Abstract
Despite ongoing advances, the vast majority of those afflicted with esophageal cancer go on to die of their disease. While some respond notably to chemoradiotherapy, others-with seemingly similar disease characteristics based on existing clinical assays-have a limited or absent response. The current climate is suitable for the development of predictive tools and novel methods of evaluation to aid in individualised patient treatment planning. In the preoperative setting our clinical staging and varied imaging modalities, although imperfect are used to determine which patients will likely receive from chemoradiotherapy. No permutation of the data currently available can predict, with a satisfactory accuracy, whether an individual patient will have a substantial response to neoadjuvant treatment with minimal morbidity. Potentially, the addition of molecular assays in tandem with standardization of radiologic data will allow the development of increasingly powerful tools to predict the likelihood of response to treatment without complication for an individual patient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Esophageal Cancer
- Standard Uptake Value
- Radiation Pneumonitis
- Esophageal Cancer Patient
- Concordance Index
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Esophageal cancer is a significant worldwide health problem, of which the incidence in the USA and Western Europe is rapidly increasing (Holmes and Vaughan 2007; Siegel et al. 2012). Patients frequently present with advanced stage disease, poor performance status, and have a poor prognosis. Over recent years, the addition of neoadjuvant chemoradiotherapy (CRT) to surgery has improved 5-year survival probability from 34 % for patients treated with surgery alone to 47 % for patients treated with chemoradiotherapy and surgery (van Hagen et al. 2012).
However, responses to neoadjuvant treatment vary, with heterogeneous responses for patients with similar clinical stage. If neoadjuvant treatment produces a pathologically complete response (pCR), outcomes are better: patients with a pCR can expect a 5-year survival rate of 48 vs. 18 % for non-responding patients (Berger et al. 2005).
Without the benefit of knowing the pathologic stage, it is difficult to select patients who will benefit from this aggressive treatment. So far, three characteristics on which prediction can be based have been identified: clinical factors, biomarkers, and imaging modalities. While these last two are promising, at this current time no combination of available clinical data is reliable enough to indicate which patients are going to have a robust response to CRT and benefit from esophagectomy, and which should be treated with only selected modalities. Thus, traditional clinical factors such as tumor stage, age and performance status are still used to select the best therapy for a particular patient.
With regard to biomarkers, new strategies for subdividing esophageal cancer patients into prognostic groups may result in patients being selected for CRT or not on the basis of molecular assays, in addition to the ‘traditional’ methods of stage and imaging modalities. While these markers are promising, the exact clinical setting in which biomarkers will routinely be of utility in guiding treatment has yet to be defined.
The third characteristic, imaging, involves the use of modalities such as endoscopic ultrasound (EUS), computed tomography (CT), positron emission tomography (PET)-CT and magnetic resonance imaging (MRI). As a group, the information these assays provide continues to play an important role in staging and re-staging and thus in patient selection for CRT.
Combining these clinical data allows for our growing field of interest, where prognostic models and nomograms attempt to predict individual prognosis and response to CRT, with the potential to individualize treatment and isolate high-risk groups for novel treatments.
The above patient and tumor characteristics are the main basis upon which multidisciplinary treatment plans are made. In this chapter we review these decision tools and their implications for prognosis, treatment response and toxicity in the progressively individualized treatment of esophageal cancer.
2 Prognosis and Treatment Response
2.1 Disease Characteristics
Currently, no single clinical parameter can be used to predict which patients will achieve a pathological complete response (pCR) following CRT or survive their disease. However, there are several clinical and oncologic factors that have consistently proven to be significantly associated with outcome.
2.1.1 Patient Characteristics
Several patient-related factors are associated with survival and response to CRT. Factors as AJCC stage, male gender, performance status, location of the tumor, high lifetime alcohol consumption, forced expiratory volume in 1 s (FEV1), number of involved lymph nodes and cigarette smoking are independent predictors of survival (Thrift et al. 2012; Situ et al. 2012). Parameters as gender, tumor grade, baseline EUS T-stage and histology are related to a pCR (Ajani et al. 2012). Achievement of pCR or any tumor response corresponds with better overall survival (Berger et al. 2005).
2.1.2 Histologic Characteristics
Histology of the tumor is an important differentiating factor, as the response at treatment differs substantially between adenocarcinomas and squamous cell carcinomas (SCC) (Rizk et al. 2007; Heath et al. 2000; Urba et al. 2001; Burmeister et al. 2005). Squamous cell carcinomas have a much higher likelihood to achieve a pCR than adenocarcinomas: a pCR in adenocarcinomas occurs in 20–30 % whereas for SCC, pCR occurs in up to 50 % of the patients (van Hagen et al. 2012). Although in general, achievement of a pCR is commonly associated with improved survival (Berger et al. 2005), this does not apply to SCC patients, since a higher rate of pCR for SCC patients does not seem to translate into better overall outcomes. Additionally, it is worth noting that while patients that have a pCR do have better prognosis when compared to all other patients with remaining invasive disease, a significant number of patients will succumb to their disease, with almost indistinguishable OS when compared to patients with stage I (Rizk et al. 2007). If one desires to predict which patients can safely go on to esophageal salvage following CRT, novel prognostic assays and use of the currently available data is required.
Histologic grade is a predictor for a pCR, long-term outcome (Ajani et al. 2012), and is implemented in initial staging for differentiating prognostic groups. However, with increasing use of neoadjuvant therapy, this grading is solely based on endoscopic biopsy. With the accuracy of grade assessment on biopsy being only 73 %, it should be interpreted with caution (Dikken et al. 2012).
2.2 Staging Systems
Esophageal cancer staging has an important role in selecting patients for the appropriate treatment strategy and is related with long-term outcomes (Talsma et al. 2012). The TNM staging system of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) for esophageal cancer is used universally and was recently revised. A major change between the 2002 (Union Internationale Contre le Cancer 2002) and the 2010 (Rice et al. 2010) editions were the development of separate stage groupings according to histology and a better description of the tumors located at the esophagogastric junction (EGJ) (Tables 1, 2, 3 and 4).
These tumors, at the esophagogastric junction (EGJ) and proximal 5 cm of the stomach that extend into the EGJ or esophagus, are staged as esophageal cancers (Table 5). While all other tumors with an epicenter in the stomach >5 cm from the EGJ, or those within 5 cm of the EGJ without extension into the esophagus are staged as gastric cancers. A sub classification of these junctional tumors can be made by the classification described by Siewert and Stein (1998) (Table 6).
A validation study of this 7th edition of the UICC-AJCC staging system was performed by Talsma et al., which showed for surgical esophageal cancer patients that the 7th edition of staging provided more accurate prognostic stratification for OS in comparison to the 6th edition (Talsma et al. 2012).
2.3 Imaging
Imaging modalities as EUS, CT, PET-CT and MRI currently play an important role in staging and in patient selection before CRT. They may also be utilized for post CRT clinical re-staging (ycTNM) after induction treatment or response monitoring during treatment, however the results should be interpreted with caution in this setting (Ribeiro et al. 2006). During these different phases of the patient care some have attempted to identify a distinctive role for each modality.
2.3.1 EUS
2.3.1.1 Staging
Endoscopic ultrasound (EUS) uses a high frequency ultrasound transducer to obtain detailed images of the tumor mass and the relationship with the five-layered structure of the esophageal wall. EUS attempts to provide measurements of tumor thickness and is regularly used to estimate tumor extension in initial staging for esophageal cancer (Ribeiro et al. 2006). The discriminatory power for distinguishing between early stage tumors and those with deeper invasion may approach 80–90 %. Some have found this gross distinction prognostic in identifying those patients at risk for a positive circumferential resection margin (CRM), if treated with isolated surgery (Reid et al. 2012). However, its exact TNM accuracy is the least prognostic of available clinical information in predicting pre operative stage (Reid et al. 2012; van Vliet et al. 2008; Thosani et al. 2012).
While, EUS remains a frequently used clinical estimate of primary tumor staging, there are technical limitations to its ubiquitous use. Not all patients are capable of receiving a complete EUS due to esophageal stenosis. Additionally, the accuracy of EUS is operator dependent and is subject to a learning curve (Fockens et al. 1996). Ultimately, preoperative staging of lymph node status is challenging. A recently proposed tool from Gaur et al. (2010) for predicting pathologic lymph node involvement based on clinical information is discussed in further detail below in Nomograms and Predictive models section.
2.3.1.2 Re-staging
For response evaluation, EUS continues to be used as the primary diagnostic modality. However, the accuracy of EUS restaging varies significantly across several recent retrospective analyses (Ribeiro et al. 2006; Giovannini et al. 1997; Chak et al. 2000).
Different methods have been proposed for response assessment with EUS. The first method is to restage according to the TNM staging system (Fockens et al. 1996), second method is to measure the relative reduction in thickness of the tumor (Gaur et al. 2010; Giovannini et al. 1997) and a third method is to measure the relative tumor shrinkage at the maximum cross-sectional area (MCSA) (Gaur et al. 2010; Chak et al. 2000). However, with these different methods the accuracy is still poor and ranges from 17 to 59 % (Sloof 2006; Hirata et al. 1997; Zuccaro et al. 1999; Bowrey et al. 1999). Even when a EUS is combined with biopsy, the accuracy does not exceed 31 % to correctly predict a pCR (Sarkaria et al. 2009). Of patients that have a negative biopsy on restaging endoscopy (cCR) less than 30 % will have a pCR (Sarkaria et al. 2009).
A possible explanation for this discrepancy between endoscopic staging and subsequent pathologic staging is that EUS may not be able to differentiate between post-treatment inflammation or fibrosis and residual tumor (Jamil et al. 2008).
2.3.2 CT
2.3.2.1 Staging
Computed tomography (CT) is usually one of the first steps in staging esophageal cancer patients and is used to evaluate the region of the primary tumor and evaluate for distant metastases. However, the accuracy for locoregional staging is limited. Accuracy for tumor staging has been reported with a range of 42–68 % (Lowe et al. 2005; Wu et al. 2003) and for regional lymph node metastases the pooled sensitivity and specificity is only 0.50 (95 % C.I. 0.41–0.6) (van Vliet et al. 2008). Also, when used for screening for distant metastases CT-scans have difficulty recognizing small distant metastases, while a PET-CT is more sensitive (van Vliet et al. 2008).
2.3.2.2 Re-staging
CT scan is the most commonly used diagnostic modality in monitoring response of nonsurgical therapy for solid tumors. For esophageal cancer restaging, however, its role remains ambiguous. CT gives good visualization of the tumor bulk in majority of the patients. However, when tumor shrinkage is correlated to pathological response following neoadjuvant treatment some have found a clear correlation (Swisher et al. 2004; Voncken et al. 2012), while others failed (Griffith et al. 1999; Jones et al. 1999).
This difference could be due to an overestimation of edema, inflammation and fibrosis for residual tumor (Westerterp et al. 2005). While, CT remains one of many modalities in re-staging after induction treatment, primary tumor response should be interpreted with caution.
2.3.3 PET
FDG-PET is a nuclear imaging modality that evaluates tumor physiology and allows for a quantitative functional assessment of the primary via the standardized uptake value (SUV). Nearly all primary esophageal cancers have high levels of cellular metabolism, increased glycolysis, and an increased number of glucose transporters. In almost all cases, SCC primary tumors have a high uptake of FDG. In adenocarcinomas this FDG accumulation is more variable, with a minority (6 %) of the tumors being non-avid, usually the mucous containing and poorly differentiated tumor types or tumors too small to detect (<5 mm) by FDG-PET (Wagner et al. 2009; Stahl et al. 2003; Wong and Chambers 2008).
2.3.3.1 Staging
FDG-PET can provide information in initial staging, especially for finding regional nodal metastases and silent distant metastases, where it has a role in selecting patients that will benefit from neoadjuvant CRT. In addition, FDG-PET can contribute to localization, size measurement and GTV definition of the primary tumor and lymph nodes (Katsoulis et al. 2007).
2.3.3.2 Restaging
PET-CT provides several pieces of clinically relevant information in restaging after induction treatment. A PET-CT can detect occult metastases after induction treatment and thus saving patients from undergoing a non-curative esophagectomy. A PET-CT after induction treatment detects metastases in 8 % of patients with a consequent adjustment in therapeutic plan (Bruzzi et al. 2007).
An additional advantage of response monitoring with PET-CT is the prognostic value in the decrease, or lack there of, in SUV of the primary tumor. While a PET-directed therapy does have the potential to change clinical practice and improve outcomes, it cannot currently be considered a standard approach in isolation. Standardization of quantitative results across facilities continues to be a technical roadblock. Several methods have been proposed as standard procedure for metabolic response analysis using the standard uptake value (SUV) as a semi quantitative measure of FDG uptake. Proposed methods are: SUV pretreatment, SUV after chemoradiation, percentage of decline of SUV, attainment of a metabolic complete response after chemoradiation and to show an early metabolic response 14 days after start of chemoradiation.
These different methods were analyzed in a systematic review of Omloo et al. evaluating 31 studies (Omloo et al. 2011). Fifteen of these studies tested the pretreatment FDG uptake as a predictive factor. On univariate analysis, SUV was a predictor of survival in 12 out of 15 studies and multivariate analysis showed only in two out of eight studies that SUV was an independent predictor of survival. SUV decrease after completion of neoadjuvant treatment was predictive in only two out of six studies. Finally, there were six studies looking at the SUV decrease and prognosis early during neoadjuvant therapy. SUV decrease was a predictor of response in all of these six studies and a predictor of survival in five of these six studies.
Comparative analysis across FDG-PET articles is challenging due the non-standardization of the image acquisition process and subsequent analytic thresholds. Since the methodology for image acquisition varies, the SUV threshold to predict prognosis varies significantly between analyses (from 3 to 10.5) and cutoff values for amount of SUV change differentiating responders from non-responders also varies depending on the publication (from −30 to −70 %) (Omloo et al. 2011). This illustrates the difficulty to translate these results to clinical practice, although the field of treatment stands to benefit from an adequately powered prospective trial evaluating the true relevance of early SUV decline during CRT. In conclusion, early SUV response assessment holds promise to potentially guide ongoing treatment, but the implementation and technical applicability have not yet developed to the extent required to find a clinical role for routine use.
2.3.4 MRI
The recent development of functional MRI imaging has opened a new window of opportunities for staging esophageal tumors, monitoring response to treatment and potentially even predicting biological behavior (Chang 2009; Riddell et al. 2007). Esophageal imaging with MRI has some technological challenges due to local cardiorespiratory motion artifact. However, with an accurately tuned sequence accurate images can be acquired.
For staging the esophageal tumor, EUS is the modality of first choice, however for 6 % of newly diagnosed patients, EUS is not possible due to a narrowing of the esophageal lumen and subsequent inability to pass the endoscope. CT is less accurate in differentiating depth of tumor invasion, thus for staging those patients, MRI could be an alternative (Riddell et al. 2007).
Staging the depth of tumor growth with MRI has an accuracy of about 60 % (Jamil et al. 2008), but with the MRI technique still under development, imaging reaches a higher level of precision, however this has not yet been correlated with accuracy of overall stage (Riddell et al. 2007). MRI cannot differentiate each layer of the esophageal wall, therefore an alternative T differentiation standard is described by Botet et al. (1991) and by Riddell et al. (2007) (Table 7).
Recently developed MRI techniques such as diffusion weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI may provide a relative increased accuracy in clinical staging and response assessment of esophageal tumors.
In diffusion-weighted magnetic resonance imaging (DWI) each voxel reflects the amount of water diffusion at that location. This diffusion process can be quantified by measuring the apparent diffusion coefficients (ADCs) of a voxel. ADC measurements have been suggested for staging or as predictive markers. However, its role for staging looks not as promising as its role as a predictor (Sakurada et al. 2009; Aoyagi et al. 2011). Further investigation is warranted to determine the exact role of DWI.
Dynamic contrast enhanced (DCE-) MRI has the ability to show alterations of vascular integrity that result from pathologic angiogenesis. Esophageal cancer is associated with a higher vascularization and an increase in vascular density, compared with normal esophageal tissue. In DCE-MRI, after a bolus of gadolinium chelate is administered intravenously, flow signal and leak can be observed. Two parameters are of importance, the contrast reagent transfer between plasma and interstitial space (Ktrans) and the volume fraction of the interstitial space (Ve). These parameters can help distinguishing normal tissue from tumor tissue. DCE-MRI could have a role in the staging phase as it distinguishes histologic subtypes (Oberholzer et al. 2008). But it also perceives tumor microvascular density changes during chemoradiotherapy and can be imaged by DCE-MRI signal (Chang et al. 2008). Therefore, for monitoring response following CRT it holds the most promise.
2.3.5 Molecular Markers/Signatures
2.3.5.1 Background
Concurrent chemoradiation with or without surgery is commonly utilized as primary management of patients with non-metastatic disease. However, there is significant heterogeneity of response, suggesting that there are sub-populations that derive differential treatment benefit from RT. For example, approximately 30 % of patients experience a complete pathological response (Berger et al. 2005; Donahue et al. 2009). A molecular diagnostic that can identify these patients could be utilized clinically to avoid surgery for these patients. In contrast for patients that are predicted to be less responsive to RT, their management could be impacted by either offering RT dose intensification and/or prioritization of surgery (without RT).
At its most basic, a molecular signature is a collection of features that attempt to explain a complex phenotype. While a single predictive molecular marker would be ideal, such an isolated predictor of response to therapy in esophageal cancer has not been documented. In lieu of such a discovery, the technique of combining multiple analytes provides an opportunity to develop a predictive molecular assay; there continue to be a relatively small number of molecular signatures that are routinely part of clinical practice.
2.3.5.2 Molecular Signature Development
Developing a molecular signature typically involves two steps. In the first step, features (genes, proteins, microRNAs etc.) are selected that define the phenotype of interest (i.e. responders to radiation therapy). Once the features are selected then an algorithm is generated to predict the phenotype in an unknown sample. A classic approach is to use samples in a dataset as a “training set” to identify the features and develop the signature. Once the signature is developed, its predictive accuracy is tested on a validation set, ideally independent of the training set. A significant problem in the field of molecular signatures has been their inherent dependence on the “training set” and thus a lack of robust validation analysis (Watanabe et al. 2006; Dalton and Friend 2006).
2.3.5.3 Radiation Therapy Molecular Signatures
The majority of molecular signatures in the literature have been developed to describe disease prognosis (independent of treatment), molecular subtypes and/or response prediction to chemotherapy. However, two independent groups have developed RT-specific signatures that have considerable clinical validation. Weichselbaum and colleagues developed an interferon-related gene signature for DNA damage, which was independently validated as a predictor of adjuvant chemotherapy efficacy and for local–regional control after RT in breast cancer (Weichselbaum et al. 2008). Separately, Eschrich and colleagues utilized a systems biology approach to identify a molecular signature of intrinsic tumor radiosensitivity (Eschrich et al. 2009a, b). Using ten specific genes they modeled a radiosensitivity index (RSI) that has been independently validated in multiple disease sites (rectal, esophagus, head and neck, breast) in over 1,000 patients. Of the two signatures, RSI has been validated in a small dataset of esophageal cancer patients (n = 12). The predicted RSI was significantly different in responders (R) vs. nonresponders (NR) in esophageal (RSI R vs. NR 0.37 vs. 0.50, p = 0.05). A low RSI value is consistent with a more radiosensitive tumor. The range of RSI values for 7 responders was 0.11–0.53 and for 5 nonresponders was 0.46–0.54. Therefore it is possible that this signature can be adjusted to support specific clinical decisions to improve clinical care for esophageal cancer patients.
2.3.5.4 Clinical Applications for an RT Molecular Signature in Esophageal Cancer
A challenge to the development of a clinically relevant radiosensitivity molecular signature stems from RT’s broad applicability as a therapeutic agent in cancer. Since RT is used in different settings depending on disease site, the clinical utility of the signature would vary depending on the clinical application. A requirement for any signature that is to be applied routinely in the clinic is the development of a standardized and reliable process for tissue acquisition, processing, RNA isolation and gene expression measurement. Recently, the National Cancer Institute selected RSI for commercial development through the recently created Clinical Assay Development Program (CADP). The purpose of the project is the development of an analytically validated, commercial-grade diagnostic platform for RSI that will be ready for testing in clinical trials.
There remains significant opportunity to improve the clinical outcomes for esophageal cancer patients by identifying biological sub-populations that will derive differential treatment benefit from RT. Tailoring RT to fit a particular molecular RT profile will lead to the development of biology-based radiation oncology and result in better RT utilization.
3 Toxicity
The evolution of treatment for locally advanced esophageal cancer from single-modality surgery or radiotherapy to multimodality therapy has resulted in improved outcomes. Unfortunately, CRT comes with a potential increase in toxicity and resultant detriment to a patient’s short-term quality of life (van Meerten et al. 2008).
The risks vs. benefits of the treatment are decisive in patient’s decision to receive CRT. To make a well-considered decision, patients should be counseled before start of treatment about the potential toxicities. We give an overview of potential risks and toxicities for patients receiving multimodality treatment or single modality radiotherapy. We provide parameters, where available, that predict toxicity.
Among the many challenges with estimating toxicity risk based on the available publications, is that toxicity scoring systems are not uniform—making direct comparison impossible. The most frequently used scoring systems for toxicity are the Common Terminology Criteria for Adverse Events (CTCAE) (Trotti et al. 2003) of the National Cancer Institute (NCI) and the toxicity criteria from the Radiation Therapy Oncology Group (RTOG) system.
3.1 Esophagitis
The most common acute and late toxicity, excluding fatigue, for esophageal cancer patients treated with (chemo-) radiotherapy is, as expected, esophagitis.
3.1.1 Acute Esophagitis
Radiation induced acute esophagitis presents as dysphagia with resultant malnutrition and dehydration, requiring nutritional support (enteral or parenteral) in 17–35 % of the patients (Ahn et al. 2005).
The incidence of acute esophagitis (any grade) ranges from 19 to 79 % and grade ≥3 esophagitis is reported in 1–43 % of patients. This broad scale of the reported esophagitis depends, primarily, on the differences in toxicity reporting and definition. Other potential risk factors for esophagitis include dose schedules and treated volume of the esophagus. Finally, the incidence of esophagitis increases with the addition of chemotherapy to radiotherapy and may vary depending on the chemotherapy schedule given (Meluch et al. 2003; Urba et al. 2003; van Meerten et al. 2006; Cooper et al. 1999; Ajani et al. 2008).
3.1.2 Late Esophageal Toxicity
Late esophagitis can present as dysphagia, stricture, necrosis or fistula of the esophagus. The incidence rates of this late toxicity are mainly based on definitive chemoradiation studies. Late esophageal toxicity of any grade occurs in about 35 % of the patients and a grade ≥3 late esophageal toxicity has been seen in 8–21 % of the patients (Cooper et al. 1999).
The strongest predictor for late esophageal toxicity is the severity of acute esophagitis, as a result of consequential late effects. Other predicting parameters for esophageal toxicity are dosimetric.
3.1.3 Parameters Predicting Esophagitis
From lung cancer series we have learned several predicting parameters for acute and late esophageal toxicity. As described earlier, the strongest parameter is the severity of the acute toxicity, but other parameters are a combination of radiation dose and treated volume of the esophagus.
A number of dosimetric parameters have been developed in an effort to reduce the continuously distributed dose-volume histogram (DVH) to a few clinically relevant indices. These relevant indices include: the percent organ volume receiving at least a certain dose (V20Gy, V30Gy, V40Gy); the surface area receiving at least a certain dose (SA20Gy, SA30Gy, SA40Gy); the length of the esophagus included in the radiation field to a threshold dose (LETT20Gy, LETT30Gy, LETT40Gy); the mean esophageal dose (MED), defined as the average dose to the esophagus; and the maximal dose, defined as the highest point-dose within the irradiated esophageal volume (Milano et al. 2007; Rose et al. 2009).
Rose et al. (2009) provided a clear review of 18 lung cancer studies reporting dosimetric parameters predicting esophagitis. They identified 83 unique dosimetric parameters, of which only 6 were evaluated in 5 or more studies that were significantly associated with radiation esophagitis: MED, V20Gy, V30Gy, V40Gy, V45Gy and V50Gy. Correlation was found for acute radiation esophagitis with MED, V20Gy, V30Gy, V40Gy and V45Gy. Correlation with the combined endpoint of acute and chronic radiation esophagitis was found with MED and V50Gy. Logistic models relating DVH parameters to clinically significant acute esophagitis were identified.
For esophageal cancer, dosimetric predictors for esophagitis have not been reviewed with this precision and one could argue that these dosimetric predictors for esophagitis could be similar for esophageal cancer patients as for lung cancer patients. However, the etiology of esophageal cancer is different from lung cancer and the esophagus of esophageal cancer patients has been subjected to other treatment modalities and received greater inflammatory insult than the esophagus of lung cancer patients. Therefore it is not certain if the relatively normal esophagus of lung cancer patients is proportionally as sensitive to radiation damage and may not respond similarly. It may not be appropriate to extrapolate these dosimetric parameters to esophageal cancer patients.
3.2 Pneumonitis
Radiation pneumonitis (RP) is a major adverse event after thoracic irradiation. While the majority of patients with RP present with mild symptoms such as a dry cough, RP can result in severe morbidity and potential mortality.
Incidences of radiation pneumonitis grade ≥2 have been reported as high as 20–22 % (Hsu et al. 2009; Nomura et al. 2012) for esophageal cancer patients treated with definitive chemoradiation.
3.2.1 Parameters Predicting Pneumonitis
Predictive factors for RP have extensively been reported for irradiated lung cancer patients (Vogelius and Bentzen 2012), but there are only a few reports of esophageal cancer available (Hsu et al. 2009; Nomura et al. 2012; Wang et al. 2008; Asakura et al. 2010; Tucker et al. 2006). Predictive parameters for RP in lung cancer patients include clinical factors such as older age, disease located in mid-lower lung (Vogelius and Bentzen 2012), and dosimetric parameters such as mean lung dose (MLD) (Kwa et al. 1998), the percent of lung volume receiving at least 20 Gy (V20Gy), 13 Gy (V13Gy), 10 Gy (V10Gy) or 5 Gy (V5Gy) (Palma et al. 2012).
For esophageal cancer patients, predictors for pulmonary complications were studied on much smaller study cohorts and mainly on studies involving neoadjuvant chemoradiation and surgery. Pulmonary complications in these studies include both radiation pneumonitis as pneumonia, atelectasis, pleural effusion and pulmonary embolism. Significant predictors for pulmonary complications were the clinical parameters: stage IV, induction chemotherapy before CRT (Wang et al. 2006) and impaired pulmonary function (FEV1) before surgery, as well as the dosimetric parameters of mean lung dose, effective dose, V10Gy and absolute volume of lung receiving <5 Gy were significant predictors for pulmonary complications.
Radiation oncologists have to balance all dosimetric and patient specific predictors for pulmonary complications of radiotherapy before approval of the treatment plan.
3.3 Hematologic Toxicity
Hematologic toxicity is the most common side effect in patients treated with chemotherapy. The severity of these adverse events depends on the chemotherapy regimen and number of cycles given.
Generally, these hematologic toxicities consist of neutropenia, anemia, and thrombocytopenia. As expected, regimens that use lower doses and fewer cycles report lower toxicity rates (Urba et al. 2001; Minsky et al. 2002; David 2008).
The most common regimens used are 5-fluorouracil (5-FU) and cisplatin based, but other regimens including carboplatin combined with paclitaxel are increasingly employed, likely due to better patient tolerance (van Hagen et al. 2012). Still, their remains a great variety of chemotherapeutic regimens, prescribed doses and number of cycles used.
In the studies that subdivide hematologic toxicity, the most common toxicity reported is neutropenia and with incidences of 9–78 % (van Hagen et al. 2012; Urba et al. 2001) with the highest incidence of myelotoxicity reported for triple agent chemotherapy regimens.
3.4 Cardiac Toxicity
The most common manifestation of late radiation injury to the heart is pericardial effusion, which may present as acute pericarditis, chronic pericardial effusion, or remain asymptomatic. Although myocardial damage is less frequent, it can result in severe toxicities, such as myocardial infarction.
From long term survivors of Hodgkin lymphoma and left sided breast cancer we have learned that radiation induced cardiac pathology leads to significant morbidity and mortality. With the overall survival of esophageal cancer patients improving, along with increasing numbers of long-term survivors, late cardiac toxicity becomes a growing concern.
In a study of 101 patients treated with definitive chemoradiation for esophageal cancer 28 % developed pericardial effusion and V30Gy was found to be the only significant predictor (Wei et al. 2008). Incidence of cardiopulmonary toxicity has been reported as high as 29 % in elderly compared to 3 % for younger patients (Morota et al. 2009).
Myocardial perfusion defects were detected in 54 % of the esophageal cancer patients treated with radiotherapy compared to 16 % of patients treated with surgery alone, 42 % had mild inferior wall ischemia compared to 4 % of the surgery only group. The perfusion defects were related to the area of the heart receiving ≥45 Gy (Gayed et al. 2006).
Other dosimetric predictors for late symptomatic cardiac toxicity besides V30Gy, V45Gy are mean heart dose (MHD) ≥ 40 Gy (Hashimoto et al. 2008) and thresholds for toxicity defined as V20Gy ≥ 70 %, V30Gy ≥ 65 % and V40Gy ≥ 60 % of the cardiac volume (Konski et al. 2012). Important risk factors for development of symptomatic cardiac toxicity is advanced age and female gender.
Validation of these predictors is necessary before these parameters can be implemented as constraints in treatment planning.
3.5 Quality of Life
It is increasingly recognized that health-related quality of life (HRQoL) is a central and increasingly quantified clinical outcome measure in oncology. Quality of life outcomes are important in new treatment regimens under evaluation or intensified regimens with small benefits in long-term outcomes. In a group of 202 patients comparing multimodality treatment with surgery alone there was a negative impact in HRQoL before surgery but postoperatively the HRQoL was similar to those who had surgery alone (Reynolds et al. 2006). This temporary negative effect of the HRQoL confirmed by the results of van Meerten et all, where the HRQoL scores were restored or even improved 1 year postoperatively (van Meerten et al. 2006). Chemoradiotherapy has a temporary negative effect on the quality of life.
In conclusion, the use of multimodality therapy of esophageal cancer results in a significant negative impact in the short-term quality of life. However, this is a temporarily effect and 1 year after surgery the HRQoL was restored and similar for patients treated with multimodality and surgery-alone.
4 Treatment Technique
Over the past several decades there has been a tremendous evolution of technological advances in radiotherapy treatment planning. Although two dimensional treatment planning was once the standard of care, the implementation of the computed tomography (CT) to treatment planning has made 3D conformal radiation therapy (3D-CRT) possible. With better anatomical visualization and target delineation, this technique created the first step to sparing normal tissue.
Recently, this ‘classic’ 3D-CRT evolved to intensity-modulated radiotherapy (IMRT) using multiple beams, allowing more concave dose distributions around the target volume and therewith avoiding normal structures. IMRT plans improve target conformity and spares organs at risks when compared with 3D-CRT.
Volumetric-arc-therapy (VMAT) is the novel form of IMRT where intensity modulated radiation is delivered during one or more gantry arcs, with continuous variable beam aperture, variable dose rates and gantry speed modulation. This has advantages in terms of simplicity of optimization and fast delivery. This fast delivery results in a shorter beam-on time. Subsequently, with a VMAT plan, as compared to IMRT plan, there is a reduction of the amount of monitor units given of 20–67 % (Vivekanandan et al. 2012; Yin et al. 2012; Van Benthuysen et al. 2011). VMAT further reduces the dose to the heart and lungs and slightly improves the dose coverage to the PTV.
4.1 Parameters to Evaluate Treatment Plans of Different Techniques
Irradiation of esophageal cancer comes with the risk of significant toxicity, with the organs at risk being the heart, lung, esophagus and spinal cord. The aim of the implementation of these novel techniques is to reduce the toxicities to these organs while retaining or improving target coverage.
4.2 Heart
VMAT and IMRT treatment plans reduce the heart dose compared to 3D-CRT. When the parameter V30Gy of the heart is measured, a significant reduction from 55 to 31 % with VMAT vs. 3DCRT (Hashimoto et al. 2008) is seen, for IMRT vs. 3DCRT this is from 61 to 24.8 % (Konski et al. 2012) and a reduction of 33.5 % in favor of VMAT over IMRT (Wei et al. 2008). This dosimetric parameter V30Gy is correlated to symptomatic cardiac disease (Vogelius and Bentzen 2012) and a reduction of the dose to the heart should lead to a reduction in late cardiac toxicity. Long-term studies are necessary to determine the contribution of this dose reduction to the incidence and severity of cardiac toxicity.
4.3 Lung
A similar reduction is also seen for lung doses, where there is a general reduction of the dosimetric parameters (V10Gy, V20Gy, V30Gy) (David 2008; Wei et al. 2008) when comparing VMAT to IMRT. However, there is a slight increase of the mean lung dose (MLD) of 2 % and an increase up to 13 % of the V5Gy in thoracic esophageal tumors (Wei et al. 2008). How this reduction of the V20Gy and increase of V5Gy and MLD will affect pulmonary toxicity remains unclear.
A retrospective review compared 676 patients treated with 3DCRT and IMRT. Treatment modality IMRT (vs. 3DCRT), in addition to known prognostic factors as stage and performance status, was associated with overall survival, locoregional control and noncancer-related death (Lin et al. 2012).
IMRT and VMAT plans result in better target dose coverage, reduces doses to the heart and high dose volumes to the lung and potentially leads to better outcomes. VMAT reduces the amount of monitor units given, reduces the high dose to heart or lung even further, but slightly increases the low radiation dose to body or lungs.
5 Nomograms and Prognostic/Predictive Models
5.1 Goals of Predictive Models in Esophageal Cancer
Clinical prediction tools used in the management of esophageal cancer aim to estimate the likelihood of specified outcomes, both dichotomous and time to event data, based on relevant clinical variables. Particularly for esophageal cancer—due to its baseline poor survival and the high morbidity of the required medical intervention, accurate and applicable estimates of treatment outcomes are essential for medical decision-making, patient counseling, and clinical trial design. Recent randomized and appropriate powered clinical data demonstrates the survival benefit of neoadjuvant chemoradiotherapy (CRT) followed by esophagectomy rather than isolated esophagectomy for specified patient populations (Berger et al. 2005). However, in this investigation, similarly, groups were stratified based on TNM staging and a heterogeneous pathologic response to CRT was observed. The survival benefit observed within the advanced stage patients is not uniformly shared, concentrated within those patients that have a pathologic response and may be entirely absent for those with no observable downstaging.
Estimates of the survival benefit derived from CRT for a particular patient given their demographic information and oncologic staging can be an aid for patient education while aiding in medical decision making and potentially improving outcomes by employing therapies predicted to be the most beneficial for each patient. It is likely that the variables included in relevant decision tools will include data beyond classical TNM staging and may ultimately involve molecular markers and novel post treatment restaging data (molecular and genetic markers were covered already in this chapter and will not be discussed in this subsection).
Standard pathologic TNM staging from the surgical specimen following neoadjuvant CRT (ypTNM) continues to be more prognostic of survival than any restaging or pre operative data—despite a, currently unpredictable, variable downstaging effect. A neoadjuvant treatment strategy makes estimating the survival benefit of chemoradiotherapy based on surgical stage difficult given that the final pathologic stage may not be the same as the stage at diagnosis. Concurrently, current techniques for clinical TNM staging and restaging techniques are unreliable.
Tools beyond predicting response and survival benefit to neoadjuvant CRT are required to model the risk for an individual patient undergoing or abstaining from some portion of trimodal therapy. Accurately predicting an individual patient’s risk of morbidity and mortality from each treatment, particularly esophagectomy, will be an important tool in recommending an individualized treatment plan.
While nomograms are available for many of the aspects of esophageal cancer treatment mentioned above, careful examination of the data used, the covariates analyzed, and the patients included is required to avoid inappropriate application of a prediction tool to a specific patient. The general technical considerations of nomogram creation, propensity weighting, regression analysis, and data interpretation are covered separately in "Statistics of Survival Prediction and Nomogram Development'' of this text and will not specifically be addressed here.
5.2 Predicting Benefit from Neoadjuvant Chemoradiotherapy
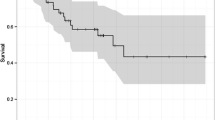
Accurately predicting the benefit for a specific patient and evaluating the response to neoadjuvant treatment continues to a challenge and potential point of controversy. Although already discussed in this chapter, it is appropriate to again address the recent evidence supporting neoadjuvant CRT in subpopulations of those affected with esophageal cancer. A recently published, appropriately powered, randomized controlled trial from van Hagen et al. (van Hagen et al. 2012) evaluating patients with disease beyond T1N0 and up to T3N1 based on clinical staging, demonstrated an overall statistically significant survival benefit, seen below in Fig. 1a (all patients) and 1b (stratified by histology). Overall, patients receiving trimodality therapy had a median survival of 49.4 vs. 24.0 months in the surgery only group (p = 0.003).
Kaplan–Meier plots of estimated overall 5-year survival. a Scohows a Kaplan–Meier plot of the estimated overall 5-year survival among patients with esophageal or esophagogastric-junction cancer who underwent neo-adjuvant chemoradiotherapy (CRT) followed by surgery (178 patients) or surgery alone (188), acrding to an intention-to-treat analysis. b Shows a Kaplan–Meier plot of the estimated overall 5-year survival among the 134 patients with adenocarcinoma (AC) treated with neoadjuvant chemoradiotherapy followed by surgery and the 141 treated with surgery alone, and the 41 patients with squamous-cell carcinoma (SCC) treated with chemoradiotherapy followed by surgery and the 43 treated with surgery alone, according to an intention-to-treat analysis. Other tumor types were excluded from this analysis. Adapted from van Hagen et al. (2012)
Traditional Kaplan–Meier survival analysis demonstrates that their selected population will have an overall survival benefit from neoadjuvant CRT. When performing subgroup survival analysis, this benefit remained statistically significant both for adenocarcinoma (AC) and for squamous cell (SCC) histology.
However, the survival benefit of chemoradiotherapy is likely not distributed evenly across all patients. Those patients who have a pathologic complete response (pCR), with no residual tumor identifiable in the surgical specimen, or even those with partial response following neoadjuvant CRT have a better prognosis than those that have no appreciable or minimal response to CRT. Identification of a molecular or histologic marker predictive of response to CRT, similar to the RSI discussed above, would provide useful adjunctive clinical information. Several institutions have attempted to predict clinical surrogates for benefit from CRT (Ajani et al. 2012). However, ypTNM remains a useful prognosticator of outcome and was recently employed in the construction of a predictive web based tool (Eil et al. 2013).
While the prognostic power of the ypTNM has been validated previously (Holmes and Vaughan 2007) the exact benefit of neoadjuvant CRT on patients by their documented ypTNM has not been examined. Eil et al. (2013) proposed a survival prediction tool applicable to resected patients with or without neoadjuvant CRT based on a SEER-Medicare database of 824 patients. The multivariate regression coefficients and OR are shown below in Table 8. A web browser based nomogram was built from this model to create individual estimates of survival and is available at http://skynet.ohsu.edu/nomograms/.
The beta coefficients and odd ratios predicted from the regression model are represented in Table 8. The predicted survival benefit from neoadjuvant CRT persisted for advanced stage disease present after treatment (Eil et al. 2013). For example, based upon the model, a 70 year old male with adenocarcinoma and 12 lymph nodes harvested with ypT4N2 stage having received neoadjuvant CRT would have a predicted 3 year OS of 29 vs. 12 % without CRT. A similar patient with ypT2N1 disease is predicted to have a 3 year OS of 64 % with neoadjuvant CRT vs. 45 % with isolated esophagectomy (Eil et al. 2013).
This analytic tool, being based on pathologic stage, is most applicable in the post-operative setting—when the ypTNM stage is available for postoperative counseling, comparison, and treatment planning. Additionally, such risk modeling is helpful in the design of research protocols for identifying homogenous high risk groups. One would expect the model to underestimate of the benefit of neoadjuvant therapy due to its expected downstaging effect on ypTNM as compared to cTNM. The ultimate goal of a predictive decision aid for designing an individualized treatment course would include early identification, or even prediction, of responders and non-responders—leading to avoidance of ineffective and dangerous application of both chemoradiotherapy and surgery.
With the above predictive tool, one can estimate the benefit of neoadjuvant CRT based on the final pathologic stage. However, this definitive information is not available when considering whether to administer neoadjuvant treatment. Pre-treatment knowledge, or at least likelihood of nodal status would aid in guiding treatment—as patients N1 or greater benefit significantly from neoadjuvant treatment (Eil et al. 2013). In 2010, with a training sample of 164 patients resected with curative intent at M.D. Anderson Cancer Center and excluding those that received neoadjuvant therapy Gaur and colleagues developed a tool for predicting nodal involvement based on preoperative clinical characteristics (Gaur et al. 2010). The nomogram was validated externally, showing a concordance index (CI) of 0.77. Their predictive tool, represented in Fig. 2 below, was adapted into a traditional point system with the strongest clinical indicator being valued at 100 points and other variables being weighed against this as described by Jasonos et al. (2008).
Nomogram to predict pathologic lymph node involvement (path N1) using clinical measurements for M. D. Anderson training set. The nomogram consists of six rows. Row 1 (points row) is the point assignment for each variable. Rows 2–4 correspond to the variables included in the model. For an individual patient, each variable in rows 2–4 is assigned a point value, which is determined by drawing a vertical line from the appropriate position on the variable row to the points row. The assigned points for all three variables are added, and the total is marked in row 5 (total points). Then, the risk of path N1 is calculated by drawing a vertical line from the appropriate position on the total-points row to the final row (predicted path N1 probability). Point scale nomogram adapted from Gaur et al. (2010)
In reviewing their point scale weighting, the most heavily weighted variable was tumor length, which the authors chose to dichotomize at 2 cm. Clinical evaluation of nodal status (cN), or EUS, was not significantly associated with pathologic nodal status on multivariate analysis, with an OR of 1.5 (0.5–5.5; p = 0.5). This predictive tool estimates that a cT2N0 patient with a tumor that measures <2 cm has only a 27 % chance of having N1 disease, while a cT2N0 patient with a tumor >2 cm has a 72 % of having N1 disease. This is troubling data given the inherent clinical confidence most place in EUS data, as well as the need for certainty in predicting nodal status—which many weight heavily when determining whether to offer neoadjuvant therapy. Additionally, the lowest risk oncologic features as measured by Gaur’s nomogram, a T2 patient maintains a 27 % risk of having nodal disease. Many would consider this a high enough likelihood to consider CRT, given the documented benefit. More precise predictive tools are required to narrow the spectrum of patients considered for trimodal treatment.
5.3 Predicting Pathologic Complete Response After Neoadjuvant Chemoradiotherapy
The ability to confidently predict which patients with an apparent clinical CR (cCR) will have a pCR would provide an opportunity to further stratify which patients may not obtain a survival benefit from esophagectomy following chemoradiotherapy. However, a pCR does not guarantee disease free survival. Many patients with a documented pCR have documented disease recurrence and ultimately succumb to their disease. However, the prognosis is significantly improved and the likelihood of a local failure is greatly reduced for patients with no residual disease when compared to all other patients with residual disease (Chirieac et al. 2005; Donahue et al. 2009; Brucher et al. 2006). Those patients that respond with a pCR, approximately 30 % of those undergoing neoadjuvant CRT, demonstrate a 55 % 5 year survival vs. 34 % for all patients treated with neoadjuvant CRT followed by surgery. The prognostic power of the pathologic response has been reproducible, with some advocating for its addition to the traditional TNM stage (Swisher et al. 2005). Of those who achieve a preoperative cCR, a small minority have a true pCR. Some are found to have a partial response, and others have no demonstrable response to neoadjuvant CRT in comparison to estimated pretreatment TNM (cTNM). However, based on the SEER-Medicare based nomogram discussed above, even patients with advanced ypTNM stage benefitted from neoadjuvant CRT (Eil et al. 2013). Given the reliable prognostic power of response to CRT, the accuracy of the clinical stage found prior to neoadjuvant treatment is critical to ultimately determining which strategy will result in the greatest survival benefit.
Several series have demonstrated post neoadjuvant CRT restaging techniques to be concerningly inaccurate. Post treatment biopsies have an accuracy approaching 30 % and are not prognostic of outcome. Post treatment endoscopic biopsy reveals no residual malignancy in approximately 80 % of patients, while the incidence of pCR is 25–30 %.
Regarding the accuracy of EUS ycTNM, Kalha and colleagues performed a retrospective review of 83 patients from MD Anderson revealed that EUS restaging correctly identified the T stage in only 29 % of patients (Kalha et al. 2004). The sensitivity for detecting nodal disease was only 51 %. Of the 22 patients who responded to the neoadjuvant chemoradiotherapy with a complete pathologic response, 19 were restaged by EUS as having residual disease. Given the above findings, some have endeavored to find modes of post treatment evaluation other than clinical restaging to estimate the patient’s state of disease and response to treatment.
Of the diagnostic modalities currently available, the change in PET standard uptake value (SUV) before and after treatment has proven to be the most reliable indicator of response (Ajani et al. 2012). In the setting of these diagnostic limitations, at the end of 2012 a predictive nomogram with an end point of pCR based on clinical parameters following CRT was released based on the institutional database of MD Anderson. The strongest predictor of pCR was the SUV after treatment. Unfortunately, PET scanning calibration is not standardized across institutions. In the face of these limitations, they produced a model with a bias-corrected area under the curve (AUC) of 0.7 (95 % CI = 0.64–0.73). Figure 3 represents their point scale nomogram.
The nomogram consists of eight rows. Row 1 (points row) is the point assignment for each variable. Rows 2–6 correspond to the variables included in the model. For an individual patient, each variable is assigned a point value, which is determined by drawing a vertical line from the appropriate position on the variable row to the points row. The demonstrates that combining variables can increase the probability of predicting pathCR to as high as 80 % if a patient scores >160 points. Among the most influential factors for attaining the highest scores for predicting pathCR were lower postchemoradiation SUVmax and the absence of cancer cells on postchemoradiation biopsy specimens. Point scale nomogram adapted from Ajani et al. (2012)
While these predictive tools hold promise for improving individualized treatment regimens, their discriminatory power is not yet such that one could depend upon it for embarking upon an esophageal preservation strategy for a specific patient. Molecular markers and oncogenetics hold promise to increase our predictive power in the future. This topic is discussed elsewhere in this chapter and will not be addressed in this section.
5.4 Predicting Survival for Patients Receiving Definitive Chemoradiotherapy
A tool predicting survival for patients unfit, unwilling, or of too advanced stage for surgery may not intuitively be of value—what decision is there to make when the planned treatment is already determined? However, when one considers the purpose of a nomogram and brings the information provided by such a tool into the larger clinical arena, it could advance all of the goals of a clinical prediction tool: aid in medical decision making, provide straightforward information for patients, supply baseline outcomes to aid in research protocol design, and potentially a baseline to compare against similar patients who did undergo CRT followed by surgery.
In contemplating the benefit of an esophageal preservation strategy for some patients based on their cCR and other appropriate variables, a predictive survival tool for those that have received definitive CRT would be immediately useful. Using their institutional clinical database including 257 patients undergoing definitive CRT Suzuki et al. (2012) produced a nomogram predicting benefit from definitive CRT. Similarly to Ajani et al., discussed above, for predicting pCR, PET SUV was a significant predictor of outcome. However, here post treatment endoscopic biopsy was the strongest predictor of OS following treatment, as no pathologic staging data was available. Their final CI was 0.7 for predicting OS. Given the baseline complex medical history, making many of them inappropriate for operative intervention, OS analysis may be confounded by deaths due to non-oncologic etiologies (Fig. 4).
Row 1 (points row) is the point assignment for each variable. Rows 2–6 correspond to the variables included in the model. For an individual patient, each variable is assigned a point value, which is determined by drawing a vertical line from the appropriate position on the variable row to the points row. Point scale nomogram adapted from Suzuki et al. (2012)
5.5 Predicting Perioperative Mortality
As part of a comprehensive strategy to correctly predict which patients will benefit from treatment, one must ultimately incorporate the potential mortality from treatment itself. Several nomograms for prediction of complications following esophagectomy have been published and are discussed below. As a general assertion one can say that these nomograms have been difficult to apply outside of their home institution due to the multifactorial etiology of the end outcomes, differing patient populations, operative techniques, and post operative management.
In 2006 Steyerberg et al. (2008) reported a predictive model for mortality following esophagectomy based on SEER-Medicare data from 1991 to 1996 and validated on several other cohorts. Perhaps due to the complex multifactorial nature of post operative outcomes, the Medicare database, and the low incidence of perioperative mortality the predictive power was low—a CI of 0.58 when externally validated.
Table 9 below shows the variables considered in their nomogram with Fig. 5 graphically demonstrating the estimated 30-day perioperative mortality.
While the above model is not independently adequate for medical decision-making in its current state with a CI of 0.58, it provides a template for a more precise prediction tool. A more accurate, perhaps institution specific, prediction tool for perioperative mortality may be of use. Such a tool could be of use in counseling high-risk patients with advanced stage to avoid esophagectomy based on a high estimate of postoperative mortality. Additionally, a perioperative mortality risk prediction tool may be used in conjunction with those predicting survival after cCR from chemoradiotherapy—identifying those patients most appropriate for esophageal salvage (Fig. 6).
Estimated surgical mortality in relation to the sum cumulative score that can be obtained from Table 9. The 95 % CIs are based on analysis of four cohorts, containing 3,592 patients undergoing surgery for esophageal cancer using the aforementioned scale and formula. Adapted from Steyerberg et al. (2008)
6 Summary
While esophageal cancer continues to claim the lives of a significant number of those that it affects, aggressive trimodal treatment strategies within a targeted population has resulted in progressive increases in survival. However, neoadjuvant CRT followed by esophagectomy has the potential to provide significant survival benefit to patients whose tumor biology is responsive to CRT, despite advanced disease. However, the oncologic response to treatment is heterogenous across and within clinically stratified treatment groups. Accurately identifying those patients that will benefit from aggressive treatment, and sparing non-responders the risks associated with trimodality treatment will depend on novel utilization of existing prognostic tools and the development of additional assays.
References
Ahn SJ, Kahn D, Zhou S et al (2005) Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys 61(2):335–347
Ajani JA, Winter K, Komaki R et al (2008) Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 26(28):4551–4556
Ajani JA, Correa AM, Hofstetter WL et al (2012) Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 23(10):2638–2642
Aoyagi T, Shuto K, Okazumi S, Shimada H, Kazama T, Matsubara H (2011) Apparent diffusion coefficient values measured by diffusion-weighted imaging predict chemoradiotherapeutic effect for advanced esophageal cancer. Dig Surg 28(4):252–257
Asakura H, Hashimoto T, Zenda S et al (2010) Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol 95(2):240–244
Berger AC, Farma J, Scott WJ et al (2005) Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 23(19):4330–4337
Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF (1991) Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 181(2):419–425
Bowrey DJ, Clark GW, Roberts SA et al (1999) Serial endoscopic ultrasound in the assessment of response to chemoradiotherapy for carcinoma of the esophagus. J Gastrointest Surg 3(5):462–467
Brucher BL, Becker K, Lordick F, Fink U, Sarbia M, Stein H, Busch R, Zimmerman F, Molls M, Hofler H, Siewart JR (2006) The clinical impact of histopathologic response assessment by residual tumor cell quantification in esopahgeal squamous cell carcinomas. Cancer 106(10):2119–2126
Bruzzi JF, Swisher SG, Truong MT et al (2007) Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer 109(1):125–134
Burmeister BH, Smithers BM, Gebski V et al (2005) Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 6(9):659–668
Chak A, Canto MI, Cooper GS et al (2000) Endosonographic assessment of multimodality therapy predicts survival of esophageal carcinoma patients. Cancer 88(8):1788–1795
Chang EY (2009) Esophageal cancer: principles and practice. Demos Medical Publisher, New York
Chang EY, Li X, Jerosch-Herold M et al (2008) The evaluation of esophageal adenocarcinoma using dynamic contrast-enhanced magnetic resonance imaging. J Gastrointest Surg 12(1):166–175
Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT (2005) Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 103:1347–1355
Cooper JS, Guo MD, Herskovic A et al (1999) Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 281(17):1623–1627
Dalton WS, Friend SH (2006) Cancer biomarkers—An invitation to the table. Science 312:1165–1168
David IH (2008) Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res 2:85–92
Dikken JL, Coit DG, Klimstra DS et al (2012) Prospective impact of tumor grade assessment in biopsies on tumor stage and prognostic grouping in gastroesophageal adenocarcinoma: relevance of the seventh edition American joint committee on cancer staging manual revision. Cancer 118(2):349–357
Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, Jatoi A, Miller RC, Wigle DA, Shen R, Deschamps C (2009a) Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enchanced survival. Ann Thoracic Surg 87(2):392–399
Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, Jatoi A, Miller RC, Wigle DA, Shen R, Deschamps C (2009b) Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enchanced survival. Ann Thoracic Surg 87(2):392–399
Eil R, Diggs BS, Wang SJ, Dolan J, Hunter JG, Thomas CR (2013) Nomogram for predicting the benefit of neoadjuvant chemoradiotherapy for esophageal cancer – A SEER – medicare analysis. Presented: ASCO Annual Meeting, 31 May to 4 June 2013, Chicago
Eschrich S, Zhang H et al (2009a) Systems biology modeling of the radiation sensitivty network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys 75(2):497–505
Eschrich S, Pramana J et al (2009b) A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys 75(2):489–496
Fockens P, Van den Brande JH, van Dullemen HM, van Lanschot JJ, Tytgat GN (1996) Endosonographic T-staging of esophageal carcinoma: a learning curve. Gastrointest Endosc 44(1):58–62
Gaur P, Sepesi B, Hofstetter WL, Correa AM, Bhutani MS, Vaporciyan AA, Watson TJ, Swisher SG (2010) A clinical nomogram predicting pathologic lymph node involvement in esophageal cancer patients. Ann Surg 252(4):611–617
Gayed IW, Liu HH, Yusuf SW et al (2006) The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med 47(11):1756–1762
Giovannini M, Seitz JF, Thomas P et al (1997) Endoscopic ultrasonography for assessment of the response to combined radiation therapy and chemotherapy in patients with esophageal cancer. Endoscopy 29(1):4–9
Griffith JF, Chan AC, Chow LT et al (1999) Assessing chemotherapy response of squamous cell oesophageal carcinoma with spiral CT. Br J Radiol 72(859):678–684
Hashimoto T, Asakura H, Zenda S et al (2008) Cardiac toxicities after concurrent chemoradiotherapy for esophageal cancer- dose volume histogram. Int J Radiat Oncol Biol Phys 72(1):S130
Heath EI, Burtness BA, Heitmiller RF et al (2000) Phase II evaluation of preoperative chemoradiation and postoperative adjuvant chemotherapy for squamous cell and adenocarcinoma of the esophagus. J Clin Oncol 18(4):868–876
Hirata N, Kawamoto K, Ueyama T, Masuda K, Utsunomiya T, Kuwano H (1997) Using endosonography to assess the effects of neoadjuvant therapy in patients with advanced esophageal cancer. AJR Am J Roentgenol 169(2):485–491
Holmes RS, Vaughan TL (2007) Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol 17(1):2–9
Hsu FM, Lee YC, Lee JM et al (2009) Association of clinical and dosimetric factors with postoperative pulmonary complications in esophageal cancer patients receiving intensity-modulated radiation therapy and concurrent chemotherapy followed by thoracic esophagectomy. Ann Surg Oncol 16(6):1669–1677
Jamil LH, Gill KR, Wallace MB (2008) Staging and restaging of advanced esophageal cancer. Curr Opin Gastroenterol 24(4):530–534
Jasonos A, Schrag D, Raj G, Panageas K (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26:1364–1370
Jones DR, Parker LA Jr, Detterbeck FC, Egan TM (1999) Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 85(5):1026–1032
Kalha I, Kaw M, Fukami N, Patel M, Singh S, Gagneja H, Cohen D, Morris J (2004) The accuracy of endoscopic ultrasound for restaging esophageal carcinoma after chemoradiation therapy. Cancer 101(5):940–947
Katsoulis IE, Wong WL, Mattheou AK, Damani N, Chambers J, Livingstone JI (2007) Fluorine-18 fluorodeoxyglucose positron emission tomography in the preoperative staging of thoracic oesophageal and gastro-oesophageal junction cancer: a prospective study. Int J Surg 5(6):399–403
Konski A, Li T, Christensen M et al (2012) Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol 104(1):72–77
Kwa SL, Lebesque JV, Theuws JC et al (1998) Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 42(1):1–9
Lin SH, Wang L, Myles B et al (2012) Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 84(5):1078–1085
Lowe VJ, Booya F, Fletcher JG et al (2005) Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 7(6):422–430
Meluch AA, Greco FA, Gray JR et al (2003) Preoperative therapy with concurrent paclitaxel/carboplatin/infusional 5-FU and radiation therapy in locoregional esophageal cancer: final results of a Minnie Pearl cancer research network phase II trial. Cancer J 9(4):251–260
Milano MT, Constine LS, Okunieff P (2007) Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol 17(2):131–140
Minsky B, Pajak T, Ginsberg R, Pisansky T, Martenson J, Komaki R, Okawara G, Rosenthal S, Kelsen D (2002) INT 0123 (radiation therapy oncology group 94–05) phase III trial of combined mortality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. JCO 20:1167–1174
Morota M, Gomi K, Kozuka T et al (2009) Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 75(1):122–128
Nomura M, Kodaira T, Furutani K, Tachibana H, Tomita N, Goto Y (2012) Predictive factors for radiation pneumonitis in oesophageal cancer patients treated with chemoradiotherapy without prophylactic nodal irradiation. Br J Radiol 85(1014):813–818
Oberholzer K, Pohlmann A, Schreiber W et al (2008) Assessment of tumor microcirculation with dynamic contrast-enhanced MRI in patients with esophageal cancer: initial experience. J Magn Reson Imaging 27(6):1296–1301
Omloo JM, van Heijl M, Hoekstra OS, van Berge Henegouwen MI, van Lanschot JJ, Sloof GW (2011) FDG-PET parameters as prognostic factor in esophageal cancer patients: a review. Ann Surg Oncol 18(12):3338–3352
Palma DA, Senan S, Tsujino K, et al (2012) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85(2):444–450
Reid TD, Chan DS, Roberts SA, Crosby TD, Williams GT, Lewis WG (2012) Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer 107(12):1925–1931
Reynolds JV, McLaughlin R, Moore J, Rowley S, Ravi N, Byrne PJ (2006) Prospective evaluation of quality of life in patients with localized oesophageal cancer treated by multimodality therapy or surgery alone. Br J Surg 93(9):1084–1090
Ribeiro A, Franceschi D, Parra J et al (2006) Endoscopic ultrasound restaging after neoadjuvant chemotherapy in esophageal cancer. Am J Gastroenterol 101:1216–1221
Rice TW, Blackstone EH, Rusch VW (2010) 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol 17(7):1721–1724
Riddell AM, Allum WH, Thompson JN, Wotherspoon AC, Richardson C, Brown G (2007) The appearances of oesophageal carcinoma demonstrated on high-resolution, T2-weighted MRI, with histopathological correlation. Eur Radiol 17(2):391–399
Rizk NP, Seshan VE, Bains MS et al (2007a) Prognostic factors after combined modality treatment of squamous cell carcinoma of the esophagus. J Thorac Oncol 2(12):1117–1123
Rizk NP, Venkatraman E, Bains MS et al (2007b) American joint committee on cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol 25(5):507–512
Rose J, Rodrigues G, Yaremko B, Lock M, D’Souza D (2009) Systematic review of dose-volume parameters in the prediction of esophagitis in thoracic radiotherapy. Radiother Oncol 91(3):282–287
Sakurada A, Takahara T, Kwee TC et al (2009) Diagnostic performance of diffusion-weighted magnetic resonance imaging in esophageal cancer. Eur Radiol 19(6):1461–1469
Sarkaria IS, Rizk NP, Bains MS et al (2009) Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg 249(5):764–767
Siegel R, DeSantis C, Virgo K et al (2012) Cancer treatment and survivorship statistics. CA Cancer J Clin 62(4):220–241
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85(11):1457–1459
Situ D, Wei W, Lin P, et al (2012) Do tumor grade and location affect survival in esophageal squamous cell carcinoma? Survival analysis of 302 cases of pT3N0M0 esophageal squamous cell carcinoma. Ann Surg Oncol 20:580–585
Sloof GW (2006) Response monitoring of neoadjuvant therapy using CT, EUS, and FDG-PET. Best Pract Res Clin Gastroenterol 20(5):941–957
Stahl A, Ott K, Weber WA et al (2003) FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 30(2):288–295
Streyerberg EW, Neville BA, Koppert LB, Lemmens VE, Tilanus HW, Coebergh JW, Weeks JC, Earle CC (2008) Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. JCO 24:4277–4283
Suzuki A, Xiao L, Hayashi Y, Blum M, Welsh J, Lin S, Lee J, Bhutani M, Weston B, Maru D, Rice D, Swisher S, Hostetter W, Erasmus J, Ajani JA (2012) Nomograms for prognostication of outcome in patients with esophageal and gastroesophageal carcinoma underoing definitive chemoradiotherapy. Oncology 82:108–113
Swisher SG, Maish M, Erasmus JJ, et al (2004) Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 78(4):1152–1160; discussion 1152–1160
Swisher S, Hofstetter W, Wu T, Correa AM, Ajani JA, Komaki RR, Chirieac L, Hunt K, Liao Z, Phan A, Rice D, Vaporciyan AA, Walsh G, Roth J (2005) Proposed revision of the esophgeal cancer staging sytem to accommodate pathologic following preoperative chemoradiation. Ann Surg 241(5):810–820
Talsma K, van Hagen P, Grotenhuis BA et al (2012) Comparison of the 6th and 7th editions of the UICC-AJCC TNM classification for esophageal cancer. Ann Surg Oncol 19(7):2142–2148
Thosani N, Singh H, Kapadia A et al (2012) Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc 75(2):242–253
Thrift AP, Nagle CM, Fahey PP et al (2012) The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer 131(5):E759–E768
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181
Tucker SL, Liu HH, Wang S et al (2006) Dose-volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 66(3):754–761
Union Internationale Contre le Cancer (2002) TNM classification of malignant tumours, 6th edn. Wiley-Liss, New York
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M (2001) Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19(2):305–313
Urba SG, Orringer MB, Ianettonni M, Hayman JA, Satoru H (2003) Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer 98(10):2177–2183
Van Benthuysen L, Hales L, Podgorsak MB (2011) Volumetric modulated arc therapy vs. IMRT for the treatment of distal esophageal cancer. Med Dosim 36(4):404–409
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
van Meerten E, Muller K, Tilanus HW et al (2006) Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br J Cancer 94(10):1389–1394
van Meerten E, van der Gaast A, Looman CW, Tilanus HW, Muller K, Essink-Bot ML (2008) Quality of life during neoadjuvant treatment and after surgery for resectable esophageal carcinoma. Int J Radiat Oncol Biol Phys 71(1):160–166
van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD (2008) Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98(3):547–557
Vivekanandan N, Sriram P, Kumar SA, Bhuvaneswari N, Saranya K (2012) Volumetric modulated arc radiotherapy for esophageal cancer. Med Dosim Spring 37(1):108–113
Vogelius IR, Bentzen SM (2012) A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 51(8):975–983
Voncken FE, Jiang H, Kim J, et al (2012) Degree of tumor shrinkage following neoadjuvant chemoradiotherapy: a potential predictor for complete pathological response in esophageal cancer? Dis Esophagus. doi: 10.1111/j.1442-2050.2012.01445.x
Wagner TD, Javie M, Yang G (2009) Esophageal cancer: principles and practice. Demos Medical Publisher, New York
Wang SL, Liao Z, Vaporciyan AA et al (2006) Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 64(3):692–699
Wang S, Liao Z, Wei X et al (2008) Association between systemic chemotherapy before chemoradiation and increased risk of treatment-related pneumonitis in esophageal cancer patients treated with definitive chemoradiotherapy. J Thorac Oncol 3(3):277–282
Watanabe T, Komuro Y, Kiyomatsu T, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Shirane M, Muto T, Nagawa H (2006) Prediction of sensitivity of rectal cancer cells in response to preoperative radio- therapy by DNA microarray analysis of gene expression profiles. Cancer Res 66:3370–3374
Wei X, Liu HH, Tucker SL et al (2008) Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 70(3):707–714
Weichselbaum RR, Ishwaranc H et al (2008) An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. PNAS 105:18490–18495
Westerterp M, van Westreenen HL, Reitsma JB et al (2005) Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology 236(3):841–851
Wong WL, Chambers RJ (2008) Role of PET/PET CT in the staging and restaging of thoracic oesophageal cancer and gastro-oesophageal cancer: a literature review. Abdom Imaging 33(2):183–190
Wu LF, Wang BZ, Feng JL et al (2003) Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 9(2):219–224
Yin L, Wu H, Gong J et al (2012) Volumetric-modulated arc therapy vs. c-IMRT in esophageal cancer: a treatment planning comparison. World J Gastroenterol 18(37):5266–5275
Zuccaro G Jr, Rice TW, Goldblum J et al (1999) Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 94(4):906–912
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Eil, R.L., Voncken, F.E.M., Torres-Roca, J., Thomas, C.R. (2013). Esophageal Cancer. In: Nieder, C., Gaspar, L. (eds) Decision Tools for Radiation Oncology. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2013_919
Download citation
DOI: https://doi.org/10.1007/174_2013_919
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37101-1
Online ISBN: 978-3-642-37102-8
eBook Packages: MedicineMedicine (R0)