Abstract
Novel heteroleptic lanthanum, praseodymium, and neodymium metal-organic coordination polymers (MOCPs) containing two types of anionic organic ligands as organic units are prepared by a two-step solvothermal synthesis in N,N-dimethylformamide. Framework derivatives with the [Ln2(CA)(fdc)2·4DMF]· ·2DMF composition are prepared (Ln = La, Pr, Nd; CA is a dianion of the chloranilic acid; fdc is a dianion of the 2,5-furandicarboxylic acid; DMF is N,N-dimethylformamide). The structure of the compounds is studied by XRD (CCDC CIF file No. 2251285 (I), 2251286 (II), 2251287 (III)).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Metal-organic coordination polymers (MOCPs) are a specific class of micro- and mesoporous solids that have been widely studied for the past decades [1]. Their structural and functional diversity opens up broad prospects for applications in the fundamental and applied science [2]. Metal-organic frameworks (MOFs) have a 3D structure with some porosity and are therefore classified as a special subclass of MOCPs.
The structure and properties of MOCPs are determined both by the metal ions and by the type of organic ligands. Di-, tri- , and tetracarboxylic acids are most commonly used as ligands to build coordination polymers [3, 4]. Other promising linkers for the construction of coordination polymers are redox-active anilate ligands, 2,5-dihydroxy-1,4-benzoquinone derivatives, containing various substituents in positions 3 and 6 (X = H, Cl, Br, I, CN, etc.) [5-7]. Being connected with metals, these ligands can exist in four different redox states (Scheme 1). Most of the currently known MOCPs include anilate ligands in the dianionic state [6].
The use of several different ligands in a MOCP allows one to obtain compounds encompassing properties of different systems, thus paving the way to the preparation of multifunctional materials. A very promising direction in the chemistry of coordination polymers is the synthesis of heteroleptic compounds containing anionic ligands of various types in the composition of their unit [8-17]. Thus, a group of Novosibirsk researchers obtained mixed-ligand zinc derivatives (NIIC-20-G) based on dicarboxylic acids and dihydric alcohols [11-13, 16]. The authors showed that the main adsorption parameters of saturated hydrocarbons (methane, ethane, and propane), which are the main components of natural gas, are affected by the size and nature of the glycol fragment in a series of zinc MOCPs [16]. These compounds also show high adsorption capacity towards various volatile organic compounds (benzene, cyclohexane, xylene isomers) [11]. Selective adsorption of NIIC-20-G compounds to various representatives of homologous series and hydrocarbon isomers allows using these frameworks for the sorption-based separation of industrially important products of petrochemical synthesis. New heteroleptic NIIC-20-G derivatives are also promising materials for air purification due to their high adsorption characteristics towards hazardous organic vapors, easy regeneration of the porous materials, and the reproducibility of their adsorption properties.
Among the huge variety of MOCPs and MOFs, lanthanide derivatives demonstrate special magnetic [18-21], luminescent [19, 22-26], sorption [27], and sensory [28, 29] properties. Recently, first examples of heteroleptic lanthanide coordination polymers based on dicarboxylate and anilate ligands were synthesized [8-10, 15]. Photophysical properties of mixed-ligand NIR-emitting Yb(III) and Er(III) derivatives make heteroleptic MOCPs [8, 10] more advantageous than their homoleptic analogs [26, 30]. The chlorocyanoanilate linker in both types of MOCPs acts as an optical antenna responsible for the Yr(III)/Er(III) luminescence sensitization as a result of efficient ligand–metal energy transfer. At the same time, the use of dicarboxylate ligands in heteroleptic systems significantly increases the efficiency of NIR radiation compared with their homoleptic counterparts. Note that the formation of end products and the phase purity of obtained derivatives directly depends on the conditions of heteroleptic MOCP synthesis [8, 9].
In the present study, we report the preparation and study of new heteroleptic lanthanide MOFs [Ln2(CA)(fdc)2·4DMF]·2DMF (Ln = La (I·2DMF), Pr (II·2DMF), Nd (III·2DMF); CA = chloranilic acid dianion; fdc = 2,5-furandicarboxylic acid dianion; DMF = N,N-dimethylformamide). The mixed-ligand derivatives were isolated from the reaction mixture in the form of finely crystalline violet samples. The structure of isostructural MOFs [Ln2(CA)(fdc)2·4DMF]·2DMF was studied by single-crystal XRD. Thermal stability of dried MOFs I–III was studied by thermogravimetric analysis (TGA).
EXPERIMENTAL
The IR spectra were recorded on a FSM-1201 FTIR spectrometer (suspensions in liquid paraffin; KBr pellets). The elemental analysis was performed on an Elementar Vario El cube analyzer. The TGA study was performed on a Mettler Toledo TGA/DSC3+ system at 30-700 °C in nitrogen (polycrystalline alumina crucible) at a heating rate of 5 °C/min. The following commercial reactants were used: LaCl3·7H2O, PrCl3·6H2O, NdCl3·6H2O, chloranilic acid H2CA; 2,5-furandicarboxylic acid H2fdc; N,N′-dimethylformamide (DMF).
Synthesis of [La2(CA)(fdc)2·4DMF]·2DMF (I·2DMF), [Pr2(CA)(fdc)2·4DMF]·2DMF (II·2DMF) and [Nd2(CA)(fdc)2·4DMF]·2DMF (III·2DMF).
A mixture of one of the lanthanide salts (LaCl3·7H2O for I·2DMF, PrCl3·6H2O for II·2DMF, NdCl3·6H2O for III·2DMF, 0.04 mmol), 2,5-furandicarboxylic acid (0.08 mmol), and chloranilic acid (0.04 mmol) was ground in a mortar for better mixing of the initial materials. The resulting mixture was heated for 24 h at 80 °C in DMF (5 mL) in a sealed glass ampoule; then the temperature was increased up to 130 °C and heated for another 24 h. The isostructural MOFs I–III were obtained as purple crystalline products that were collected on a glass filter and washed with 3 mL of DMF. When dried in air, the [Ln2(CA)(fdc)2·4DMF]·2DMF compounds rapidly lose their crystallinity due to the gradual release of the “guest” DMF solvent from the MOF pores. For the elemental analysis, IR spectroscopy, and TGA experiments, dried samples of MOFs I–III containing no “guest” solvent were used.
Yield of MOF I: 72%. IR spectrum (ν, cm–1): 1658 s (–C=O (DMF)), 1565 s (group –C(O)O 2,5-furandicarboxylic acid), 1487 s (–CO - CA2–), 1379 s (–CO - CA2–), 1295 m, 1228 w, 1200 w, 1154 w, 1112 s, 1104 s, 1066 m, 1020 s, 967 m, 867 m, 832 s, 823 s, 789 s, 976 s, 670 s, 625 m, 598 s, 576 s, 520 w, 497 s. Found (%): C 33.01, H 3.12, N 5.57; for C30H32La2Cl2N4O18 calculated (%): C 33.20, H 2.97, N 5.16.
Yield of MOF II: 70%. IR spectrum (ν, cm–1): 1657 s (–C=O (DMF)), 1565 s (–C(O)O - fdc2–), 1490 s (–CO - CA2–), 1380 s (–CO - CA2–), 1294 m, 1254 m, 1227 w, 1201 w, 1166 w, 1154 w, 1110 s, 1103 s, 1065 m, 1022 m, 991 s, 966 m, 866 m, 840 s, 823 s, 788 s, 679 s, 670 s, 625 m, 598 s, 576 s, 522 w, 498 s. Found (%): C 32.60, H 3.04, N 5.62; for C30H32Pr2Cl2N4O18 calculated (%): C 33.08, H 2.96, N 5.14.
Yield of MOF III: 63%. IR spectrum (ν, cm–1): 1652 s (–C=O (DMF)), 1565 s (–C(O)O - fdc2–), 1490 s (–CO - CA2–), 1378 s (–CO - CA2–), 1292 m, 1252 m, 1219 w, 11492 w, 1110 s, 1104 s, 1089 w, 1063 w, 1018 w, 994 m, 963 w, 866 m, 842 s, 823 s, 887 s, 678 s, 669 s, 624 m, 597 s, 577 s, 521 w, 495 s. Found (%): C 32.42, H 3.08, N 5.58; for C30H32Pr2Cl2N4O18 calculated (%): C 32.88, H 2.94, N 5.11.
XRD. The XRD studies of MOCPs I–III were conducted on a Bruker D8 Quest diffractometer (MoKα radiation, ω-scanning, λ = 0.71073 Å, T = 100.0(2) K). The measurements and integrations of experimental intensities, absorption corrections, structure determination and refinements were carried out using APEX3 [31], SADABS [32], and SHELX [33] packages. The structures were determined using the dual-space algorithm [34] and refined by full-matrix least squares on \(F_{\,hkl}^{2}\) anisotropically for the non-hydrogen atoms. All hydrogen atoms in I–III were calculated geometrically and refined isotropically with fixed thermal parameters U(H)iso = 1.2U(C)eq (U(H)iso = 1.5U(C)eq for methyl groups).
The topology of coordination polymers was analyzed using the ToposPro software [35].
The structures were deposited with the Cambridge Crystallographic Data Centre (CCDC numbers 2251285 (I), 2251286 (II), 2251287 (III)) and are available at ccdc.cam.ac.uk/structures.
RESULTS AND DISCUSSION
Heteroleptic lanthanide MOFs [Ln2(CA)(fdc)2·4DMF]·2DMF (Ln = La (I·2DMF), Pr (II·2DMF), Nd (III·2DMF)) were prepared by a previously developed technique [9] using two-stage solvothermal synthesis (Scheme 2). At the first stage, the reaction mixture was heated up to 80 °C for 24 h; at the second stage, the thermostat temperature increased to 130 °C, and the reaction mixture was heated for another 24 h. As a result, purple crystalline mixed-ligand MOCPs were obtained. The purple color of the crystals is not unusual for such derivatives and is typical for compounds containing anilate ligands in the dianion state [14].
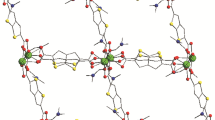
The structure of I·2DMF, II·2DMF, and III·2DMF was determined by XRD. The I·2DMF, II·2DMF, and III·2DMF MOCPs are isostructural, so their structure was considered on the example of the I·2DMF derivative. The molecular structure of I·2DMF is shown in Fig. 1. The crystal data and parameters of XRD experiments for isostructural MOCPs [Ln2(CA)(fdc)2·4DMF]·2DMF (La, Pr, Nd) are summarized in Table 1; the selected bond lengths are listed in Table 2.
According to the XRD data, the I·2DMF, II·2DMF, and III·2DMF derivatives are isostructural and crystallize in the P21/c monoclinic space group. The formal coordination number of metal centers in these compounds is nine: La3+ (I), Pr3+ (II), and Nd3+ (III) cations are bonded to five oxygen atoms of four fdc2− dianions, two oxygen atoms of CA2− dianions, and two oxygen atoms of two coordinated DMF molecules (Fig. 1). The structural unit of each MOCP is formed by two metal centers connected to each other by means of two μ2–κ1:κ1-bridging COO groups of furandicarboxylic acids and participates in the formation of secondary building units (Fig. 2a). The distance between two Ln atoms in the secondary building units of I·2DMF, II·2DMF, and III·2DMF is 4.0713(3) Å, 4.0044(4) Å, and 3.9790(5) Å, respectively. There are two uncoordinated solvate DMF molecules per each MOCP unit. The MOCP 2D layers in I·2DMF, II·2DMF, and III·2DMF are built due to the μ2–κ1:κ2 bonds between free COO groups of the fdc2− dianions and metal cations of the neighboring units (Fig. 2b). Finally, the chloranilic acid dianions “crosslink” the layers into a 3D non-interpenetrating structure with the xah topology [35-37] (Fig. 2c).
MOCPs with anilate [38-40] and dicarboxylate [41] ligands can show different types of coordination for the metal atom; most common of them are shown in Scheme 3 on the example of chloranil and 2,5-furandicarboxylic acids. The types of ligand coordination in I·2DMF, II·2DMF, and III·2DMF are shown in the scheme by rectangles.
The structure of chloranilic acid dianions in I·2DMF, II·2DMF, and III·2DMF can be represented as two delocalized π-electronic OCCCO systems connected by single C–C bonds. The lengths of single C–C bonds range from 1.537(3) Å to 1.546(4) Å (Table 2), while other C–C distances of the six-membered rings fall within a wider range of 1.382(7)-1.408(7) Å. The lengths of the C–O bonds (1.249(4)-1.257(5) Å) are intermediate between single and double C–O bonds [42].
As already shown, dicarboxylate ligands in all derivatives act as bridging μ2–κ1:κ1 and μ2–κ1:κ2 ligands connecting four Ln3+ ions. The La–O (2.509(2)-2.695(2) Å). Pr–O (2.462(2)-2.652(2) Å), and Nd–O (2.462(2)-2.632(2) Å) bonds fall within the intervals typical for La, Pr, and Nd MOCPs with 2,5-furandicarboxylic acid [43, 44].
The pores in the crystal structures of I·2DMF, II·2DMF, and III·2DMF have a volume of 22% and are occupied by DMF guest molecules (Fig. 3) [45]. The crystal lattice destroys when the guest molecules are released and the pores are emptied. For example, when stored without mother solvent in air for 24 h, the I·2DMF, II·2DMF, and III·2DMF samples lose their crystallinity to the extent that their powder XRD spectra show no distinct diffraction peaks. The elemental analysis and TGA data confirm the absence of DMF guest molecules in the dried samples and show agree well with the results for the simplest formula [Ln2(CA)(fdc)2·4DMF] (La, Pr, Nd).
Secondary building unit in I·2DMF (a). View of the I·2DMF framework along direction (100) (b) and (010) (c). Color code: La (cyan), chloranilic acid dianion (red), 2,5-furandicarboxylic acid dianion (blue), DMF (gray) (see the electronic version). Thermal ellipsoids are set at a 50% probability level Hydrogen atoms and “guest” DMF molecules are not shown.
The TGA data indicate that the anionic framework of [Ln2(CA)(fdc)2·4DMF] (I–III) is thermally quite stable (Fig. 4). The TGA curve shows that the dried MOFs contain no guest solvent. All the derivatives show four successive steps of weight loss corresponding to the stepwise removal of four coordinated DMF molecules from the lanthanide ions. In the case of the lanthanum MOF (I), the steps 2-4 are not pronounced, but the weight loss of 21% corresponds to three coordinated DMF molecules. More exact data on the temperature intervals and weight losses for all the compounds are presented in Table 3. The anionic framework of the derivatives begins to destroy above 320 °C. Thermal stability of the heteroleptic MOCPs I–III is close to that of homoleptic (anilate and carboxylate) derivatives of lanthanides [43, 46].
CONCLUSIONS
New mixed-ligand MOFs of lanthanides (La, Pr, Nd) containing two types of anionic ligands (anilate and dicarboxylate) in the composition of their units were described. According to the XRD data, the obtained compounds are isostructural 3D coordination polymers containing solvate dimethylformamide molecules in their pores. The loss of the latter during the drying of crystalline samples results in the crystallinity loss. The TGA data indicate that the anionic framework of the synthesized lanthanide MOFs destroys above 320 °C.
ADDITIONAL INFORMATION
The study was carried out using the equipment of the Analytical Center of IOMC RAS.
REFERENCES
K. A. Kovalenko, A. S. Potapov, and V. P. Fedin. Micro- and mesoporous metal-organic frameworks for hydrocarbon separation. Russ. Chem. Rev., 2022, 91, RCR5026. https://doi.org/10.1070/RCR5026
M. A. Agafonov, E. V. Alexandrov, N. A. Artyukhova, G. E. Bekmukhamedov, V. A. Blatov, V. V. Butova, Y. M. Gayfulin, A. A. Gharibyan, Z. N. Gafurov, Y. G. Gorbunova, L. G. Gordeeva, M. S. Gruzdev, A. N. Gusev, G. L. Denisov, D. N. Dybtsev, Y. Y. Enakieva, A. A. Kagilev, A. O. Kantyukov, M. A. Kiskin, K. A. Kovalenko, A. M. Kolker, D. I. Kolokolov, Y. M. Litvinova, A. A. Lysova, N. V. Maksimchuk, Y. V. Mironov, Y. V. Nelyubina, V. V. Novikov, V. I. Ovcharenko, A. V. Piskunov, D. M. Polyukhov, V. A. Polyakov, V. G. Ponomarev, A. S. Poryvaev, G. V. Romanenko, A. V. Soldatov, M. V. Solovyov, A. G. Stepanov, I. V. Terekhov, O. Y. Trofimova, V. P. Fedin, M. V. Fedin, O. A. Holdeeva, A. Y. Tsivadze, W. V. Chervonova, A. I. Cherevko, V. F. Shulgin, E. S. Shutov, and D. G. Yakhvarov. Metal-organic coordination polymers in Russia: From synthesis and structure to functional properties and materials. J. Struct. Chem., 2022, 63(5), 671. https://doi.org/10.26902/JSC_id93211
B. Li, H.-M. Wen, Y. Cui, W. Zhou, G. Qian, and B. Chen. Emerging multifunctional metal-organic framework materials. Adv.Mater., 2016, 28, 8819. https://doi.org/10.1002/adma.201601133
H. Jiang, D. Alezi, and M. Eddaoudi. A reticular chemistry guide for the design of periodic solids. Nat. Rev. Mater., 2021, 6, 466. https://doi.org/10.1038/s41578-021-00287-y
S. Kitagawa and S. Kawata. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chemi. Rev., 2002, 224, 11. https://doi.org/10.1016/S0010-8545(01)00369-1
N. Monni, M. Oggianu, S. A. Sahadevan, and M. L. Mercuri. Redox activity as a powerful strategy to tune magnetic and/or conducting properties in benzoquinone-based metal-organic frameworks. Magnetochemistry, 2021, 7, 109. https://doi.org/10.3390/magnetochemistry7080109
N. Monni, M. S. Angotzi, M. Oggianu, S. A. Sahadevan, and M. L. Mercuri. Redox-active benzoquinones as challenging “non-innocent” linkers to construct 2D frameworks and nanostructures with tunable physical properties. J. Mater. Chem. C, 2022, 10, 1548. https://doi.org/10.1039/d1tc05335c
S. A. Sahadevan, F. Manna, A. Abhervé, M. Oggianu, N. Monni, V. Mameli, D. Marongiu, F. Quochi, F. Gendron, B. L. Guennic, N. Avarvari, and M. L. Mercuri. Combined experimental/theoretical study on the luminescent properties of homoleptic/heteroleptic erbium(III) anilate-based 2D coordination polymers. Inorg. Chem., 2021, 60, 17765. https://doi.org/10.1021/acs.inorgchem.1c02386
O. Y. Trofimova, A. V. Maleeva, I. V. Ershova, A. V. Cherkasov, G. K. Fukin, R. R. Aysin, K. A. Kovalenko, and A. V. Piskunov. Heteroleptic LaIII anilate/dicarboxylate based neutral 3D-coordination polymers. Molecules, 2021, 26, 2486. https://doi.org/10.3390/molecules26092486
S. A. Sahadevan, N. Monni, M. Oggianu, A. Abhervé, D. Marongiu, M. Saba, A. Mura, G. Bongiovanni, V. Mameli, C. Cannas, N. Avarvari, F. Quochi, and M. L. Mercuri. Heteroleptic NIR-emitting YbIII/anilate-based neutral coordination polymer nanosheets for solvent sensing. ACS Appl. Nano Mater., 2020, 3, 94. https://doi.org/10.1021/acsanm.9b01740
A. A. Lysova, K. A. Kovalenko, D. N. Dybtsev, S. N. Klyamkin, E. A. Berdonosova, and V. P. Fedin. Hydrocarbon adsorption in a series of mesoporous metal-organic frameworks. Microporous Mesoporous Mater., 2021, 328, 111477. https://doi.org/10.1016/j.micromeso.2021.111477
A. A. Lysova, D. G. Samsonenko, K. A. Kovalenko, A. S. Nizovtsev, D. N. Dybtsev, and V. P. Fedin. A series of mesoporous metal-organic frameworks with tunable windows sizes and exceptionally high ethane over ethylene adsorption selectivity. Angew. Chem., Int. Ed., 2020, 59, 20561. https://doi.org/10.1002/anie.202008132
A. A. Lysova, D. G. Samsonenko, P. V. Dorovatovskii, V. A. Lazarenko, V. N. Khrustalev, K. A. Kovalenko, D. N. Dybtsev, and V. P. Fedin. Tuning the molecular and cationic affinity in a series of multifunctional metal-organic frameworks based on dodecanuclear Zn(II) carboxylate wheels. J. Am. Chem. Soc., 2019, 141, 17260. https://doi.org/10.1021/jacs.9b08322
O. Y. Trofimova, A. V. Maleeva, K. V. Arsenyeva, A. V. Klimashevskaya, I. A. Yakushev, and A. V. Piskunov. Glycols in the synthesis of zinc-anilato coordination polymers. Crystals, 2022, 12, 370. https://doi.org/10.3390/cryst12030370
M. Oggianu, F. Manna, S. A. Sahadevan, N. Avarvari, A. Abhervé, and M. L. Mercuri. Metal-organic framework vs. coordination polymer–influence of the lanthanide on the nature of the heteroleptic anilate/terephtalate 3D network. Crystals, 2022, 12, 763. https://doi.org/10.3390/cryst12060763
A. A. Lysova, K. A. Kovalenko, A. S. Nizovtsev, D. N. Dybtsev, and V. P. Fedin. Efficient separation of methane, ethane and propane on mesoporous metal-organic frameworks. Chem. Eng. J., 2023, 453, 139642. https://doi.org/10.1016/j.cej.2022.139642
S. Benmansour, C. Pintado-Zaldo, J. Martínez-Ponce, A. Hernández-Paredes, A. Valero-Martínez, M. Gómez-Benmansour, and C. J. Gómez-García. The versatility of ethylene glycol to tune the dimensionality and magnetic properties in DyIII-anilato-based single-ion magnets. Cryst. Growth Des., 2023, 23, 1269. https://doi.org/10.1021/acs.cgd.2c01409
P. J. Saines and N. C. Bristowe. Probing magnetic interactions in metal-organic frameworks and coordination polymers microscopically. Dalton Trans., 2018, 47, 13257. https://doi.org/10.1039/c8dt02411a
S. Benmansour, C.J. Gómez-García. Lanthanoid-anilato complexes and lattices. Magnetochemistry, 2020, 6, 71. https://doi.org/10.3390/magnetochemistry6040071
P. Gómez-Claramunt, S. Benmansour, A. Hernández-Paredes, C. Cerezo-Navarrete, C. Rodríguez-Fernández, J. Canet-Ferrer, A. Cantarero, and C. J. Gómez-García. Tuning the structure and properties of lanthanoid coordination polymers with an asymmetric anilato ligand. Magnetochemistry, 2018, 4, 6. https://doi.org/10.3390/magnetochemistry4010006
K. Bondaruk and C. Hua. Effect of counterions on the formation and structures of Ce(III) and Er(III) chloranilate frameworks. Cryst. Growth Des., 2019, 19, 3338. https://doi.org/10.1021/acs.cgd.9b00233
T. Gorai, W. Schmitt, and T. Gunnlaugsson. Highlights of the development and application of luminescent lanthanide based coordination polymers, MOCPs and functional nanomaterials. Dalton Trans., 2021, 50, 770. https://doi.org/10.1039/d0dt03684f
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk. Luminescent metal–organic frameworks. Chem. Soc. Rev., 2009, 38, 1330. https://doi.org/10.1039/b802352m
M. Huangfu, M. Wang, C. Lin, J. Wang, and P. Wu. Luminescent metal–organic frameworks as chemical sensors based on “mechanism–response”: a review. Dalton Trans., 2021, 50, 3429. https://doi.org/10.1039/D0DT04276E
P. A. Demakov, A. A. Vasileva, V. A. Lazarenko, A. A. Ryadun, and V. P. Fedin. Crystal structures, thermal and luminescent properties of gadolinium(III) trans-1,4-cyclohexanedicarboxylate metal-organic frameworks. Crystals, 2021, 11, 1375. https://doi.org/10.3390/cryst11111375
S. A. Sahadevan, N. Monni, A. Abhervé, D. Marongiu, V. Sarritzu, N. Sestu, M. Saba, A. Mura, G. Bongiovanni, C. Cannas, F. Quochi, N. Avarvari, and M. L. Mercuri. Nanosheets of two-dimensional neutral coordination polymers based on near-infrared-emitting lanthanides and a chlorocyananilate ligand. Chem. Mater., 2018, 30, 6575. https://doi.org/10.1021/acs.chemmater.8b03399
C. J. Kingsbury, B. F. Abrahams, J. E. Auckett, H. Chevreau, A. D. Dharma, S. Duyker, Q. He, C. Hua, T. A. Hudson, K. S. Murray, W. Phonsri, V. K. Peterson, R. Robson, and K. F. White. Square grid metal–chloranilate networks as robust host systems for guest sorption. Chem. Eur. J., 2019, 25, 5222. https://doi.org/10.1002/chem.201805600
H.-H. Zeng, W.-B. Qiu, L. Zhang, R.-P. Liang, and J.-D. Qiu. Lanthanide coordination polymer nanoparticles as an excellent artificial peroxidase for hydrogen peroxide detection. Anal. Chem., 2016, 88, 6342. https://doi.org/10.1021/acs.analchem.6b00630
H.-J. Chen, L.-Q. Chen, L.-R. Lin, L.-S. Long, and L.-S. Zheng. Doped luminescent lanthanide coordination polymers exhibiting both white-light emission and thermal sensitivity. Inorg. Chem., 2021, 60, 6986. https://doi.org/10.1021/acs.inorgchem.1c00740
F. Artizzu, M. Atzori, J. Liu, D. Mara, K. V. Hecke, and R. V. Deun. Solution-processable Yb/Er 2D-layered metallorganic frameworks with high NIR-emission quantum yields. J. Mater. Chem. C, 2019, 7, 11207. https://doi.org/10.1039/c9tc03698a
APEX3; SAINT. Madison, Wisconsin, USA: Bruker AXS Inc., 2018.
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr., 2015, 48, 3. https://doi.org/10.1107/S1600576714022985
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
V. A. Blatov, A. P. Shevchenko, and D. M. Proserpio. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des., 2014, 14, 3576. https://doi.org/10.1021/cg500498k
E. V. Alexandrov, V. A. Blatov, A. V. Kochetkov, and D. M. Proserpio. Underlying nets in three-periodic coordination polymers: topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm, 2011, 13, 3947. https://doi.org/10.1039/c0ce00636j
E. V. Alexandrov, A. P. Shevchenko, N. A. Nekrasova, and V. A. Blatov. Topological methods for analysis and design of coordination polymers. Russ. Chem. Rev., 2022, 91, RCR5032. https://doi.org/10.1070/RCR5032
S. Benmansour, G. López-Martínez, J. Canet-Ferrer, and C. J. Gómez-García. A family of lanthanoid dimers with nitroanilato bridges. Magnetochemistry, 2016, 2, 32. https://doi.org/10.3390/magnetochemistry2030032
L. A. Dubraja, K. Molcanov, D. Zilic, B. Kojic-Prodic, and E. Wenger. Multifunctionality and size of the chloranilate ligand define the topology of transition metal coordination polymers. New J.Chem., 2017, 41, 6785. https://doi.org/10.1039/c7nj01058c
V. Vuković, K. I. Molčanov, C. Jelsch, E. Wenger, A. Krawczuk, M. Jurić, L. A. Dubraja, and B. Kojić-Prodić. Malleable electronic structure of chloranilic acid and its species determined by X-ray charge density studies. Cryst. Growth Des., 2019, 19, 2802. https://doi.org/10.1021/acs.cgd.9b00033
H.-Y. Cao, Q.-Y. Liu, M.-J. Gao, Y.-L. Wang, L.-L. Chen, and Y. Liu. Ionothermal syntheses, crystal structures and luminescence of three three-dimensional lanthanide-1,4-benzenedicarboxylate frameworks. Inorg. Chim. Acta, 2014, 414 226. https://doi.org/10.1016/j.ica.2014.02.014
S. N. Brown. Metrical oxidation states of 2-amidophenoxide and catecholate ligands: Structural signatures of metal–ligand π bonding in potentially noninnocent ligands. Inorg. Chem., 2012, 51, 1251. https://doi.org/10.1021/ic202764j
H. Wang, R.-M. Wen, and T.-L. Hu. Two series of lanthanide metal-organic frameworks constructed from crown-ether-like secondary building units. Eur. J. Inorg. Chem., 2014, 2014, 1185. https://doi.org/10.1002/ejic.201301324
Ya-PingWang, X.-Y. Li, H.-H. Li, H.-Z. Zhang, H.-Y. Sun, Q. Guo, H. Li, and Z. Niu. A novel 3D Nd(III) metal-organic frameworks based onfuran-2,5-dicarboxylic acid exhibits new topology and rare near-infrared luminescence property. Inorg. Chem. Commun., 2016, 70, 27. https://doi.org/10.1016/j.inoche.2016.05.008
L. J. Barbour. Crystal porosity and the burden of proof. Chem. Commun., 2006, (11), 1163. https://doi.org/10.1039/B515612M
A. D. Kharitonov, O. Yu. Trofimova, I. N. Meshcheryakova, G. K. Fukin, M. N. Khrizanforov, Y. H. Budnikova, A. S. Bogomyakov, R. R. Aysin, K. A. Kovalenko, and A. V. Piskunov. 2D-Metal-organic coordination polymers of lanthanides (La(III), Pr(III) and Nd(III)) with redox-active dioxolene bridging ligand. CrystEngComm, 2020, 22, 4675. https://doi.org/10.1039/d0ce00767f
Funding
This work was funded the Russian Science Foundation (project No. 22-23-00750).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 6, 112229.https://doi.org/10.26902/JSC_id112229
Rights and permissions
About this article
Cite this article
Trofimova, O.Y., Maleeva, A.V., Arsenyeva, K.V. et al. Heteroleptic Lanthanide (La, Pr, Nd) Metal-Organic Frameworks Based on Chloranilic and Furandicarboxylic Acids. J Struct Chem 64, 1070–1080 (2023). https://doi.org/10.1134/S0022476623060100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623060100