Abstract
Recent investigations indicate that β2-adrenergic receptor (β2-AR) signaling may facilitate the progression of various tumors, whose underlying mechanisms remain largely elusive. In the present study, we showed that β2-AR recruited Cdc42 in response to isoproterenol (ISO, a β-AR selective agonist) exposure in pancreatic ductal adenocarcinoma (PDAC) cells. The association of β2-AR and Cdc42 promoted the activation of Cdc42, as revealed by increased levels of Cdc42-GTP, and co-incubation with β2-AR antagonist abrogated ISO-induced activation of Cdc42. β2-AR-mediated Cdc42 activation further led to the phosphorylation of downstream PAK1, LIMK1 and Merlin. Furthermore, we showed that the activation of β2-AR/Cdc42 signaling facilitated the migration and invasion of PDAC cells. In addition, β2-AR and Cdc42 were overexpressed in PDAC specimens, compared with adjacent non-tumor tissues. High expression of β2-AR and Cdc42 were correlated with lymph node metastasis and TNM stage in PDAC patients. Finally, we showed that overexpression of β2-AR and Cdc42 were indicative of unfavorable prognosis in PDAC patients. Taken together, our findings suggested that β2-AR might facilitate Cdc42 signaling to drive the migration and invasion of PDAC cells, consequently resulting in the metastasis and dismal prognosis of PDAC. These studies highlight targeting β2-AR/Cdc42 signaling as a therapeutic strategy against PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) ranks one of the most common cancer types worldwide, especially in developed countries. In the United States, PDAC is the 9th and 10th most frequently-diagnosed cancer in female and male, respectively (Rawla et al. 2019). PDAC has been widely viewed as one of the most deadly cancer type, with a 5-year overall survival of approximate 9%. PDAC represents the 4th leading cause of cancer-related death in the United States. One of the most important factors leading to unfavorable prognosis of PDAC is the lack of effective therapies for patients diagnosed at advanced stages. The development of PDAC involves complex genetic and environmental determinants (Goral 2015). The clarification of these factors and molecular mechanisms underlying their roles in the progression of PDAC may provide novel therapeutic strategies for the prevention and management of PDAC.
Studies in recent years have revealed that psychological disorders, including depression, anxiety and stress, played vital roles in the initiation and progression of PDAC (Huang et al. 2013; Kennedy et al. 2014; Sugimoto et al. 2016). It is assumed that psychological factors may affect the development of multiple cancers, especially PDAC, through the secretion of stress-related hormones and neurotransmitters (Shin et al. 2016). In agreement, multiple studies have explicitly shown that neurotransmitter pathways, particularly β-adrenergic pathways, play critical roles in stress-induced PDAC development in animal models (Eng et al. 2015; Kim-Fuchs et al. 2014). Experimental chronic stress may facilitate PDAC development and worsened prognosis in tumor-bearing animals, and these effects can be ameliorated by the treatment of beta-adrenergic receptor blocker propranolol (Partecke et al. 2016). These findings drew attention to an integral role of β-adrenergic signaling in the development of PDAC.
Beta-adrenergic signaling pathways may contribute to PDAC progression through various mechanisms. Catecholamines, include norepinephrine and adrenaline, may modulate cancer immune microenvironments, resulting in immune suppression and tumor immune evasion (Huan et al. 2017; Repasky et al. 2015). Apart from a role in immune regulation, norepinephrine and adrenaline can directly promote the proliferation, invasion and chemoresistance of PDAC cells through the activation of beta-adrenergic receptors that reside on tumor cells. β2-AR may upregulate the expression of HIF-1α to promote the proliferation and invasion of pancreatic cancer cells (Zhang et al. 2016). β2-AR forms a positive feedback loop with NGF/Trk pathways to drive PDAC progression and poor prognosis (Renz et al. 2018). Many investigations have indicated that the sympathetic nervous system played an integral role in PDAC development (Demir et al. 2012). Hara MR et al. reported that β2-AR and β-arrestin-1 facilitated the activation of MDM2 through direct interaction, leading to the degradation of p53 and DNA damage (Hara et al. 2011). Our previous study also indicated that β2-AR promoted PDAC proliferation through facilitating PCBP2-initiated c-myc expression (Wan et al. 2016). These findings implicated a complex mechanism by which β2-AR signaling drives PDAC development.
Because recent investigations revealed a variety of novel molecular mechanisms underlying the tumor-promoting role of β2-AR signaling in cancer development, we explored novel downstream signaling of β2-AR in PDAC. Using immunoprecipitation-mass spectrometry analysis, we identified Cdc42 as a novel binding protein of β2-AR. Given that Cdc42 was typically recruited following G-protein-coupled receptors (GPCRs) activation, we speculated that β2-AR could modulate Cdc42 pathway activation. In the present study, we showed that β2-AR activated Cdc42 pathway through direct recruitment in PDAC cells. Furthermore, we showed that PAK1, LIMK1 and Merlin, three of downstream effectors of Cdc42, were phosphorylated following isoproterenol (ISO, a β-AR selective agonist) exposure. ISO-mediated β2-AR/Cdc42 signaling promoted the migration and invasion of PDAC cells. High expression of β2-AR and Cdc42 predicted significantly worsened prognosis in patients with PDAC. These findings implicate that β2-AR signaling may drive the metastasis and invasion of PDAC via activating Cdc42.
Material and methods
Cell culture and treatment
PANC-1 and BxPC-3 cells were obtained from the Cell Bank of Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM glutaMAX™ medium or RPMI 1640 medium (Gibco, Thermo Fisher, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% penicillin–streptomycin (Gibco) at 37 °C in a humidified 5% CO2 incubator. ISO and ICI 118 551 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lentivirus vectors containing the small hairpin RNA (shRNA) against Cdc42 (NM_001039802.2) and the small hairpin negative control (shNC) were constructed and generated by Vigene Biosciences Company (Shandong, China). The target sequences for Cdc42 were as follows: shRNA#1, 5′-CAG ATG TAT TTC TAG TCT GTT TCA AGA GAA CAG ACT AGA AAT ACA TCT GTT TTT T-3′; shRNA#2, 5′-CGG AAT ATG TAC CGA CTG TTT CAA GAA AAC AGT CGG TAC ATA TTC CGT TTT TT-3′; shRNA#3, 5′-CGG AAT ATG TAC CGA CTG TTT CAA GAA AAC AGT CGG TAC ATA TTC CGT TTT TT-3′. The sequence of control shRNA was 5′-TTC TCC GAA CGT GTC ACG TTT CAA GAG AAC GTG ACA CGT TCG GAG AAT TTT TT-3′, which was the random sequence that was not related to the above mRNA.
Mass spectrometry analysis
Mass spectrometry analysis was performed as described previously (Wan et al. 2016). Briefly, after preprocessing, the fresh-frozen PDAC tissue was immunoprecipitated with anti-β2-AR antibody (ab182136, Abcam, Cambridge, UK). The immune complexes were separated by 10% SDS-PAGE and stained with coomassie brilliant blue. The proteins were detected using an LTQ mass spectrometer (Thermo, San Jose, CA). Protein identifications were accepted if they could be established at 95.0% probability and contained at least two unique identified peptides.
Immunoprecipitation (IP) and Western blot analysis
PDAC cells were lysed using an IP lysis buffer (50 mM Tris–Cl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1× Protease inhibitor cocktail (Roche, Basel, Switzerland) and 1× phosphatase inhibitor cocktail (Roche)). After centrifugation at 13,000 rpm, 4 °C for 15 min, the samples were transferred and pre-cleaned using 40 μL protein G sepharose. The samples were incubated with 4 μg anti-β2-AR antibody (ab182136, Abcam, Cambridge, UK) or control IgG (Bioworld) over night. Thereafter, 30μL protein G sepharose was added into each sample and incubated for an additional 2 h. The immunocomplexes were washed using IP lysis buffer for 5 times. The resulting samples were loaded for 10% SDS-PAGE separation. The Western blot experimental procedures were conducted as reported (Wan et al. 2016). The primary antibodies were as follows: anti-β2-AR antibody (ab182136, Abcam), anti-Cdc42 (ab64533, Abcam), anti-PAK1 (ab223849, Abcam), anti-PAK1 (phospho T212) (ab75599, Abcam), anti-Merlin (ab88957, Abcam), anti-Merlin (phospho S518) (ab2478, Abcam), anti-LIMK1 (ab108507, Abcam), anti-LIMK1 (phospho T508) (ab38508, Abcam).
Cdc42 activation detection assay
After ISO stimulation, the cells were washed with cold PBS and lysed using the lysis buffer for 5 min on ice. Then, active form of Cdc42 in the cell lysates was detected using an active Cdc42 Detection Kit (#8819, Cell Signaling Technology) in accordance with the manufacturer's protocol.
Immunofluorescence assay
Following ISO exposure, cells were washed with cold PBS and fixed using 4% paraformaldehyde in PBS for 30 min. Next, cells were permeabilized with 1% Triton X-100 in PBS for 15 min and blocked using 1% BSA in PBS for 1 h. Subsequently, cells were incubated with anti-β2-AR antibody (sc-271322, Santa Cruz Biotechnology) and anti-Cdc42 antibody (ab64533, Abcam) over night at 4 °C. After washing with PBS for 3 times, cells were incubated with Alexa Fluor 568-conjugated goat anti-rabbit or Alexa Fluor 488-conjugated goat anti-mouse IgG (Thermo Fisher) for 2 h. The slides were washed and stained with 10 μg/ ml DAPI for 5 min. Finally, the slides were mounted and observed under a Nikon confocal microscope (Nikon, NY, USA).
Wound healing, transwell migration assay and matrigel invasion assay
For the wound healing assay, the Panc-1 and BxPC-3 cells were seeded into 6-well culture plates. Cells progressed to confluency of 80%, and wounds were subsequently scratched in the monoculture using a 10-µl pipette tip. Three reference marks were made on the wells, and light-phase images were captured at 0 and 24 h post-wounding. The healing percentages were calculated based on the amount of decreased wound area using the ImageJ program. For the invasion assay, the cells were cultured in 24-well plates, which were exposed to 10 μM ISO or 100 µM ICI 118 551. The cells suspended in 600 µl serum-free medium were seeded into an insert chamber pre-coated with Matrigel matrix (BD Biosciences, San Jose, CA, USA), and 0.5 ml 10% FBS medium was added to the matched lower chamber. In an atmosphere containing 5% CO2, the chambers were incubated for 36 h at 37˚C. Invasive cells on the lower surface of the membrane, which had invaded the Matrigel and migrated through the polycarbonate membrane, were fixed in 4% formaldehyde for 1 h and stained with 0.05% crystal violet for 10 min. Five microscopic fields were randomly chosen to count the invasive cells. A similar procedure was utilized for the migration assay with no Matrigel used.
Patients and tissue specimens
The tissue microarray of 71 matched pairs of primary PDAC samples and adjacent normal tissues and 28 primary PDAC specimens was obtained from Shanghai Zuocheng Biotechnology Co., Ltd. All of the human tissues were collected using protocols that had been approved by the Ethics Committee of Soochow University. The study population consisted of 63 males and 36 females, and the age ranged from 34 to 85 years. The main clinical and pathologic variables were summarized in Table 2.
Immunohistochemical analysis
The tissue microarray was deparaffinized using a graded ethanol series, and then the endogenous peroxidase activity was blocked by soaking in 0.3% hydrogen peroxide for 30 min. After heating to 120 °C in an autoclave for 30 min to retrieve the antigen, the sections were rinsing in 10% goat serum (Gibco, Thermo Fisher, Carlsbad, CA) for 40 min at room temperature to block any nonspecific reactions. Thenceforth, the slides were incubated with β2-AR antibody (sc-271322, Santa Cruz Biotechnology) and Cdc42 antibody (ab187643, Abcam) at 4 °C overnight. The negative control slides using a nonspecific immunoglobulin IgG (Sigma Chemical Co., St. Louis, USA) as primary antibody were included in all assays. After incubating with diaminobenzidine (DAB) solution, the sections were stained with hematoxylin, and dehydrated with graded alcohol and cover slipped. For analyses, at least five high-power fields were randomly chosen and at least 500 cells were counted. The expression index was determined by staining intensity and immunoreactive cell percentage. For intensity evaluation, a score of 0 was for no staining, 1 was for weak staining, 2 was for moderate staining, and 3 was for strong staining. Also, the extent of staining was recorded: 1, < 10%; 2, 10–40%; 3, 40–70% and 4, > 70%. The scores from the two scales were combined, and each section was classified as low/no expression (0 to 4) or high expression (> 4) based on the final score. All immunostained sections were randomly examined by two independent pathologists using a Leica fluorescence microscope (Germany).
Statistical analysis
The data of all detections were performed from three independent experiments and presented as mean ± SEM. All of the statistical analyses were performed using the SPSS 21.0 software package. For analysis of the survival data, Kaplan–Meier curves were constructed, and the log-rank test was performed. The association between β2-AR and Cdc42 and the clinicopathological factors was analyzed using the Chi-square (χ2) test. Multivariate analysis was performed by Cox proportional hazards model and the 95% CI was recorded for every marker. The quantification for the colocalization was analyzed using Fiji software, and the Pearson correlation coefficient test was performed. For all statistical analyses, P < 0.05 was considered to be statistically significant.
Results
Cdc42 was identified as a binding protein of β2-AR in PDAC
To decipher the molecular mechanism underpinning β2-AR-mediated PDAC progression, we performed β2-AR immunoprecipitation (IP) using PDAC tissues, and sent the samples for mass spectroscopy, as described (Wan et al. 2016). Using this strategy, we identified multiple novel potential binding proteins of β2-AR and subsequently conducted immunoprecipitation to validate their interactions. As such, we identified GTPase Cdc42 as a novel binding protein of β2-AR. Cdc42 was detectable in β2-AR-immunoprecipitated samples in both PANC-1 and BxPC-3 PDAC cells (Fig. 1A). To further validate this interaction, we performed immunofluorescence analysis to determine the co-localization between β2-AR and Cdc42 in PDAC cells. As shown in Fig. 1B, partial co-localization of β2-AR and Cdc42 was observed in ISO-treated PDAC cells, as indicated by appearance of yellow color, and the Pearson correlation coefficient analysis confirmed the co-localization (Fig. 1C). These findings suggested that β2-AR might recruit Cdc42 to transmit its downstream signaling following ISO treatment.
β2-AR associated with Cdc42 in response to ISO treatment. a Immunoprecipitation of β2-AR and Cdc42 in the absence or presence of 10 μM ISO. PANC-1 and BxPC-3 cells were treated with vehicle (ethanol) or 10 μM ISO for 30 min and subjected to immunoprecipitation using an anti-β2-AR antibody. 10% for Input. b Immunofluorescence analysis shows the co-localization of β2-AR and Cdc42 in ISO-treated PDAC cells. PANC-1 and BxPC-3 cells were exposed to 10 μM ISO for 30 min, and then subjected to immunofluorescence assay. DAPI was used to stain the nuclei. The yellow color in merged images indicates the co-localization between β2-AR and Cdc42. c Graphic representation of the relationship between β2-AR and Cdc42 localization. Statistical analyses were carried out using Pearson correlation coefficient test. Pearson's R value for PANC-1 cells was 0.68, and for BxPC-3 cells was 0.70
ISO-induced β2-AR activation facilitated Cdc42 signaling in PDAC cells
Cdc42 is a Rho-GTPase that plays a central role in the beginning of variety of cellular responses including cellular transformation, cell division, cell invasion, migration and cell polarity in cells(Arias-Romero and Chernoff 2013; Qadir et al. 2015). Like other GTPases, Cdc42 cycles between an inactive, GDP-bound state and an active, GTP-bound state. Once activated, Cdc42 can interact with a variety of downstream effectors leading to their activation, including p21-activated kinase 1 (PAK1), LIMK1 and Merlin(Szczepanowska 2009). Thus, we determined whether ISO treatment could activate Cdc42 in a β2-AR-dependent manner. To this end, PANC-1 and BxPC-3 cells were exposed to ISO and subjected to Western blot analysis to examine the levels of Cdc42-GTP. The result showed that Cdc42-GTP was increased dramatically following ISO exposure (Fig. 2A), whereas the total levels of Cdc42 were unchanged. Importantly, co-treatment with β2-AR selective antagonist ICI 118 551 abrogated ISO-induced Cdc42 activation. The results suggested that the binding between the two proteins could be induced by the activation of β2-AR signaling.
β2-AR activated Cdc42 signaling pathway. a PANC-1 and BxPC-3 cells were treated with ISO and β2-AR antagonist ICI 118 551 for 6 h. The cells samples were subjected to Western blot analysis to determine the expression of the indicated proteins. The bar chart showed the quantitative analysis of the expression of Cdc42-GTP/total Cdc42 in the groups. Mean ± SEM of three independent experiments. *P < 0.05. b Western blot analysis of Cdc42 and GAPDH (loading control) in wide-type, control shRNA and Cdc42 shRNAs in Panc-1 and BxPC-3 cells. The bar chart showed the quantitative analysis of the expression of Cdc42 /GAPDH in the groups. Mean ± SEM of three independent experiments. *P < 0.05.c PANC-1 and BxPC-3 cells were treated with ISO and β2-AR antagonist ICI 118 551 for 6 h. The cells samples were subjected to Western blot analysis to determine the expression of the indicated proteins. Quantitative analysis of the expression of p-PAK1/PAK1, p-LIMK1/LIMK1, p-Merlin/Merlin, E-cadherin and Vimentin in the groups. Mean ± SEM of three independent experiments. *P < 0.05
Subsequently, to further verify the impact of β2-AR on Cdc42 signaling pathway, we detected the expression of Cdc42 downstream effectors. To this end, the expression of Cdc42 was knocked down in Panc-1 and BxPC-3 cells using three different chemically synthesized shRNAs. The knockdown efficiencies were examined using Western blot analysis, and shRNA#3 exhibited the best efficacy in silencing Cdc42 expression, compared with the negative control (Fig. 2B). As such, shRNA#3 was employed in the following study. As shown in Fig. 2C, ISO treatment significantly increased the expression of phosphorylated PAK1 (p-PAK1), phosphorylated LIMK1 (p-LIMK1) and phosphorylated Merlin (p-Merlin) in Panc-1 and Bxpc3 cells. In addition, treatment with β2-AR selective antagonist ICI 118 551, or knockdown of Cdc42 could reverse the phosphorylation of Cdc42 downstream effectors caused by ISO exposure, suggesting that the effect of ISO was mediated by β2-AR and Cdc42. Meanwhile, we discovered that the β2-AR/Cdc42 signaling was correlated with the expression of E-cadherin and Vimentin, two marker proteins of cell metastasis and invasion. These results indicated that β2-AR might activate Cdc42 signaling cascade in PDAC cells.
β2-AR/Cdc42 signaling induced the migration and invasion of PDAC cells
Based on the fact that Cdc42/PAK1/LIMK1 and Cdc42/PAK1/Merlin signaling both play a profound role in regulating cell mobility (Dummler et al. 2009), and our findings that β2-AR-mediated Cdc42 activation was correlated with the expression of E-cadherin and Vimentin, we speculated that β2-AR/Cdc42 signaling might have an influence on PDAC cell migration and invasion. Therefore, PANC-1 and BxPC-3 cells were subjected to wound healing assay and transwell migration assay in the absence or presence of ISO. As revealed in Fig. 3A-B, treatment with ISO led to significantly enhanced migration of PANC-1 and BxPC-3 cells compared with the control groups. Of note, co-incubation with ICI 118 551 or knockdown of Cdc42 with shRNA diminished ISO-induced migration of PDAC cells. We further assessed the impact of ISO on PDAC cell invasion using matrigel invasion assay. As predicted, ISO treatment facilitated apparent invasion of PDAC cells, whereas co-treatment with ICI 118 551 or knockdown of Cdc42 abrogated the invasion of PDAC cells (Fig. 3C). These data supported the assumption that β2-AR/Cdc42 signaling might contribute to the migration and invasion of PDAC cells.
β2-AR/Cdc42 signaling promoted the migration and invasion of PDAC cells. a PANC-1 and BxPC-3 cells were exposed to 10 μM ISO coupled with 100 µM ICI 118 551, and then subjected to wound healing assay. The migration of cells is determined by the width of the wound at 24 h time point. b Transwell migration assay was conducted to determine the ability of migration for PDAC cells following ISO and ICI 118 551 treatment. c The ability of invasion for PDAC cells was assessed using Matrigel invasion assay. All data are representative of three independent experiments (mean ± SEM) (*P < 0.05)
β2-AR and Cdc42 expression were correlated with invasive phenotype and unfavorable prognosis in PDAC patients
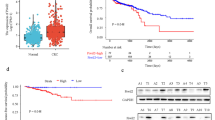
Our aforementioned data indicated that β2-AR might trigger PDAC cells migration and invasion via Cdc42 signaling. To address whether this molecular mechanism may contribute to the metastasis of PDAC in clinical setting, we performed immunohistochemistry analysis to determine the expression profiles of β2-AR and Cdc42 in PDAC patients. Immunohistochemistry results indicated that β2-AR and Cdc42 were both highly expressed in PDAC specimens, as compared with adjacent non-tumor tissues and normal pancreatic tissues (Fig. 4). The expression of β2-AR in PDAC tissues was positively correlated with that of Cdc42 (Table 1). Next, the correlations among β2-AR and Cdc42 expression and clinicopathological parameters were analyzed. Both of β2-AR and Cdc42 expression were associated with lymph node metastasis and TNM stage in PDAC patients (Table 2). In addition, we performed univariate and multivariate analyses using a proportional Cox regression hazard model to determine which parameters might serve as independent prognostic factors in predicting the survival of PDAC patients. As shown in Table 3, univariate Cox regression analysis indicated that histological differentiation, lymph node metastasis, TNM stage, β2-AR and Cdc42 expression might be prognosis-related. Further study using multivariate Cox regression analyses showed that histological differentiation and β2-AR might serve as independent prognostic factors in PDAC patients. Furthermore, Kaplan–Meier analysis indicated that high expression of β2-AR and Cdc42 both were associated with unfavorable prognosis in PDAC patients (Fig. 5A-B). Of note, high expression level of both β2-AR and Cdc42 indicated a poor outcome of patients with PDAC (Fig. 5C). These findings implicated that β2-AR/Cdc42 signaling might be a crucial pathway leading to dismal prognosis in PDAC patients.
β2-AR and Cdc42 were overexpressed in PDAC specimens. a Representative images of β2-AR and Cdc42 immunohistochemistry in PDAC and adjacent normal tissues. b The chart showed the expression profiles of β2-AR and Cdc42 in PDAC and non-tumor tissues. Statistical analyses were carried out using Pearson χ2 test
Cumulative survival curves according to β2-AR and Cdc42 expression in 99 PDAC patients. a Overall survival curves of low β2-AR expression patients (score ≤ 4) vs high β2-AR expression patients (score > 4). b Overall survival curves of low Cdc42 expression patients (score ≤ 4) vs high Cdc42 expression patients (score > 4). c Overall survival curves of high β2-AR and Cdc42 expression (β2-AR score > 4 and Cdc42 score > 4) patients vs the other patients
Discussion
Mounting studies in recent years implicated a critical involvement of the peripheral nervous system and neurotransmitter-mediated signaling in tumor progression. In the present study, we investigated the potential mechanisms underlying β2-AR signaling in PDAC cells and its involvement in PDAC progression. We for the first time showed that active β2-AR might recruit Cdc42, leading to consequent activation of Cdc42 and its downstream effectors. This molecular pathway may result in the migration and invasion of PDAC cells. We further showed that both β2-AR and Cdc42 were highly expressed in PDAC tissues and associated with worsened prognosis of PDAC patients. These findings together point to a pivotal role of β2-AR-mediated Cdc42 activation in PDAC metastasis and progression, shedding new light on the involvement of neurotransmitter signaling in PDAC development.
The neurotransmitter such as catecholamines are widely documented to be implicated in various physiological and pathological conditions, especially cancer development. For instance, social isolation may increase the level of tumor norepinephrine in patients with ovarian carcinoma (Lutgendorf et al. 2011). Thus, these neurotransmitters may be linked to cancer development caused by emotional and behavioral disorders. Because catecholamines are widely regarded as stress-associated hormones, elevated concentrations of catecholamines may directly contribute to the initiation and progression of tumors via catecholamine receptors, especially β2-AR. Multiple studies demonstrated that β2-AR activated a variety of tumor-promoting signaling pathways, such as AP-1, STAT3, HIF-1α, Her2 and CREB, to drive malignant phenotypes, such as uncontrolled proliferation, invasion and chemoresistance (Liu et al. 2020; Shan et al. 2013; Shi et al. 2010; Zhang et al. 2019, 2020). Our previous study also suggested that β2-AR signaling promoted c-myc expression in PDAC cells via PCBP2-dependent RNA translation (Wan et al. 2016). Intriguingly, previous reports mostly focused on the role of β2-AR signaling in transcriptional and translational regulation. Our present study implied that β2-AR signaling might also drive PDAC progression via a mechanism that is largely independent from gene expression. These studies infer that β2-AR may mediate cancer development through assorted mechanisms.
Cdc42 has been reported to be involved in multiple kinds of cancer, such as hepatocellular carcinoma, esophageal cancer and neuroblastoma (Lee et al. 2014; Tseng et al. 2015; Wang et al. 2014). In this study, we discovered that β2-AR activated Cdc42 signaling pathway to facilitate the migration and invasion ability of PDAC cells. A variety of studies suggested that Cdc42 might be recruited by a variety of neuronal receptors to facilitate cell motility. For instance, ligand-bound serotonin receptor subtype 7 (5-HT7R) may promote Cdc42 activation to induce actin filaments dynamics and neurite elongation (Speranza et al. 2015). EphB receptors may activate Cdc42 to regulate spine morphogenesis (Irie and Yamaguchi 2002). Neurotrophin receptors may regulate the migration of Schwann cells via the activation of Cdc42 (Yamauchi et al. 2004). Further studies to clarify the mechanisms underpinning β2-AR-mediated Cdc42 recruitment and the binding motif of each protein are beneficial to the understanding of β2-AR signaling in cancer cells.
In conclusion, we reported that liganded β2-AR recruited Cdc42 to facilitate the migration and invasion ability of PDAC cells. β2-AR-mediated Cdc42 signaling may contribute to the metastasis and worsened prognosis in patients with PDAC. These findings indicate that β2-AR and its downstream Cdc42 pathways may serve as therapeutic targets in PDAC treatment. Further studies are required to dissect the regulatory roles of β2-AR in PDAC development.
References
Arias-Romero LE, Chernoff J (2013) Targeting Cdc42 in cancer. Expert Opin Ther Targets 17:1263–1273. https://doi.org/10.1517/14728222.2013.828037
Demir IE, Friess H, Ceyhan GO (2012) Nerve-cancer interactions in the stromal biology of pancreatic. Cancer Front Physiol 3:97. https://doi.org/10.3389/fphys.2012.00097
Dummler B, Ohshiro K, Kumar R, Field J (2009) Pak protein kinases and their role in cancer. Cancer Metastasis Rev 28:51–63. https://doi.org/10.1007/s10555-008-9168-1
Eng JW et al (2015) Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nat Commun 6:6426. https://doi.org/10.1038/ncomms7426
Goral V (2015) Pancreatic cancer: pathogenesis and diagnosis. Asian Pac J Cancer Prev 16:5619–5624. https://doi.org/10.7314/apjcp.2015.16.14.5619
Hara MR et al (2011) A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477:349–353. https://doi.org/10.1038/nature10368
Huan HB et al (2017) Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav Immunity 59:118–134. https://doi.org/10.1016/j.bbi.2016.08.016
Huang J, Valdimarsdottir U, Fall K, Ye W, Fang F (2013) Pancreatic cancer risk after loss of a child: a register-based study in Sweden during 1991–2009. Am J Epidemiol 178:582–589. https://doi.org/10.1093/aje/kwt045
Irie F, Yamaguchi Y (2002) EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci 5:1117–1118. https://doi.org/10.1038/nn964
Kennedy B, Valdimarsdottir U, Sundstrom K, Sparen P, Lambe M, Fall K, Fang F (2014) Loss of a parent and the risk of cancer in early life: a nationwide cohort study. Cancer Causes Control 25:499–506. https://doi.org/10.1007/s10552-014-0352-z
Kim-Fuchs C et al (2014) Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40:40–47. https://doi.org/10.1016/j.bbi.2014.02.019
Lee S, Craig BT, Romain CV, Qiao J, Chung DH (2014) Silencing of CDC42 inhibits neuroblastoma cell proliferation and transformation. Cancer Lett 355:210–216. https://doi.org/10.1016/j.canlet.2014.08.033
Liu D et al (2020) beta2-AR activation promotes cleavage and nuclear translocation of Her2 and metastatic potential of cancer cells. Cancer Sci 111:4417–4428. https://doi.org/10.1111/cas.14676
Lutgendorf SK et al (2011) Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 25:250–255. https://doi.org/10.1016/j.bbi.2010.10.012
Partecke LI et al (2016) Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 16:423–433. https://doi.org/10.1016/j.pan.2016.03.005
Qadir MI, Parveen A, Ali M (2015) Cdc42: role in cancer management. Chem Biol Drug Des 86:432–439. https://doi.org/10.1111/cbdd.12556
Rawla P, Sunkara T, Gaduputi V (2019) Epidemiology of pancreatic cancer: global trends, Etiology and risk factors. World J Oncol 10:10–27. https://doi.org/10.14740/wjon1166
Renz BW et al (2018) beta2 Adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 34:863–867. https://doi.org/10.1016/j.ccell.2018.10.010
Repasky EA, Eng J, Hylander BL (2015) Stress, metabolism and cancer: integrated pathways contributing to immune suppression. Cancer J 21:97–103. https://doi.org/10.1097/PPO.0000000000000107
Shan T et al (2013) beta2-AR-HIF-1alpha: a novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr Mol Med 13:1023–1034. https://doi.org/10.2174/15665240113139990055
Shi M et al (2010) Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer 9:269. https://doi.org/10.1186/1476-4598-9-269
Shin KJ et al (2016) Molecular mechanisms underlying psychological stress and cancer. Curr Pharm Des 22:2389–2402
Speranza L et al (2015) Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci 9:62. https://doi.org/10.3389/fnbeh.2015.00062
Sugimoto H et al (2016) The prognostic factors and trajectory of HRQOL in patients with pancreatic cancer who received psychiatric intervention. J Gastroenterol Hepatol 31:685–690. https://doi.org/10.1111/jgh.13172
Szczepanowska J (2009) Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol 56:225–234
Tseng RC et al (2015) Deregulation of SLIT2-mediated Cdc42 activity is associated with esophageal cancer metastasis and poor prognosis. J Thorac Oncol 10:189–198. https://doi.org/10.1097/JTO.0000000000000369
Wan C et al (2016) beta2-adrenergic receptor signaling promotes pancreatic ductal adenocarcinoma (PDAC) progression through facilitating PCBP2-dependent c-myc expression. Cancer Lett 373:67–76. https://doi.org/10.1016/j.canlet.2016.01.026
Wang B, Xu T, Liu J, Zang S, Gao L, Huang A (2014) Overexpression of activated Cdc42-associated kinase1 (Ack1) predicts tumor recurrence and poor survival in human hepatocellular carcinoma. Pathol Res Pract 210:787–792. https://doi.org/10.1016/j.prp.2014.09.014
Yamauchi J, Chan JR, Shooter EM (2004) Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci USA 101:8774–8779. https://doi.org/10.1073/pnas.0402795101
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z (2019) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis 10:788. https://doi.org/10.1038/s41419-019-2030-2
Zhang B et al (2020) The stress hormone norepinephrine promotes tumor progression through beta2-adrenoreceptors in oral cancer. Arch Oral Biol 113:104712. https://doi.org/10.1016/j.archoralbio.2020.104712
Zhang D et al. (2016) beta2-adrenogenic signaling regulates NNK-induced pancreatic cancer progression via upregulation of HIF-1alpha Oncotarget 7:17760–17772. doi:https://doi.org/10.18632/oncotarget.5677
Acknowledgements
This work was supported by the National Natural Scientific Foundation of China (No.81802378, 81902406), the Suzhou Kejiaoxingwei Youth Science and Technology Program (No. KJXW2017066) and the Taicang Science and Technology Program (No. TC2017YYJC04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, C., Hu, B., Chen, H. et al. β2-adrenergic receptor drives the metastasis and invasion of pancreatic ductal adenocarcinoma through activating Cdc42 signaling pathway. J Mol Histol 53, 645–655 (2022). https://doi.org/10.1007/s10735-022-10076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10076-8