Abstract
This review covers the recent recyclable protocols for the C–N bond forming reactions between aromatic, heterocyclic and aliphatic amines such as imidazoles, benzimidazoles, benzylamines, piperidine, pyrrole, imides, anilines, hexyl, cyclohexyl amines, and amides as coupling partners with aryl iodides, bromides, chlorides, and arylboronic acids employing copper-mediated systems. The physical properties and characterization of the catalysts and their use in organic synthesis will be outlined. Most importantly, these recyclable versions developed by many groups in the recent years are potential candidates for commercial exploitation. The effect of additives, solvents, temperature, base, the nature of aryl halides on reactivity, and recycle studies of the heterogeneous catalysts are included in this review. We believe that this information is beneficial for the people who are doing similar studies in this field. Catalyst optimization is of critical importance to catalyst development, thus the information we have included in this review contains very valuable information for the newcomers to the field. To our knowledge this is the first review that covers the title chemistry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aryl halides
- C-N bond formation
- Copper catalyst
- Cross-Coupling
- Heterocycles
- Heterogeneous
- Reusable catalysts
1 Heterogeneous or Reusable Catalysis
Organic synthesis employing heterogeneous catalysts has several beneficial effects to the environment. Because it involves the reuse of precious metal catalysts, it forms less waste and results in lower metal contamination in the final pharmaceutically important molecules. However, solid-supported catalysts are complex assemblies. Their preparation and characterization are challenging tasks. Minor changes to their preparation conditions can significantly influence the delicate balance of conflicting demands: high activity, high selectivity, and a long lifetime [1]. Several varieties of heterogeneous or reusable catalytic systems are known depending on the type of application.
2 Green Chemistry of Coupling Reactions
The coupling reactions were carried out mostly under homogeneous conditions [2]. Although homogeneous reactions have tremendous benefits in terms of ready availability of the catalyst and defined structure, the separation of the catalyst from the reaction products poses a problem. Some copper-catalyzed homogeneous methods require stoichiometric amounts of additional base that generates copious amounts of solid waste as a by-product. In general, few reports are available on heterogeneous cross-coupling reactions compared to the homogeneous reactions. Some of the heterogeneous methods have also been considered as atom economic [3].
3 Copper-Catalyzed Coupling Reactions in Organic Synthesis
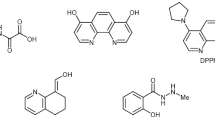
Homogeneous copper-catalyzed coupling reactions have been successfully applied in the natural product synthesis. An excellent up-to-date review on the application of copper-catalyzed methods in medicinal chemistry was recently published by Evano, Blanchard, and Toumi [4]. Some selected examples are listed in Fig. 1, wherein C–N bond formation was mediated by copper species.
Copper-catalyzed C–N bond formation was first applied in the total synthesis by Ma et al. [5] for the synthesis of benzolactam 1 (Fig. 1), a protein kinase C inhibitor, involving a coupling reaction between valine and aryl iodide. The compound structure 2 shown is an intermediate for the synthesis of lotrafiban, a potent glycoprotein IIb/IIIa receptor antagonist [6]. Copper-catalyzed methodology was successfully utilized for the synthesis of compound 3, a potential intermediate for the synthesis of tetrahydroquinoline alkaloid and martinellic acid [7]. Coupling of cyclic aliphatic amines with iodoarenes was reported for the synthesis of cyclopropanated iprodione, an analogous intermediate compound shown in 4 [8]. Compounds 5 and 6 are active intermediates for the synthesis of antibiotics, namely, linezolid and toloxatone [9], prepared by the coupling of carbamate with aryl bromides in the presence of copper and ligand. Furstner et al. have successfully employed the copper-catalyzed protocol for the synthesis of compound 7, which is an intermediate for the total synthesis of macrocyclic spermidine alkaloid isooncinotine [10]. Ghosh et al. developed a method for the synthesis of compound 8 on a large scale using a copper catalyst and a ligand from oxazolidinone [11]. Buchwald and Chae reported the total synthesis of U86192, a potent hypertensive agent via a step involving the copper catalyst for the synthesis of compound 9 using aryl bromide and N-aryl, N-Boc hydrazide [12]. A biologically active compound Nilotinib, (AMN107) useful for chronic myelogenous leukemia, shown in structure 10 was prepared by employing a copper-catalyzed protocol [13]. Compound 11 is an intermediate for the synthesis of the naphthalenoid H3 antagonist prepared in multi-gram scale from the corresponding bromo derivative and pyridazinone using a copper catalyst. This method was quite impressive since palladium-based catalytic systems failed to produce compound 11 [14].
4 CuI/l-Proline/[Bmim][BF4] Ionic Liquids with Aryl/Heteroaryl Bromides
Room temperature ionic liquids (RTILs) are a class of nonmolecular ionic solvents. The intrinsic properties of RTILs such as low vapor pressure, high polarity, solubility of metal salts, and reusability have made them more attractive candidates for solvents in the last two decades [15]. Bao et al. reported the use of RTILs in C–N bond forming reactions of aryl/heteroaryl bromides with imidazoles and benzimidazoles using a combination of CuI and l-proline at 105–115°C in the presence of K2CO3 to give the coupled products in good yields. They found the use of RTILs, for example, [Bmim]BF4 gave slightly improved yields over bench top organic solvents (Table 1).

The scope of the method was thoroughly explored using a variety of aryl bromides with imidazole, and the selected examples are listed in Table 2. In most cases, good yields of products were obtained, except in the case of o-substituted heterocycle bromides such as 2-bromo-4-chloro-3-methylbenzo[b]thiophene which is much more difficult to couple with imidazole (see Table 2, right column first entry), probably because the methyl group on the 3-position hinders the coplanarity of the imidazole and the thiophene moieties which gives a negative effect on product formation (19% yield). Prolonging the reaction time from 48 to 72 h did not substantially improve the yield. As can be seen from Table 2, aryl halides containing electron-withdrawing groups gave slightly lower yields compared to those containing electron-donating groups. This method is also applicable to the coupling of benzimidazole moieties with a variety of heterocyclic halides under the conditions mentioned in the Scheme of Table 3.
5 CuI/l-Proline/[Bmim]BF4 Ionic Liquids with Vinyl Halides
Owing to the usefulness of N-vinylimidazoles in organic synthesis as valuable intermediates, Bao and coworkers developed a method for the coupling of vinyl bromides with imidazoles [16] using a combination of reagents, l-proline, and potassium carbonate in ionic liquids in the presence of CuI catalyst (Scheme 1) at 110°C. They found that l-proline has a significant effect in cross-coupling reactions, for example, only 9% (entry 2 of Scheme 1) of isolated product was obtained in the absence of l-proline compared to 87% when l-proline is added (Scheme 1, entry 1). Several other ionic liquids were investigated, and [Bmim]BF4 was found to be the best solvent. Conventional organic solvents such as DMSO and DMF were incompatible for the formation of coupling products; only 25% of coupled product was isolated using CH3CN as solvent. It was also reported that Z-vinyl bromides were ineffective under these conditions due to their increased level of steric hindrance.
Product yields were in the range of 60–91% in the coupling of a variety of vinyl bromides with simple and substituted imidazoles and benzimidazoles (Scheme 2). Bao et al. have also reported the reusability of the copper/l-proline/[Bmim]BF4 system by adding additional base quantities for each successive cycle. The system was quite capable of performing at least four catalytic cycles using β-bromostyrene and imidazole, albeit with a mild decrease in yield.
6 CuO Nanoparticles
In 2007, Punniamurthy et al. [17] reported the use of commercially available CuO nanoparticles (Aldrich, particle size 33 nm and surface area 29 m2/g) for C–N coupling of halobenzene with simple aniline derivatives (Scheme 3). CuO nanoparticles were found to be the best performing copper source under the conditions given in Scheme 3. Under these reaction conditions, bromo- and chloroarenes gave moderate yields of the coupled products. The scope of the catalyst was well examined, for example, amines containing electron-donating groups showed greater reactivity compared to those possessing electron-withdrawing groups when coupled with iodobenzene (Scheme 4).
Aliphatic amines are known to be difficult substrates in C–N coupling reactions, however, using CuO nanoparticles, good to excellent yields of coupled products were obtained employing the reactions conditions shown in Scheme 5.
CuO nanoparticles were also found to be effective for the coupling of heterocyclic amines such as imidazole, 2-methylimidazole, and benzimidazole with iodobenzene using 2.5 mol% of catalyst, whereas 1.26 mol% of catalyst was sufficient for pyrrole and indole (Fig. 2).
The coupling of substituted iodobenzenes containing an electron-donating group such as 4-iodoanisole with aniline gave 22% yield using 1.26 mol% of catalyst. CuO nanoparticles were recycled three times efficiently (first cycle 95%, third cycle 91%), with a catalyst recoverability of 95% in the first cycle and 81% in the third cycle by simple centrifugation.
7 Cu2O Catalyst
In 2008, Xu et al. [18] reported the use of simple Cu2O (10 mol%) as an efficient reusable catalyst for the N-arylation of various heterocycles such as imidazole, benzimidazole, indole, pyrazole, and pyrrole with aryl iodides, whereas bromides and chlorides react with imidazole in the presence of two equivalents of a base, typically KOH. Other bases such as Cs2CO3, K3PO4, and K2CO3 resulted in moderate to poor yields. Significantly, poor conversion (ca. 24%) was observed under these reaction conditions when using CuO as catalyst.
The efficacy of Cu2O for general N-arylation of imidazole with diverse aryl halides was examined, and selected examples are listed in Table 4. Interestingly, imidazole can be selectively arylated in the presence of a free –NH2 group (Table 4, entry 1). Cyano and ester functionalized aryl iodides (Table 4, entry 4 and 5) equally participated in the coupling reaction by simply replacing KOH with Cs2CO3. Other imidazoles such as benzimidazole, indole, pyrazole, and pyrrole were successfully coupled with iodobenzene to give the corresponding N-arylated products in good yields employing 10 mol% of Cu2O. The catalyst was reused for four times with consistent activity for the coupling of iodobenzene with imidazole at 120°C. In the first cycle 90% yield was obtained while in the fourth cycle 88% yield was obtained.
8 Copper-Exchanged Fluorapatite
Choudary et al. have chosen [19] a weakly amphoteric apatite as support, since various kinds of cations and anions can be readily introduced into their framework due to their large ion exchange ability. Such exchanged apatites are already in use for several organic transformations [20]. A schematic representation of copper-exchanged fluorapatite (1) by incorporating the basic species F− in apatite in situ by co-precipitation and subsequent exchange with Cu (II) is shown (Scheme 6). N-Arylation of imidazoles and other heterocycles with chloroarenes and fluoroarenes to afford good to excellent yields of aryl heterocycles by using CuFAP catalyst is described.
The method is general and amenable to the N-arylation of imidazole with a wide range of chloro- and fluoroarenes using catalyst 1 (Table 5). As illustrated in Table 5, chloroarenes (electron withdrawing), 2-chloropyridine, and 2-chloropyrimidine provided excellent yields in shorter reaction times than chlorobenzene and chloroarenes (electron donating) (entries 1–11). Cyano, nitro, and trifluoromethyl groups are well tolerated (entries 1, 3, and 4). The N-arylation results of deactivated chloroarenes using K2CO3 as a base are quite impressive over the unreactive system using nanocopper and Cs2CO3 base [21]. Another significant feature is that under similar conditions, the N-arylation of imidazole with chlorobenzene afforded moderate yields, which could be further improved by the addition of KOtBu (entry 9). N-Arylation of heterocycles developed here using the less expensive chloroarenes is more attractive than the methods using the expensive bromo- and iodoarenes in terms of economic feasibility. The catalyst is recycled four times with a slight decrease in activity (entry 1). Interestingly, fluoroarenes composed of several o- or p-electron-withdrawing (EW) groups (entries 12–15) are also coupled with imidazole to afford the corresponding N-arylated products in excellent yields. Faster reactivity over the chloroarenes (entries 1, 5, 13 and 14) and selective coupling involving C–F activation only in chlorofluoroarene (entry 15) are reported.

As shown in Table 6, benzimidazole, pyrrole, pyrazole, and piperidine are also coupled with 1-chloro-4-nitrobenzene and 1-fluoro-4-nitrobenzene to give the corresponding N-arylated products in excellent yields.

To understand the mechanism of the N-arylation of imidazole, a series of experiments were conducted. The reaction of catalyst (1) with imidazole gives a deep blue Cu-imidazole complex (3), which is considered to be the first step of the catalytic cycle as described in Scheme 7, since there was no reaction between the catalyst and chloro- or fluorobenzaldehyde. The XPS of N 1s (3) lines appear at 401.8 and 399.6 eV, which are assigned to the Cu–N and C=N bonds, respectively [22]. The FTIR of 3 shows a P-OH vibration at 867 cm−1 which is in agreement with the similar hydride(enolato)ruthenium(II)apatite complex reported earlier [23]. Subsequent reaction of complex 3 with chloro- or fluorobenzaldehyde afforded the final product N-arylimidazole instantly, leaving the [Cu(II)] catalyst in normal conditions and Cu(I) in nitrogen atmosphere. The FTIR spectrum of the used catalyst 1, obtained in normal conditions, shows the disappearance of the P-OH stretching indicating the regeneration of 1 to initiate another cycle. Although we are unable to isolate any intermediate complexes, the identification of Cu(I) complex in a nitrogen atmosphere presumes copper assisted nucleophilic displacement of X− of the arene by N−-Het providing the coupled product via transient 4. In normal conditions, the formed Cu(I) may be reoxidized to 1. Similarly, in a reaction with the chloroarene, the deep blue complex 3 obtained from 2 also provides the coupling product. The deep blue complex formed on the treatment of a simple copper hydroxyapatite with imidazole is inert in the coupling reaction with the chlorobenzaldehyde. The above results and the identification of intermediates provide a better understanding of the mechanism and the necessity of strong basic sites in the apatite for the transformation of 3 to give the coupled product.
Possible mechanism for the N-arylation of heterocycles. Reproduced with permission from [19]. American Chemical Society
The same catalyst showed good activity for the N-arylation of bromo- and iodoarenes using K2CO3 as base (Table 7) [24]. To identify the best system for N-arylation of imidazole with bromobenzene, a variety of bases were screened and it was found that the CuFAP catalyst with K2CO3 (2 eq) afforded good yields (90%) in DMSO at 110°C. A control reaction conducted under identical conditions devoid of CuFAP gave no coupled product. CuFAP was recovered quantitatively by simple filtration and reused, which gave consistent activity even after the fourth cycle (Table 7, entry 5). Moreover the absence of copper in the filtrate was confirmed by AAS, which reiterates that no leaching of copper occurred during the reaction and provides evidence for heterogeneity throughout the reaction.
The generality of the CuFAP promoted N-arylation of imidazole with K2CO3 as base is shown in Table 7. 4-Bromopyridine, 2-bromopyrimidine, and bromobenzenes with electron-withdrawing groups, such as 4-nitrobromobenzene and 4-bromobenzaldehyde, gave excellent yields of coupled products in short reaction times (Table 7, entries 8–11) compared with bromobenzene. Bromobenzenes with electron-donating groups such as 4-bromotoluene and 4-bromoanisole required longer reaction times and gave moderate yields (Table 7, entries 6 and 7). Among the iodoarenes tested, iodobenzenes with electron-withdrawing groups (Table 7, entry 4) underwent smooth reaction with excellent yields compared to iodobenzenes with electron-donating groups (Table 7, entries 2 and 3). As expected, iodobenzene provided good yields in a shorter time than bromobenzene (Table 7, entry 1).
In order to expand the scope of the reaction, a variety of other nitrogen-containing heterocycles such as benzimidazole, pyrrole, pyrazole, and piperidine were successfully coupled with bromobenzene to give the corresponding N-arylated products in good yields. Among these heterocycles, piperidine and pyrrole gave the corresponding N-arylated products in good yields in short periods (Table 8, entries 3 and 4).
9 CuI Nanoparticles
In 2009, Sreedhar et al. reported [25] the use of CuI nanoparticles for the coupling of aryl bromides and chlorides with various azoles in the absence of both ligands and strong bases in DMF solvent. Specifically, this method uses inexpensive aryl chlorides [26].
Aryl chlorides possessing electron-donating (ED) or electron-withdrawing (EW) groups are coupled using CuI nanoparticles with imidazole, and the results are shown in Scheme 8. Aryl chlorides with ED group show poor activity (entry 2) compared to EW groups.
Various other substituted azoles such as pyrazoles, benzimidazoles, and primary and secondary aliphatic cyclic amines were also successfully coupled with chlorobenzenes to afford N-arylated products in fair yields (Scheme 9, entries 1–10).
Impressively, many azoles such as pyrazoles, pyrrole, and benzimidazoles reacted equally well with substituted chlorobenzenes to yield the corresponding N-arylated products in good yields (Scheme 9, entries 1–4). 1H-benzimidazole seemed to be a little sluggish to react with aryl chloride compared to imidazole (Scheme 9, entry 2). The authors have observed that air is necessary in the coupling process, which involves oxidative addition followed by reductive elimination; however, more data is required to propose a reaction pathway.
10 Cu(II)-NaY Zeolite
Zeolites are very useful catalysts for a large variety of reactions, from acid to base and redox catalysis [27]. Hutchings et al. reported that bis(oxazoline)-modified Cu(II)-HY catalysts are effective for asymmetric carbonyl- and imino-ene reactions and aziridination of styrene [28, 29]. Recently Djakovitch and Kohler [30–34] found that Pd(II)-NaY zeolite activates aryl halides towards Heck olefination, α-arylation of malonate, and amination reactions. It is well known that alkali-exchanged faujasite zeolites are solid base catalysts [35]. Owing to the usefulness of zeolites in organic chemistry, and our interest, we recently reported the use of modified alkali-exchanged zeolite Y, NaY zeolite [36] with electron rich copper catalyst in the N-arylation of nitrogen heterocycles with aryl halides to afford N-arylheterocycles in excellent yields under mild conditions without the use of any additive.
Catalyst preparation: Cu(II)-NaY zeolite was prepared by ion exchange of zeolite NaY (10 g) with a solution of copper(II) acetate (2.86 g, 15.75 mmol in deionized water 150 mL) for 24 h at room temperature. The material was recovered by filtration, dried (110°C), and calcined (550°C) in a flow of air. Atomic absorption spectroscopy analysis showed that the zeolite contained 6.84 wt% of Cu.
As can be seen from Table 9, N-arylation of imidazole with chloroarenes containing electron-withdrawing groups such as nitro-, cyano-, and trifluoromethyl- gave quantitative yields (entries 1–3) in shorter reaction times when compared with 4-chloroacetophenone (entry 4). Bromoarenes containing electron-donating groups afforded the corresponding N-arylated products in high yields after 36 h (entries 5–8). The reaction with iodoarenes resulted in complete conversion in shorter reaction times (entries 9–11). Reaction of 1-chloro-4-iodobenzene with imidazole gave selectively N-(4-chlorophenyl)imidazole (entry 12). The catalyst was used for four cycles successfully with minimal loss of activity (Table 9, entry 7).
After completion of the reaction, the catalyst was separated by simple filtration and washed with DMF and then with acetone and dried in an oven. AAS results of the used Cu(II)-NaY catalyst indicate leaching of 1.8% of copper in the N-arylation reaction of imidazole with 4-bromotoluene after the first cycle and 6.2% leaching after the fourth cycle. When a fresh reaction was conducted with the filtrate obtained at the end of the N-arylation reaction, no product formation was observed.
The application of this catalytic system for the coupling of nitrogen-containing heterocycles with 4-bromo- and 4-iodotoluene was also explored and the desired N-arylated products were obtained in excellent yields (Table 10). The reaction of 4-chlorotoluene with nitrogen heterocycles provided the products in only trace amounts even after 48 h.
11 Nanocrystalline Copper(II) Oxide
Nanocrystalline metal oxides find excellent applications as active adsorbents for gases, the destruction of hazardous chemicals [37, 38], and catalysts for various organic transformations [39–43]. Recently, we reported the use of nano-CuO as a catalyst for C–N bond forming reactions [44]. Nano-CuO samples were obtained from NanoScale Materials, Inc., having a surface area of 136 m2/g and crystallite size of 7–9 nm.
As illustrated in Table 11, the catalytic system (10 mol% of nano-CuO, K2CO3, DMF at 120°C) proved to be highly efficient with other activated chloroarenes. When o-, m-, and p-nitrochlorobenzenes were used, the corresponding N-arylated products were obtained in excellent yields (Table 11, entries 1, 2 and 3). Cyano, nitro, and aldehyde groups are well tolerated (Table 11, entries 1, 4, and 6).
2-Chloropyridine, 4-chloropyridine, and 2-chloropyrimidine also provided excellent yields as can be seen from Table 11 (entries 7, 8, and 10). The sterically bulky 4-chlorobenzophenone was converted to the corresponding N-arylated product (Table 11, entry 9) with excellent yields. This catalytic system is completely inactive in the case of deactivated chloroarenes such as chlorobenzene (Table 11, entry 12). The activity of nano-CuO is lower than the Cu(II) fluoroapatite catalyst previously reported by us [19] and comparable with Cu2O-coated Cu nanoparticles reported by Hyeon [21].
Particularly noteworthy is that fluoroarenes composed of o- or p-electron-withdrawing groups are also coupled with imidazole to afford the corresponding N-arylated products in excellent yields (Table 12). Similar behavior has been observed for greater reactivity of C–F over C–Cl bonds for fluoroarenes in the Suzuki reaction [45].
Chloro-4-nitrobenzene, 4-chlorobenzonitrile, 2-chlorobenzonitrile, 2-chloropyrimidine, and 2-chloropyridine are coupled with indole to give the corresponding N-arylated products in good to excellent yields (Table 13, entries 1, 2, 3, 4, and 5). The N-arylated product was obtained in 20% yield by coupling indole with 4-chloroacetophenone using nano-CuO catalyst, while a complex mixture was obtained in the case of Cu2O-coated Cu nanoparticles [21].
As can be seen from Table 14, other nitrogen-containing heterocycles like benzimidazole, pyrrole, and pyrazole gave the corresponding N-arylated products with 1-chloro-4-nitrobenzene and 1-fluoro-4-nitrobenzene in excellent yields.

The nano-CuO catalyst was recovered by centrifugation and reused for several cycles without any significant loss of activity. Transmission electron microscopy (TEM) studies of both fresh and used catalysts were carried out to determine the shape and size of the particles. Interestingly, it is observed that the shape and size of the particles remain unchanged. This confirms that the morphology of the catalyst remains same even after recycling.
12 Cu2O-Coated Cu Nanoparticles
Hyeon [21] synthesized uniform Cu2O-coated Cu nanoparticles from the thermal decomposition of copper acetylacetone followed by air oxidation and used these nanoparticles as catalysts for Ullmann-type amination coupling reaction of aryl chlorides.
Ullmann-type amination reaction of imidazole with various aryl chlorides having electron-withdrawing groups were conducted using the Cu2O coated copper nanoparticles as catalysts, the reactions proceeded very well (Table 15, entries 1–8). The high catalytic activity of the Cu2O-coated Cu nanoparticles seems to result from the high surface area derived from the nanoparticles. In the case of un-activated aryl chlorides such as chlorobenzene and 4-methoxychlorobenzene, there is no reaction (Table 15, entries 9 and 10). Various amines were screened to obtain the coupled products with 4-chloroacetophenone (Table 16). In the case of benzimidazole, pyrazole, and pyrrole, the yield of the product is good to moderate.
13 Resin-Supported Sulfonato-Cu-(salen) Complex
Salen ligands are recognized as efficient auxiliaries, and many metallosalen complexes have been found to serve as excellent catalysts for various organic transformations [46]. Styring et al. have reported the Suzuki–Miyaura cross-coupling reaction by using a polymer-supported salen-type palladium complex [47, 48]. Taillefer et al. examined the efficiency of salen ligands in the N-arylation of imidazole with bromobenzene in moderate yields [49, 50]. We synthesized a resin-supported sulfonato-Cu(salen) catalyst 1 (Scheme 10) from 2 (Scheme 11) [51] and tested for the N-arylation of heterocycles with chloro- and fluoroarenes in the presence of an inexpensive and mild base K2CO3.
After optimizing the reaction conditions (see foot note a of Table 17), the reaction scope was extended with various aryl halides and azoles. A variety of substituted chloro- and fluoroarenes possessing a wide range of functional groups are examined. As illustrated in Table 17, when o- and p-chloronitrobenzenes were used, the corresponding N-arylated products were obtained in excellent yields (entries 1 and 2). Cyano, nitro, and aldehyde groups are well tolerated. 2-Chloropyridine and 2-chloropyrimidine also provided excellent yields, as can be seen from Table 17 (entries 7 and 9). The sterically bulky 4-chlorobenzophenone was converted to the corresponding N-arylated product (entry 8) with excellent yields. This catalytic system is completely inactive in the case of un-activated chloroarenes such as chlorobenzene.
Particularly, noteworthy is that fluoroarenes containing o- or p-electron-withdrawing groups are also coupled with imidazole to afford the corresponding N-arylated products in excellent yields (Table 18).
As can be seen from Table 19, other nitrogen-containing heterocycles like pyrrole, pyrazole, indole, and piperidine gave the corresponding N-arylated products with 1-chloro-4-nitrobenzene and 1-fluoro-4-nitrobenzene in excellent yields. Lower reaction rates but comparable yields were observed with resin supported 1 compared to its homogeneous counterpart. The resin supported 1 can be recovered by simple filtration and reused for three cycles with consistent activity.
14 Silica Immobilized Copper Complexes
Silica modified with different functionalities such as -NH2, -SH, diamines, and amino acids is reported to be a good support for various metals like Pd, Cu, Sc, Ru, Pt, and V for different organic transformations [52]. Recently, Likhar et al. [52] reported the N-arylation of N(H)-heterocycles and benzylamines with aryl halides and arylboronic acids using silica immobilized copper complexes (Scheme 12).
The FTIR spectrum of chemically modified silica (imine) shows a peak due to the C=N bond around 1,640 cm−1, which on complexation with copper shifts to a lower value. The lowering in frequencies of the C=N peak is indicative of the formation of the metal–ligand bond. The difference in the values of the C=N stretching band before and after complexation for all the catalysts is shown in Table 20.
XPS analysis of the catalysts shows that in catalysts cat1-cat5, two intense peaks appear at 933–935 eV and 952–954 eV. These peaks are attributable to the Cu 2p3/2 and Cu 2p1/2 levels of Cu0-δ+ species, which may be due to the formation of the mono/bi/multidentate copper complexes with nitrogen ligands [53, 54]. A simplified catalyst structure is shown in Scheme 12. The copper content of the catalysts were estimated by using ICP-AES, and the results are shown in Table 20.
To find the best catalyst for the N-arylation reaction, all the catalysts (5 mol% Cu) were screened for the reaction of iodobenzene with imidazole using Cs2CO3 at 100°C under nitrogen atmosphere in toluene, and the results are summarized in Table 21. From Table 21, it can be seen that the catalysts derived from pyridine-2-carboxaldehyde (Cat2) and 2-bipyridyl ketone (Cat5) were equally good; Cat3 showed moderate activities whereas Cat1 and Cat4 gave poor yields. Interestingly, when neat Cu(OAc)2, was used there was almost no reaction (Table 21, entry 6).

To explore the scope and limitations of the current catalyst (Cat2), several haloarenes were used for the arylation of the imidazole under the optimized conditions, and the results are summarized in Table 22. It was observed that iodoarenes with an electron-withdrawing group (entries 3 and 4) reacted at a faster rate than iodoarenes with an electron-donating group (entry 2). Decreasing the amount of catalyst from 5 mol% to 2 mol% gave good yields only after 24 h (entry1). Interestingly, heterogeneous Cat2 afforded a comparable yield in shorter duration when compared with the Buchwalds CuOTf-1,10-phenanthroline system (entry 3) [55]. To expand the scope of the catalyst, a variety of other nitrogen-containing heterocycles such as benzimidazole, pyrrole, and pyrazole were successfully coupled with iodoarenes to give the corresponding N-arylated products in good yields. Next, benzylamine was reacted with iodobenzene and a good yield was obtained after 14 h. Benzylamine with electron-donating moieties was found to be more active than simple benzylamine (entry 9). When dibenzylamine was employed in the reaction, no product formation was observed (entry 10). This may be due to the steric hindrance of the benzylamine. Bromoarenes were found to be unreactive but an increase in temperature from 100°C to 135°C and a change in solvent from toluene to xylene gave excellent yield of product for bromoarenes with an electron-withdrawing group (entries 13 and 14). Bromobenzene gave a satisfactory yield after 24 h (entry 12), whereas bromoarenes with an electron-donating group gave low yield (entry 15).

Catalyst recyclability: For any heterogeneous system, it is important to know the ease of catalyst separation and possible reuse. The catalyst (Cat2) can easily be separated by filtration. The recovered catalyst after washing with acetone followed by drying at 60°C was used in the next run and minimal decrease in activity was observed (first cycle: 92%, fifth cycle: 86%) (Fig. 3). This decrease in activity may be due to the leaching or deactivation of the metal center. To see whether the reaction was occurring mainly due to the leached metal or the supported catalyst, a reaction between imidazole and iodobenzene was terminated after a small conversion (45 min, 18% conversion), the catalyst was filtered off by hot filtration, and the reaction was continued with the filtrate for 10 h. Almost no change in the conversion of iodobenzene was observed. These studies and the non-activity of Cu(OAc)2 in N-arylation of imidazole clearly prove that the reaction occurred heterogeneously. Lipshutz et al. [56] also reported that significant amount of supported metal can be dissolved during the reaction and would redeposit quickly on the solid during the filtration as a result of unavoidable minor temperature differences during the filtration. It also may be assumed in this case that during filtration, the solid matrix (silica) traps the copper species leached from the catalyst during the reaction.
Reusability study of Cat2 for the N-arylation of imidazole with iodobenzene. Reproduced with permission from [52]. Copyright 2007, Elsevier
15 Bio-degradable Cellulose-Supported Copper(0) Catalyst
Cellulose is a natural biopolymer, which is biodegradable, environmentally safe, widely abundant, inexpensive, and easy to handle [57]. Cellulose and its derivatives are widely used in chemical and bio-chemical applications and also as supports for the synthesis of organic molecules [58]. Interestingly, the cellulose fibers also act as a nanoreactor for the stabilization of metal nanoparticles [59]. However, its use as a support for catalytic applications is not well explored. Recently, Choplin and coworkers reported cellulose as the support for water soluble Pd(OAc)2/5 TPPTS system in the Trost–Tsuji allylic alkylation reaction [60]. To corroborate the above concept in the cross coupling of aryl halides and boronic acids, we reported N-arylation of imidazoles with aryl halides using a cellulose-supported Cu(0) catalyst (CELL-Cu(0) [61] . The prepared catalyst was well characterized using various instrumental techniques. For example, the X-ray diffraction pattern of CELL-Cu(0) catalyst clearly indicates the presence of Cu (111) and Cu (200) phases which are attributed to Cu(0) [46]. Further, the high resolution XPS narrow scan spectrum of the fresh CELL-Cu(0) catalyst shows a Cu 2p3/2 peak at 932.72 ev, which is attributed to Cu (0) [22].
The scope of the cellulose-supported copper catalyst has been examined in the case of aryl halides, and the results are summarized in Table 23. A very good yield (89%) was observed for the reaction of imidazole with 4-iodoanisole (Table 23, entry 2). On the other hand, 2-iodoanisole gave lower yields, which may be because of steric factors (Table 23, entry 3). In the case of bromoderivatives, 4-bromoacetophenone and bromobenzene afforded 70% of the coupled product (Table 23, entries 4 and 5). Further, the catalyst was screened for chloroderivatives (Table 23, entries 6–8) for the N-arylation of imidazole. Among them p-nitrochlorobenzene with a strong electron-withdrawing group resulted in quantitative yield (Table 23, entry 6), whereas 4-chloroacetophenone only gave low yield (Table 23, entry 7). N-Arylation of imidazole with chlorobenzene gave no coupled product under the above conditions. However, an increase of catalyst loading (0.1 g) with a longer reaction time provided 20% yield of the coupled product (Table 23, entry 8). The catalytic couplings of benzimidazole and indole resulted in good yields of products (Table 23, entries 9–11).
Reusability of the catalyst: The catalyst was recovered by simple filtration and washed with acetone and oven dried. The recovered catalyst was reused for N-arylation of imidazole and aryl halide (Table 23, entries 1, 2 and 6). These results indicate that the CELL-Cu(0) catalyst can be used for several cycles successfully with minimal loss of activity. The high resolution XPS narrow scan spectrum of the used CELL-Cu(0) catalyst shows two peaks at 932.72 and 935.25 for Cu 2p3/2, which are attributed to Cu(0) and Cu(II), respectively. ICP-AES results of the used CELL-Cu(0) catalyst indicate leaching of 0.8% of copper in the N-arylation reaction of imidazole with iodobenzene after the first cycle and 3.5% after the fourth cycle.
16 Polyaniline-Cu Catalyst
Polyaniline (PANI) is one of the most widely studied conducting polymers for electronic and optical applications. It is also receiving considerable attention in modern organic synthesis as a support and a promoter for metal-catalyzed organic transformations due to its easy preparative protocol from inexpensive starting material, high environmental stability, easy acid–base doping–dedoping, and redox properties [62]. Recently, Choudary et al. have reported polyaniline-supported Os-, Sc-, In-, and Pd-catalyzed organic transformations [63]. Later, we reported [64] the preparation, characterization, and catalytic properties of polyaniline-supported cuprous iodide (Scheme 13) in the N-arylation of N(H)-heterocycles and benzylamines with aryl halides and arylboronic acids.
PANI-Cu was prepared by stirring CuI and polyaniline in acetonitrile for 24 h followed by filtration. The prepared PANI-Cu was fully characterized using FTIR, XPS, ICP-AES, SEM, and EDAX. The most important bands in the FTIR spectrum of PANI are located at 1,584, 1,494, 1,376, 1,308, 1,163, and 830 cm−1. They are attributed to the stretching vibrations of quinoid (υC=N + υC=C), benzenoid (υC=C) units of the polymer, deformations of the C–N bond, stretching vibrations of the C–N bond, in-plane deformations of CH bonds present in the aromatic rings of the undoped polymer, and the out-of-plane deformations of CH bonds in the 1,4-substituted aromatic ring, respectively [65]. Upon incorporation of CuI into PANI, no appreciable shifts in the quinoid or benzenoid ring bands positions have been observed. XPS analysis of PANI-Cu showed a Cu 2p3/2 line at 932.4 eV and Cu 2p1/2 line at 952 eV, which confirmed the oxidation state of copper in PANI-Cu to be +I [22]. The PANI catalyst was analyzed by XPS for the N 1s, and it showed three types of N, namely, -N (398 eV, proportion 45%), -NH− (399.4 eV, proportion 45%), and -N+ (402 eV, proportion 10%). Similarly, PANI-Cu showed the presence of -N (397.7 eV, proportion 13%), -NH− (400 eV, proportion 65%), and -N+ (401.9 eV, proportion 22%). The decrease of imine nitrogen indicates that more of the copper was bound with the PANI via imine nitrogen than the amine nitrogen.
To explore the scope and limitations of the current catalytic protocol for N-arylation, several haloarenes and nitrogen-containing heterocycles were allowed to react under the optimized conditions, and the results are summarized in Table 24. It was observed that iodoarenes with electron-withdrawing groups (Table 24, entries 4 and 5) reacted at a faster rate than iodoarenes with electron-donating groups (Table 24, entries 2 and 3). Sterically hindered 2-iodotoluene took a longer time to afford a good yield (Table 24, entry 6). Benzylamine with electron-donating moieties was found to be more active than the simple benzylamine (Table 24, entries 11 and 12). When dibenzylamine was employed, no product formation was observed (Table 24, entry 13). This is may be due to steric hindrance of the benzylamine. Bromoarenes react at 100°C in DMF to give good yields of products (Table 24, entries 16–22). The recovered catalyst, after washing with acetone followed by drying at 80°C, was used in the next run. Almost consistent activity was observed over five cycles in the reaction of iodobenzene with imidazole (Table 24, entry 1). The difference between the copper content of the fresh catalyst and the used catalyst (fifth cycle) is only 2.4%.
17 Copper–Aluminum Hydrotalcite
Hydrotalcites are a class of layered materials of current interest. They are represented by a general formula [M(II)1−x M(III)x (OH)2]n− An− x/n.y H2O, where M(II) and M(III) are divalent and trivalent cations such as Cu2+, Mg2+, and Al3+, respectively. The compensating anions may be OH−, Cl−, NO3 −, CO3 2−, etc. (see for a recent review [66]). Copper-containing LDHs act as the best heterogeneous catalysts for the production of substituted amines via Ullmann-type cross-coupling reactions of unreactive aryl chlorides with aryl and aliphatic amines under ligand-free conditions [67]. This method tolerates a variety of functional groups and does not require any additive (Schemes 14 and 15).
Chlorobenzenes with electron-withdrawing groups substituted at para and ortho provided moderate to good yields (Scheme 14, entries 1–7). In the case of 2,4-dichlorobenzaldehyde, the chloro group at the ortho position was N-arylated (Scheme 14, entry 4). The C–N coupling product bearing an aldehyde group is oxidized to an acid (Scheme 14, entry 3) while in the case of 2,4-dichlorobenzaldehyde, no oxidation of the aldehyde group occurred (Scheme 14, entry 3 vs. 4). Electron-neutral and electron-donating chloroarenes such as chlorobenzene and 4-chloroanisole (Scheme 14, entries 8 and 9) did not form any coupled products under these conditions.
This protocol was successfully applied to cycloalkylamines (Scheme 15) using the amine as self-solvent, with good isolated yields and negligible amount of Cu leaching. Cu/Al-HT was recovered quantitatively by simple filtration and reused. Consistent activity is observed even after the fourth cycle (91% in the first cycle and 90% in the fourth cycle) (Scheme 15, entry 1) for the coupling reaction between o-nitrochlorobenzene and cyclohexylamine. A similar observation was made when a C–N coupling product bearing an aldehyde in the para-position was oxidized to an acid, while in the case of 2,4-dichlorobenzaldehyde, no oxidation of the aldehyde group occurred (Scheme 15, entry 3 vs. 6).
In 2009, Sreedhar et al. [68] expanded the scope of amination with various amines and aryl halides using Cu/Al-HT. In this study, the authors had chosen a coupling reaction between octylamine and 2-nitro-chlorobenzene (Scheme 16) with a catalytic amount of Cu catalyst under ligand-free conditions. From Table 25, it can be seen that Cu/Al-HTB is the best catalyst among the catalysts screened (Table 25, entry 6). The catalysts screened were (Cu-(HAP) copper hydroxyapatite, Cu-(FAP) copper flouroapatite, and Cu/Al-HTs (HT, Hydrotalcite of Cu/Al in different ratios) Cu/Al-HTA, 3:1(Cu: Al); Cu/Al-HTB, 2.5:1; and Cu/Al-HTC, 2:1)

The scope of the methodology was examined on many aryl chlorides using the Cu/Al-HTB as catalyst. Selected examples are listed in Table 26. Although this method works for variety of amines, some of the aryl chlorides, for example chlorobenzene and 2-methyl chlorobenzene, did not participate in the coupling of cyclopentyl and octylamine under the same reaction conditions. As can be seen, aryl chlorides with electron-donating and electron-withdrawing groups coupled with equal ease in many cases.

Using the same catalyst system, Cu/Al-HTB, the scope was extended in the case of heterocyclic amines such as imidazole, benzimidazole, pyrazole, and morpholine with aryl chlorides, bromides, and iodides in good to excellent isolated yields. Selected examples are listed in Table 27. However, some of the chlorides either participated sluggishly or were unreactive towards coupling reactions under the same reaction conditions. Many examples of the methodology were studied for coupling partners on both sides.

18 C–N Bond Forming Cross-Coupling Reactions Using Arylboronic Acids
Significant improvement in copper-catalyzed N-arylation is realized after the introduction of arylboronic acids as the aryl donors independently by Lam et al. [69, 70], Chan et al. [71], and Evans et al. [72] utilizing stoichiometric amount of Cu(OAc)2.
Combs et al. [73] reported the first examples of polymer-supported aryl-heteroaryl C–N cross-coupling reactions and dramatically decreased reaction times upon microwave irradiation. The methodology provides easy access to N-arylated heterocycles from heterocycles bearing an N–H bond and readily available arylboronic acids in the presence of copper(II) acetate and pyridine.
Later Combs et al. [74] reported a general and mild method for the N-arylation of sulfonamides on solid supports. Copper acetate, triethylamine-mediated coupling of arylboronic acids at room temperature to solid-supported sulfonamides gave good to excellent yields of the desired N-arylsulfonamides. Sulfonamide bond cleavage of the o, p-dinitrobenzene(N-aryl) sulfonamide provides a route to N-arylated secondary amine products.
N-arylated primary and secondary aliphatic amines are important substituents in many biologically active compounds. The predominance of arylpiperidines and arylpiperazines in CNS drugs is particularly noteworthy [75–77].
Copper acetate, triethylamine-mediated CN cross-coupling reaction of arylboronic acids at room temperature to solid-supported primary and secondary amines gave good to excellent yields of the desired N-arylated products [78]. This method demonstrates the generality of this methodology for the solid-phase synthesis of combinatorial libraries (Scheme 17).
Chiang et al [79] reported the preparation of polymer-supported copper complex by immobilization of copper on to modified Wang resin. This catalyst is effective in cross-coupling reactions between N- or O-containing substrates and arylboronic acids. The copper catalyst is air stable and can be recycled with minimal loss of activity. A series of N-containing substrates and phenols were tested (Table 28), and the reactions were complete after 24 h, with yields varying between 48% and 93%. The reaction is not general for amides and only starting material was recovered. (entries 8 and 9). Both electron-withdrawing and –donating phenols worked well (entries 11–13) and the phenolic OH group can be arylated in the presence of an amide group (entry 14).
We have reported [80] the coupling of imides with various arylboronic acids using Cu–Al hydrotalcite in refluxing methanol with continuous bubbling of air through the mixture without employing base or ligand to afford N-arylated products in very good yields (Scheme 18, Table 29). Cu–Al hydrotalcite is used for four cycles successfully with minimal loss of activity.
Wang Lei et al. [81] reported the Ar–N coupling of arylboronic acids with imidazoles by using 3-(2-aminoethylamino)propyl functionalized silica gel immobilized copper(II) catalyst (10 mol%) in methanol without any additives and bases. The reactions generated the corresponding cross-coupling products in good yields. Furthermore, silica-supported copper can be recovered and recycled by a simple filtration procedure and used for five consecutive trials without decrease in activity.
We reported [82] the N-arylation of imidazoles and amines with arylboronic acids with copper-exchanged fluorapatite (Cu-FAP) in methanol at room temperature. The products N-arylimidazoles and N-arylamines were isolated in good to excellent yields. A variety of arylboronic acids were converted to the corresponding N-arylimidazoles and N-arylamines, demonstrating the versatility of the reaction.
In an effort to evolve a better catalytic system, various catalysts were screened for N-arylation of imidazole and phenylboronic acid in methanol at room temperature. The results are summarized in Table 30. Homogeneous Cu catalyst, Cu(OAc)2 and CuI gave very low yield (Table 30, entries 1–2). Copper-exchanged hydroxyapatite (CuHAP) also gave a very low yield (Table 30, entry 4), while CuFAP afforded a very good yield (Table 30, entry 5). Moreover, the controlled N-arylation reaction conducted under identical conditions devoid of CuFAP gave no coupled product despite prolonged reaction time (Table 30, entry 6). CuFAP was recovered quantitatively by simple filtration and was reused several times, with consistent activity even after the fourth cycle (Table 30, entry 5). The absence of copper in the filtrate was confirmed by Atomic Absorption Spectroscopy which confirms no leaching of copper during the reaction and provides evidence for heterogeneity throughout the reaction.

Our method was successfully amenable to a wide range of arylboronic acids, allowing preparation of N-arylimidazoles and N-arylbenzimidazoles in high yield and the results are shown in Table 31. Phenylboronic acids with an electron-donating group afforded better yields (Table 31, entries 2–4) than with electron-withdrawing groups (Table 31, entries 5–7). Similar observation was made when benzimidazoles were used in place of imidazoles to obtain the corresponding N-arylbenzimidazoles (Table 31, entries 8–10), but the reactions took longer time compared to imidazoles.
After achieving excellent results with imidazoles, we further applied this catalytic system for the N-arylation of aromatic amines and aliphatic amines. The results are shown in Table 32 and Table 33. Table 32 shows the results of N-arylation of aniline with several arylboronic acids.

It is clear from Table 32 that N-arylation proceeds very effectively and afforded the corresponding N-arylated products in good to excellent yields under very mild conditions. No spectacular electronic effects were observed in N-arylation of aniline; only a slight decrease in the reaction rate was noted with the 3-nitrophenylboronic acid. Next we examined the N-arylation of various primary amines such as aliphatic, cyclohexyl and heterocyclic amines with phenylboronic acid using CuFAP catalyst at room temperature, and the results are listed in Table 33. All the reactions proceeded very efficiently at room temperature and yielded the corresponding N-arylated products. It was interesting to note that the formation of the conceivable diarylated product is not observed in our conditions.
We [83] reported the N-arylation of nitrogen heterocycles with a variety of arylboronic acids to afford the corresponding coupled products in good to excellent yields without using external ligands or additives as promoters using cellulose-supported Cu(0) catalyst. The catalyst was recovered by simple filtration and reused for several cycles.
Preliminary experiments are carried out by taking phenylboronic acid as a test molecule for the N-arylation of imidazole (Scheme 19). In order to determine the best reaction medium, we tested different solvents and methanol is found to be the best solvent for the N-arylation of imidazole (98%). The nature of base has a pronounced effect in these reactions. Reaction of imidazole with phenylboronic acid in presence of K2CO3 and KOtBu gave no coupled product, while triethylamine and pyridine provided good yield. However, we continued the reactions in triethylamine instead of pyridine because of toxicity reasons.
The reaction temperature plays an important role, the reaction at room temperature afforded 80% of the coupled product after 18 h (87%, 24 h), while the duration of the reaction is drastically decreased to 2.5 h under reflux conditions to provide quantitative yields (Table 34, entry 1). After optimizing the reaction conditions, different arylboronic acids were coupled with imidazole using CELL-Cu(0) catalyst, triethylamine as base and methanol as solvent under refluxing conditions, and the results are summarized in Table 34. Various structurally and electronically diverse arylboronic acids gave the corresponding N-arylated products in high yields. However, methyl-, acetyl-, and methoxy-substituted boronic acids required longer reaction times (Table 34, entries 5, 6 and 8) compared to chloro-, fluoro-, and trifluoromethyl-substituted boronic acids (Table 34, entries 2, 3 and 4). o- and p-substituted arylboronic acids were equally effective for the coupling with imidazoles (Table 34, entries 7 and 8).
Later we reported [84] the N-arylation of imidazoles, imides, amines, amides, and sulfonamides with arylboronic acids using a recyclable Cu(OAc)2·H2O/[bmim][BF4] system in the absence of a base or additive to afford the corresponding N-arylated products in good to excellent yields.
Similarly, the cross-coupling reaction between imidazole and phenylboronic acid was performed with different ILs, and the results are grouped in Fig. 4. Among the ILs tested, hydrophilic [bmim][BF4] was found to be superior (95% yield) than that of hydrophobic [bmim][PF6] (80% yield), whereas the cross-coupling reaction was not successful in other molten salts such as n-tetrabutylammonium bromide (n-Bu4Br) and 1-n-butyl-3-methylimidazolium bromide, [bmim][Br], under similar reactions conditions. These results clearly indicate that both the cation and anion play a significant role in this cross-coupling reactions.
Under optimized reaction conditions, a wide range of structurally diverse arylboronic acids were coupled with imidazole (Table 35, entries 1–7) using Cu(OAc)2⋅H2O/[bmim][BF4] system to produce the corresponding substituted N-aryl imidazoles in good to excellent yields. Finally, upon completion of the reaction, the ionic liquid phase containing [bmim][BF4] and catalyst was almost quantitatively recovered by simple extraction of the product with Et2O. The recovered ionic liquid phase containing the catalyst was reused for several cycles with consistent activity (Table 35, entry 1).
In an endeavor to expand the scope of the above methodology, the catalytic system was applied to imides, amines, amides, and sulfonamides. A series of substituted arylboronic acids were coupled with phthalimide (Table 36, entries 1–6) and succinamide (Table 36, entries 7 and 8) under the generalized reaction conditions to afford the corresponding N-aryl imides in good to excellent yields.
Similarly, aniline was subjected to cross coupling with substituted arylboronic acids and o-, p-substituted anilines with phenylboronic acid, and the corresponding N-aryl amines were obtained in satisfactory yields (Table 37, entries 1–9). The coupling of alkylamines with phenylboronic acid gave the N-alkyl anilines in moderate yields (Table 37, entries 10–14). However, the reaction of amides and sulfonamides with phenylboronic acid afforded the corresponding products albeit in lower yields (Table 37, entries 15 and 16).
In general, the reactions are facile, clean for the synthesis of a variety of N-arylated products. Several functional groups such as Cl, CF3, F, CH3, COCH3, and OCH3 remain unaffected under the present reaction conditions.
We have used silica tethered copper complexes as reusable catalysts in the N-arylation of N(H)-heterocycles and benzylamines with arylboronic acids [85] (Table 38). N-arylated products were isolated in good to excellent yields, demonstrating the versatility of the protocol. Moreover the catalyst was easily recovered by simple filtration and reused for several cycles with consistent activity.
19 Recently Reported Copper Heterogeneous Systems
Recently Nageswar et al. reported that copper iodide along with trans-1,2-diaminocyclohexane as a ligand (reported by Buchwald in homogeneous conditions) acts in water, as a simple, efficient, cheap, and recyclable catalytic system (Scheme 20) [86].
N-heterocycles like indole, substituted indoles, pyrazole, imidazole, benzamide, morpholine, benzimidazole, thiobenzamide, aniline, benzylaniline, octylaniline, heptylaniline, and cyclohexylaniline couple with aryl iodides and bromides in the presence of K2CO3 as base and water as solvent to give the N-arylated products in good to excellent yields. For aryl iodides, the reaction proceeded at 80°C, whereas a higher reaction temperature of 95–100°C was needed to activate aryl bromides. Ortho-substituted aryl iodides afforded poor yields of the product owing to steric reasons.
This copper catalyst system along with the aqueous phase were recyclable up to four times without loss of catalytic activity, and FT-IR spectrum on the used catalyst did not indicate any copper oxide formation.
Chaudhari et al. reported encapsulation of copper complexes in zeolite-Y and MCM-41 or by tethering of copper complexes on various supports like zeolite-Y, silica, charcoal, or clay [87]. These materials were then characterized by a plethora of sophisticated analytical techniques like EPR, diffused reflectance UV–vis, XRD, IAS, ICPES, SEM, and TEM and employed for the amination of aryl iodide to synthesize diphenyl aniline or triphenyl amine (Scheme 21).
For encapsulated catalysts, amination reaction was carried out in a high pressure autoclave containing aniline, iodobenzene, catalyst, and potassium tert-butoxide (KOt-Bu) at 408 K for 14 h. For tethered catalysts, the amination reaction was carried out in a two necked flask under reflux at 385 K for 10–12 h.
The results of amination reaction as obtained by different copper catalysts are tabulated in Table 39.
Recyclability and stability studies were carried out for both encapsulated and tethered copper catalysts. For encapsulated Cu(Phen)–Y catalyst, the catalyst activity and selectivity remained constant even after five reaction cycles. Encapsulated Cu(Bipy)-MCM-41 lost its catalytic activity with every reuse indicating leaching of copper from the support. This is justified from the fact that wide openings of pore and channel diameter of MCM-41 (~35 A˚) results in some washing of copper complex under the reaction conditions. Thus Cu(Bipy)-MCM-41 loses its heterogeneity and performs as a homogeneous catalyst during the course of the reaction. Recyclability experiments on tethered Cu(Phen)(PPh3)Br-PTA-Y catalyst revealed that the catalyst was stable even after fifth recycle, and no leaching of copper or tungsten occurred. The catalyst was found to give a higher turnover number (TON) of 69 compared to its homogeneous counterparts (TON 27).
The authors also presented a plausible reaction pathway for the amination of aryl iodide catalyzed by zeolite-supported copper complex, wherein they experimentally demonstrated the formation of PhNHK type of salt as an intermediate.
The various types of immobilized copper complexes described in this work aim to eliminate the problems encountered in homogeneous amination of aryl halides. The catalysts displayed good activity and recyclability depending upon the nature of the support.
Islam et al. reported, in 2011, the synthesis of a heterogeneous polystyrene-supported Cu(II) catalyst (Cu-PAR) for the N-arylation of N–H heterocycles with aryl halides as well as arylboronic acids to afford the corresponding coupled products in good to excellent yields [88].
Cu-PAR catalyst was employed for the N-arylation of imidazole with various aryl halides under N2 atmosphere at 100°C using DMSO as the solvent and K2CO3 as the base (Scheme 22). Iodoarenes with electron-withdrawing groups reacted faster than that with electron-donating groups. Sterically hindered 2-iodotoluene afforded good yield of the corresponding of the N-arylated product albeit at longer reaction time (20 h). Bromoarenes as well as other N-containing cycles such as benzimidazole, pyrazole, and pyrrole also reacted well under these reaction conditions.
Cu-PAR catalytic system was also found to be applicable for N-arylation of N–H heterocycles with arylboronic acids (Scheme 23) under mild conditions in less time. Methanol was found to be the best solvent as other solvents gave trace amount of the coupled product under identical conditions. No organic co-solvent or additive or base was needed to promote the reaction. Arylboronic acids with electron-withdrawing groups reacted at a slower rate yielding moderate to good yield of the corresponding product. 3-nitrophenylboronic acid, a relatively tough substrate, was also found to be reactive under these reaction conditions. Cu-PAR catalytic system also worked well for imide, amide, phthalimide, and sulfonamide yielding decent yield of the N-arylated product.
The scope of this catalytic system was even extended for the amination of aromatic amines with boronic acids using DMSO as solvent and KOH as base at 120°C for 12 h (Scheme 24). The amination reaction proceeded smoothly and good to excellent yields of the products were recorded in all cases.
Cu-PAR is a very versatile catalyst and offers excellent stability, activity, and reusability for multiple reaction cycles.
Wang et al. in 2011 [89] developed a polystyrene immobilized phenanthroline–Cu(I) catalyst and applied it successfully for the synthesis of 2-aminobenzothiazoles from 2-halobenzeneamines and phenyl isothiocyanates (Scheme 25).
The optimum reaction conditions were found to be 2.5 mol% copper loading in the catalyst with DABCO as base and toluene as the solvent at 60°C for 8 h. The efficiency of the catalytic system was then tested by using 2-chloroaniline, 2-iodoaniline, and various substituted 2-bromoanilines possessing electron-withdrawing as well as electron-donating groups along with phenyl isocyanate. Even substituted phenyl isocyanates bearing methoxy, chloro, methyl, nitro, and trifluoromethyl groups were found to react appreciably with 2-bromoaniline as well as 2, 4-dibromoaniline. In all cases good to excellent yields (69–95%) of the corresponding 2-aminobenzothiazoles were obtained. Even a less active substrate, 2-bromo-5-trifluoromethylaniline, reacted surprisingly well with phenyl isothiocyanate to give a decent yield (71%) of the corresponding product. The catalytic system was even found to tolerate sterically hindered substrate like 2,4-dibromo-6-fluoroaniline to give an excellent yield (84%) of the corresponding 2-aminobenzothiazole product.
Recyclability studies of the catalyst using 2-iodoaniline and phenyl isothiocyanate as the model substrates demonstrated that the catalyst can be separated from the reaction mixture simply by filtration, washed dried, and reused for at least ten reaction cycles with almost no leaching of copper from the support (<0.20 ppm).
20 Summary and Outlook
In summary, we have gathered information from the recent published methodologies, wherein heterogeneous or reusable copper-catalyst systems were used for the C(aryl)–N bond formation employing aryl halides and arylboronic acids as coupling partners. Among the methods we discussed here for the arylation of aryl halides, most of the reactions were conducted at 100–160°C. To our knowledge no room-temperature reports have appeared under heterogeneous conditions.
The challenges and opportunities in copper-catalyzed heterogeneous catalysis are enormous as less research was carried out compared to homogeneous systems [4, 90]. The development of less expensive and robust new catalysts with higher activities and turnover numbers is desirable. It is also important to exploit these heterogeneous catalysts for the synthesis of biologically important molecules.
References
Yin LX, Liebscher J (2007) Chem Rev 107:133
Thomas AW, Ley SV (2009) In: Ackerman L (ed) Modern arylation methods, 4th edn. Wiley-VCH, Weinheim, p 121 (Chapter 4)
Sheldon RA (2008) Chem Commun 3352
Evano G, Blanchard N, Toumi M (2008) Chem Rev 108:3054
Ma D, Zhang Y, Yao J, Wu S, Tao F (1998) J Am Chem Soc 120:12459
Clement JB, Hayes JF, Sheldrake HM, Sheldrake PW, Wells AS (2001) Synlett 1423
Ma D, Xia C, Jiang J, Zhang J, Tang W (2003) J Org Chem 68:442
Brackman F, Es-Sayed M, de Meijere A (2005) Eur J Org Chem 2250
Mallesham B, Rajesh BM, Reddy PR, Srinivas D, Trehan S (2003) Org Lett 5:963
Scheiper B, Glorius F, Leitner A, Furstner A (2004) Proc Natl Acad Sci USA 101:11960
Ghosh A, Sieser JE, Caron S, Couturier M, Dupont-Gaudet K, Girardin M (2006) J Org Chem 71:1258
Chae J, Buchwald SL (2004) J Org Chem 69:3336
Huang WS, Shakespeare WC (2007) Synthesis 2121
Pu YM, Ku YY, Grieme T, Black LA, Bhatia AV, Cowart M (2007) Org Proc Res Dev 11:1004
Lv X, Wang Z, Bao W (2006) Tetrahedron 62:4756
Wang Z, Bao W, Jiang Y (2005) Chem Commun 2849
Rout L, Jammi S, Punniamurthy T (2007) Org Lett 9:3397
Huang YZ, Miao H, Zhang QH, Chen C, Xu J (2008) Catal Lett 122:344
Choudary BM, Sridhar CH, Lakshmi Kantam M, Venkanna GT, Sreedhar B (2005) J Am Chem Soc 127:9948
Mori K, Hara T, Mizugaki T, Ebitani K, Kaneda K (2004) J Am Chem Soc 126:10657, and cited therein
Son SU, Park IK, Park J, Hyeon T (2004) Chem Commun 778
Moulder JF, Stickle WF, Sobol PE, Bomden KD (1992) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp, Eden Prairie, MN
Mori K, Hara T, Mizugaki T, Ebitani K, Kaneda K (2003) J Am Chem Soc 125:11460
Lakshmi Kantam M, Venkanna GT, Sreedhar C, Kumar KBS (2006) Tetrahedron Lett 47:3897
Sreedhar B, Arundhathi R, Reddy PL, Lakshmi Kantam M (2009) J Org Chem 74:7951
Reetz MT, Lohmer G (1996) Chem Commun 1921
Corma A (2003) J Catal 216:298
Caplan NA, Hancock FE, Bulman Page PC, Hutchings GJ (2004) Angew Chem Int Ed Engl 43:1685
Gullick J, Taylor S, Ryan D, McMorn P, Coogan M, Bethell D, Bulman Page PC, Hancock FE, King F, Hutchings GJ (2003) Chem Commun 22:2808
Djakovitch L, Heise H, Köhler K (1999) J Organomet Chem 584:16
Djakovitch L, Köhler K (1999) J Mol Catal A Chem 142:275
Djakovitch L, Köhler K (2001) J Am Chem Soc 123:5990
Djakovitch L, Köhler K (2000) J Organomet Chem 606:101
Djakovitch L, Wagner M, Köhler K (1999) J Organomet Chem 592:225
Davis RJ (2003) J Catal 216:396
Lakshmi Kantam M, Rao BPC, Choudary BM, Reddy KS (2006) Synlett 2195
Jiang Y, Decker S, Mohs C, Klabunde KJ (1998) J Catal 180:24
Lucas E, Decker S, Khaleel A, Seitz A, Fultz S, Ponce A, Li W, Carnes C, Klabunde KJ (2001) Chem Eur J 7:2505
Schlogl R, Abd Hamid SB (2004) Angew Chem Int Ed 43:1628
Lakshmi Kantam M, Kumar KBS, Sridhar C (2005) Adv Synth Catal 347:1212
Lakshmi Kantam M, Laha S, Yadav J, Choudary BM, Sreedhar B (2006) Adv Synth Catal 348:867
Choudary BM, Lakshmi Kantam M, Ranganath KVS, Mahendar K, Sreedhar B (2004) J Am Chem Soc 126:3396
Choudary BM, Ranganath KVS, Pal U, Lakshmi Kantam M, Sreedhar B (2005) J Am Chem Soc 127:13167
Lakshmi Kantam M, Yadav J, Laha S, Sreedhar B, Jha S (2007) Adv Synth Catal 349:1938
Widdowson DA, Wilhelm R (2003) Chem Commun 578
Larrow JF, Jacobsen EN (2004) Asymmetric processes catalyzed by chiral (salen)metal complexes. Top Organomet Chem 6:123
Phan NTS, Brown DH, Styring P (2004) Tetrahedron Lett 45:7915
Phan NTS, Khan J, Styring P (2005) Tetrahedron 61:12065
Cristau HJ, Cellier PP, Spindler JF, Taillefer M (2004) Eur J Org Chem 695
Cristau HJ, Cellier PP, Spindler JF, Taillefer M (2004) Chem Eur J 10:5607
Lakshmi Kantam M, Ramani T, Chakrapani L (2008) Synth Commun 38:626
Likhar PR, Roy S, Roy M, Lakshmi Kantam M, De RL (2007) J Mol Catal A Chem 271:58, and cited therein
Jones CW, McKittrick MW, Nguyen JV, Yu K (2005) Top Catal 34:67
Cao W, Zhang H, Yuan Y (2003) Catal Lett 91:243
Kiyomori A, Marcoux JF, Buchwald SL (1999) Tetrahedron Lett 40:2657
Lipshutz BH, Tasler S (2003) J Org Chem 68:1190
Klemm D, Heublein B, Fink HP, Bohn A (2005) Angew Chem Int Ed 44:3358
Porcheddu A, Giacomelli G, Chighine A, Masala S (2004) Org Lett 6:4925
He J, Kunitake T, Watanabe T (2005) Chem Commun 795
Quignard F, Choplin A (2001) Chem Commun 21
Reddy KR, Kumar NS, Sreedhar B, Lakshmi Kantam M (2006) J Mol Catal A Chem 252:136
Velusamy S, Ahamed M, Punniyamurthy T (2004) Org Lett 6:4821
Choudary BM, Roy M, Roy S, Lakshmi Kantam M, Sreedhar B, Kumar KV (2006) Adv Synth Catal 348:1734
Lakshmi Kantam M, Roy M, Roy S, Sreedhar B, De RL (2008) Catal Commun 9:2226, and cited therein
Ping Z, Nauer GE, Neugebauer H, Theiner J, Neckel A (1997) J Chem Soc Faraday Trans 93:121
Figueras F, Lakshmi Kantam M, Choudary BM (2006) Curr Org Chem 10:1627
Likhar PR, Arundhati R, Lakshmi Kantam M (2007) Tetrahedron Lett 48:3911
Sreedhar B, Arundhathi R, Reddy PL, Reddy MA, Lakshmi Kantam M (2009) Synthesis 2517
Lam PYS, Clark CG, Saubern S, Adams J, Winters MP, Chan DMT, Combs A (1998) Tetrahedron Lett 39:2941
Lam PYS, Clark CG, Saubern S, Adams J, Averill KM, Chan DMT, Combs A (2000) Synlett 674
Chan DMT, Monaco KL, Wang RP, Winters MP (1998) Tetrahedron Lett 39:2933
Evans DA, Katz JL, West TR (1998) Tetrahedron Lett 39:2937
Combs AP, Saubern S, Rafalski M, Lam PYS (1999) Tetrahedron Lett 40:1623
Combs AP, Rafalski M (2000) J Comb Chem 1:29
Evrard DA, Harrison BL (1999) Recent approaches to novel antidepressant therapy. Annu Rep Med Chem 34:1
Robichaud AJ, Largent BL (2000) Recent advances in selective serotonin receptor modulation. Annu Rep Med Chem 35:11 (Chapter 2)
Schaus JM, Bymaster FP (1998) Dopaminergic approaches to antipsychotic agents. Annu Rep Med Chem 33:1
Combs AP, Tadesse S, Rafalski M, Haque TS, Lam PYS (2002) J Comb Chem 4:179
Chiang GCH, Olsson T (2004) Org Lett 6:3079
Kantam ML, Prakash BV, Redy CV (2005) J Mol Catal A Chem 241:162
Zhang LY, Wang L (2006) Chin J Chem 24:1605
Kantam ML, Venkanna GT, Sridhar C, Sreedhar B, Choudary BM (2006) J Org Chem 71:9522
Reddy KR, Kumar NS, Sreedhar B, Kantam ML (2006) J Mol Catal A Chem 252:136
Kantam ML, Neelima B, Reedy CH, Neeraja V (2006) J Mol Catal A Chem 249:201
Likhar PR, Roy S, Roy M, Kantam ML, De RL (2007) J Mol Catal A Chem 271:57
Swapna K, Murthy SN, Nageswar YVD (2010) Eur J Org Chem 6678
Patil NM, Gupte SP, Chaudhari RV (2010) Tetrahedron Lett 372:73
Islam M, Mondal S, Mondal P, Roy AS, Tuhina K, Mobarok M, Paul S, Salam N, Hossain D (2011) Catal Lett 141:1171
Yang J, Li P, Wang L (2011) Tetrahedron 67:5543
Monnier F, Taillefer M (2009) Angew Chem Int Ed 48:6954
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kantam, M.L., Reddy, C.V., Srinivas, P., Bhargava, S. (2013). Recent Developments in Recyclable Copper Catalyst Systems for C–N Bond Forming Cross-Coupling Reactions Using Aryl Halides and Arylboronic Acids. In: Taillefer, M., Ma, D. (eds) Amination and Formation of sp2 C-N Bonds. Topics in Organometallic Chemistry, vol 46. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3418_2012_58
Download citation
DOI: https://doi.org/10.1007/3418_2012_58
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40545-7
Online ISBN: 978-3-642-40546-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)