. Front Oncol 6:3
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014:1–19
Lazennec G, Lam PY (2016) Recent discoveries concerning the tumor-mesenchymal stem cell interactions. Biochim Biophys Acta Rev Cancer 1866(2):290–299
Lu J, Getz G, Miska EA et al (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834
Melzer C, Yang Y, Hass R (2016) Interaction of MSC with tumor cells. Cell Commun Signal 14(1):20
Mohammadi A, Mansoori B, Baradaran B (2016) The role of microRNAs in colorectal cancer. Biomed Pharmacother 84:705–713
O’Malley G, Heijltjes M, Houston AM et al (2016) Mesenchymal stromal cells (MSCs) and colorectal cancer: a troublesome twosome for the anti-tumour immune response? Oncotarget 7(37):60752
Peddareddigari VG, Wang D, DuBois RN (2010) The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron 3(1):149–166
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437
Rhee KJ, Lee J, Eom Y (2015) Mesenchymal stem cell-mediated effects of tumor support or suppression. Int J Mol Sci 16(12):30015–30033
Ribatti D, Mangialardi G, Vacca A (2006) Stephen Paget and the ‘seed and soil’theory of metastatic dissemination. Clin Exp Med 6(4):145–149
Sansom SE, Nuovo GJ, Martin MM, Kotha SR, Parinandi NL, Elton TS (2010) miR-802 regulates human angiotensin II type 1 receptor expression in intestinal epithelial C2BBe1 cells. Am J Physiol Gastrointest Liver Physiol 299(3):G632–G642
Schee K, Fodstad Ø, Flatmark K (2010) MicroRNAs as biomarkers in colorectal cancer. Am J Pathol 177(4):1592–1599
Strubberg AM, Madison BB (2017) MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech 10(3):197–214
Tauriello DV, Batlle E (2016) Targeting the microenvironment in advanced colorectal cancer. Trends Cancer 2(9):495–494
Wang B, Shen ZL, Gao ZD, Zhao G, Wang CY, Yang Y, Zhang JZ, Yan YC, Shen C, Jiang KW, Ye YJ (2015) MiR-194, commonly repressed in colorectal cancer, suppresses tumor growth by regulating the MAP 4K4/c-Jun/MDM2 signaling pathway. Cell Cycle 14(7):1046–1058
Wang M, Zhao J, Zhang L et al (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8(5):761
Xu K, Chen Z, Qin C, Song X (2014) miR-7 inhibits colorectal cancer cell proliferation and induces apoptosis by targeting XRCC2. Onco Targets Ther 7:325
Zhang H, Li M, Han Y, Hong L, Gong T, Sun L, Zheng X (2010) Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig Dis Sci 55(9):2545–2551
Zhang Y, Guo L, Li Y, Feng GH, Teng F, Li W, Zhou Q (2018) MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol Cancer 17(1):1
Zucker S, Vacirca J (2004) Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 23(1–2):101–117
Acknowledgement
The authors are thankful to Chettinad Academy of Research and Education for providing the infrastructural support and to SERB, DST, Govt. of India for providing the financial support. The authors are thankful to Mr. Jaganth Arunachalam for technical help in formulating the table.
Funding
This work was supported fully by the grants sanctioned to Dr. Antara Banerjee (PI) from the SERB-DST Govt. of India with the sanction file no ECR/2017/001066.
Disclosure of Interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Banerjee, A. et al. (2019). Role of Tumor Specific niche in Colon Cancer Progression and Emerging Therapies by Targeting Tumor Microenvironment. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 13. Advances in Experimental Medicine and Biology(), vol 1341. Springer, Cham. https://doi.org/10.1007/5584_2019_355
Download citation
DOI: https://doi.org/10.1007/5584_2019_355
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79057-8
Online ISBN: 978-3-030-79058-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)