Abstract
Kidney disease is commonly found in heart failure (HF) patients. They share many risk factors and common pathophysiological pathways which often lead to mutual dysfunction. Both haemodynamic and non-haemodynamic mechanisms are involved in the development of renal impairment in heart failure patients. Moreover, the presence of a chronic kidney disease is a significant independent predictor of worse outcome in chronic as well as in acute decompensated HF. As a consequence, an accurate evaluation of renal function plays a key role in the management of HF patients. Serum creatinine levels and glomerular filtration rate (GFR) estimates are the corner stones of renal function evaluation in clinical practice. However, to overcome their limits, several emerging glomerular and tubular biomarkers have been proposed over the last years. Alongside the renal biomarkers, imaging techniques could complement the laboratory data exploring different pathophysiological pathways. In particular, Doppler evaluation of renal circulation is a highly feasible technique that can effectively identify HF patients prone to develop renal dysfunction and with a worse outcome. Finally, some classes of drugs currently used in heart failure treatment can affect renal function and their use can be influenced by the presence of chronic kidney disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Biomarkers
- Cardio-renal syndrome
- Chronic kidney disease
- Doppler
- Haemodynamics
- Heart failure
- Prognosis
- Renal function
- Renal resistance index
- Venous Doppler
1 Introduction
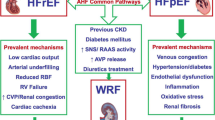
Renal dysfunction is a common condition in cardiovascular diseases. The burden of renal impairment is especially relevant in the heart failure (HF) population, regardless of the phenotype of cardiac dysfunction and the acute or chronic clinical setting, because of the close relationship between heart and kidney function (Chong et al. 2015). Renal and heart diseases share common risk factors and pathophysiological pathways which can lead to an acute or chronic mutual dysfunction, termed cardiorenal syndrome (Table 1). Cardiorenal syndrome has been classified in five subtypes: type 1 refers to acute heart failure as the cause of acute kidney disease; type 2 refers to chronic heart failure as the cause of chronic kidney disease; in type 3 the acute worsening of kidney function causes heart dysfunction; in type 4, chronic kidney disease (CKD) leads to heart failure; in type 5 there is a simultaneous injury of heart and kidneys caused by systemic diseases (Ronco et al. 2008). About half of the patients with heart failure suffers from at least moderate chronic kidney disease, defined as a glomerular filtration rate (GFR) <60 ml/min/1.73 m2, compared to the 10.6% of the general population (Damman et al. 2014a; Hill et al. 2016). The prevalence of CKD is higher among the patients with acute decompensated heart failure (ADHF), ranging from 30 to 60%, depending on the definition used (Adams et al. 2005). Similarly, in-hospital worsening renal function (WRF) is observed in 23% of HF patients, even if the optimal definition for WRF is still debated, leading to some heterogeneity in the results from the various cohorts (Damman et al. 2014a; Smith et al. 2006; Metra et al. 2008). Among patients with chronic heart failure (CHF), up to 42% have CKD (Damman et al. 2014a). Renal impairment is an independent risk factor for adverse outcome in subjects with ADHF and CHF (Smith et al. 2006; Hillege et al. 2000; Damman et al. 2009a; Chang et al. 2010).
2 Pathophysiology
Heart and kidney are two organs tightly linked in physiological and pathological conditions, because they both contribute to preserve the homeostasis of water and electrolytes and the cardio-circulatory function. Heart is greatly dependent on fluid homeostasis regulated by the kidney and renal function is subordinated to perfusion pressure through hemodynamic, neuro-hormonal, inflammatory and local mechanisms (Damman and Testani 2015). Moreover, heart and kidney diseases frequently coexist because they share pathophysiological pathways and risk factors (such as hypertension, diabetes and atherosclerosis). In heart failure several mechanisms are involved in the pathogenesis of renal impairment, e.g. haemodynamic alterations due to reduced cardiac output and renal venous congestion, neurohormonal deregulations, systemic and local inflammation (Colombo et al. 2012), high-dose of diuretics.
Renal haemodynamic factors are main determinants of renal impairment (and GFR reduction) in HF patients (Damman and Testani 2015). GFR is dependent on renal blood flow and filtration fraction and it is maintained in a normal range by autoregulation despite significant reduction of cardiac output. When these mechanisms are exhausted, as in HF, GFR declines with cardiac output (Braam et al. 2012). Renal hypoperfusion with “forward” underfilling triggers renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system activation which causes arteriolar vasoconstriction both at glomerular and tubular level. Especially in the acute setting, relevant drop of cardiac output can lead to tubular hypoxia and acute tubular necrosis (Schefold et al. 2016). However, the haemodynamic interaction between heart and kidney is even more complex and other mechanisms are involved, considering the modest association between renal impairment and left ventricular systolic function in a large registry of HF patients (Heywood et al. 2007). Emerging data on the interaction between venous congestion and renal dysfunction in HF have lead to a reappraisal of this relationship (Nohria et al. 2008). High central venous pressure and renal venous hypertension can impair GFR through an increase in interstitial pressure and a reduction of artero-venous gradient (Mullens et al. 2009).
Among the non-haemodynamic factors, the hyperactivation of RAAS and sympathetic nervous system has an important role in HF. In addition to the direct effect on the arteriolar tone, at renal level RAAS increases in the long term the secretion of proinflammatory molecules, promotes fibrosis and leads to reduced response to natriuretic peptides (Ruiz-Ortega et al. 2002; Charloux et al. 2003). Moreover, the sympathetic hypertone provokes renin release from juxtaglomerular cells and RAAS activation, oxidative stress and endothelial dysfunction (together with RAAS) (Damman and Testani 2015; Goldsmith et al. 2010).
On the other hand, chronic kidney disease is an established and increasingly recognized risk factor for cardiovascular disease, particularly heart failure. The risk of cardiac impairment increases gradually with kidney dysfunction. In particular, a 15 times higher cardiovascular mortality has been observed in end-stage renal disease (ESRD) patients compared to general population (Granata et al. 2016). In this group of patients, the myocardial structural changes are mainly characterized by left ventricular hypertrophy and fibrosis responsible for diastolic dysfunction; however also dilatation of the left ventricle and impairment of systolic function can occur (Alhaj et al. 2013). Among the several mechanisms involved in the development of heart failure in ESRD patients, an increased preload, caused by fluid overload and a high flow state, related to arterio-venous fistulas, can promote the eccentric remodeling of the left ventricle. On the other hand, an increased afterload, due to high arterial systemic resistances (systolic and diastolic hypertension) and a reduced arterial compliance (vascular calcification), can promote a ventricular concentric remodeling. In addition to the changes in preload and afterload, myocardial fibrosis can be enhanced by a number of non-haemodynamic factors as uremic toxins, oxidative stress, inflammatory status, hyperparathyroidism, hypovitaminosis D and hyperphosphatemia (Alhaj et al. 2013; Tumlin et al. 2013).
3 Markers of Glomerular Function
Taking into account its relevant prognostic role, the evaluation of kidney function in subjects with heart failure is extremely important. A number of laboratory examinations are available to assess renal function and, in particular, the filtration ability of the kidney (Table 2).
3.1 Serum Creatinine and Glomerular Filtration Rate
Serum creatinine levels and GFR estimation are the corner stone for renal function evaluation and are routinely recommended in HF patients management for assessing renal dysfunction and filtration impairment (Ponikowski et al. 2016). Serum creatinine is the product of creatine phosphate breakdown at muscular level. It is produced at relatively constant rate and eliminated by the kidney through glomerular filtration and partially by active secretion in the tubules (Levey et al. 2006). Empirical formulas can be used to easily estimate GFR based on serum creatinine levels, age, gender and weight. The most commonly used formulas are Cockroft-Gault, simplified Modification of Diet in renal disease (MDRD) and, recently, Chronic Kidney Disease Epidemiology Collaboration (CKD EPI) (Stevens et al. 2006). These equations have been validated in CKD populations with good results. Among these formulas, CKD EPI has been shown to be less biased in GFR estimation, especially within the population with preserved renal function (Stevens et al. 2010). In the setting of CHF patients, it leads to a more accurate classification of renal functional stages and a better risk stratification, particularly in those patients with normal or near normal renal function (McAlister et al. 2012; Valente et al. 2014). For this reason, it has recently become the most used estimate of GFR in clinical practice. Serum creatinine levels can also be used to identify patients with WRF, who are at higher risk for events (Smith et al. 2006; Metra et al. 2008). In HF patients, it is generally defined as an absolute increase of >0.3 mg/dl and/or a relative increase of >25% in serum creatinine levels within two-time points (Damman et al. 2009a). In the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, CKD progression has been defined as a decline in the stage of CKD accompanied by a 25% or greater drop in eGFR from baseline (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2013).
Creatinine concentration and GFR estimation are currently recommended to assess renal impairment in HF and to classify renal dysfunction based on GFR category (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2013). However, some limitations in the use of serum creatinine exist, as its levels are biased due to age, diet, gender and body mass. A decrease in creatinine levels (with overestimation of GFR) is observed in muscle wasting, a common condition seen in advanced HF. Additionally, GFR estimation can be hampered when filtration function decreases as the relative effect of active secretion is more pronounced, leading to an overestimation of GFR (Damman et al. 2012). On the basis of these limitations, new biomarkers have been proposed to better evaluate glomerular function.
3.2 Blood Urea Nitrogen (BUN)
Urea is produced by protein catabolism, filtered at glomerular level and reabsorbed in the collecting ducts by an AVP-mediated system. In conditions of low cardiac output, the increased RAAS and adrenergic stimulation lead to enhanced fluid and sodium retention. Consequently, distal flow is reduced and urea reabsorption increases. Urea has been shown to be a strong prognostic marker in both acute and chronic HF populations, being an important predictor of morbidity and mortality (Damman et al. 2012; Aronson et al. 2004; Cauthen et al. 2008). In the acute setting, blood urea nitrogen, together with blood pressure and serum creatinine, has been used to estimate in-hospital mortality with a tree algorithm in the ADHERE registry (Fonarow et al. 2005). The prognostic value of urea could also be greater than serum creatinine (Klein et al. 2008), probably because urea levels take into account other factors than glomerular filtration, as protein intake (and thus nutritional status), catabolic state and reabsorption coupled with sodium at tubular level as a marker of low flow (Schrier 2008). Urea levels are therefore dependent both on glomerular function and on a number of factors related to HF severity. This could explain its strong prognostic role, but it cannot be considered a reliable marker of filtration function. The ratio between urea and creatinine has been extensively used for the differentiation of prerenal renal dysfunction from intrinsic renal parenchymal disease. In prerenal renal dysfunction significant renal neurohormonal activation causes a disproportionate reabsorption of urea compared to creatinine, leading to an urea/creatinine ratio > 100 (or a BUN/creatinine ratio > 20). In patients with HF and renal dysfunction, an elevated urea/creatinine ratio could differentiate a renal impairment due to hypoperfusion from CKD. Furthermore, high urea/creatinine ratio has been associated with congestion and long-term mortality in HF patients (Parrinello et al. 2015).
3.3 Cystatin C
Cystatin C belongs to cysteine proteinase inhibitor family and is secreted by all nucleated cells. It is filtered freely through the glomerulus and then reabsorbed but not secreted by tubular cells, where it is catabolised. As opposed to creatinine, it is relatively independent from age, body mass, nutritional status and cachexia (Newman et al. 1995). The only concerns are inflammation and thyroid dysfunction which could influence Cystatin C levels (Fricker et al. 2003; Singh et al. 2007). Empirical formulas have been proposed to estimate GFR, with better results compared to serum creatinine in different clinical settings and particularly in normal and near normal renal function (Damman et al. 2012; Dharnidharka et al. 2002). Cystatin C has also shown to be accurate in stratifying the risk in the elderly, in diabetic patients and in patients affected by coronary artery disease (Shlipak et al. 2005a; Ix et al. 2006; de Boer et al. 2009). Limited data exist on the role of Cystatin C in HF; however, it appears to be a promising prognostic marker both in acute and chronic HF (Arimoto et al. 2005; Shlipak et al. 2005b; Lassus et al. 2007). Despite these interesting proprieties, Cystatin C is not widely used in clinical practice, perhaps because of its relative high cost compared to creatinine. However, current KDIGO guidelines for CKD state that Cystatin C could be used as a confirmatory test in circumstances when GFR estimation based on creatinine alone is less accurate (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2013)
3.4 Albuminuria
Albumin is the most abundant protein in blood. Because of its size, its negative electric charge (the same as the glomerular basement membrane) and the tubular absorption, it is normally present at very low level in the urine. The urinary excretion of albumin is generally evaluated by the ratio between urinary albumin and creatinine (UACR). Albuminuria is present when UACR is >30 mg/g, an UACR between 30 and 300 mg/g is termed microalbuminuria, an UACR >300 mg/g is defined macroalbuminuria (Miller et al. 2009). Albuminuria is the effect of glomerular membrane damage due to endothelial dysfunction, inflammation, increased glomerular pressure and atherosclerosis (Comper et al. 2008). For this reason, it should be considered a marker of glomerular permeability rather than an estimate of filtration. Microalbuminuria is highly prevalent in CHF (van de Wal et al. 2005). In this setting other mechanisms could be involved, i.e. renal venous congestion and reduced renal perfusion (Damman et al. 2009b). It has been shown to be associated mortality and HF related hospitalization, independently from creatinine levels and GFR. This association remains significant even in CHF patients with normal GFR, suggesting that microalbuminuria could be an early sign of renal impairment (Jackson et al. 2009; Masson et al. 2010). In the management of patients with renal dysfunction, albuminuria classification, together with GFR category, is recommended as a marker of kidney damage in CKD definition and risk stratification (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2013).
4 Markers of Tubular Damage
Another component of acute kidney injury is tubular cell damage, which may be damaged earlier than the glomerulus. Therefore, different markers of tubular damage are being studied to better evaluate and predict renal impairment.
4.1 Neutrophil Gelatinase Associated Lipocalin (NGAL)
NGAL is a small protein produced by the kidney and other organs. It is freely filtered through the glomerulus and completely reabsorbed in the proximal part of the tubule. In normal conditions very low urinary and blood concentrations are observed (Schmidt-Ott et al. 2007). When tubular damage occurs, NGAL cannot be completely reabsorbed, leading to increased urinary levels. In renal diseases, higher plasma levels are also common but relatively less specific, being also found in sepsis, inflammation and cancer (Schmidt-Ott et al. 2007). In case of acute kidney injury, NGAL precedes the rise of serum creatinine by at least 24 h, as the other tubular markers (Mishra et al. 2005). In the acute decompensated heart failure setting, it is associated with the occurrence of WRF and with adverse clinical outcome (Collins et al. 2012; Alvelos et al. 2013). In CHF patients, NGAL levels are markedly increased compared to controls, but they were not able to predict adverse events. Thus, NGAL should be considered as a marker of tubular damage in the acute rather than in the chronic setting (Damman et al. 2010).
4.2 N-Acetyl Beta Glucosaminidase (NAG)
NAG is a lysosomal protein of the proximal tubule, excreted into urine in case of tubular damage (Bazzi et al. 2002). NAG levels are associated with tubular injury in acute kidney disease where it predicts worse prognosis (Liangos et al. 2007). In the intensive care setting, NAG is related to the risk of acute kidney injury (Westhuyzen et al. 2003). In patients with CHF it showed to predict an increased risk of death or HF-related hospitalizations, independently from GFR (Damman et al. 2010). These results were also confirmed in the large cohort of CHF patients of the GISSI HF trial, where it was the only tubular marker related to prognosis and risk of WRF (Damman et al. 2011). Few data are available on the prognostic role of NAG in acute decompensated heart failure.
4.3 Kidney Injury Molecule (KIM1)
KIM1 is a transmembrane glycoprotein which is expressed in proximal tubule cells after hypoxic tubular injury almost 1 day before the increase in serum creatinine (Han et al. 2002). KIM1 urinary levels have high sensitivity for early detection of acute kidney injury. CHF patients have higher KIM1 levels compared to controls. In these setting, urinary KIM1 predicts poor prognosis independently from GFR (Damman et al. 2010).
4.4 Fatty Acid-Binding Proteins(FABPs)
FABPs are proteins that bind free fatty acids. Different tissue-specific FABs have been identified (Veerkamp et al. 1990). In the kidney, liver specific FABP (FABP-1) and heart specific FABP (FABP-3) have been respectively expressed in the proximal and in the distal tubule (Maatman et al. 1991). Urinary FABP-1 and FABP-3 levels have been associated with ischemic tubular injury and risk for AKI (Noiri et al. 2009). In CHF patients persistently high FABP-3 levels are associated with cardiovascular events (Niizeki et al. 2008).
4.5 TIMP-2 and IGFBP7
Tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor–binding protein 7 (IGFBP7) are cell-cycle arrest biomarkers found to be elevated in renal tubule damage (Niizeki et al. 2008). The product of the urinary concentrations of these two biomarkers, [TIMP-2] × [IGFBP7], has been demonstrated to predict short term acute kidney injury in acute decompensated heart failure with good sensitivity and specificity (Schanz et al. 2017). Furthermore, patients with AKI and elevated [TIMP-2] × [IGFBP7] show a trend toward reduced survival within 1 year (Schanz et al. 2017).
5 Imaging
Several different imaging techniques can explore renal function in terms of anatomy and haemodynamics (Table 2). Ultrasound and Doppler examinations represent an easy and widespread technique used to evaluate parenchymal abnormalities and estimate perfusion pressures with parameters correlated with prognosis in the setting of HF patients. On the other hand, there are several promising second level techniques, especially involving nuclear and magnetic resonance imaging (MRI), which are confined only to an experimental role, so far.
5.1 Renal Ultrasonography
Bidimensional renal ultrasound is a low cost and easy to perform technique which allows an anatomical examination of renal parenchyma. Unfortunately, there are no morphological abnormalities specifically related to heart failure. The observed renal anatomy is greatly dependent on the stage of heart failure and on the severity of the concurrent kidney disease, so the bidimensional examination mostly differentiate an acute from a chronic renal impairment and can exclude an obstructive uropathy as a cause for renal dysfunction. Renal dimensions are related to body surface area, and are generally between 10 and 12 cm in length (Emamian et al. 1993).
In case of renal dysfunction associated with chronic heart failure, there is a non-specific sonographic appearance, common with other causes of CKD: kidneys are smaller, with reduced cortical thickness and cortex-medulla ratio (Moghazi et al. 2005). The parenchyma appears relatively echogenic compared to the liver, due to interstitial fibrosis related to chronic disease (Page et al. 1994). During de novo acute decompensated heart failure renal anatomy is frequently preserved, provided no prior CKD is present. Kidneys have normal dimension and cortex-medulla ratio, with a fairly echolucent appearance of the parenchyma compared to the liver. An echogenic appearance can be rarely observed in case of long-standing renal hypoperfusion (Di Lullo et al. 2012). Both in the acute and in the chronic setting signs of urinary obstruction are generally absent, unless urologic co-morbidities occur.
5.2 Vascular Renal Doppler
The Doppler study of renal arterial flow allows a more comprehensive evaluation of the complex mechanisms involved in the relationship between renal dysfunction and HF, considering renal haemodynamics and, in particular, arterial resistances. Renal resistances are determined by a number of factors: arteriolar tone, parenchymal anatomy and renal venous congestion.
Glomerular filtration is strictly related to glomerular (blood) hydrostatic pressure and, in turn, to renal blood flow, together with capsular hydrostatic pressure and colloid osmotic pressure. In normal conditions, glomerular filtration and renal perfusion are in parallel stabilized by intrinsic and extrinsic regulation mechanisms with low changes despite wide fluctuations of systemic blood pressure between 70 and 180 mmHg. Out of this range filtration is highly dependent on blood pressure. Renal blood flow is mainly regulated by intrinsic autoregulation (namely myogenic reflex and tubulo-glomerular feedback) and neurohormonal factors as opposed to perfusion of other organs, which are primarily dependent on oxygen demand (Braam et al. 2012; Smilde et al. 2009). Renal blood flow, in theory, is dependent on perfusion pressure (and therefore on cardiac output) and on arterial resistances. In case of a normal autoregulatory system, a reduced cardiac output will not affect net filtration because autoregulation will determine a decrease of arterial resistances ensuring a normal renal blood flow. However, in the setting of heart failure, autoregulatory mechanisms could be altered, being depleted or overruled by sympathetic activation and leading to high renal resistances. Therefore, a reduced cardiac output may cause a disproportional fall of glomerular filtration (Braam et al. 2012). A long-standing increase of renal resistances due to hyperstimulation of arteriolar tone can lead to a functional and then to a structural vascular rarefaction. The former is caused by a prolonged vasoconstriction which causes a reduced number of perfused vessels without anatomical vascular abnormalities. A sustained vasoconstriction may lead to local ischemia, endothelial dysfunction, release of both growth factors and enzymes and, finally, to fibrosis and vascular remodeling. This condition, labeled as “structural rarefaction”, refers to an actual reduction in the number of anatomical vessels embedded in the tissue. The vascular remodeling could be considered a cause of persistent increase of renal resistances (Prewitt et al. 1982; Chade 2013). Whatever the mechanism involved, functional rarefaction due to neurohormonal activation or anatomical rarefaction due to remodeling, it is clear that high resistances are associated with reduced renal blood flow and could eventually identify a subset of HF patients prone to renal dysfunction.
Renal arterial haemodynamics could be quantitatively evaluated through renal resistive index (RRI) of Doppler arterial waveform. RRI is derived from Pourcelot’s formula: (peak systolic velocity – end diastolic velocity) / peak systolic velocity. The relationship between RRI and arterial resistances is not completely linear. A number of other variables are involved in this interaction, such as vascular stiffness and rhythm disturbances (Mostbeck et al. 1990; Bude and Rubin 1999; Tublin et al. 1999; Murphy and Tublin 2000). Moreover, central venous pressure (CVP) has shown to be positively correlated with RRI: an increase in central venous and intra-abdominal pressure could be transmitted to the renal parenchyma by the stiff capsule leading to an increase in renal interstitial pressure and consequently in arterial resistances (Ciccone et al. 2014). Doppler evaluation of arterial circulation is performed with an anterior approach and a convex probe or with a posterior approach in sitting position and an echocardiographic phased-array probe. Renal arteries are sampled at the level of segmental arteries with a good feasibility. The median value of RRI in healthy adults is 0.60, with slight variations between right and left kidneys (Darmon et al. 2010). RRI evaluation is generally reproducible, with low intraobserver and interobserver variability (Lubas et al. 2014). RRI values above 0.70 are considered abnormal.
The prognostic role of RRI has been evaluated first in patients with chronic nephropathy, where higher values have been shown to correlate with greater degree of parenchymal fibrosis, an increased risk for progression of kidney disease and a generally poor outcome (Platt et al. 1990; Parolini et al. 2009; Hanamura et al. 2012). In the population of patients with HF, RRI has proven to be useful in risk stratifying patients with both preserved (HFpEF) and reduced (HFrEF) ejection fraction. In the HFpEF population, it has been correlated with poor prognosis, i.e. a greater risk for death and HF related hospitalization (Ennezat et al. 2011). Later on, RRI has been also evaluated in the cohort of outpatients with HFrEF. In this setting, it was associated with a number of endpoints related to progression of heart failure. Patients with higher RRI values are considered at higher risk for HF related hospitalization, all cause death, death due to HF and urgent heart transplantation (Ciccone et al. 2014). RRI could also identify CHF patients prone to poorer renal prognosis, such as those at risk for diuretic resistance (considered as the need for higher diuretic doses) and WRF, during a mid and long-term follow up (Iacoviello et al. 2015; Iacoviello et al. 2016). The prognostic role of RRI in HF has been demonstrated to be independent and incremental, when compared to renal function evaluated with serum creatinine and GFR (Ciccone et al. 2014). RRI could be used together with established laboratory renal markers to better characterize renal function of HF patients, thus integrating information provided by estimated GFR and microalbuminuria (Leone et al. 2016). However, further studies are needed to confirm clinical usefulness of this imaging approach.
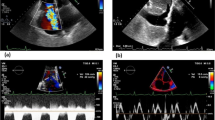
In a similar way, scientific efforts should be made to clarify the possible role of renal venous Doppler pattern, which has been recently proposed as a tool to better evaluate renal congestion. Indeed, the renal venous congestion plays a key role in kidney physiology. This has been already demonstrated in the first decades of the last century in an experimental ex-vivo model. High renal venous pressure more than low renal perfusion pressure was associated with a reduction of glomerular filtration rate (Winton 1931). This has been recently observed also in acute as well as in chronic heart failure (Mullens et al. 2009; van de Wal et al. 2005). Renal congestion can lead to a fall in glomerular filtration in two ways. High venous pressure can be transmitted upstream to capillaries at glomerular and tubular level, causing in turn a reduced artero-venous gradient through renal circulation. Moreover, because of the tight renal capsule, high pressure can be transmitted to renal interstitial and then to tubular space. Tubular hypertension can affect upstream capsular hydrostatic pressure, one of the driving forces of glomerular filtration (Tublin et al. 2003). According to this pathophysiological background, the evaluation of renal venous flow could provide information about the presence of increased renal pressure due to peripheral congestion (Jeong et al. 2011). Venous impedance index (VII) has been used as a measure of venous pulsatility. It is calculated as peak maximum flow velocity minus maximum flow velocity at nadir, divided by peak maximum flow velocity (Ishimura et al. 1997). However, as minimum velocity is considered, VII is useful unless intermittent flow is observed. On the other hand, intrarenal venous flow pattern has been shown to be significantly associated with central venous pressure. In the setting of HF patients, venous flow has been classified as continuous or discontinuous, and among those as monophasic or biphasic discontinuous. Discontinuous patterns identify the subset of patients with worse renal function and high central venous pressure. Finally, they were associated with poor prognosis, considered as cardiovascular death and HF hospitalization (Iida et al. 2016). In other series of patients, venous flow has been divided into five groups: continuous with a normal velocity decrease in diastole, continuous with reduced modulation, monophasic with short presystolic interruption or reversal (corresponding to atrial systole), polyphasic intermittent and monophasic intermittent flow. Even in this cohort of patients, the last two intermittent flow patterns are observed in more compromised patients who show worse outcome. Interestingly, the pattern with short presystolic interruption represents a group at intermediate risk, with mild increase in central venous pressure and probably an early renal involvement (Puzzovivo et al. 2016).
5.3 Other Imaging Techniques
Other imaging techniques have been explored for the functional evaluation of the kidneys. Nuclear imaging is based on the use of radioisotopes bound to non-metabolized molecules with known pharmacokinetics. Among the different radioisotopes, Technetium (99mTc) is the molecule of choice because of its short half-life and low radiation exposure. For assessment of GFR, 99mTc–DTPA (diethylenetriaminepentaacetic acid) is used. DTPA is eliminated by filtration without entering the cells, and being neither secreted nor reabsorbed by the tubules. GFR values obtained with 99mTc–DTPA are slightly lower compared to inulin (Haufe et al. 2006). 99mTc–MAC3 (mercaptoacetyltriglycine) is used for renal blood flow estimation. In the setting of pre-renal azotemia (as in HF), 99mTc–MAC3 uptake is generally normal in the first 1–2 min, whereas it is reduced in vascular and parenchymal causes and in acute tubular necrosis. After 20 min, MAC3 uptake is increased in pre-renal azotemia, vascular causes and acute tubular necrosis, while it is reduced in parenchymal and obstructive diseases (Kalantarinia 2009). Positron emission tomography (PET), especially combined with CT to enhance spatial resolution, allows a functional and molecular evaluation of the kidney. It could be useful to determine GFR, renal blood flow and to assess the tissue activity of transporters, enzymes and receptors. However, this technology is not widely available for clinical purposes and at the moment it is limited only to the experimental setting (Haufe et al. 2006; Kalantarinia 2009).
Computed tomography (CT) is considered the gold standard for the diagnosis of nephrolithiasis, renal masses and parenchymal diseases. Triphasic helical CT after contrast injection can be used to evaluate functional parameters, as GFR and renal blood flow (Hackstein et al. 2004). However, in the clinical setting of HF, especially in patients with renal impairment, it has limited feasibility and scarce literature because of the high risk of contrast induced nephrotoxicity.
Magnetic resonance imaging (MRI) is a fast growing field in the setting of kidney disease especially because it combines anatomical and functional evaluation of the kidney. The main limitations to its widespread use is the lack of standardized sequences and postprocessing softwares, and for this reason it is confined, so far, to the research field (Michaely et al. 2007). MRI could be used to estimate classical parameters such as GFR and renal blood flow. GFR quantification is based on the kinetics of a gadolinium-containing contrast medium which is eliminated only through glomerular filtration, being not reabsorbed nor secreted by the tubules (Hackstein et al. 2003; Boss et al. 2007). On the other hand, renal blood flow can be evaluated with phase-contrast MRI of the renal artery. This method relies on magnetically excited arterial water molecules used as endogenous tracer for perfusion imaging (Roberts et al. 1995). Together with these approaches, used to assess these conventional parameters, some other techniques are under development for further functional evaluations. With this regard, BOLD (blood oxygenation level dependent) MRI seems to be a promising tool; it works by exploiting the magnetic properties of haemoglobin in order to evaluate the degree of tissue oxygenation of renal parenchyma. Deoxygenated haemoglobin has paramagnetic activity as opposed to oxygenated haemoglobin which is diamagnetic. The increase in tissue deoxygenated haemoglobin during oxygen extraction leads to a decrease in T2* signal, corresponding to the degree of tissue oxygenation. Since renal parenchyma shows a physiological heterogeneous oxygenation with the medulla working at lower oxygen levels, in the setting of HF, BOLD MRI of this region could be especially sensitive to early prerenal kidney injury (Simon-Zoula et al. 2006; Artunc et al. 2011).
6 Renal Dysfunction and HF Treatment
Current recommended therapies for HF patients have been shown to improve functional status and survival but, in some cases, they could negatively interact with renal function. Patients with HF and renal dysfunction are usually underrepresented in randomized controlled trials on cardiovascular diseases and there are relatively limited data on the baseline renal function and on the effects of treatment on renal disease (Coca et al. 2006). The subgroup of patients with severely decreased renal function (GFR <30 mL/min/1.73m2) has been generally excluded from clinical trials and therefore there is lack of evidence-based therapies in this population (Ponikowski et al. 2016).
6.1 ACE Inhibitors and Angiotensin II Receptor Blockers
The favourable effect of Angiotensin-converting enzyme inhibitors (ACE-I) and Angiotensin II receptor blockers (ARB) on cardiac function and survival in patients with HF has been well established (Ponikowski et al. 2016). They have shown to improve symptoms and exercise capacity, reduce the risk of hospitalization and mortality (Chang et al. 2010; Garg and Yusuf 1995). ACE-I are recommended, unless contraindicated, in all symptomatic HF patients, while ARB are recommended in patients who are ACE-I intolerant (Chang et al. 2010). In the last European guidelines, ACE-I are also recommended in patients with asymptomatic LV systolic dysfunction to reduce the risk of symptoms development, hospitalization and death (Ponikowski et al. 2016). In the subgroup of patients with HF and moderate renal dysfunction (GFR 30–59 ml/min/1.73 m2) there is evidence of favorable outcome with ACE-I therapy (Bowling et al. 2012; Massie et al. 2001; Tokmakova et al. 2004). ACE-I might also be beneficial and considered in patients with severe renal dysfunction (GFR <30 ml/min/1.73 m2), however there are no conclusive data (Bowling et al. 2012). Analogously, there is moderate evidence for the use of ARB in the population of patients with HF and moderate renal dysfunction, whereas there are no data available on the effect of ARB in severe renal disease (Granger et al. 2003). ACE-I and ARB are contraindicated in pregnancy, history of angioedema, bilateral renal artery stenosis and previous allergy to ACE-I/ARB. Recommended dose of ACE-I/ARB should be adjustment according to GFR (Table 3) (Damman et al. 2014b). Caution is advised in patients who experience hyperkalemia (>5 mmol/l) and/or severe renal dysfunction. An early increase in serum creatinine is relatively common during initiation and uptitration of ACE-I/ARB therapy (10–35% of patients). This deterioration in renal function is usually small and does not have the same adverse prognostic implications as other kind of WRF in HF natural history (Testani et al. 2011). However, an extreme decrease in renal filtration function is dangerous. In patients with severe renal dysfunction, the renin-angiotensin system blockade is known to impair the autoregulatory mechanisms and make glomerular filtration critically dependent on renal blood flow (Metra et al. 2012). The degree of creatinine increase that should warrant therapy discontinuation is uncertain. The European guidelines suggest that an increase in creatinine of up to 50% above baseline or an increase in potassium to ≤5.5 mmol/L is acceptable (Ponikowski et al. 2016). If greater rises in creatinine or potassium are observed, the dose of ACE-I/ARB should be reduced. If creatinine increases by >100% or to >3.5 mg/dl and/or GFR <20 ml/min/1.73 m2, therapy should be discontinuated. Renal function and electrolytes should be checked before initiation of ACE-I/ARB therapy, 1–2 weeks after initiation and final uptitration, and every 4 months after the final dosage is achieved. Blood chemistry should be also checked after dose reduction in case of WRF.
6.2 Mineralcorticoid Receptor Antagonists
Mineralcorticoid receptor antagonists (MRA) act by blocking the receptor that binds aldosterone. Two MRAs, spironolactone and eplerenone, have been demonstrated to reduce mortality and hospitalization in HF with reduced ejection fraction (Pitt et al. 1999, 2003; Zannad et al. 2011). Therefore, MRA are recommended in all symptomatic patients with HF and reduced ejection fraction (LVEF ≤35%) despite treatment with an ACE-I/ARB and beta-blockers (Ponikowski et al. 2016). The beneficial effects of spironolactone and eplerenone have shown to be consistent irrespective of baseline renal function. There is convincing evidence for the use of MRA in patients with HF and moderate renal dysfunction. However, no data are available the setting of severe renal dysfunction because these patients were excluded from the randomized clinical trials (Vardeny et al. 2012). Therefore, MRA are contraindicated in patients with baseline GFR <30 ml/min/1.73 m2 and/or potassium >5.0 mmol/l and in patients with previous allergic reaction. Spironolactone and eplerenone should be initiated at a dose of 25 mg daily, increasing to 50 mg daily. For patients with moderate renal dysfunction or concerns of hyperkalemia an initial regimen of every-other-day dosing is advised (Table 3). As for ACE-I/ARB, MRA can determine an initial increase in serum creatinine. In the RALES trial, an increase in creatinine was more common in the spironolactone group compared to placebo. However, WRF was not associated with worse outcome in this group, in contrast to WRF of the placebo group (Vardeny et al. 2012). The main concern associated with use of MRA is hyperkalemia, which is relatively common in the clinical practice (25% of patients) and occurs mostly in patients with reduced baseline GFR. The development of potassium >5.5 mmol/l and/or creatinine >2.5 mg/dl and/or GFR <30 ml/min/1.73 m2 should trigger dose reduction of MRA. In case of potassium levels >6.0 mmol/l and/or creatinine >3.5 mg/dl and/or GFR <30 ml/min/1.73 m2, MRA should be discontinuated. Renal function and electrolytes should be checked at 1 and 4 weeks after initiation/uptitration, monthly for the first 3 months and every 3–4 months thereafter.
6.3 Angiotensin Receptor Neprilysin Inhibitors
Angiotensin receptor neprilysin inhibitors (ARNI) is a new class of drugs recently developed. LCZ696 is the first agent of this class and combines the neprilysin inhibitor prodrug sacubitril and the ARB valsartan. The active metabolite of sacubitril inhibits neprilysin, leading to a slowed degradation of natriuretic peptides and bradikinin. Higher blood levels of vasoactive peptides are responsible for the favourable cardiovascular effects, such as increased diuresis and natriuresis, vasodilatation and myocardial anti-remodeling effect (McCormack 2016). In the recent PARADIGM-HF trial, sacubitril/valsartan has demonstrated to be superior over enalapril in reducing mortality and hospitalization in a population of ambulatory HFrEF patients with LVEF <40% (later amended to <35%) and high BNP levels (McMurray et al. 2014a). Sacubitril/valsartan is therefore recommended as a replacement for ACE-I in patients with these characteristics who remained symptomatic despite optimal medical treatment (Ponikowski et al. 2016). In the PARADIGM-HF, 37% of enrolled patients had a baseline GFR <60 ml/min/1.73 m2 (McMurray et al. 2014b). Reduced GFR at the enrolment did not modify the effect of sacubitril/valsartan on mortality and morbidity, as shown in a subgroup analysis (McMurray et al. 2014a). Up to now, there is very limited clinical experience in patients with severe renal dysfunction, as those patients were excluded from the previous studies. Sacubitril/valsartan is contraindicated with concomitant use of an ACE inhibitor. Other contraindications related to valsartan should also be considered. The initial recommended dose of sacubitril/valsartan is 49/51 mg twice daily, increased to 97/103 mg after 2–4 weeks. An initial dose of 24/26 mg twice daily should be considered in patients with moderate renal dysfunction. The effect of sacubitril on renal dysfunction is of interest because neprilysin inhibition is expected to have favourable effect on kidney function (Judge et al. 2015). This renoprotective effect is probably related to the slower degradation of natriuretic peptides and it has been observed in both the studies on sacubitril/valsartan. In the PARAMOUNT trial, GFR decreased to a greater extent in the valsartan group compared to the sacubitril/valsartan group (Solomon et al. 2012). In the PARADIGM-HF, significant increase in creatinine levels (>2.5 mg/dl) and discontinuation of the treatment because of renal impairment were less frequent in the sacubitril arm in comparison to the enalapril group (McMurray et al. 2014a). Further data on the renoprotective role of sacubitril/valsartan will come from the ongoing Heart and Renal Protection III (UK HARP-III) trial, which will compare sacubitril/valsartan with irbesartan in a population of CKD patients with proteinuria and GFR between 20 and 60 ml/min/1.73m2.
6.4 Hydralazine and Nitrates
In CHF hydralazine is generally used in combination with high doses of oral nitrates, as isosorbide dinitrate (ISDN). Both these molecules are vasodilator drugs with a complementary “nitroprusside-like” hemodynamic effect, caused by the predominant venodilatory action of ISDN and the arterial-dilatory effect of hydralazine (Bauer and Fung 1991). The first data on the clinical effects of the association of hydralazine and isosorbide dinitrate (H-ISDN) come from a small RCT on 642 men treated with digoxin and diuretics. In this population, H-ISDN was associated with a trend towards mortality reduction compared to placebo (Cohn et al. 1986). In a subsequent study, the African-American Heart Failure Trial (A-HeFT), the addition of H-ISDN to conventional therapy significantly reduced mortality and HF hospitalizations in African-American patients affected by HF (Taylor et al. 2004). The results of A-HeFT are difficult to translate to non-African-American patients, therefore in this population the mortality benefit remains uncertain. In the HF European guidelines, H-ISDN is recommended in self-identified African-American patients with HFrEF, symptomatic despite standard treatment. H-ISDN may also be considered in symptomatic patients intolerant or with contraindications to RAAS blockers (Ponikowski et al. 2016). In patients with advanced HF, the acute treatment with intravenous hydralazine showed positive changes on the renal hemodynamics. Hydralazine decreased total renal resistance and significantly increased renal blood flow, with preservation of GFR (Cogan et al. 1980). Similar results were observed after oral hydralazine therapy. The favorable hemodynamic effect was associated with a significant increase in creatinine clearance (Pierpont et al. 1980). Data from a post-hoc analysis of the A-HeFT, demonstrate that the beneficial effects of H-ISDN on event-free survival were consistent across subgroups, in particular in patients with and without history of chronic heart failure (Taylor et al. 2007). Because of its neutral effect in kidney disease, H-ISDN is frequently prescribed, in clinical practice, to patients with HF and renal failure or hyperkalemia, in whom ACE-I/ARB use could be complicated or contraindicated (Ziaeian et al. 2017; Giamouzis et al. 2011).
6.5 Diuretics
Despite the beneficial effects on renal haemodynamics due to the RAAS inhibition, the sodium excretion capacity of kidneys remains often poor, leading to sodium retention and fluid overload. The goal of diuretic therapy in HF is to relieve signs and symptoms of congestion by stimulating diuresis and natriuresis. Diuretics act by inhibiting the reabsorption of sodium at tubular level. The loop diuretics (furosemide, bumetanide and torasemide) operate at the level of the ascending limb of the loop of Henle and are the cornerstone of symptoms management in HF patients. On the other hand, thiazide diuretics act in the distal convoluted tubule and are mainly used as antihypertensive agents. Diuretic treatment is generally initiated with the minimum effective dose and then adjusted according to the therapeutic response. The symptomatic benefit of diuretics is universally accepted; nevertheless, no randomized controlled trial has prospectively evaluated their effects on morbidity and mortality. A small meta-analysis on loop and thiazide diuretics showed a possible favorable effect on mortality and hospitalization in the HF general population (Faris et al. 2012), but no prognostic data are available in the specific setting of HF and renal dysfunction (Damman et al. 2014b).
However, diuretics may have detrimental effects of renal function. Because of their mechanism of action, a prolonged use has been shown to worsen renal function in patients with both normal and reduced filtration capacity (Bayliss et al. 1987). The mechanisms involved are hypovolemia with renal hypoperfusion which, in turn, causes a reduction of glomerular perfusion pressure and renal arteriolar constriction mediated by neurohormonal upregulation (Bayliss et al. 1987). The degree of renal dysfunction has been shown to influence the variability of diuretic response among HF patients. Indeed, renal impairment is associated with a downward and right shift of the dose-response curve, leading to higher doses required to achieve the same level of sodium excretion (Ellison 2001). An altered dose-response relationship could lead to a self-perpetuating vicious circle with higher loop diuretic dosages needed for symptoms relief, which have been demonstrated to be independently associated with mortality (Eshaghian et al. 2006).
Furthermore, renal disease is one of the main causes of diuretic resistance, i.e. the lack of treatment response and the inability to achieve a negative fluid balance. In patients with impaired renal function, poor kidney perfusion as well as low protein levels are responsible for the reduced concentration of loop diuretics that reach peritubular capillaries. Moreover, high levels of urate and other endogenous organic anions compete with loop diuretics for the organic anion transporter. Finally, filtered albumin can bind loop diuretics in the tubular lumen, preventing their effect at their site of action (Verbrugge et al. 2016). Diuretic resistance frequently requires an adequate fluid and/or sodium restriction and increasing loop diuretic dose is often a solution to the problem. Furthermore, adding either thiazides or metolazone, and eventually MRA, on top of loop diuretic therapy could be of further help in case of severely impaired renal function. Indeed, the sequential nephron blockade may be useful in order to overcome the upregulation of thiazide-sensitive sodium transporter and the renal adaptation of distal tubules that increase the capacity to reabsorb sodium (Verbrugge et al. 2016). Unfortunately, the combined diuretic therapy is associated with enhanced risk of electrolytes disturbances (hypokalemia, hypomagnesemia, hyponatremia), hypovolemia and renal impairment. Blood chemistry and volume status should be periodically checked in order to adjust the diuretic dose and maintain euvolemia. In case of significant hypokalemia (<2.5 mmol/l) and/or severe renal dysfunction a dose reduction or the discontinuation of thiazide diuretics should be considered.
7 Conclusion
Kidney disease is highly prevalent in subjects affected by heart failure and has a negative prognostic value independently of the degree of cardiac dysfunction. Therefore, an accurate renal function evaluation is relevant to identify patients prone to worse outcome. GFR estimation based on serum creatinine levels is considered the easiest way to assess overall kidney function in clinical practice and it is routinely used in HF patients management. However, several limitations in the use of serum creatinine exist. To overcome some of the caveats related to creatinine, several new glomerular and tubular markers have been studied. Emerging renal biomarkers have demonstrated to carry incremental diagnostic and prognostic information in renal diseases and identify renal impairment at an earlier stage. In addition to laboratory data, imaging techniques have shown to give complementary information in HF-related renal dysfunction. In particular, Doppler evaluation can explore renal arterial and venous haemodynamics, giving independent and incremental prognostic information. Finally, the management of patients with significant renal impairment and concomitant heart failure is still a major challenge. Unfortunately, because of the underrepresentation of patients with renal dysfunction in HF clinical trials, limited data are available for this specific subgroup. With this regard, current evidences support the use of HF drugs in patients with moderate renal dysfunction, but data on the population with severe and end-stage renal dysfunction are still lacking. Lastly, the new ARNI class showed a promising renoprotective effect in recent clinical trials, but further data are expected about its safety in patients with severe renal dysfunction.
References
Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT et al (2005) ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry. Am Heart J 149:209–216
Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M (2013) Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Fail 19:E40–E45
Alvelos M, Lourenço P, Dias C, Amorim M, Rema J, Leite AB et al (2013) Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol 165:51–55
Arimoto T, Takeishi Y, Niizeki T, Takabatake N, Okuyama H, Fukui A et al (2005) A novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 11:595–601
Aronson D, Mittleman MA, Burger AJ (2004) Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med 116:466–473
Artunc F, Rossi C, Boss A (2011) MRI to assess renal structure and function. Curr Opin Nephrol Hypertens 20:669–675
Bauer JA, Fung HL (1991) Concurrent hydralazine administration prevents nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Circulation 84:35–39
Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P (1987) Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J 57:17–22
Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M et al (2002) Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant 17:1890–1896
Boss A, Martirosian P, Gehrmann M, Artunc F, Risler T, Oesingmann N et al (2007) Quantitative assessment of glomerular filtration rate with MR gadolinium slope clearance measurements: a phase I trial. Radiology 242:783–790
Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB et al (2012) Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD treatment trial. Int J Cardiol 167:151–156
Braam B, Cupples WA, Joles JA, Gaillard C (2012) Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev 17:161–175
Bude RO, Rubin JM (1999) Relationship between the resistive index and vascular compliance and resistance. Radiology 211:411–417
Cauthen CA, Lipinski MJ, Abbate A, Appleton D, Nusca A, Varma A (2008) Relation of blood urea nitrogen to long-term mortality in patients with heart failure. Am J Cardiol 101:1643–1647
Chade AR (2013) Renal vascular structure and rarefaction. Compr Physiol 3:817–883
Chang SM, Granger CB, Johansson PA, Kosolcharoen P, McMurray JJ, Michelson EL, CHARM Investigators et al (2010) Efficacy and safety of angiotensin receptor blockade are not modified by aspirin in patients with chronic heart failure: a cohort study from the Candesartan in Heart Failure–Assessment of Reduction in Mortality and Morbidity (CHARM) programme. Eur J Heart Fail 12:738–745
Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B (2003) Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur J Clin Invest 33:769–778
Chong VH, Singh J, Parry H, Saunders J, Chowdhury F, Mancini DM et al (2015) Management of noncardiac comorbidities in chronic heart failure. Cardiovasc Ther 33:300–315
Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A et al (2014) The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 16:210–216
Coca SG, Krumholz HM, Garg AX, Parikh CR (2006) Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296:1377–1384
Cogan JJ, Humphreys MH, Carlson CJ, Rapaport E (1980) Renal effects of nitroprusside and hydralazine in patients with congestive heart failure. Circulation 61:316–323
Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE et al (1986) Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 314:1547–1552
Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF et al (2012) Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail 14:1020–1029
Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE et al (2012) Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev 17:177–190
Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM (2008) Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 295:F1589–F1600
Damman K, Testani JM (2015) The kidney in heart failure: an update. Eur Heart J 36:1437–1444
Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ (2009a) Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail 11:847–854
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL (2009b) Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53:582–588
Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD et al (2010) Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 96:1297–1302
Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C et al (2011) Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J 32:2705–2712
Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL (2012) Current and novel renal biomarkers in heart failure. Heart Fail Rev 17:241–250
Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL (2014a) Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 35:455–469
Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H et al (2014b) Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol 63:853–871
Darmon M, Schnell D, Zeni F (2010) Doppler based renal resistive index: a comprehensive review. In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Heidelberg, pp 331–338
de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K et al (2009) Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 32:1833–1838
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Di Lullo L, Floccari F, Granata A, D’Amelio A, Rivera R, Fiorini F et al (2012) Ultrasonography: Ariadne’s thread in the diagnosis of the Cardiorenal syndrome. Cardiorenal Med 2:11–17
Ellison DH (2001) Diuretic therapy and resistance in congestive heart failure. Cardiology 96:132–143
Emamian SA, Nielsen MB, Pedersen JF, Ytte L (1993) Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 160:83–86
Ennezat PV, Maréchaux S, Six-Carpentier M, Pinçon C, Sediri I, Delsart P et al (2011) Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant 26:3908–3913
Eshaghian S, Horwich TB, Fonarow GC (2006) Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 97:1759–1764
Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ (2012) Diuretics for heart failure. Cochrane Database Syst Rev 2:CD003838
Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ, ADHERE, Scientific Advisory Committee, Study Group, and Investigators (2005) Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293:572–580
Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C (2003) Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63:1944–1947
Garg R, Yusuf S (1995) Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 273:1450–1456
Giamouzis G, Butler J, Triposkiadis F (2011) Renal function in advanced heart failure. Congest Heart Fail 17:180–188
Goldsmith SR, Sobotka PA, Bart BA (2010) The sympathorenal axis in hypertension and heart failure. J Card Fail 16:369–373
Granata A, Clementi A, Virzì GM, Brocca A, de Cal M, Scarfia VR et al (2016) Cardiorenal syndrome type 4: from chronic kidney disease to cardiovascular impairment. Eur J Intern Med 30:1–6
Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B et al (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet 362:772–776
Hackstein N, Heckrodt J, Rau WS (2003) Measurement of single-kidney glomerular filtration rate using a contrast-enhanced dynamic gradient-echo sequence and the Rutland-Patlak plot technique. J Magn Reson Imaging 18:714–725
Hackstein N, Wiegand C, Rau WS, Langheinrich AC (2004) Glomerular filtration rate measured by using triphasich elical CT with a two-point Patlak plot technique. Radiology 230:221–226
Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV (2002) Kidney injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62:237–244
Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T (2012) The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol 2012:139565
Haufe SE, Riedmüller K, Haberkorn U (2006) Nuclear medicine procedures for the diagnosis of acute and chronic renal failure. Nephron Clin Pract 103:c77–c84
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, ADHERE Scientific Advisory Committee and Investigators (2007) High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 13:422–430
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS et al (2016) Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 11:e0158765
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A et al (2000) Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102:203–210
Iacoviello M, Doronzo A, Paradies V, Antoncecchi V, Monitillo F, Citarelli G et al (2015) The independent association between altered renal arterial resistance and loop diuretic dose in chronic heart failure outpatients. IJC Heart & Vasculature 7:119–123
Iacoviello M, Monitillo F, Leone M, Citarelli G, Doronzo A, Antoncecchi V et al (2016) The renal arterial resistance index predicts worsening renal function in chronic heart failure patients. Cardiorenal Med 7:42–49
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T et al (2016) Clinical implications of Intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail 4:674–682
Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S et al (1997) Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int 51:1920–1927
Ix JH, Shlipak MG, Chertow GM, Whooley MA (2006) Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 115:173–179
Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, CHARM Investigators and Committees et al (2009) Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 374:543–550
Jeong SH, Jung DC, Kim SH, Kim SH (2011) Renal venous Doppler ultrasonography in normal subjects and patients with diabetic nephropathy: value of venous impedance index measurements. J Clin Ultrasound 39:512–518
Judge P, Haynes R, Landray MJ, Baigent C (2015) Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant 30:738–743
Kalantarinia K (2009) Novel imaging techniques in acute kidney injury. Curr Drug Targets 10:1184–1189
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:1–150
Klein L, Massie BM, Leimberger JD, O’Connor CM, Piña IL, Adams KF Jr et al (2008) Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the outcomes of a prospective trial of intravenous Milrinone for exacerbations of chronic heart failure (OPTIME-CHF). Circ Heart Fail 1:25–33
Lassus J, Harjola VP, Sund R, Siirilä-Waris K, Melin J, Peuhkurinen K, FINN-AKVA Study Group et al (2007) Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 28:1841–1847
Leone M, Doronzo A, Paradies V, Monitillo F, Rizzo C, Lattarulo MS et al (2016) Renal resistance index and microalbuminuria as multiparametric approach to assess renal function in heart failure: prognostic aspects. Eur J Heart Fail 18:368. Abstract
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H et al (2007) Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 18:904–912
Lubas A, Kade G, Niemczyk S (2014) Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int Urol Nephrol 46:395–402
Maatman RG, Van Kuppevelt TH, Veerkamp JH (1991) Two types of fatty acid-binding protein in human kidney. Isolation, characterization and localization. Biochem J 273:759–766
Massie BM, Armstrong PW, Cleland JG, Horowitz JD, Packer M, Poole-Wilson PA et al (2001) Toleration of high doses of angiotensin-converting enzyme inhibitors in patients with chronic heart failure: results from the ATLAS trial. The assessment of treatment with Lisinopril and survival. Arch Intern Med 161:165–171
Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, GISSI-HF Investigators et al (2010) Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail 3:65–72
McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A et al (2012) Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group Formula. Circ Heart Fail 5:309–314
McCormack PL (2016) Sacubitril/valsartan: a review in chronic heart failure with reduced ejection fraction. Drugs 76:387–396
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR et al (2014a) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371:993–1004
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR et al (2014b) Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail 16:817–825
Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R et al (2008) Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 10:188–195
Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA (2012) The role of the kidney in heart failure. Eur Heart J 33:2135–2142
Michaely HJ, Sourbron S, Dietrich O, Attenberger U, Reiser MF, Schoenberg SO (2007) Functional renal MR imaging: an overview. Abdom Imaging 32:758–771
Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ et al (2009) National Kidney Disease Education Program-IFCC working group on standardization of albumin in urine current issues in measurement and reporting of urinary albumin excretion. Clin Chem 55:24–38
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C et al (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA et al (2005) Correlation of renal histopathology with sonographic findings. Kidney Int 67:1515–1520
Mostbeck GH, Gössinger HD, Mallek R, Siostrzonek P, Schneider B, Tscholakoff D (1990) Effect of heart rate on Doppler measurements of resistive index in renal arteries. Radiology 175:511–513
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor D, Starling RC et al (2009) Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53:589–596
Murphy ME, Tublin ME (2000) Understanding the Doppler RI: impact of renal arterial distensibility on the RI in hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med 19:303–314
Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO et al (1995) Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47:312–318
Niizeki T, Takeishi Y, Arimoto T, Nozaki N, Hirono O, Watanabe T et al (2008) Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ J 72:109–114
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M et al (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51:1268–1274
Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T et al (2009) Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injursssssy. Am J Physiol Renal Physiol 296:F669–F679
Page JE, Morgan SH, Eastwood JB, Smith SA, Webb DJ, Dilly SA et al (1994) Ultrasound findings in renal parenchymal disease: comparison with histological appearances. Clin Radiol 49:867–870
Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G (2009) Renal resistive index and long term outcome in chronic nephropathies. Radiology 252:888–896
Parrinello G, Torres D, Testani JM, Almasio PL, Bellanca M et al (2015) Blood urea nitrogen to creatinine ratio is associated with congestion and mortality in heart failure patients with renal dysfunction. Intern Emerg Med 10:965–972
Pierpont GL, Brown DC, Franciosa JA, Cohn JN (1980) Effect of hydralazine on renal failure in patients with congestive heart failure. Circulation 61:323–327
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A et al (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341:709–717
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B et al (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321
Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB (1990) Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol 154:1223–1227
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18:891–975
Prewitt RL, Chen II, Dowell R (1982) Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Phys 243:H243–H251
Puzzovivo A, Iacoviello M, Monitillo F, Leone M, Rizzo C, Lattarulo MS et al (2016) Renal venous pattern: a new parameter for predicting cardiorenal syndrome progression. Eur J Heart Fail 18:162–163. (Abstract)
Roberts DA, Detre JA, Bolinger L, Insko EK, Lenkinski RE, Pentecost MJ et al (1995) Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology 196:281–286
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R (2008) Cardiorenal syndrome. J Am Coll Cardiol 52:1527–1539
Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S (2002) Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82:S12–S22
Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M (2017) Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 40:485–491
Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S (2016) Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 12:610–623
Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P et al (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18:407–413
Schrier RW (2008) Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail 1:2–5
Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB et al (2005a) Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352:2049–2060
Shlipak MG, Katz R, Fried LF, Jenny NS, Stehman-Breen C, Newman AB et al (2005b) Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol 45:268–271
Simon-Zoula SC, Hofmann L, Giger A, Vogt B, Vock P, Frey FJ et al (2006) Non-invasive monitoring of renal oxygenation using BOLD-MRI: a reproducibility study. NMR Biomed 19:84–89
Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG (2007) Association of cystatin C and estimated GFR with inflammatory biomarkers: the Heart and Soul Study. Nephrol Dial Transplant 22:1087–1092
Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA et al (2009) Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol 98:121–129
Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P et al (2006) Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 47:1987–1996
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E et al (2012) Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 380:1387–1395
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M et al (2010) Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56:486–495
Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Ferdinand K (2004) Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 351:2049–2057
Taylor AL, Ziesche S, Yancy CW, Carson P, Ferdinand K, Taylor M et al (2007) African-American Heart Failure Trial Investigators. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: consistency across subgroups in the African-American Heart Failure trial. Circulation 115:1747–1753
Testani JM, Kimmel SE, Dries DL, Coca SG (2011) Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 4:685–691
Tokmakova MP, Skali H, Kenchaiah S, Braunwald E, Rouleau JL, Packer M et al (2004) Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the survival and ventricular enlargement (SAVE) study. Circulation 110:3667–3673
Tublin ME, Tessler FN, Murphy ME (1999) Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology 213:258–264
Tublin ME, Bude RO, Platt JF (2003) Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol 180:885–892
Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA et al (2013) Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182:158–173
Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ et al (2014) The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the modification of diet in renal disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail 16:86–94
van de Wal RM, Asselbergs FW, Plokker HW, Smilde TD, Lok D, van Veldhuisen DJ (2005) High prevalence of microalbuminuria in chronic heart failure patients. J Card Fail 11:602–606
Vardeny O, DH W, Desai A, Rossignol P, Zannad F, Pitt B et al (2012) Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 60:2082–2089
Veerkamp JH, Paulussen RJ, Peeters RA, Maatman RG, van Moerkerk HT, van Kuppevelt TH (1990) Mol Cell Biochem 98:11–18
Verbrugge FH, Mullens W, Tang WH (2016) Management of Cardio-Renal Syndrome and Diuretic Resistance. Curr Treat Options Cardiovasc Med 18:11
Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ (2003) Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant 18:543–551
Winton FR (1931) The influence of venous pressure on the isolated mammalian kidney. J Physiol 72:49–61
Zannad F, McMurray JJV, Krum H, Van Veldhuisen DJ, Swedberg K, Shi H et al (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364:11–21
Ziaeian B, Fonarow GC, Heidenreich PA (2017) Clinical effectiveness of hydralazine-Isosorbide Dinitrate in African-American patients with heart failure. JACC Heart Fail 5:632–639
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Grande, D., Gioia, M.I., Terlizzese, P., Iacoviello, M. (2017). Heart Failure and Kidney Disease. In: Islam, M. (eds) Heart Failure: From Research to Clinical Practice. Advances in Experimental Medicine and Biology(), vol 1067. Springer, Cham. https://doi.org/10.1007/5584_2017_126
Download citation
DOI: https://doi.org/10.1007/5584_2017_126
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-78279-9
Online ISBN: 978-3-319-78280-5
eBook Packages: MedicineMedicine (R0)