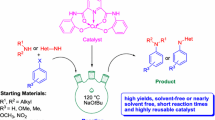
Abstract
Polymer supported Cu(II) catalyst was prepared, characterized and employed for the N-arylation and amination reaction of N–H heterocycles with aryl halides as well as arylboronic acids to afford the corresponding coupled products in good to excellent yields. This catalyst can be used several times with consistent catalytic activity.
Graphical Abstract
R1 = H, Me, OMe, COMe, NO2; R2 = H, Me, OMe, Cl, F, CF3, NO2.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
C–N bond formation via transition metal catalysis is currently a subject of great interest, and intensive research is being carried out to ensure useful organic transformations [1]. The synthesis of N-arylimidazoles and N-arylamines has attracted significant interest because of the frequent occurrence of these structural units in biologically active inhibitors [2–5]. The most straightforward route to N-arylimidazoles involves the direct formation of the aryl-nitrogen bond. Usually these compounds were synthesized via SNAr substitution with aryl halides bearing electron-withdrawing substituent or via the Ullmann type coupling at high temperatures [6]. After the initial reports of Chan and Lam, the Cu-catalyzed cross coupling between N–H heterocycles and arylboronic acids has become an important synthetic methodology in modern organic synthesis [7, 8]. Later the discovery and development of the catalytic path for N-arylation of heterocycles by Buchwald with bromo- and iodoarenes using copper in the presence of basic ligands [9–12] generated greater interest in industry. Afterwards Taillefer and coworkers reported oxime type as well as Schiff base ligands [13, 14] and Ma and coworkers α- and β-amino acids as ligands for effective N-arylation of N–H heterocycles with aryl halides [15]. By using certain copper precatalysts and ligands, successful N-arylation of anilines [16–18], amides [19], hydrazides [20], oxazolidinones [21] and various N–H heterocycles [22] with aryl halides as arylating reagents was reported. Despite the synthetic elegance these coupling protocols suffer from serious limitations of (1) non-reusability of the catalysts, (2) possible contamination of the product with metal and (3) using toxic and/or expensive ancillary ligands. One of the most promising solutions to this problem seems to be the immobilization of the soluble catalysts onto an insoluble matrix using a simplified protocol. However, only limited papers have contributed to N-arylation of alkylamines (not chelating substrates) and various N–H heterocycles in heterogeneous condition [4, 23, 24]. Homogeneous catalysts have some disadvantages, such as they may easily be destroyed during the course of the reaction and they cannot be easily recovered after the reaction for reuse. These disadvantages can be overcome by anchoring metal on suitable supports which will allow easy separation and recyclability of the catalyst with minimal amount of product contamination with metal. These studies confirm that the anchoring of metal on solid support not only exhibits improved catalyst activity, stability and selectivity of the product but also enables easy recovery and reuse of the catalyst.
Herein, we report the synthesis and characterization of a new polymer supported Cu(II) catalyst. This catalyst show facile N-arylation of N–H heterocycles with aryl halides at 100 °C in DMSO medium using K2CO3 as well as arylboronic acid at 40 °C in MeOH without any base. This catalyst was also effective in amination reaction of primary amines with arylboronic acids at 120 °C in DMSO medium using KOH. This polymer supported Cu(II) catalyst was more active than the homogeneous analogue. The catalyst is air stable and can be reused several times without noticeable loss of catalytic activity.
2 Experimental
2.1 Materials and Instruments
Analytical grade reagents and freshly distilled solvents were used throughout. All reagents and substrates were purchased from Merck. Liquid substrates were predistilled and dried by molecular sieve and solid substrates were recrystallized before use. Distillation, purification of the solvents and substrate were done by standard procedures [25]. Chloromethylated polystyrene and 4-(2-pyridylazo)resorcinol were purchased from Aldrich Chemical Company, USA. Copper acetate was procured from Merck and used without further purification.
The FTIR spectra of the samples were recorded from 400 to 4000 cm−1 on a Perkin–Elmer FTIR 783 spectrophotometer using KBr pellets. UV–Vis spectra were recorded using a Shimadzu UV-2401PC doubled beam spectrophotometer having an integrating sphere attachment for solid samples. Thermogravimetric analysis (TGA) was carried out using a Mettler Toledo TGA/DTA 851e. Surface morphology of the samples was measured using a scanning electron microscope (SEM) (ZEISS EVO40, England) equipped with EDX facility. Copper content in the catalyst was determined using a Varian AA240 atomic absorption spectrophotometer (AAS). All spectra were recorded at 400 MHz for 1H NMR and 100 MHz for 13C NMR, respectively. The characterizations of the products were carried out by 1H NMR spectroscopy using Bruker DPX-400 in CDCl3 with TMS as internal standard. Chemical shifts were given as δ value with reference to tetramethylsilane (TMS) as the internal standard. The reaction products were quantified (GC data) by Varian 3400 gas chromatograph equipped with a 30 m CP-SIL8CB capillary column and a flame ionization detector and identified by Trace DSQ II GC–MS equipped with a 60 m TR-50MS capillary column.
2.2 Synthesis of Metal Complexes
The preparation procedure followed to obtain the catalyst is given in Scheme 1. Catalyst was readily prepared in two steps. At first dimethylformamide (DMF) containing little amount of sodium hydride (NaH) was placed in a round bottom flask and stirred for 30 min at 0–5 °C. Then 4-(2-Pyridylazo) resorcinol (PAR) (2.5 g) was added slowly in small portions and the color of the solution was changed from light blue to deep blue instantly. After 30 min chloromethylated polystyrene (1 g) was added to the above solution and stirred overnight at room temperature. The deep violet polymer anchored PAR-ligand was filtered out, washed thoroughly with methanol and dried under vacuum. This Polymer anchored PAR-ligand (1 g) in acetic acid (20 mL) was treated with 5 mL 1% (w/v) acetic acid solution of copper acetate over a period of nearly 30 min under constant stirring. Then the reaction mixture was refluxed for 24 h. The deep brown copper(II) catalyst (Cu-PAR) thus formed was filtered and washed thoroughly with ethanol and dried in room temperature under vacuum.
2.3 General Procedure for N-Arylation of N–H Heterocycles with Aryl Halides
In an oven dried 100 mL RB flask, Cu-PAR (0.05 g, 0.0129 mmol), aryl halide (1 mmol), N–H heterocycles (1.2 mmol), K2CO3 (2 mmol), and 10 mL DMSO were stirred under nitrogen atmosphere, at 100 °C. The reaction mixtures were collected at different time interval and identified by GCMS and quantified by GC analysis. After the completion of the reaction, the catalyst was filtered off and washed with water followed by acetone and dried in oven. The filtrate was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were dried with anhydrous Na2SO4 by vacuum. The filtrate was concentrated by vacuum and the resulting residue was purified by column chromatography on silica gel to provide the desired product.
2.4 General Procedure for N-Arylation of N–H Heterocycles with Arylboronic Acids
In a 100 mL RB flask, Cu-PAR (0.05 g, 0.0129 mmol), arylboronic acid (1 mmol), N–H heterocycles (1.2 mmol), and 10 mL methanol were stirred under nitrogen atmosphere, at 40 °C. The reaction mixtures were collected at different time interval and identified by GCMS and quantified by GC analysis. After the completion of the reaction, the catalyst was filtered off and washed with water followed by acetone and dried in oven. The filtrate was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were dried with anhydrous Na2SO4 by vacuum. The filtrate was concentrated by vacuum and the resulting residue was purified by column chromatography on silica gel to provide the desired product.
2.5 General Procedure for Amination of Aromatic Amines with Arylboronic Acids
In an oven dried 100 mL RB flask, Cu-PAR (0.05 g, 0.0129 mmol), arylboronic acid (1.5 mmol), aromatic amines (1.2 mmol), KOH (1 mmol), and 10 mL DMSO were stirred under nitrogen atmosphere, at 120 °C. The reaction mixtures were collected at different time interval and identified by GCMS and quantified by GC analysis. After the completion of the reaction, the catalyst was filtered off and washed with water followed by acetone and dried in oven. The filtrate was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were dried with anhydrous Na2SO4 by vacuum. The filtrate was concentrated by vacuum and the resulting residue was purified by column chromatography on silica gel to provide the desired product.
2.6 Spectral Data of all Products
1-Phenyl-1H-imidazole (A) [26]:1H NMR (400 MHz, CDCl3) δ 7.85 (s, 1H), 7.53–7.44 (m, 2H), 7.42–7.35 (m, 3H), 7.28 (bs, 1H), 7.21 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 136.1, 134.5, 130.1, 129.3, 127.4, 121.3, 118.2.
1-(4-Methylphenyl)-1H-imidazole (B) [26]: 1H NMR (400 MHz, CDCl3) δ 7.76 (s, 1H), 7.24 (m, 4H), 7.19 (bs, 1H), 7.13 (bs, 1H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 137.4, 135.5, 134.8, 130.3, 130.0, 121.3, 118.3, 20.90.
1-(4-Methoxyphenyl)-1H-imidazole (C) [27]: 1H NMR (400 MHz, CDCl3) δ 7.71 (s, 1H), 7.28 (d, 2H, J = 9.0 Hz), 7.15 (bs, 1H), 7.11 (bs, 1H), 6.94 (d, J = 9.0 Hz, 2H), 3.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.0, 135.8, 130, 130.8, 123 (2C), 118.5, 114.6, (2C), 55.2.
1-(4-(1H-Imidazol-1-yl)phenyl)ethanone(D) [26]: 1H NMR (400 MHz, CDCl3) δ 8.08 (d, 2H), 7.95 (bs, 1H), 7.49 (d,2H), 7.35 (bs, 1H), 7.24 (bs, 1H), 2.63 (s, 3H);13C NMR (100 MHz, CDCl3) δ 196.4, 140.7, 135.7, 135.4, 131.0,130.3, 120.6, 117.7, 26.5.
1-(4-Nitrophenyl)-1H-imidazole(E) [26]:1H NMR (400 MHz, CDCl3) δ 8.39 (d, 2H), 7.99 (bs, 1H), 7.58 (d, 2H), 7.38 (bs, 1H), 7.28 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 146.3, 141.9, 135.4, 131.7, 125.7, 121.0, 117.6.
1-o-Tolyl-1H-imidazole (F) [26]: 1H NMR (400 MHz, CDCl3) δ 7.59 (bs, 1H), 7.34–7.27 (m, 3H), 7.23–7.20 (m, 2H), 7.06 (bs, 1H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 137.4, 136.5, 133.8, 131.2, 129.2,128.7, 126.8, 126.4, 120.4, 17.5.
1-phenyl-1H-benzimidazole (G) [23]: 1H NMR (400 MHz, CDCl3) δ 8.07 (s, 1H), 7.82–7.86 (m, 1H), 7.44–7.60 (m, 6H), 7.5–7.3 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 143, 142.5, 136.7, 133.6, 130, 127.8, 126, 123.8, 122.9(2C), 120.3, 110.1, (2C).
1-Phenyl-1H-pyrazole (H) [28]: 1H NMR (400 MHz, CDCl3) δ 7.88 (bs, 1H), 7.68–7.63 (m, 3H), 7.43 (t, 2H), 7.23 (m, 1H), 6.42 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 140.7, 140.0, 129.1, 126.7, 126.4, 119.0, 107.5.
1-Phenyl-1H-pyrrole (I) [28]: 1H NMR (400 MHz, CDCl3) δ 7.46–4.42 (m, 4H), 7.32–7.25 (m, 1H), 7.11 (s, 2H), 6.39 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 141.0, 129.8, 126.0, 121.3, 117.3, 111.2.
1-(3,4-dimethoxyphenyl)-1H-imidazole (J) [23]: 1H NMR (400 MHz, CDCl3) δ 7.78 (s, 1H), 7.13 (br s, 1H), 7.10 (br s, 1H), 6.86 (br s, 2H), 6.85 (br s, 1H), 3.92 (s, 6H).
1-(4-Chlorophenyl)-1H-imidazole (K) [26]: 1H NMR (400 MHz, CDCl3) δ 7.85 (bs, 1H), 7.46–7.42 (m, 2H), 7.35–7.26 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 135.7, 133.0, 131.9, 130.5, 129.9, 122.6, 118.1.
1-(3-nitrophenyl)-1H-imidazole (L) [27]: 1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 8.24–8.02 (m, 2H), 7.91 (d, J = 9.4 Hz, 1H), 7.74 (t, J = 8.6 1H), 7.52 (br s, 1H), 7.18 (br s, 1 H).
1-(4-Fluorophenyl)-1H-imidazole (M) [23]: 1H NMR (400 MHz, CDCl3) δ 7.76 (br s, 1H), 7.33–7.37 (m, 2H), 7.15–7.21 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 163.3, 162, 133.7, 130.2, 123.0 (2C), 118.1, 116.3 (2C).
1-(4-Trifluoromethylphenyl)-1H-imidazole (N) [23]: 1H NMR (400 MHz, CDCl3) δ 7.81 (br s, 1H), 7.65 (d, J = 8.91 Hz, 2H), 7.47 (d, J = 8.91z, 2H), 7.21 (br s, 1H), 7.14(br s, 1H).
1-(4-Methylphenyl)-1H-benzimidazole (O) [23]: 1H NMR (400 MHz, CDCl3) δ 8.01 (br s, 1H), 7.77–7.85 (m, 1H), 7.40–7.47 (m, 1H), 7.20–7.33 (m, 6H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 144, 142, 138, 135 (2C), 130.6, 124, 123.9, 122.8 (2C), 120.3, 110.1(2C), 21.
1-(4-Methoxyphenyl)-1H-benzimidazole (P) [23]: 1H NMR (400 MHZ, CDCl3) δ 8.06 (br s, 1H), 7.85–7.89 (m, 1H), 7.45–7.46 (m, 1H), 7.40 (d, J = 9.0 Hz, 2H), 7.30–7.32 (m, 2H), 7.07 (d, 2H, J = 9.0 Hz), 3.90 (s, 3H).
2-Phenylisoindoline-1,3-dione (Q) [29]: 1H NMR (400 MHz, CDCl3) δ 7.41–7.46 (m, 3H), 7.49–7.55 (m, 2H), 7.79–7.83 (m, 2H), 7.97 (m, 2H).
2-p-Tolylisoindoline-1,3-dione (R) [29]: 1H NMR (400 MHz, CDCl3) δ 2.40 (s, 3H), 7.31 (m, 4H), 7.77–7.82 (m, 2H), 7.93–7.95 (m, 2H).
N-Phenylbenzamide (S) [30]: 1H NMR (400 MHz, CDCl3) δ 7.13–7.17 (m, 1H), 7.36–7.40 (m, 2H), 7.48–7.52 (m, 2H), 7.55–7.57 (m, 1H), 7.64–7.65 (m, 2H), 7.81 (s, 1H), 7.86–7.88 (m, H).
N-phenylsulfonamide (T) [31]: 1H NMR (400 MHz, CDCl3) δ 7.79–7.76 (m, 2H), 7.52 (t, J = 7.4 Hz, 1H), 7.40 (t, J = 7.4 Hz, 2H), 7.21 (t, J = 7.8 Hz, 2H), 7.09–7.06 (m, 4H).
N-(Phenyl)aniline (U) [32].1H NMR (400 MHz, CDCl3) δ 7.27–7.22 (m, 4H), 7.07–7.04 (d, J = 7.2 Hz, 4H), 6.93–6.89 (m, 2H), 5.69 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 143.33, 129.55, 121.21, 118.01.
N-(4-Methylphenyl)aniline (V) [33]: 1H NMR (400 MHz, CDCl3) δ 2.35 (s,3H), 5.62 (s, 1H), 6.92 (t, J = 7.3 Hz, 1H), 7.00–7.07 (m, 4H), 7.12 (d, J = 8.3 Hz, 2H), 7.26 (dd, J = 7.3, 8.7 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 20.88, 117.04, 120.44, 129.78, 129.02, 131.09, 139.48, 144.04.
N-(4-Methoxyphenyl)aniline (W) [32]: 1HNMR (400 MHz, CDCl3) δ 7.42 (d, J = 8.4 Hz, 2H), 7.24–7.12 (m, 5H) 6.90 (d, J = 8.4 Hz, 2H) 5.51 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 153.54, 145.79, 135.58, 129.10, 123.42, 120.10, 115.15, 55.32.
N-(4-Chlorophenyl)aniline(X) [34]: 1HNMR (400 MHz, CDCl3) δ 7.27–7.16 (m, 5H), 7.03–6.91 (m, 4H), 5.66 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 147.29, 146.30, 132.38, 129.66, 121.35, 119.21, 115.43.
N- (3-nitrophenyl)aniline (Y)[35]:1H NMR (400 MHz, CDCl3) δ 7.82 (s, 1H), 7.67 (d, J = 7.63 Hz, 1H), 7.4–7.22 (m, 4H), 7.16–7.0 (m, 3H), 5.9 (br, s 1H).
N-Phenylbenzylamine (Z) [36]: 1NMR (400 MHz, CDCl3) δ 7.34–7.22 (m, 5H), 7.15 (t, J = 7.2 Hz, 2H), 6.71 (t, J = 7.6 Hz, 1H), 6.61 (d, J = 7.63 Hz, 2H), 4.31 (s, 2H), 4.09 (s, 1H); 13C NMR (100 MHz,CDCl3) δ 149.56, 139.54, 130.08, 129.05, 128.70, 127.38, 118.32, 113.31, 48.56.
3 Results and Discussion
3.1 Characterization of the Cu-PAR Catalyst
Due to insolubilities of the polymer anchored copper catalyst in all common organic solvents, its structural investigation was limited to its physicochemical properties, chemical analysis, SEM, TGA, IR and UV–Vis spectroscopic data. Elemental analysis of the free ligand (C = 71.05%, H = 4.6% and N = 13.81%) and polymer anchored copper catalyst (C = 56.27%, H = 3.98% and N = 9.85%) supports the formulation of the Cu-PAR catalyst. The metal content in the catalyst determined by atomic absorption spectroscopy suggests 1.65% Cu in the immobilized metal complex.
The modes of attachment of copper metal onto the support were confirmed by comparison of the FTIR spectral bands of the copper catalyst over the polymer in various steps of its synthesis. The IR spectrum of pure chloromethylated polystyrene has shown absorption band at 1261 cm−1, due to the presence of C–Cl group in polymer beads, which reduced in intensity in the polymer anchored PAR-ligand. The N=N stretching frequency for the ligand at 1451 cm−1 is shifted to 1442 cm−1 in the Cu(II) complex [37]. In the PAR-ligand, the presence of the bands at 3411 and 1368 cm−1 have been assigned to the stretching vibrational frequency of phenolic –OH and free C–O stretching. The FTIR spectra of the PAR-ligand shows one medium to sharp band around at 1245 cm−1, which may be attributed to the stretching vibration of phenolic oxygen attached with polymer. In the Cu-PAR catalyst, a new peak appears at 1351 cm−1. This could be attributed to a shift in free C–O stretching as a result of coordination of phenolic oxygen with copper metal [38]. In addition, the bands at 621, 533 and 548 cm−1 may be assigned to the Cu–O, Cu–Nazo and Cu–Npyridine [39–41] stretching vibration respectively. In this catalyst a medium intensity band observed at 1318 cm−1 suggests the monodentate coordination of the acetate groups [42]. Monodentate acetate usually shows two bands at 1630 and 1310 cm−1 due to antisymmetric and symmetric stretching, respectively, [43]. The acetate oxygen involved in the complexation with copper which is clearly evident from the appearance of a new medium intensity band at 430 cm−1 assignable to ν Cu–O in the IR spectra [43].
The electronic spectrum of the polymer anchored PAR-ligand and the immobilized Cu-PAR catalyst has been recorded in the diffuse reflectance mode. The spectrum of the Cu-PAR catalyst exhibits few spectral bands below 260 nm, which may be assigned to π–π* intra-ligand charge transfer and π–π* transition of the benzenoid moiety. The shoulder peaks nearer to 310 nm are attributed to the n–π* transition of the azo (–N=N–) group [44]. The high intensity band in the visible region, at 400–425 nm involving the whole electronic system of the Cu-PAR catalyst and a broad band from 600 to 720 nm are assigned to the π–π* transition [37]. This envelop is due to the presence of conjugated chromophoric groups in the azo ligand.
The scanning electron micrographs of PAR-ligand (Fig. 1a) and supported Cu-PAR catalyst (Fig. 1b) clearly show the morphological change which occurred on the surface of polystyrene after loading of metal on it. Energy dispersive spectroscopy analysis of X-rays (EDX) data for PAR-ligand and supported Cu-PAR catalyst are given in Fig. 2a and b. The EDX data also inform that the attachment of copper metal on the surface of the polymer matrix.
Thermal stability of the complex was investigated using TGA-DTA at a heating rate of 10 °C/min in air over a temperature range of 30–600 °C. TGA-DTA curves of the polymer anchored PAR-ligand and supported Cu-PAR catalyst are shown in Fig. 3. The PAR-ligand is stable up to 300 °C and thermal decomposition of the ligand is start above this temperature. Initial decomposition of the catalyst is occurred at 250 °C. Finally degradation of the Cu-PAR catalyst is occurred above the temperature 350–380 °C. So, we can suggest that the complex is stable up to 250 °C and above this temperature it starts decomposes. Thermo gravimetric study suggests that the polymer anchored Cu-PAR catalyst degrades at considerably higher temperature.
3.2 Catalytic Activity of the Cu(II)-PAR Catalyst
The N-arylation reaction is a convenient method for the C–N bond formation between N–H heterocycles with aryl halides and arylboronic acids. To test the applicability of the polymer anchored copper(II) catalyst, we examined the N-arylation reaction of imidazole with various aryl halides and arylboronic acids. Considering that N-arylimidazoles have been recurrent templates in medicinal chemistry, a preliminary survey of the reaction conditions is conducted with iodobenzene and imidazole as model arylating agents (Scheme 2). Solvents such as DMF, ACN, MeOH, DMSO, Toluene and water are investigated and it is found that polar solvents are more favored (Table 1, entries 1–6). With DMF, ACN, and MeOH, yields are comparatively low. Consequently DMSO is chosen as the medium of choice for this coupling. Bases such as KOH, Cs2CO3, Et3N, Na2CO3, K3PO4 and K2CO3 are found to facilitate this coupling reaction and among them K2CO3 is the best (Table 1, entries 1 and 7–11). This arylation is also found to be highly sensitive to reaction temperature and time. At lower temperatures (50 and 70 °C) only low to moderate yield is obtained (Table 1, entries 12–13). It is noteworthy that when homogeneous copper catalyst is used instead of supported copper catalyst, very low amount of product formation is observed (Table 1, entry 14). With optimized conditions now in hand; we explored the scope of this process with respect to aryl iodide structure. To our delight, the N-arylation of imidazole is smoothly performed with the extensive pool of aryl iodides to afford the corresponding products in good to excellent yields. It is observed that iodoarenes with electron-withdrawing group (Table 2, entry 4 and 5) reacted at a faster rate than iodoarenes with electron donating group (Table 2, entry 2 and 3). Sterically hindered 2-iodotoluene took longer duration to afford a good yield (Table 2, entry 6). Further experiments with different nitrogen containing heterocycles such as benzimidazole, pyrazole, and pyrrole are carried out under these optimized conditions (Table 2, entries 7–9). In the case of bromo-derivatives, bromobenzene and 4-methylbromobenzene afforded lower yield of the coupled product (Table 2, entries 10 and 11). Bromobenzene with electron-withdrawing group gave excellent yield of the coupled product (Table 2, entry 12).
For any supported catalyst, it is important to know its ease of separation and possible reuse. Polymer anchored Cu-PAR catalyst can be easily separated by filtration. For the recycling study, N-arylation reaction was performed with imidazole and iodobenzene maintaining the same reaction conditions using the recovered catalyst. Each time after completion of the reaction catalyst was recovered by simple filtration, washed thoroughly with DMSO to remove any excess reagents and finally with methanol, dried under vacuum and reused under the same reaction conditions as for the initial run without any regeneration. The recycling efficiency of the catalyst up to five successive runs are shown in Table 2, entry 1. From the results, it is seen that the catalyst retained its high catalytic activity in these five repeating cycles.
N-arylation of N–H heterocycles with arylboronic acids is complementary to the N-arylation with aryl halides as it requires very mild conditions and less time but uses expensive arylboronic acids. Preliminary experiments are carried out by taking phenylboronic acid as a test molecule for the N-arylation of imidazole (Scheme 3). In order to determine the best reaction medium, we tested different solvents such as methanol, DMSO, acetonitrile, DMF and toluene. Methanol is found to be the best solvent for the N-arylation of imidazole (97%). On the other hand, reaction in other solvents gives trace amount of coupled product under identical conditions. N-arylation reactions of N–H heterocycles with phenylboronic acid are carried out in methanol with Cu-PAR catalyst, without the need of any organic co-solvent or additives such as phase transfer catalysts. To check the generality and scope of the catalyst, we tried the N-arylation of N–H heterocycles with several other arylboronic acids in MeOH without using any base and the results are summarized in Table 3. The reaction of imidazole with phenylboronic acid is completed within 12 h at lower temperature (40 °C). Imidazole is successfully applied to couple electron rich arylboronic acids with in high yields (Table 3, entries 2–5). Electron withdrawing arylboronic acids reacted at a slower rate with moderate to good yields (entries 6–9). The coupling of 3-nitrophenylboronic acid which is a difficult substrate also proceeded smoothly under the present conditions (Table 3, entry 7). Similar observation is made when benzimidazole is used in place of imidazole to obtain the corresponding N-arylbenzimidazoles, but the reactions took longer time compared to that in the reactions of imidazole (Table 3, entry 10–12). In an endeavor to expand the scope of the above methodology, the catalytic system is also applied to imides, amides and sulfonamides. Such coupling was found to give the desired N-arylation products in moderate yields, as shown in Table 3, except for sulfonamide, which afforded the corresponding products in lower yield (Table 3, entry 17). A series of substituted arylboronic acids (Table 3, entries 13–15) are coupled with phthalimide under the generalized reaction conditions to afford the corresponding products in excellent yields. However, the reaction of benzamides with phenylboronic acid afforded the corresponding products in good yields (Table 3, entry 16). The catalyst is recovered by simple filtration and reused for several cycles with consistent activity (Table 3, entry 1).
After achieving excellent results with N–H heterocycles, we further applied this catalytic system for the amination of aromatic amines. Preliminary experiments are carried out by taking phenylboronic acid as a test molecule for the amination of aniline (Scheme 4). In order to determine the best reaction medium, we tested different solvents such as methanol, DME, toluene, DMF and DMSO for the amination reaction of aniline with phenylboronic acid (Table 4). DMSO is found to be the best solvent for the amination of aniline provided 91% yield (Table 4, entry 1). On the other hand, reaction in other solvents gives low to moderate coupled product (Table 4, entries 2–5). The nature of base has a pronounced effect in these reactions. Reaction of aniline with phenylboronic acid in presence of K3PO4, K2CO3 and Cs2CO3 give moderate coupled product, while triethylamine and KOH provided good yield (Table 4, entries 1 and 6–9). However, we continued the reactions in KOH instead of triethylamine because of toxicity reasons. Temperature is proved to be very crucial, when the reaction is carried out at lower temperature (70 °C), 58% product formation is observed, by raising the temperature (120 °C), 91% yield of coupled product is achieved (Table 4, entry 10 and 1). Further experiments with different arylboronic acids are carried out under these optimized conditions and results are summarized in Table 5. It is clear from Table 5 that amination proceeds very effectively and affords the corresponding products in good to excellent yields under our reaction conditions. No spectacular electronic effects are observed in the amination of aniline; only a slight decrease in the reaction rate is noted with the 3-nitrophenylboronic acid. Benzyl amine gives 87% yield of the desired product when reacted with phenylboronic acid. The catalyst is recovered by simple filtration and reused for several cycles with consistent activity (Table 5, entry 1).
3.3 Comparison with Other Reported System
Table 6 provides a comparison of the results obtained for our present catalytic system with those reported in the literature. From Table 6, it is seen that the present catalyst exhibited higher yields compared to the other reported system [3, 23, 27, 45–48].
3.4 Heterogeneity Tests
To determine whether the catalyst is actually functioning in a heterogeneous manner, a hot-filtration test was performed in the N-arylation reaction of imidazole with phenylboronic acid. The solid catalyst was filtered out after the reaction proceeded for 4 h and the determined yield was 69%. The liquid phase of the reaction mixture is collected at the reaction temperature. Atomic absorption spectrometric analysis of the liquid phase of the reaction mixtures thus collected by filtration confirms that Cu is absent in the reaction mixture. The obtained filtrate was continually stirred under the reaction conditions. After 8 h the conversion was determined to still be 69%. This result indicated that the catalytic reaction was caused by the solid catalyst. Cu is also not detected in the liquid phase of the reaction mixture after the completion of the reaction. It is noteworthy that the methanol remains completely colorless on addition of Cu-PAR catalyst. These results suggest that the Cu is not being leached out from the catalyst during N-arylation reactions.
4 Conclusions
In summary, we have developed an experimentally simple and mild Cu-PAR catalyzed N-arylation and amination reaction of nitrogen-containing heterocycles with a variety of aryl iodides as well as arylboronic acids. This catalyst offers a number of advantages, such as excellent stability, easy separation from the reaction mixture by filtration, reusability for several times with often minimal loss of activity, and wide accessibility of chloromethylated polystyrene. Further more this catalyst can be successfully applied in a number of N-arylation and amination reactions and gives better results than other reported copper catalysts. The easy workup procedure provides a method that is well suited toward the synthesis of parallel libraries based upon this type of transformation.
References
Muci AR, Buchwald SL (2002) Top Curr Chem 219:131
Chang JWW, Xu X, Hong Chan PW (2007) Tetrahedron Lett 48:245
Rout L, Jammi S, Punniyamurthy T (2007) Org Lett 9:3397
Reddy KR, Kumar NS, Sreedhar B, Kantam ML (2006) J Mol Catal A: Chem 252:136
Bhanushali MJ, Nandurkar NS, Bhor MD, Bhanage BM (2006) J Mol Catal A: Chem 259:46
Jacobs C, Frotscher M, Dannhardt G, Hartmann RW (2000) J Med Chem 43:1841
Lam PYS, Clark CG, Saubern S, Adams J, Winters MP, Chan DMT, Combs A (1998) Tetrahedron Lett 39:2941
Chiang GCH, Olsson T (2004) Org Lett 6:3079
Kiyomori A, Marcoux JF, Buchwald SL (1999) Tetrahedron Lett 40:2657
Klapars A, Antilla JC, Huang X, Buchwald SL (2001) J Am Chem Soc 123:7727
Antilla JC, Klapars A, Buchwald SL (2002) J Am Chem Soc 124:11684
Antilla JC, Baskin JM, Barder TE, Buchwald SL (2004) J Org Chem 69:5578
Cristau HJ, Cellier PP, Spindler JF, Taillefer M (2004) Eur J Org Chem 695
Cristau HJ, Cellier PP, Spindler JF, Taillefer M (2004) Chem Eur J 10:5607
Cai Q, Zhu W, Zhang H, Zhang Y, Ma D (2005) Synthesis 496
Kelar AA, Patil NM, Chaudhari RV (2002) Tetrahedron Lett 43:7143
Haider J, Kunz K, Scholz U (2004) Adv Synth Catal 346:717
AS Gajare, Toyota K, Yoshifuji M, Ozawa F (2004) Chem Commun 1994
Deng W, Wang Y, Zou Y, Liu L, Guo Q (2002) Tetrahedron Lett 44:2311
Wolter M, Klapars A, Buchwald SL (2001) Org Lett 3:3803
Mallesham B, Rajesh M, Rajmohan-Reddy P, Srinivas D, Trehan S (2003) Org Lett 5:963
Tao M, Lei W (2007) Tetrahedron Lett 48:95
Kantam ML, Venkanna GT, Sridhar C, Sreedhar B, Choudary BM (2006) J Org Chem 71:9522
Kantam ML, Rao BPC, Choudary BM, Reddy RS (2006) Synlett 2195
Vogel AI (1989) Text book of practical organic chemistry (quantitative analysis), 5th edn. Longman, London
Sl Ashton PR, Koniger R, Stoddart JF (1996) J Org Chem 61:903
Choudary BM, Sridhar C, Kantam ML, Venkanna GT, Sreedhar B (2005) J Am Chem Soc 127:9948
Palaniswamy S, Kasi P (2008) J Org Chem 73:9121
Yadav LDS, Yadav BS, Rai VK (2006) Synthesis 1868
Correa A, Bolm C (2007) Angew Chem Int Ed 46:8862
Hosseinzadeh R, Tajbakhsh M, Mohadjerani M, Alikaramj M (2010) J Chem Sci 122:143
Zhang H, Cai Q, Ma D (2005) J Org Chem 70:5164
Wolfe JP, Buchwald SL (1997) J Org Chem 62:6066
Anderson KW, Mendez-Perez M, Priego J, Buchwald SL (2003) J Org Chem 68:9563
Zhang Z, Mao J, Zhu D, Wu F, Chen H, Wan B (2006) Tetrahedron 62:4443
Kwong FY, Klapars A, Buchwald SL (2002) Org Lett 4:581
Islam M, Mondal P, Mondal S, Mukherjee S, Roy AS, Mubarak M, Paul M (2010) J Inorg Organomet Polym 20:87
Marvel CS, Aspey SA, Dudley EA (1956) J Am Chem Soc 78:4905
Sallam SA, Orabi AS (2002) Transit Metal Chem 27:447
Kandil SS, El-Hefnawy GB (2003) Transit Metal Chem 28:168
Zhou Y, Zhong C, He Y, Xiao L, Liu Y, Zhang H (2009) J Inorg Organomet Polym 19:328
Krishnankutty K, Ummathur MB, Sayudevi P (2009) J Indian Chem Soc 86:325
Nakamoto N (1997) Infrared spectra and Raman spectra of inorganic and coordination compounds. Wiley, New York
Kirkan B, Gup R (2008) Turk J Chem 32:9
Huang Y–Z, Miao H, Zhang Q–H, Chen C, Xu J (2008) Catal Lett 122:344
Kantam ML, Roy M, Roy S, Sreedhar B, Lal De R (2008) Catal Commun 9:2226
Singh BK, Stevens CV, Acke DRJ, Parmar VS, Van der Eycken EV (2009) Tetrahedron Lett 50:15
Antilla JC, Buchwald SL (2001) Org Lett 3:2077
Acknowledgments
We thank the Department of chemistry, University of Calcutta, for providing us the instrumental support. We gratefully acknowledge DST, New Delhi, for award of grant under its FIST program to the Department of Chemistry, University of Kalyani. SMI acknowledge the following agencies for funding: DST, CSIR and UGC, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M., Mondal, S., Mondal, P. et al. An Efficient Recyclable Polymer Supported Copper(II) Catalyst for C–N Bond Formation by N-Arylation. Catal Lett 141, 1171–1181 (2011). https://doi.org/10.1007/s10562-011-0606-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0606-2