Abstract
Distress, or negative stress, is known to considerably increase the incidence of several diseases, including cancer. There is indeed evidence from pre-clinical models that distress causes a catecholaminergic overdrive that, mainly through the activation of β-adrenoceptors (β-ARs), results in cancer cell growth and cancer progression. In addition, clinical studies have evidenced a role of negative stress in cancer progression. Moreover, plenty of data demonstrates that β-blockers have positive effects in reducing the pro-tumorigenic activity of catecholamines, correlating with better outcomes in some type of cancers as evidenced by several clinical trials. Among β-ARs, β2-AR seems to be the main β-AR subtype involved in tumor development and progression. However, there are data indicating that also β1-AR and β3-AR may be involved in certain tumors. In this chapter, we will review current knowledge on the role of the three β-AR isoforms in carcinogenesis as well as in cancer growth and progression, with particular emphasis on recent studies that are opening new avenues in the use of β-ARs as therapeutic targets in treating tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cancer cell proliferation
- Carcinogenesis
- Catecholamines
- Dedifferentiation
- Immune-tolerance
- Stress
- Tumor growth
- Tumor infiltration
- Tumor microenvironment
1 Introduction
From an evolutionary point of view, animals need to develop strategies to face environmental changes that may impact on their lives. In particular, the exposure to a stressful environment triggers homeostatic responses aiming at facing the deriving perturbation. In this respect, it is known that the nature of the stress may influence the nature of the response, with acute stressors mainly inducing positive effects while chronic stressors leading to deleterious outcomes (Jessop 2019). David Livingstone, in 1857, had a direct experience of positive responses to stress: “[…] I heard a shout. Starting, and looking half round, I saw the lion just in the act of springing upon me. I was upon a little height; he caught my shoulder as he sprang, and we both came to the ground below together. Growling horribly close to me ear, he shook me as a terrier dog as a rat. The shock produced a stupor similar to that which seems to be felt by a mouse after the first shake of the cat. It caused a sort of dreaminess, in which there was no sense of pain nor feeling of terror, though quite conscious of all that was happening. It was like what patients partially under the influence of chloroform describe, who see all the operation, but feel not the knife. This singular condition was not the result of any mental process. The shake annihilated fear, and allowed no sense of horror in looking round at the beast. This peculiar state is probably produced in all animals killed by the carnivora and, if so, is a merciful provision by our benevolent Creator for lessening the pain of death” (Livingstone 1857). In the case of Dr. Livingstone (we presume, of course), the stressful condition acted on pain receptors, enkephalins and possible additional players that are not part of the present story, which is instead based on adrenoceptors. And, particularly, on the response that adrenoceptors evoke when an individual is exposed to chronic stress conditions, as chronic stress (distress), may induce illness states.
2 Stress and Cancer
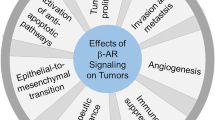
The homeostatic response to stressors involves two different, although inter-related, systems: the hypothalamus-pituitary-adrenal axis and the sympathetic nervous system. Perceiving stress results in the activation of these pathways, whose dysregulation is responsible for an increased risk of developing diseases, including cancer (Flaherty et al. 2019). As recently reviewed, preclinical data seem to point on a pro-carcinogenic role of stress hormones, although clinical studies remain inconclusive about this point, suggesting instead a role of stress in cancer progression (Mravec et al. 2020a). Among stress hormones, there is extensive evidence that norepinephrine and epinephrine may modulate both cancer cell biology and the tumor microenvironment, whose strict relationship with cancer cells is paramount in cancer progression (Mravec et al. 2020b). It is of note that catecholamines may modulate tumor cells with opposite effects, giving rise to the recently defined “cancer catecholamine conundrum” (Wackerhage et al. 2022). In fact, it has been suggested that, for instance in the context of exercise, catecholamines may have a positive effect on cancer, possibly linked to the induction of a eustress condition, that is a stress condition having beneficial effects on health. In this respect, mice bearing liver cancer raised in condition of enriched environment, a condition known to produce eustress, showed an increased antitumor immunity and reduced malignant progression with respect to mice raised in standard condition (Liu et al. 2021). Similarly, exercise training in mice reduced the growth of melanoma xenotransplant by about 60% with respect to untrained mice, due to induction of migration and activation of immune cells into the tumor mass (Pedersen et al. 2016). In contrast, chronic distress, such as psychosocial stress, has been associated to tumor development or to tumor progression, as evidenced both in pre-clinical models and in humans (see for Ref. Wackerhage et al. 2022). In particular, many studies indicate that catecholamines stimulate cancer cell growth and cancer progression mainly acting at β-adrenoceptors (β-ARs) (Mravec et al. 2020b). The first evidence indicating a role of β-ARs in tumor growth dates back to the late ‘80s, when Schuller and Cole showed that human lung adenocarcinoma cells proliferate when stimulated with isoprenaline, an effect blunted by propranolol (Schuller and Cole 1989). After that, plenty of data demonstrated that norepinephrine stimulates the proliferation of different types of cancer cells and induces several hallmarks of cancer, including cell proliferation, cell migration and angiogenesis. In addition, β-blockers reduce the pro-tumorigenic effect of stress hormones, decreasing tumor growth in preclinical models and reducing mortality and recurrence in tumor patients (see for Refs. Mravec et al. 2020a; b, c; Gosain et al. 2020; Dal Monte et al. 2019). However, in clinical trials the use of β-blockers correlates with better outcomes only in specific types of cancer, such as melanoma and ovarian cancer, but not in breast, colorectal or lung cancer (Musselman et al. 2018; Yap et al. 2018). Given that differential β-AR subtype expression is found in cancer cells, and that the activation of these receptors in different cancer types has diverse effects on tumor proliferation, migration, and invasion (Tang et al. 2013), one could speculate that the effectiveness of β-blockers should depend not only on the tumor subtype, but also on the specific β-blocker. In this context, it is easy to understand the importance of deeper investigations on the usage of specific β-AR antagonists/agonists in order to achieve the best possible outcome with the minimum risk of adverse events. To accomplish this goal, it is crucial to unravel the role of each single β-AR in the examined tumor type. Although β2-AR seems to be the main β-AR involved in tumor development and progression, there are data indicating that also β1-AR and β3-AR may be involved in certain tumors. Therefore, in this article, we review literature data, referring to both pre-clinical and clinical studies, about the involvement of the three β-AR isoforms in cancer. Figure 1 summarizes the effects that catecholamines, acting at the three different β-ARs, exert on tumor cells.
Effects of stress-induced catecholamine overdrive on β-ARs expressed by cancer cells. The increased levels of epinephrine and/or norepinephrine acting at β1-, β2-, and/or β3-ARs promote tumor cell viability, proliferation, and invasion, also inducing the dedifferentiation of cancer cells (green arrows). There is however some evidence that in specific cancers β1- and β2-AR activation may have protective effects against tumor growth (red dashed arrows)
3 β1-ARs
Since most studies rely on the use of β-AR agonists/antagonists that target both the β1 and β2-AR subtypes, it is often difficult to extrapolate the specific role of each subtype in tumor biology over that of β2-ARs. However, evidence has been provided that β1-ARs may play a role in cancer. The potential involvement of β1-ARs in tumor progression was first demonstrated in 1990 when Hough and Chuang showed that β1- and β2-AR mRNAs s were downregulated in C6 rat glioma cells after exposure to the non-selective β-AR agonist isoproterenol, although its effect on protein levels of β1- and β2-ARs was not investigated (Hough and Chuang 1990). In addition, the authors observed that in growing C6 cells β-AR transcripts are downregulated with time of culture and that β-AR downregulation is accompanied by contact inhibition, suggesting a possible role of β-ARs in glioma cell proliferation. In line with this study, Hosoda et al. demonstrated that exposure of C6 cells to isoproterenol caused a biphasic modulation of β1-AR mRNA expression, with transcript levels raised by short-term treatment, and decreased by long-term exposure (Hosoda et al. 1994). In particular, it was shown that β1-AR transcriptional regulation is mediated by cAMP through binding to cAMP responsive elements present in the human and rat β1-AR gene (Collins et al. 1993; Hosoda et al. 1994). In addition, the expression of β1-ARs have been found in human melanoma cell lines and biopsies of benign naevi and melanomas, with a higher expression level in malignant tumors, suggesting that blockade of β1-ARs may represent a target to slow down melanoma progression (Moretti et al. 2013). Moreover, Gao et al. in a clinical cohort study showed that autoantibodies against β1-ARs were higher in de novo multiple myeloma patients than in normal participants, suggesting that β1-AR autoantibodies may be used as predictors to identify multiple myeloma patients (Gao et al. 2018). A recent in silico study concerning functional network analysis has evidenced that atenolol, a commonly used “cardio-selective” β1-AR blocker that in the rat is three- to fourfold more potent on β1-ARs than on β2-ARs (Minneman et al. 1979) and that shows a profile of inverse agonist (Baker et al. 2003; Hopkinson et al. 2000; Michel et al. 2020), may target several signaling pathways involved in pancreatic cancer development, suggesting that atenolol may be repurposed as a novel therapy for this type of cancer (Hermawan et al. 2020).
The specific targeting of β1-ARs has recently proved its efficacy in the treatment of infantile hemangiomas, a benign vascular tumor in which the pharmacologic treatment accelerates the shrink away of the tumor in respect with its natural history. Indeed, even though propranolol is currently the most common treatment for infantile hemangiomas (Pam et al. 2021), atenolol has lately risen interest in this field (Alexopoulos et al. 2018; Bayart et al. 2017). In particular, a recent prospective, multicenter, randomized clinical trial has shown that oral atenolol is equally effective as propranolol in the treatment of problematic infantile hemangiomas. Nevertheless, different from propranolol, atenolol can be administered as a daily therapy and, because of its hydrophilic nature, it is less prone to produce central nervous system-related adverse events compared to the lipophilic propranolol. In addition, it is also less likely to produce bronchial related adverse events than propranolol, suggesting that oral atenolol may be a valid alternative treatment in infantile hemangioma patients requiring systemic therapy (Ji et al. 2021). However, since atenolol is not so selective towards β1-ARs, it is not clear whether the atenolol-induced regression of infantile hemangioma is a β1-AR-mediated response or, rather, a more general β-AR-mediated phenomenon. Among new chemicals designed to have a more specific targeting of β1-ARs, landiolol hydrochloride is a new generation, ultra-short acting β1-selective antagonist that has been developed in Japan, with a selectivity for β1-ARs 255 times higher than for β2-ARs and whose short half-life (4 min) enables rapid recovery after cessation of administration if side effects occur (Iguchi et al. 1992). Its putative preventive effect against early recurrence after curative surgery for non-small cell lung cancer is currently being evaluated in a phase III, multicenter, randomized trial, which was expected to be completed in May 2023. In this study, landiolol has been continuously infused intravenously at 2.5 μg kg−1 min−1 for 72 h from just before surgery (Yamamoto et al. 2019). In addition, landiolol hydrochloride has already been proven to be effective in improving relapse-free survival rate, prolonging relapse-free survival and overall survival when administered at low doses during lung resection surgery for lung malignancies, suggesting that targeting β1-ARs with landiolol-based therapies may be an adjuvant to current therapies in combating any resectable cancer (Sakamoto et al. 2019).
Besides most part of the paper investigating the role of β1-ARs in cancer point on a pro-tumorigenic role of their activation, some studies report a possible anti-tumorigenic role of β1-AR agonism For instance, in surgically resected gastric cancer specimens, a negative correlation between β1-AR expression and the number of metastatic lymph nodes has been recently reported, suggesting that a reduced β1-AR expression is associated with an aggressive behavior and that β1-AR activation may inhibit tumor progression in gastric cancer (Bae et al. 2019). A similar role of β1-AR agonism in the tumor microenvironment has also been proposed. For instance, in sub-population of T cells endowed with a potent antitumor activity and expressing β-ARs, the β1-AR antagonist bisoprolol partially reduced their cytotoxicity, suggesting that the cytotoxic activity of these cells at least in part relies on β1-AR signaling (Baker et al. 2020).
Overall, these studies suggest that β1-AR activation by endogenous catecholamines may have tumor-inhibiting or -promoting effects depending on tumor type. What is certain is that, given the encouraging findings coming not only from pre-clinical studies but also from clinical trials, the possible clinical usage of specific β1-AR blockers in the treatment of some cancers deserves to be further investigated. The controversial effects resulting from the activation of β1-ARs expressed by cancer cells and by T cells belonging to the tumor microenvironment are summarized in Fig. 2.
Schematic diagram depicting the effects of β1-AR activation in cancer cells and in T cells of the tumor microenvironment. The activation of β1-ARs expressed by cancer cells leads to different results in different cancers, ranging from the induction of cell proliferation and tumor growth (as for instance in pancreatic cancer or in lung cancer) to the reduction of tumor progression, which seems to be also reduced by the activation of β1-ARs expressed by T cells of the tumor microenvironment
4 β2-ARs
There are studies highlighting the crucial role that β2-ARs exert in cancer cells. The role of selective and non-selective β-blockers has been studied in many preclinical models of cancer, showing that, in many cases, the capability of non-selective β-blockers in reducing tumor growth and tumor cell migration is replicated by the selective blockade of β2-ARs but not of β1-ARs, thus suggesting a major role of β2-ARs over β1-ARs in tumorigenesis. For instance, in colon carcinoma cells norepinephrine (NE) stimulates cell migration, an effect that is inhibited by propranolol but not by atenolol, suggesting that in these cells the locomotor phenotype is mediated by β2-ARs (Masur et al. 2001). In addition, in prostate carcinoma cells expressing both β1- and β2-ARs, the NE-induced cell migration is abolished by the β2-AR antagonist ICI-118,551 but only partially prevented by atenolol, indicating that in these cells NE acts mainly through β2-AR-activated signaling (Lang et al. 2004). Moreover, in primary cells derived from clear cell renal cell carcinoma β2-AR blockade with either propranolol or ICI-118,151 similarly interferes with two central aspects of cancer progression, that is inflammation and oxidative stress (Albiñana et al. 2022). Furthermore, in triple-negative brain-metastatic breast cells, which are characterized by high expression of β2-ARs and low expression of β1-ARs, proliferation, migration and invasion are stimulated by selective β2-AR agonism and are blunted by propranolol, indicating that the metastatic features of these cells mainly rely on β2-AR activation (Choy et al. 2016). Recently, a fundamental role of β2-ARs in gastric cancer progression and metastasis has been demonstrated both in vitro, in several gastric cancer cell lines, and in vivo, in nude mice implanted with human gastric cancer cells. In vitro, propranolol and ICI-118,551 decreased NE-induced cancer cell proliferation, invasion and viability, while in vivo they reduced tumor growth and metastasis. On the contrary, atenolol had almost no effect either in vitro or in vivo; in particular, atenolol reduced gastric cancer cell proliferation by about 12% only at 50 μM, a concentration that is not selective. Overall, these finding suggest that pathways downstream β2-AR activation play a major role in progression and metastasis of gastric cancer and indicate that β2-AR blockers may represent a new paradigm in complementing the armamentarium presently used against gastric cancer (Zhang et al. 2019a). Similarly, β2-AR activation seems to be mainly involved in promoting tumorigenesis, proliferation, invasiveness, and angiogenesis in lung cancer (see for Ref. Huang et al. 2018) and in hemangioblastomas from von Hippen-Lindau disease patients (Cuesta et al. 2019).
Besides their expression by tumor cells, β2-ARs also represent the main β-AR subtype expressed by cells of the tumor microenvironment, in particular by immune cells, which are known to be inhibited by catecholamines (Ben-Eliyahu et al. 2000). Catecholamines may indeed decrease the activation of antitumor natural killer cells and the overall T cell response, while they may increase the activity of immunosuppressive cells (see for Ref. Silva et al. 2022). For instance, in human and murine macrophages, catecholamines induce the phenotypic shift towards an M2 state, which characterizes the tumor-associated macrophages, and increase the expression of pro-tumorigenic genes. In these cells, either propranolol or β2-AR silencing equally prevented the effect of catecholamines, suggesting that the phenotypic shift of tumor-associated macrophages that promotes cancer progression may be, at least in part, associated to β2-AR activation (Qin et al. 2015). In addition, myeloid-derived suppressor cells, characterized by an immunosuppressive activity that favors the tumor immune escape, were stimulated by β2-AR activation and inhibited by either β2-AR blockade or β2-AR deletion. The same study also demonstrated that co-injecting breast cancer cells and myeloid-derived suppressor cells in β2-AR knockout mice resulted in a decreased expression of immunosuppressive genes, an increased expression of antitumor cytokines and a reduced tumor growth with respect to wild type mice, suggesting a major role of β2-ARs in promoting the pro-tumorigenic functions of immunosuppressive cells (Mohammadpour et al. 2019). On the other hand, a recent bioinformatics analysis investigating the crosstalk between β2-AR expression and breast cancer-infiltrating immune cells, revealed that β2-AR expression is positively related with T cells endowed with antitumor activity and negatively correlated with T cells endowed with pro-tumorigenic activity. The same study also reported a functional analysis showing an enrichment in pathways related to the activation of the immune system, including those downstream β2-AR-regulated transcription factors, suggesting that β2-AR activation may have promising protective effects in breast cancer and indicating them as a possible target for boosting immunotherapy (Wei et al. 2021). In the same line, a clinical study has shown that a high β2-AR expression may be a favorable prognostic factor in patients with human epidermal growth factor receptor 2 positive breast cancer (Caparica et al. 2020). Overall, this apparent contradiction about a role that depresses or, on the contrary, stimulates the activity of the immune system suggests that further preclinical as well as controlled trials using selective β2-AR agonists are required.
If in some instances the expression of β2-AR has been proposed as a favorable prognostic factor in breast cancer (Wei et al. 2021; Caparica et al. 2020), there are also studies indicating that this receptor could be considered a marker associated with poor prognosis in other tumors. For instance, a bioinformatic analysis performed on a dataset containing 300 different gastric cancer samples has revealed that β2-ARs are highly expressed in diffuse type gastric cancer, a type associated with an unfavorable prognosis, and that β2-AR expression level is negatively correlated with disease prognosis (Li et al. 2021). In the same line, β2-AR levels have been negatively associated with poor overall survival and/or recurrence-free survival in patients suffering from hepatocellular carcinoma (Chen et al. 2012), oral squamous cell carcinoma (Krishna et al. 2022), pancreatic ductal adenocarcinoma (Gong et al. 2022), colorectal cancer (Ogawa et al. 2020), estrogen receptor-negative breast cancer (Kurozumi et al. 2019) and malignant melanoma (Shimizu et al. 2016), among others. In addition, there is growing evidence that single nucleotide polymorphisms (SNPs) of the ADRB2 gene, the gene encoding β2-ARs, may be associated to cancer susceptibility, prognosis, and response to medical treatment in patients suffering from some cancers, mainly lung, breast, and pancreatic cancers (see for Ref. Wang and Jiang, 2021). For instance, in the SNP rs1042711, in which the replacement of a Cys residue with and Arg leads to β2-AR downregulation (McGraw et al. 1998), the minor allele C has been found to be associated with an increased risk by about 67% of developing lung cancer (Mei et al. 2019) and with a worse drug response in acute lymphoblastic leukemia, characterized by a statistically significant worse two-year overall survival of about 10% as compared with the major allele T (Pottier et al. 2010). In addition, the SNP rs1042713 has been found to be associated with the increased risk of developing lung adenocarcinoma (by about 42%) and breast cancer (by about 16%) in Chinese populations (Du et al. 2019; Wang et al. 2006), or pancreatic cancer (by about 52%), as evidenced in a population-based case-control study in Minnesota (Zhang et al. 2014). The same SNP has also been associated to progression and metastasis of pancreatic cancer, which is almost doubled than in subjects suffering from pancreatic cancer but not expressing the SNP (Wenjuan et al. 2013). Interestingly, this SNP is associated with an increased expression of β2-AR and with its increased agonist sensitivity (Large et al. 1997; Wenjuan et al. 2013), suggesting a direct role of β2-AR activation by catecholamines in development and progression of some cancers. On the other hand, the GG and AG genotypes of the SNP rs1042713 have been found to be associated to a reduced risk of developing breast cancer in a Chinese population (by about 28%), in a Japanese cohort (by about 33%), and in Hispanic but not in non-Hispanic white women in the southwestern United States (by about 26%) (Connor et al. 2012; Du et al. 2019; Huang et al. 2001). Overall, these data suggest that the possibility to consider β2-AR expression and/or the presence of β2-AR SNPs as a negative or positive prognostic factor may depend on the type of tumor, its progression state and ethnicity. However, most of the studies rely on epidemiological data, therefore further elucidation of the molecular mechanisms activated downstream the different ADRB2 haplotypes coming from preclinical investigations is needed to validate β2-ARs as a possible biomarker in cancer.
A novel frontier about the role of β2-ARs in cancer is the possible use of promising combinatory approaches in which β2-AR antagonists, either non-selective or selective, are associated to conventional anticancer therapies to synergize with them and overcome phenomena of drug resistance. For instance, in non-small cell lung cancer, the treatment with the VEGF receptor 2 inhibitor apatinib led to β2-AR upregulation, while activation of the receptor downstream signaling caused the therapeutic resistance to apatinib. However, the treatment of human non-small cell lung cancer cells with a combination of apatinib and either ICI-118,551 or propranolol enhanced cell sensitivity to apatinib, thus increasing its antitumor effect. The same approach has shown that, in nude mice xenografted with human non-small cell lung cancer cells, the combination of apatinib and propranolol greatly enhances the efficacy of apatinib, leading to a reduction of the xenograft volume that is about threefold larger than that following apatinib or propranolol alone (Xu et al. 2022). Propranolol has also been demonstrated to be effective in enhancing the effect of the chemotherapeutic drug Irinotecan in counteracting the growth of colorectal cancer in a syngeneic mouse model (Lin et al. 2023) and in sensitizing human chemotherapy-resistant prostate cancer cells reducing the resistance to docetaxel (Zhang et al. 2023). Similar results have been obtained in human head and neck squamous cell carcinoma cell lines, in which the combined treatment with the mitogen activated protein kinase (MAPK) inhibitor U0126 and ICI-118,551 was more effective than the single treatments in inducing cell death, thus suggesting that the most adopted therapy for this cancer, which relies on MAPK inhibition and often leads to drug resistance, may be complemented by β2-AR antagonists (Mele et al. 2020). These findings suggest that in comparison with traditional monotherapy, the combination with β2-AR blockers may represent a promising therapeutic strategy, by improving the efficacy of classic chemotherapeutics and reducing drug toxicity. However, whether the combinatorial approach with β2-AR blockers and conventional chemotherapeutic agents may be used to enhance the anticancer effects in a wide range of malignancies requires further preclinical studies before translation in the clinics. In the meantime, supported by preclinical findings, the combination of propranolol with the checkpoint inhibitor pembrolizumab has been tested in a phase I clinical trial that demonstrated the safety of the combination and gave preliminary results on the antitumor efficacy in patients with metastatic melanoma (Gandhi et al. 2021). The effects resulting from the activation of β2-ARs expressed by cancer cells and by immune cells belonging to the tumor microenvironment are summarized in Fig. 3.
Schematic diagram depicting the effects of β2-AR activation in cancer cells and in immune cells of the tumor microenvironment. The activation of β2-ARs expressed by cancer cells, through the stimulation of oxidative stress and inflammatory processes, induces cell proliferation and angiogenesis, contributing to cancer cell survival, which is also directly stimulated by activated β2-ARs. Overall, all these processes trigger tumor growth. In addition, β2-AR activation leads to the acquisition of a locomotor phenotype by cancer cells that migrate and spread to distant sites, acquiring metastatic features. Moreover, the activation of β2-ARs expressed by immune cells of the tumor microenvironment participates, by inducing phenomena of immune-tolerance, to cancer cell invasion of surrounding tissues
5 β3-ARs
Although the interest regarding the role of the adrenergic system in the progression of tumors has been focused mainly on β2-ARs, in recent years awareness of a possible involvement of β3-ARs has progressively grown. On the other hand, while the use of beta blockers as co-adjuvant in treating cancer patients gave evidence supporting the role of β2-ARs in several malignancies (Gales et al. 2022), the possible involvement of β3-ARs is mainly based on preclinical results obtained in vitro and animal models.
The first reports concerned the identification of β3-AR mRNA in different tumors including colon cancer (Perrone et al. 2008), vascular tumors (Chisholm et al. 2012), and human leukemia cells (Lamkin et al. 2012). In addition, the Trp64Arg β3-AR polymorphism was associated to an increased susceptibility in developing colon or endometrial cancer by about 1.5–3 times (Babol et al. 2004; Takezaki et al. 2001).
Alongside studies exploring the role of stress and the involvement of the adrenergic system in the progression of human melanoma, in vitro and in vivo experiments demonstrated the presence of β3-ARs in mouse melanoma cells and explored a possible contribution of β3-ARs in melanoma growth and vascularization in a mouse model. This idea arose after demonstrating that β3-ARs were involved in hypoxia-induced vascularization processes (Dal Monte et al. 2013a).
The presence of β3-ARs on the cellular surface, the up-regulation of their expression under hypoxia (a strategy to reproduce the environment of the growing melanoma in vivo) and their involvement in the induction of VEGF production were demonstrated in mouse melanoma B16F10 cells. The blockade of β3-ARs with SR59230A or L-748,337, or their silencing with selective siRNAs reduced melanoma cell proliferation, induced their apoptosis, and prevented hypoxia-induced VEGF up-regulation. Moreover, in mice bearing mouse melanoma B16F10 cells, the pharmacologic antagonism of β3-ARs with the same drugs reduced melanoma growth and its vascularization thanks to a significant downregulation of VEGF (Dal Monte et al. 2013b). Although SR59230A, the widely used β3-AR antagonist, is not selective for β3-ARs (Vrydag and Michel 2007), the results obtained with the selective antagonist L-748,337 in vivo and with the siRNA approach in vitro point on a specific functional role of β3-ARs in melanoma growth. These effects of SR59230A and L-748,337 were mediated by the inhibition of the expression of the inducible form of nitric oxide synthase and the promotion of apoptosis (Dal Monte et al. 2013b, 2014). These results were confirmed in β1/2-AR knockout mice bearing melanoma, where the treatment based on L-748,337 was again particularly effective in reducing tumor proliferation and vascularization. Interestingly, in this model intratumor level of NE was statistically higher than in controls suggesting a synergy between β3-ARs and catecholamines in melanoma growth (Sereni et al. 2015). β3-AR expression in tumor cells was demonstrated to be a poor prognostic factor also in different human cancers, such as melanoma (Calvani et al. 2015), non-small cell lung carcinoma (Zheng et al. 2020) and in breast cancer (Zhou et al. 2022).
In melanoma, the expression of β3-ARs was demonstrated not only in cancer cells, but also on the membrane of many cells constituting the tumor microenvironment, such as cancer-associated fibroblasts, endothelial progenitor cells, mesenchymal stem cells, and monocytes. In all these human cells β3-ARs were upregulated by hypoxia and, for the first-time, specific functions were attributed to β3-ARs such as the ability to stimulate the NE-mediated recruitment of circulating stromal cell precursors to favor the invasiveness of melanoma cells and to promote cancer stemness. Indeed, in human melanoma cells, a catecholaminergic stimulus increased both the expression of stemness markers, such as CD20 and CD133, and the ability to form melanospheres through the activation of β3-ARs (Calvani et al. 2015).
In a series of subsequent studies, some of the functions of β3-ARs were better elucidated. β3-ARs were demonstrated to be involved in the metabolic rearrangement of human melanoma stem cells by promoting an accelerated glycolysis (Warburg effect), as suggested by the increased glucose uptake and lactate export (Calvani et al. 2018). Interestingly, β3-AR activation with the agonist BRL37344 can promote this metabolic switch by upregulating the expression of some key-enzymes involved in glycolysis such as hexokinase 2, or transmembrane proteins such as monocarboxylate transporter-4, but also by reducing mitochondrial activity through the induction of the specific uncoupling protein 2 (UCP-2), which uncouples the activity of the respiratory chain from ATP synthesis (Calvani et al. 2018). In fact, UCP2 activation by β3-ARs simultaneously induces a significant reduction of ATP synthesis, a decrease of mitochondrial reactive oxygen species (ROS) content, and an increase of lactate production/export in the microenvironment. Limiting ROS production preserves the cancer cells from oxidative stress that causes cell death (Aggarwal et al. 2019), while the reduction of extracellular pH promotes the disaggregation of surrounding tissues and facilitates the infiltration of the tumor (De la Cruz-López et al. 2019).
A recent study suggested the involvement of β3-ARs in the induction of chemoresistance. In this study performed on human myeloid leukemia cell lines, the exposition of a leukemic doxorubicin-resistant cell line to hypoxia increased at the same time the expression of β3-ARs and the cell chemoresistance. On the other hand, SR59230A reverted such doxorubicin resistance, suggesting that the levels of β3-ARs and chemoresistance were not simply associated but closely related phenomena (Calvani et al. 2020a). Although this preliminary study needs further confirmation, some mechanisms promoting chemoresistance have been suggested: in K562 human myeloid leukemia cells β3-ARs modulate the expression of P-glycoprotein (an efflux protein encoded by the multiple drug resistance gene), UCP-2 levels, and hypoxia-inducible factor-1 (HIF-1) expression (Calvani et al. 2020a), proteins that are actively involved in chemoresistance induction in myeloid neoplasms (Zhang et al. 2019b). Additional mechanisms involved in chemoresistance are likely to be under regulation of β3-ARs. In this respect, it is important to note that NE, through the activation of β3-ARs, increases intracellular concentration of glutathione (Yoshioka et al. 2016), whose major function is the detoxification of xenobiotics in cancer (Traverso et al. 2013).
Considering that cancer relies on a hypoxic immune-tolerant context (Facciabene et al. 2011), the assumption that hypoxic induction of β3-ARs in tumor infiltrating lymphocytes could affect tumor immunoediting was investigated in a syngeneic mouse model of melanoma, with the hypothesis that β3-ARs should be able to promote an immune-tolerance confined to the site of intense proliferation, without systemic immunological effects. The data showed that the treatment with SR59230A or β3-AR silencing reduced tumor growth promoting the switch from an immunosuppressive (rich in regulatory T cells, myeloid-derived suppressor cells, M2 macrophages and N2 neutrophils) to an immunocompetent tumor microenvironment (with higher presence of natural killer cells, CD8 cells, M1 macrophages, and N1 neutrophils), within the tumor mass. These data supported the hypothesis that β3-ARs play a role in the promotion of immune-tolerance of cancer (Calvani et al. 2019).
Considering that β3-AR expression is modulated by oxygen levels and that hypoxia promotes immune-tolerance (Facciabene et al. 2011), our hypothesis is that hypoxia may promote the shift towards a tolerant immunophenotype through the upregulation of β3-ARs, which may be the trick adopted by cancer cells to create an aura of immune-tolerance in an immune-competent environment (Calvani et al. 2019). The observation that many of the functions exerted by β3-ARs in tumor models were replicated in embryonic cells (Calvani et al. 2020a) and in placental tissues (Calvani et al. 2020b) suggested the hypothesis that the tumor microenvironment reactivates fetal competences, including local immunosuppression, predominantly through the activation of β3-ARs (Filippi et al. 2022). In essence, β3-ARs, hypoxia and stemness appear to be closely related, as confirmed by the recent demonstration of the genetic link that binds HIF-1 and β3-ARs (Amato et al. 2022). In the earliest stages of fetal development, the low oxygen tension is necessary to initiate the embryonic stem cell proliferation, and this physiologic hypoxia is strictly associated with high levels of HIF-1 and β3-ARs. At the same time, it is well-known that during embryo development oxygen levels represent the signal to induce tissue differentiation (Fathollahipour et al. 2018; Simon and Keith 2008). The close relationship between oxygen, HIF-1 and β3-ARs suggested that oxygen might regulate embryo differentiation through the modulation of β3-ARs. As pregnancy progresses, the progressively increasing levels of oxygen could induce a gradual down-regulation of β3-ARs during embryogenesis (Fujinaga and Scott 1997) favoring embryonic differentiation. Therefore, in light of this hypothesis, β3-AR antagonism of highly undifferentiated tumors expressing high levels of β3-ARs was hypothesized to be the biological sign able to promote cancer differentiation. However, even considering the different role that β3-ARs exert in adult mice and in humans, the translational perspective of studies performed in preclinical models needs to be further assessed.
In a recent study performed in a syngeneic mouse model of melanoma, SR59230A was able to reduce the expression of cancer stem cell markers and induce a differentiated phenotype of hematopoietic subpopulations and mesenchymal stem cells within the tumor (Calvani et al. 2020c). In detail, the study showed the development of a hematopoietic niche within the tumor mass, following the recruitment of hematopoietic progenitor cells that had already started the differentiation process in the bone marrow. Within the tumor mass it was also possible to demonstrate a process of trans-differentiation from mesenchymal stem cell to pre-adipocytes, which explains the yellowish and greasy tumor appearance. This finding was in line with the effect of the treatment of infantile hemangiomas with propranolol, where β-blockade promoted the adipogenic trans-differentiation of hemangioma stem cells (Ma et al. 2014). A similar effect was demonstrated in the human breast cancer MCF-7 cell line where β3-AR activation prevented the trans-differentiation of MCF-7 cells into adipocyte-like cells (Zhou et al. 2022).
In a study performed in mice bearing murine Neuro2A neuroblastoma cells, treatments with SR59230A or with β3-AR siRNAs inhibited the growth of neuroblastoma and its progression (Bruno et al. 2020). These data were in agreement with a previous study demonstrating the ability of SR59230A and of β3-AR silencing to inhibit neuroblastoma cell proliferation through the suppression of the mTOR pathway (Deng et al. 2019). Experiments performed on human neuroblastoma cells demonstrated that SR59230A reduced the expression of stemness markers, such as the capability to form neurospheres and the levels of the stem cell marker CD34, while it increased neurite formation. Similar results were observed in mice bearing syngeneic neuroblastoma tumor cells, where SR59230A decreased the expression levels of the early neuronal differentiation markers and increased the intermediate and late neuronal differentiation markers (Bruno et al. 2020). More recently, in a murine syngeneic model of neuroblastoma, SR50230A was demonstrated to be effective in reactivating the host immune response in the tumor microenvironment, leading to a decrease in tumor growth through the involvement of the programmed death 1/programmed death ligand-1 signaling axis. The same study, also showed that in specimens from neuroblastoma patients, the high expression of the ADRB3 gene is associated with a reduction in event-free survival probability and in overall survival probability in respect to the low expression of the receptor (from 70% to 50% and from 80% to 60%, respectively) (Bruno et al. 2023). In conclusion, these data suggest a strong relationship between the expression of β3-ARs and the undifferentiated state of cancer, and the possibility to promote tumor cell differentiation antagonizing these receptors. This possibility opens very promising therapeutic scenarios because the differentiation grade of tumors is closely correlated with the biology of their malignancies, being the undifferentiated tumors the most aggressive and malignant (Bao et al. 2013). At the same time, these results confirm the role played by β3-ARs in promoting stemness and undifferentiated state, both in embryo and in cancer.
Currently, the antagonism of β3-ARs may represent a new therapeutic approach to counteract the proliferation of cancer, its metabolic shift, chemoresistance, immune-tolerance and to promote its differentiation. The effects resulting from the activation of β3-ARs expressed by cancer cells and by cells belonging to the tumor microenvironment are summarized in Fig. 4.
Schematic diagram depicting the effects of β3-AR activation in cancer cells and in cells of the tumor microenvironment. The activation of β3-ARs expressed by cancer cells, through the induction of Warburg effect leads to the acidification of the surrounding tissue that favors tumor infiltration and growth. Through: (i) the reduction of oxidative stress-dependent apoptosis, which is a consequence of the Warburg shift, (ii) the induction of chemoresistance derived from an increase in the activity of drug efflux pumps, (iii) The activation of angiogenic processes, (iv) the dedifferentiation of cancer cells and (v) the induction of stemness-related immune-tolerance, β3-AR agonism favors cell survival and tumor growth. In addition, also the activation of β3-ARs expressed by cells of the tumor microenvironment participates, directly and indirectly, to tumor growth
6 Conclusions and Future Perspectives
Distress conditions may importantly affect the development of cancer and its progression. In particular, stress-induced catecholamine overdrive stimulates carcinogenesis and tumor growth, as shown by results from pre-clinical and clinical studies indicating that β-ARs expressed by tumor cells and in the tumor microenvironment are the target mediating these effects of epinephrine/norepinephrine. Although β2-ARs have been recognized as the main β-AR subtype involved in the pro-tumorigenic effects of catecholamines, there is growing evidence that also β1- and β3-ARs may have a role in tumor biology, thus indicating the perspective of β-ARs as intriguing targets to fight cancer.
Although some reports indicating that β1-AR activation may have an anticancer potential, these β-AR subtypes seem to have a role in the growth of certain tumors, such as infantile hemangiomas, highlighting the role of β1-AR blockers in the treatment of specific malignancies. However, additional studies are required to better define the potential tumorigenic role of these receptors.
A paramount role of β2-ARs in many tumors has been recognized, and β2-AR blockade has been demonstrated to be effective in counteracting tumor growth in pre-clinical models. In addition, several studies have shown that the previous use of β-AR blockers in tumor patients increases survival and reduces recurrence and metastasis rates. In this respect, several studies have demonstrated that β-AR blockers targeting both β1- and β2-ARs exert their antitumor effects acting mainly at β2-ARs. The finding that β2-ARs are expressed not only by tumor cells but also by cells of the tumor-microenvironment, the possibility that β2-ARs or particular SNPs of these receptors may be recognized as biomarkers of specific tumors, and the evidence that β2-AR blockade may synergize with conventional antitumor drugs in a combinatorial approach to tumor treatment reveal that there may be still unexplored or only partially understood uses of β2-AR-targeting molecules, which may be useful to counteract cancer growth and progression. Then, although further investigations are required to clarify the molecular mechanisms mediating β2-AR blocker effects in different tumors and to assess the importance of a minority of studies, based on bioinformatics, reporting a possible protective role of β2-ARs in some tumors, the use of β2-AR blockers seems to be not so far from moving from the bench to the bedside.
Regarding the less studied β-ARs, β3-ARs, during the last decade they have been demonstrated to be involved in tumor growth to the point that their expression can be considered a poor prognostic factor in specific human cancers such as neuroblastoma. Being expressed by tumor cells, as well as in the tumor microenvironment, blocking these receptors in animal models has been proven to be effective in reducing the growth of melanoma and neuroblastoma, suggesting a potential use of β3-AR blockers in tumor treatment. In this respect, the restricted expression in the human body of β3-ARs with respect to that of β1- and β2-ARs should be of advantage in treating tumor patients since off-target effects of β3-AR blockers may be, in principle, less than those of β1- and β2-AR antagonists. However, it is difficult to imagine the use of β3-AR blockers in tumor patients in a near future, since the currently available β3-AR blockers have problems of selectivity and specificity and are not marketed for human use. On the other hand, the finding obtained from pre-clinical studies are so encouraging that they may pave the way to future clinical trials essaying the available β3-AR blockers (and, hopefully, newly synthetized ones) as treatment for selected cancers. Of note, the finding that β3-AR activation stimulates tumor cell dedifferentiation, reactivating embryo competences, is opening a new way that may be of importance in studying tumor biology. On the other hand, the fact that β3-AR blockade is effective in hampering tumor growth and that β3-AR activation has an opposite effect, may represent the other side of the coin of the increasing use of β3-AR agonists in the treatment of overactive bladder, the only use for which β3-AR-acting drugs are approved in humans.
7 Antitumor Effect of the Catecholaminergic System Beyond β-ARs: Is There a Role for α2-ARs?
Among ARs, β-ARs are the main subtypes studied for their role in tumor biology. However, some evidence about a role for α-ARs has been produced and, although this role has not been thoroughly examined, the expression level of α-ARs has been linked to poor prognosis in human breast cancer (Powe et al. 2011). Among α-ARs, α2-ARs have been identified for a potential role in regulating the growth of different tumors, although results from different studies seem to be contradictory. In fact, it has been demonstrated that α2-AR agonism with dexmedetomidine or clonidine promotes proliferation and migration in human breast cancer cell lines (Castillo et al. 2017; Vazquez et al. 2006; Xia et al. 2016). In addition, dexmedetomidine treatment results in an increase in tumor growth and metastasis formation in syngeneic mouse models of breast cancer (Lavon et al. 2018; Szpunar et al. 2013), as well as in syngeneic mouse models of lung carcinoma and colon adenocarcinoma (Lavon et al. 2018). On the contrary, the α2-AR agonist UK14,304 inhibits the growth of human cholangiocarcinoma cells (Kanno et al. 2002), while α2-AR agonism with ST91 attenuates tumor growth in a syngeneic mouse model of melanoma (Maccari et al. 2022). A possible explanation of these conflicting results may lie in the models, in the tumors and/or in the drug and in their doses used in the different studies, and points on the need of additional studies in order to obtain definitive data about the pro- or anti-tumorigenic role of α2-AR activation. In this respect, a very recently published paper seems to put a full stop on the matter. Zhu and co-authors (2023) indeed demonstrated that α2-AR agonists (guanabenz, clonidine, and guanfacine) exert an impressive antitumor effect in either syngeneic or allogeneic mouse models of different cancers. The effects of α2-AR agonists were blocked by α2-AR antagonists and were not observed in α2-AR knockout mice, indicating (i) the selectivity of these effects and (ii) that these effects are not exerted on tumor cells but on host cells belonging to the tumor microenvironment. Overall, this work demonstrated that α2-AR agonism acts directly on macrophages that, in turn, would stimulate the adaptive immune response of T lymphocytes. Of note, α2-AR agonists not only strongly reduced tumor growth when used as monotherapy but were also able to synergize with immune checkpoint blockers leading to a complete tumor rejection in many mice. Finally, the authors showed that in patients suffering from lung adenocarcinoma there is a high statistically significant association between a high expression of α2-ARs and both the progression-free survival and the overall survival, suggesting the translatability of the results of this study to patients. It is obvious that the translational implications of this study need to be carefully verified, and the definition of the doses of α2-AR agonists to be used in humans may be only the starting point of this path. However, the fact that some α2-AR agonists are clinically available, that their safety profile is known and that they have been used for many years in treating hypertension, may accelerate the development of treatments (either mono- or combined therapies) for specific human cancers.
References
Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G (2019) Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomol Ther 9(11):735
Albiñana V, Recio-Poveda L, González-Peramato P, Martinez-Piñeiro L, Botella LM, Cuesta AM (2022) Blockade of β2-adrenergic receptor reduces inflammation and oxidative stress in clear cell renal cell carcinoma. Int J Mol Sci 23(3):1325
Alexopoulos A, Thanopoulou I, Dakoutrou M, Georgiadou E, Chrousos GP, Kakourou T (2018) Atenolol treatment for severe infantile hemangiomas: a single-centre prospective study. J Eur Acad Dermatol Venereol 32(3):e117–e119
Amato R, Pisani F, Laudadio E, Cammalleri M, Lucchesi M, Marracci S, Filippi L, Galeazzi R, Svelto M, Dal Monte M, Bagnoli P (2022) HIF-1-dependent induction of β3 adrenoceptor: evidence from the mouse retina. Cells 11(8):1271
Babol K, Przybylowska K, Lukaszek M, Pertynski T, Blasiak J (2004) An association between the Trp64Arg polymorphism in the beta3-adrenergic receptor gene and endometrial cancer and obesity. J Exp Clin Cancer Res 23(4):669–674
Bae GE, Kim HS, Won KY, Kim GY, Sung JY, Lim SJ (2019) Lower sympathetic nervous system density and β-adrenoreceptor expression are involved in gastric cancer progression. Anticancer Res 39(1):231–236
Baker JL, Hall IP, Hill SJ (2003) Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol 64(6):1357–1369
Baker FL, Bigley AB, Agha NH, Pedlar CR, O'Connor DP, Bond RA, Bollard CM, Katsanis E, Simpson RJ (2020) Systemic β-adrenergic receptor activation augments the ex vivo expansion and anti-tumor activity of Vγ9Vδ2 T-cells. Front Immunol 10:3082
Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH (2013) Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol. Chapter 14:Unit 14.25
Bayart CB, Tamburro JE, Vidimos AT, Wang L, Golden AB (2017) Atenolol versus propranolol for treatment of infantile hemangiomas during the proliferative phase: a retrospective noninferiority study. Pediatr Dermatol 34(4):413–421
Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K (2000) Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation 8(3):154–164
Bruno G, Cencetti F, Pini A, Tondo A, Cuzzubbo D, Fontani F, Strinna V, Buccoliero AM, Casazza G, Donati C, Filippi L, Bruni P, Favre C, Calvani M (2020) β3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene 39(2):368–384
Bruno G, Nastasi N, Subbiani A, Boaretto A, Mannurita SC, Mattei G, Nardini P, Della Bella C, Magi A, Pini A, De Marco E, Tondo A, Favre C, Calvani M (2023) β3-adrenergic receptor on tumor-infiltrating lymphocytes sustains IFN-γ-dependent PD-L1 expression and impairs anti-tumor immunity in neuroblastoma. Cancer Gene Ther 30(6):890–904
Calvani M, Pelon F, Comito G, Taddei ML, Moretti S, Innocenti S, Nassini R, Gerlini G, Borgognoni L, Bambi F, Giannoni E, Filippi L, Chiarugi P (2015) Norepinephrine promotes tumor microenvironment reactivity through β3-adrenoreceptors during melanoma progression. Oncotarget 6(7):4615–4632
Calvani M, Cavallini L, Tondo A, Spinelli V, Ricci L, Pasha A, Bruno G, Buonvicino D, Bigagli E, Vignoli M, Bianchini F, Sartiani L, Lodovici M, Semeraro R, Fontani F, De Logu F, Dal Monte M, Chiarugi P, Favre C, Filippi L (2018) β3-Adrenoreceptors control mitochondrial dormancy in melanoma and embryonic stem cells. Oxidative Med Cell Longev 2018:6816508
Calvani M, Bruno G, Dal Monte M, Nassini R, Fontani F, Casini A, Cavallini L, Becatti M, Bianchini F, De Logu F, Forni G, la Marca G, Calorini L, Bagnoli P, Chiarugi P, Pupi A, Azzari C, Geppetti P, Favre C, Filippi L (2019) β3-Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br J Pharmacol 176(14):2509–2524
Calvani M, Dabraio A, Bruno G, De Gregorio V, Coronnello M, Bogani C, Ciullini S, Marca G, Vignoli M, Chiarugi P, Nardi M, Vannucchi AM, Filippi L, Favre C (2020a) β3-Adrenoreceptor blockade reduces hypoxic myeloid leukemic cells survival and chemoresistance. Int J Mol Sci 21(12):4210
Calvani M, Dabraio A, Subbiani A, Buonvicino D, De Gregorio V, Ciullini Mannurita S, Pini A, Nardini P, Favre C, Filippi L (2020b) β3-adrenoceptors as putative regulator of immune tolerance in cancer and pregnancy. Front Immunol 11:2098
Calvani M, Bruno G, Dabraio A, Subbiani A, Bianchini F, Fontani F, Casazza G, Vignoli M, De Logu F, Frenos S, Filippi L, Favre C (2020c) β3-Adrenoreceptor blockade induces stem cells differentiation in melanoma microenvironment. Int J Mol Sci 21(4):1420
Caparica R, Richard F, Brandão M, Awada A, Sotiriou C, de Azambuja E (2020) Prognostic and predictive impact of Beta-2 adrenergic receptor expression in HER2-positive breast cancer. Clin Breast Cancer 20(3):262–273.e7
Castillo LF, Rivero EM, Goffin V, Lüthy IA (2017) Alpha2-adrenoceptor agonistss trigger prolactin signaling in breast cancer cells. Cell Signal 34:76–85
Chen D, Xing W, Hong J, Wang M, Huang Y, Zhu C, Yuan Y, Zeng W (2012) The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann Surg Oncol 19(11):3556–3565
Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE (2012) β-Adrenergic receptor expression in vascular tumors. Mod Pathol 25(11):1446–1451
Choy C, Raytis JL, Smith DD, Duenas M, Neman J, Jandial R, Lew MW (2016) Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: the potential benefit of perioperative β-blockade. Oncol Rep 35(6):3135–3142
Collins S, Ostrowski J, Lefkowitz RJ (1993) Cloning and sequence analysis of the human beta 1-adrenergic receptor 5′-flanking promoter region. Biochim Biophys Acta 1172(1–2):171–174
Connor A, Baumgartner RN, Kerber RA, O'Brien E, Rai SN, Wolff RK, Slattery ML, Giuliano AR, Risendal BC, Byers TE, Baumgartner KB (2012) ADRB2 G-G haplotype associated with breast cancer risk among Hispanic and non-Hispanic white women: interaction with type 2 diabetes and obesity. Cancer Causes Control 23(10):1653–1663
Cuesta AM, Albiñana V, Recio-Poveda L, de Rojas-P I, Gómez V, de Las HK, Aguirre DT, Botella LM (2019) The β2-adrenergic receptor antagonist ICI-118,551 blocks the constitutively activated HIF signalling in hemangioblastomas from von Hippel-Lindau disease. Sci Rep 9:10062
Dal Monte M, Filippi L, Bagnoli P (2013a) Beta3-adrenergic receptors modulate vascular endothelial growth factor release in response to hypoxia through the nitric oxide pathway in mouse retinal explants. Naunyn Schmiedeberg’s Arch Pharmacol 386(4):269–278
Dal Monte M, Casini G, Filippi L, Nicchia GP, Svelto M, Bagnoli P (2013b) Functional involvement of β3-adrenergic receptors in melanoma growth and vascularization. J Mol Med (Berl) 91(12):1407–1419
Dal Monte M, Fornaciari I, Nicchia GP, Svelto M, Casini G, Bagnoli P (2014) β3-adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn Schmiedeberg’s Arch Pharmacol 387(6):533–543
Dal Monte M, Calvani M, Cammalleri M, Favre C, Filippi L, Bagnoli P (2019) β-Adrenoceptors as drug targets in melanoma: novel preclinical evidence for a role of β3 -adrenoceptors. Br J Pharmacol 176(14):2496–2508
De la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J (2019) Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol 9:1143
Deng J, Jiang P, Yang T, Huang M, Qi W, Zhou T, Yang Z, Zou Y, Gao G, Yang X (2019) Targeting β3-adrenergic receptor signaling inhibits neuroblastoma cell growth via suppressing the mTOR pathway. Biochem Biophys Res Commun 514(1):295–300
Du Y, Lin Y, Yin K, Zhou L, Jiang Y, Yin W, Lu J (2019) Single nucleotide polymorphisms of let-7-related genes increase susceptibility to breast cancer. Am J Transl Res 11(3):1748–1759
Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475(7355):226–230
Fathollahipour S, Patil PS, Leipzig ND (2018) Oxygen regulation in development: lessons from embryogenesis towards tissue engineering. Cells Tissues Organs 205(5–6):350–371
Filippi L, Pini A, Cammalleri M, Bagnoli P, Dal Monte M (2022) β3-adrenoceptor, a novel player in the round-trip from neonatal diseases to cancer: suggestive clues from embryo. Med Res Rev 42(3):1179–1201
Flaherty RL, Falcinelli M, Flint MS (2019) Stress and drug resistance in cancer. Cancer Drug Resist 2(3):773–786
Fujinaga M, Scott JC (1997) Gene expression of catecholamine synthesizing enzymes and beta adrenoceptor subtypes during rat embryogenesis. Neurosci Lett 231(2):108–112
Gales L, Forsea L, Mitrea D, Stefanica I, Stanculescu I, Mitrica R, Georgescu M, Trifanescu O, Anghel R, Serbanescu L (2022) Antidiabetics, Anthelmintics, statins, and beta-blockers as co-adjuvant drugs in cancer therapy. Medicina (Kaunas) 58(9):1239
Gandhi S, Pandey MR, Attwood K, Ji W, Witkiewicz AK, Knudsen ES, Allen C, Tario JD, Wallace PK, Cedeno CD, Levis M, Stack S, Funchain P, Drabick JJ, Bucsek MJ, Puzanov I, Mohammadpour H, Repasky EA, Ernstoff MS (2021) Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res 27(1):97–95
Gao W, Guo WJ, Hou DY, Yang GZ, Wu Y, Li YC, Leng Y, Tang Y, Xu L, Liu JM, Wang H, Wang X, Zhang J, Zhao WS, Chen WM, Zhang L (2018) Autoantibodies against β1-adrenergic receptor: response to induction therapy with bortezomib-containing regimens for multiple myeloma patients. Leuk Lymphoma 59(3):717–724
Gong C, Hu B, Chen H, Zhu J, Nie J, Hua L, Chen L, Fang Y, Hang C, Lu Y (2022) β2-adrenergic receptor drives the metastasis and invasion of pancreatic ductal adenocarcinoma through activating Cdc42 signaling pathway. J Mol Histol 53(4):645–655
Gosain R, Gage-Bouchard E, Ambrosone C, Repasky E, Gandhi S (2020) Stress reduction strategies in breast cancer: review of pharmacologic and non-pharmacologic based strategies. Semin Immunopathol 42(6):719–734
Hermawan A, Putri H, Utomo RY (2020) Functional network analysis reveals potential repurposing of β-blocker atenolol for pancreatic cancer therapy. Daru 28(2):685–699
Hopkinson HE, Latif ML, Hill SJ (2000) Non-competitive antagonism of beta(2)-agonist-mediated cyclic AMP accumulation by ICI 118551 in BC3H1 cells endogenously expressing constitutively active beta(2)-adrenoceptors. Br J Pharmacol 131(1):124–130
Hosoda K, Feussner GK, Rydelek-Fitzgerald L, Fishman PH, Duman RS (1994) Agonist and cyclic AMP-mediated regulation of beta 1-adrenergic receptor mRNA and gene transcription in rat C6 glioma cells. J Neurochem 63(5):1635–1645
Hough C, Chuang DM (1990) Differential down-regulation of beta 1- and beta 2-adrenergic receptor mRNA in C6 glioma cells. Biochem Biophys Res Commun 170(1):46–52
Huang XE, Hamajima N, Saito T, Matsuo K, Mizutani M, Iwata H, Iwase T, Miura S, Mizuno T, Tokudome S, Tajima K (2001) Possible association of beta2- and beta3-adrenergic receptor gene polymorphisms with susceptibility to breast cancer. Breast Cancer Res 3(4):264–269
Huang Q, Tan Q, Mao K, Yang G, Ma G, Luo P, Wang S, Mei P, Wu F, Xu J, Guo M, Lv Z, Fan J, Zhang S, Wang X, Jin Y (2018) The role of adrenergic receptors in lung cancer. Am J Cancer Res 8(11):2227–2237
Iguchi S, Iwamura H, Nishizaki M, Hayashi A, Senokuchi K, Kobayashi K, Sakaki K, Hachiya K, Ichioka Y, Kawamura M (1992) Development of a highly cardioselective ultra short-acting beta-blocker, ONO-1101. Chem Pharm Bull (Tokyo) 40(6):1462–1469
Jessop DS (2019) The power of positive stress and a research roadmap. Stress 22(5):521–523
Ji Y, Chen S, Yang K, Zhang X, Zhou J, Li L, Xiang B, Qiu T, Dai S, Jiang X, Lu G, Qiu L, Kong F, Zhang Y (2021) Efficacy and safety of propranolol vs atenolol in infants with problematic infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 147(7):599–607
Kanno N, Lesage G, Phinizy JL, Glaser S, Francis H, Alpini G (2002) Stimulation of α2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology 35(6):1329–1340
Krishna A, Singh V, Singh N, Singh S, Mohanty SK, Singh R, Kumar V, Singh US, Singh RK (2022) Expression pattern and clinical significance of beta 2-adrenergic receptor in oral squamous cell carcinoma: an emerging prognostic indicator and future therapeutic target. Clin Transl Oncol 24(11):2191–2199
Kurozumi S, Kaira K, Matsumoto H, Hirakata T, Yokobori T, Inoue K, Horiguchi J, Katayama A, Koshi H, Shimizu A, Oyama T, Sloan EK, Kurosumi M, Fujii T, Shirabe K (2019) β2-adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res Treat 177(3):603–610
Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, Morizono K, Cole SW (2012) Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun 26(4):635–641
Lang K, Drell TL 4th, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, Entschladen F (2004) Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 112(2):231–238
Large V, Hellström L, Reynisdottir S, Lönqvist F, Eriksson P, Lannfelt L, Arner P (1997) Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest 100(12):3005–3013
Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, Elbaz E, Cata JP, Gottumukkala V, Ben-Eliyahu S (2018) Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth 120(1):188–196
Li S, Yu C, Cheng Y, Du F, Wen G (2021) Bioinformatics analysis identifies biomarkers associated with poor prognosis in diffuse type gastric cancer. Mol Med Rep 23(3):193
Lin Y, Liu Y, Gao Z, Jing D, Bi R, Cui X, Cao Q, Zhao Q, Gao R, Su Y, Liu S, Zhao M, Yang Y, Chen A, Dai B, Gao X (2023) Beta-adrenergic receptor blocker propranolol triggers anti-tumor immunity and enhances irinotecan therapy in mice colorectal cancer. Eur J Pharmacol 949:175718
Liu C, Yang Y, Chen C, Li L, Li J, Wang X, Chu Q, Qiu L, Ba Q, Li X, Wang H (2021) Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat Commun 12(1):5725
Livingstone D (1857) Missionary travels and researches in South Africa: including a sketch of sixteen years’ residence in the interior of Africa. Jonh Murray Publisher, London
Ma X, Zhao T, Ouyang T, Xin S, Ma Y, Chang M (2014) Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int J Clin Exp Pathol 7(7):3809–3817
Maccari S, Buoncervello M, Ascione B, Stati T, Macchia D, Fidanza S, Catalano L, Matarrese P, Gabriele L, Marano G (2022) α-Adrenoceptor stimulation attenuates melanoma growth in mice. Br J Pharmacol 179(7):1371–1383
Masur K, Niggemann B, Zanker KS, Entschladen F (2001) Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 61(7):2866–2869
McGraw DW, Forbes SL, Liggett SB (1998) Polymorphisms of the 5′ leader cistron of the human beta2-adrenergic receptor regulate receptor expression. J Clin Invest 102(11):1927–1932
Mei L, Huang C, Wang A, Zhang X (2019) Association between ADRB2, IL33, and IL2RB gene polymorphisms and lung cancer risk in a Chinese Han population. Int Immunopharmacol 77:105930
Mele L, Del Vecchio V, Marampon F, Regad T, Wagner S, Mosca L, Bimonte S, Giudice A, Liccardo D, Prisco C, Schwerdtfeger M, La Noce M, Tirino V, Caraglia M, Papaccio G, Desiderio V, Barbieri A (2020) β2-AR blockade potentiates MEK1/2 inhibitor effect on HNSCC by regulating the Nrf2-mediated defense mechanism. Cell Death Dis 11(10):850
Michel MC, Michel-Reher MB, Hein P (2020, 1923) A systematic review of inverse agonism at adrenoceptor subtypes. Cells 9(9)
Minneman KP, Hegstrand LR, Molinoff PB (1979) The pharmacological specificity of Beta-1 and Beta-2 adrenergic recepotors in rat heart and lung in vitro. Mol Pharmacol 16(1):21–33
Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA (2019) β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129(12):5537–5552
Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P (2013) β-Adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Investig 93(3):279–290
Mravec B, Tibensky M, Horvathova L (2020a) Stress and cancer. Part I: mechanisms mediating the effect of stressors on cancer. J Neuroimmunol 346:577311
Mravec B, Horvathova L, Hunakova L (2020b) Neurobiology of cancer: the role of β-adrenergic receptor signaling in various tumor environments. Int J Mol Sci 21(21):7958
Mravec B, Tibensky M, Horvathova L (2020c) Stress and cancer. Part II: therapeutic implications for oncology. J Neuroimmunol 346:577312
Musselman RP, Bennett S, Li W, Mamdani M, Gomes T, van Walraven C, Boushey R, Al-Obeed O, Al-Omran M, Auer RC (2018) Association between perioperative beta blocker use and cancer survival following surgical resection. Eur J Surg Oncol 44(8):1164–1169
Ogawa H, Kaira K, Motegi Y, Yokobori T, Takada T, Kato R, Osone K, Takahashi R, Suga K, Ozawa N, Katayama C, Oyama T, Shimizu A, Yao T, Asao T, Saeki H, Shirabe K (2020) Prognostic significance of β2-adrenergic receptor expression in patients with surgically resected colorectal cancer. Int J Clin Oncol 25(6):1137–1144
Pam N, Kridin K, Khamaysi Z (2021) Propranolol for infantile hemangioma: evaluating efficacy and predictors of response and rebound growth. Dermatol Ther 34(3):e14936
Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P (2016) Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab 23(3):554–562
Perrone MG, Notarnicola M, Caruso MG, Tutino V, Scilimati A (2008) Upregulation of beta3-adrenergic receptor mRNA in human colon cancer: a preliminary study. Oncology 75(3–4):224–229
Pottier N, Paugh SW, Ding C, Pei D, Yang W, Das S, Cook EH, Pui CH, Relling MV, Cheok MH, Evans WE (2010) Promoter polymorphisms in the β-2 adrenergic receptor are associated with drug-induced gene expression changes and response in acute lymphoblastic leukemia. Clin Pharmacol Ther 88(6):854–861
Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, Ellis IO, Entschladen F (2011) Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat 130(2):457–463
Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, Wang Y (2015) Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep 48(5):295–300
Sakamoto A, Yagi K, Okamura T, Harada T, Usuda J (2019) Perioperative administration of an intravenous beta-blocker landiolol hydrochloride in patients with lung cancer: a Japanese retrospective exploratory clinical study. Sci Rep 9(1):5217
Schuller HM, Cole B (1989) Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 10(9):1753–1755
Sereni F, Dal Monte M, Filippi L, Bagnoli P (2015) Role of host β1- and β2-adrenergic receptors in a murine model of B16 melanoma: functional involvement of β3-adrenergic receptors. Naunyn Schmiedeberg’s Arch Pharmacol 388(12):1317–1331
Shimizu A, Kaira K, Mori K, Kato M, Shimizu K, Yasuda M, Takahashi A, Oyama T, Asao T, Ishikawa O (2016) Prognostic significance of β2-adrenergic receptor expression in malignant melanoma. Tumour Biol 37(5):5971–5978
Silva D, Quintas C, Gonçalves J, Fresco P (2022) Contribution of adrenergic mechanisms for the stress-induced breast cancer carcinogenesis. J Cell Physiol 237(4):2107–2127
Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9(4):285–296
Szpunar MJ, Burker KA, Dawes RP, Brown EB, Madden KS (2013) The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 6(12):1262–1272
Takezaki T, Hamajima N, Matsuo K, Tanaka R, Hirai T, Kato T, Ohashi K, Tajima K (2001) Association of polymorphisms in the beta-2 and beta-3 adrenoceptor genes with risk of colorectal cancer in Japanese. Int J Clin Oncol 6(3):117–122
Tang J, Li Z, Lu L, Cho CH (2013) β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol 23(6 Pt B):533–542
Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C (2013) Role of glutathione in cancer progression and chemoresistance. Oxidative Med Cell Longev 2013:972913
Vazquez SM, Mladovan AG, Perez C, Bruzzone A, Baldi A, Lüthy IA (2006) Human breast cell lines exhibit functional alpha(2) adrenoceptors. Cancer Chemother Pharmacol 58:50–61
Vrydag W, Michel MC (2007) Tools to study beta3-adrenoceptors. Naunyn Schmiedeberg’s Arch Pharmacol 374(5–6):385–398
Wackerhage H, Christensen JF, Ilmer M, von Luettichau I, Renz BW, Schönfelder M (2022) Cancer catecholamine conundrum. Trends Cancer 8(2):110–122
Wang Y, Jiang S (2021) The role of ADRB2 gene polymorphisms in malignancies. Mol Biol Rep 48(3):2741–2749
Wang H, Hao B, Chen X, Zhao N, Cheng G, Jiang Y, Liu Y, Lin C, Tan W, Lu D, Wei Q, Jin L, Lin D, He F (2006) Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: a case-control study in a Chinese population. Cancer Lett 240(2):297–305
Wei X, Chen L, Yang A, Lv Z, Xiong M, Shan C (2021) ADRB2 is a potential protective gene in breast cancer by regulating tumor immune microenvironment. Transl Cancer Res 10(12):5280–5294
Wenjuan Y, Yujun L, Ceng Y (2013) Association of single nucleotide polymorphisms of β2-adrenergic receptor gene with clinicopathological features of pancreatic carcinoma. Acta Histochem 115(3):198–203
Xia M, Ji N-N, Duan M-L, Tong J-H, Xu J-G, Zhang Y-M, Wang S-H (2016) Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur Rev Med Pharmacol Sci 20(16):3500–3506
Xu Y, Wang J, Wang X, Zhou X, Tang J, Jie X, Yang X, Rao X, Xu Y, Xing B, Li Z, Wu G (2022) Targeting ADRB2 enhances sensitivity of non-small cell lung cancer to VEGFR2 tyrosine kinase inhibitors. Cell Death Discov 8(1):36
Yamamoto H, Hamasaki T, Onda K, Nojiri T, Aragaki M, Horie N, Sato N, Hida Y (2019) Landiolol, an ultra-short acting beta-1 blocker, for preventing postoperative lung cancer recurrence: study protocol for a phase III, multicenter randomized trial with two parallel groups of patients. Trials 20(1):715
Yap A, Lopez-Olivo MA, Dubowitz J, Pratt G, Hiller J, Gottumukkala V, Sloan E, Riedel B, Schier R (2018) Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 121(1):45–57
Yoshioka Y, Kadoi H, Yamamuro A, Ishimaru Y, Maeda S (2016) Noradrenaline increases intracellular glutathione in human astrocytoma U-251 MG cells by inducing glutamate-cysteine ligase protein via β3-adrenoceptor stimulation. Eur J Pharmacol 772:51–61
Zhang J, Dhakal IB, Zhang X, Prizment AE, Anderson KE (2014) Genetic variability in energy balance and pancreatic cancer risk in a population-based case-control study in Minnesota. Pancreas 43(2):281–286
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z (2019a) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis 10(11):788
Zhang J, Gu Y, Chen B (2019b) Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther 12:1937–1945
Zhang M, Chen F, Sun X, Huang Y, Zeng Y, Chen J, Wu S, Xu C (2023) Sympathetic β2-adrenergic receptor blockade overcomes docetaxel resistance in prostate cancer. Biochem Biophys Res Commun 657:69–79
Zheng M, Zhou Z, Tian X, Xiao D, Hou X, Xie Z, Liang H, Lin S (2020) ADRB3 expression in tumor cells is a poor prognostic factor and promotes proliferation in non-small cell lung carcinoma. Cancer Immunol Immunother 69(11):2345–2355
Zhou Z, Zhan J, Luo Q, Hou X, Wang S, Xiao D, Xie Z, Liang H, Lin S, Zheng M (2022) ADRB3 induces mobilization and inhibits differentiation of both breast cancer cells and myeloid-derived suppressor cells. Cell Death Dis 13(2):141
Zhu J, Naulaerts S, Boudhan L, Martin M, Gatto L, Van den Eynde BJ (2023) Tumor immune rejection triggered by activation of α2-adrenergic receptors. Nature 618(7965):607–615
Acknowledgments
The authors would like to thank Prof. Giovanni Casini for his critical reading of the manuscript. Over the past years, the studies in the MDM laboratory focusing on the role of β-ARs in cancer have been supported by Italian Ministry of Health (RF-2011-02351158), Azienda Ospedaliera-Universitaria Meyer, Fondazione Meyer, Ente Cassa di Risparmio di Firenze and intramural funds at the University of Pisa.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Amato, R., Lucchesi, M., Marracci, S., Filippi, L., Dal Monte, M. (2023). β-Adrenoceptors in Cancer: Old Players and New Perspectives. In: Baker, J.G., Michel, M.C., Summers, R.J. (eds) Adrenoceptors. Handbook of Experimental Pharmacology, vol 285. Springer, Cham. https://doi.org/10.1007/164_2023_701
Download citation
DOI: https://doi.org/10.1007/164_2023_701
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-66775-6
Online ISBN: 978-3-031-66776-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)