Abstract
Human activities are causing major negative environmental impacts, and the development of sustainable processes for production of commodities is a major urgency. Plant biomass represents a valuable alternative to produce energy and materials, but exploiting present crops for commodities production would however require massive resources (i.e. land, water and nutrients), raising serious sustainability concerns. In addition to efforts to improve plant, land and resource use efficiency, it is thus fundamental to look for alternative sources of biomass to complement crops. Microalgae are unicellular photosynthetic organisms that show a huge, yet untapped, potential in this context.
Microalgae metabolism is powered by photosynthesis and thus uses sunlight, a renewable energy source, and the exploitation of microalgae-based products has the potential to provide a beneficial environmental impact. These microorganisms have the ability to synthesize a wide spectrum of bioactive compounds, with many different potential applications (e.g. nutraceutics/pharmaceutics and biofuels). Several, still unresolved, challenges are however present such as the lack of cost-effective cultivation platforms and biomass-harvesting technologies. Moreover, the natural metabolic plasticity of microalgae is not optimized for a production at scale, and low biomass productivity and product yields affect competitiveness. Tuning microalgae metabolism to maximize productivity thus represents an unavoidable challenge to reach the theoretical potential of such organisms.
Communicated by Ulrich Lüttge
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Microalgae Biomass Applications
1.1 Exploitation of Microalgae Biomass to Mitigate Environmental Consequences of Human Activities
Human activities are responsible for the massive release of greenhouse gases (GHG) and other pollutants in the environment (Moss et al. 2010). GHG accumulation in the atmosphere is causing serious environmental consequences, leading to global temperature increase, extreme weather events, oceans acidification and deoxygenation and is magnifying the pressures on food and water security, as well as on forests and other ecosystems functional biodiversity (Levy and Patz 2015; Allison and Bassett 2015). Available models indeed foresee a 30% net increase in anthropogenic emissions by the end of the century, if global economy is assumed to follow historical trends, with a consequent rise in the average global temperature up to 3°C (Walsh et al. 2015). According to the Paris agreement, net anthropogenic carbon emissions must be gradually reduced to zero in order to limit the temperature increase to less than 2°C and thus avoid the worst-case scenario (Schellnhuber et al. 2016; Rockström et al. 2017).
GHG emissions are not only connected with fuel combustion and energy production but are also due to other human activities. As example, agricultural productivity needs to increase to follow the growing world population, but intensive practices can have negative consequences such as eutrophication (United Nations 2017; Ewel et al. 2018). A short-term remodelling of human activities towards sustainability is thus a strong urgency. Replacing part of industrial processes currently supporting the global economy with more sustainable alternatives would enable to preserve opportunities of economic development without constraining human welfare in the long term.
In such context, plant biomass represents a promising alternative to fulfill the demand of commodities. The photosynthetic metabolism requires only sustainable energy/carbon sources (i.e. sunlight and atmospheric CO2) to fuel production of biomass, which can ultimately be converted into biofuels or valuable materials. However, scaling up crop cultivation comes at the expense of the natural environment, rising several concerns. Crop-derived biomass needs arable land/freshwater and nutrients, and redirecting part of the agricultural practice to fuel the production of other commodities besides food is thus expected to increase the pressure on land/resource use (i.e. increased fertilization and consequent nutrient run-off into environment causing eutrophication), with negative effects that could outweigh the benefits (Khanna et al. 2017). In fact, in order to replace the biomass diverted into the production of alternative bio-commodities, farmers worldwide would need to increase arable land thus converting present forests and grasslands to new croplands (Fargione et al. 2008; Whitaker et al. 2018). Since soils and plant biomass are the two largest biologically active stores of terrestrial carbon (Schlesinger and Bernhardt 2013), such practice would release a huge amount of CO2 in the atmosphere, as a result of burning and consequent microbial decomposition of the biomass. In such a scenario, thus, the increase of arable land would cause an increase in GHG emissions, leading to a massive carbon debt only repairable in a long term (Searchinger et al. 2008).
It is thus necessary to find alternatives for the sustainable production of bio-commodities, beyond the simple expansion of area dedicated to crop cultivation. Production of plant-biomass feedstocks from marginal lands might be helpful in preserving the advantages of a photosynthesis-driven economy while minimizing the negative impacts. As example, the cultivation of perennial grasses and short-rotation trees would not require new arable land, thus providing a positive impact on GHG emissions also by reducing fertilizers and water usage. However, implementing standard crops with perennial grass cultivation on a global scale is not a trivial task (Robertson et al. 2017). In this context an emerging promising alternative is to use other biomass feedstocks such as microalgae.
Microalgae, as plants, support their metabolisms through photosynthesis, and they could thus represent an additional source of biomass to complement crops. Microalgae also present several other potentially interesting advantages. Their whole biomass is photosynthetically active with a potentially higher photon-to-biomass conversion efficiency (PBCE) with respect to plants (Melis 2009; Ooms et al. 2016). A larger PBCE would allow minimizing the pressure on environmental resources (i.e. land/water) to generate the biomass. Moreover, microalgae cultivation does not require arable land and the use of marine species would also reduce the pressure on the demand of freshwater (Usher et al. 2014). As a consequence, most of the resources needed to sustain microalgae cultivation do not compete with agriculture, not substantially perturbing crop productivity and food/feed production chain. Another consequence of a larger PBCE is that microalgae photosynthesis is highly effective in carbon sequestration, leading to the ability to withstand high CO2 concentrations, up to 90% (Salih 2011) and enabling their cultivation in connection to industrial sites, to mitigate overall anthropogenic GHG emissions.
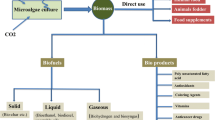
Fertilizer input to sustain microalgae cultivation is instead expected to be substantial, given most species consist of ≈7% of nitrogen (N) and ≈1% of phosphorus (P) (Slade and Bauen 2013). Fertilizers currently come from finite resources/environmentally unsustainable processes (Haber-Bosch process and phosphate rocks for N and P, respectively), and large-scale microalgae cultivation would increase the demand for such feedstocks. However, current research efforts aim to tackle such issue exploiting the natural ability of some species to harvest nutrients from civil and industrial wastewaters, where they have been found to be particularly efficient in removing nitrates and phosphates (Ramos Tercero et al. 2014). Such feature leaves also room to implement microalgae as valuable tools to curb nutrient run-off into the environment as a consequence of intensive agricultural and industrial practices (Santos and Pires 2018) (Fig. 1a). It is thus possible to design highly sustainable processes where biomass production is combined with wastewater treatment and or water/nutrient recycling (Fig. 1). The high potential in sustainability has indeed been shown by economic models where microalgae-derived biomass exploited to satisfy at least 40% of global energy/feed demands would result in massive environmental benefits and a drop in fossil fuel exploitation (Walsh et al. 2015).
Microalgae biomass-based economy. (a) Microalgae metabolism is powered by renewable energy/carbon sources (i.e. sunlight and CO2) and can be supported by nutrients from civil, agricultural and industrial wastewaters, further improving environmental sustainability. CO2 used for the cultivation can originate from an industrial plant, sequestering the emissions. (b) Biomass is feedstock for production of several molecules, finding application in various markets. Low-value compounds can be converted to liquid biofuels to fulfill part of the global energy demand, while high-value bioactive compounds find wide applications in both nutraceutical and pharmaceutical markets as food or feed additives. Biomass serves also for the production of energy to support water/nutrient recycling to the cultivation phase, potentially allowing energetic sustainability
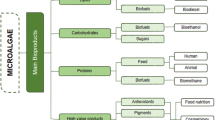
A second main characteristic of algae biomass beyond high sustainability is the large spectrum of its potential applications. Microalgae are a highly diverse group of organisms, including species adapted to far different ecological niches, which shaped also their metabolic versatility. Some microalgae species can naturally accumulate high concentration of energy dense storage molecules, i.e. starch and triacylglycerols (Nascimento et al. 2013; Simionato et al. 2013b; Fazeli Danesh et al. 2018), that can be used as liquid biofuel precursors. As a consequence, microalgae could contribute to satisfy part of the primary energy demand on a global scale to sustain current anthropogenic activities. The global primary energy demand is currently fulfilled by fuels of fossil origin, which are finite and major contributors to the accumulation of greenhouse gases in the atmosphere. The constant growth in global population has increased the pressure on fossil fuel feedstocks, also increasing the environmental consequences to dramatic levels. Diversifying the feedstocks for energy production, including more sustainable alternatives, might allow fulfilling an increasing energy demand while also lowering the environmental impact. Microalgae are renewable feedstocks for energy production and have the potential to decrease the dependence from fossil fuels, with an expected twofold advantage: (1) lower GHG emissions and (2) develop a more stable energy market based on feedstocks less susceptible to price fluctuations (Milano et al. 2016).
Different algae species can also synthesize a wide spectrum of bioactive compounds (Appeltans et al. 2012; Maeda et al. 2018), potentially exploitable for the production of several commodities, such as food/feed, dyes, cosmetics and drugs in nutraceutical and pharmaceutical markets (Vanthoor-Koopmans et al. 2013; Gimpel et al. 2015; Bilal et al. 2017; Koutra et al. 2018; Renuka et al. 2018) (Fig. 1b). The synthesis of many bioactive compounds currently commercialized by biopharmaceutical and chemical industries relies on environmentally unsustainable chemical reactions (i.e. toxic catalysers) and thus the development of alternative processes based on naturally occurring enzymes would provide a further advantage in the long term (Ullrich et al. 2015).
All these potential advantages clearly open several challenges for their realization. As example, the generation of microalgae biomass to fulfill the demand of different commodities at scale would clearly require the development of a completely sustainable and economically competitive production chain. As example, the fraction of the biomass not converted into the desired product should not be discarded as waste but be exploited, for example, to generate energy via anaerobic digestion (Gonzalez-Fernandez et al. 2015). Logistics of the process will need to be highly integrated to maximize the advantages. As example, if cultivation occurs in marginal lands far from biorefineries where products are extracted, this could have a negative impact on the carbon balance (Robertson et al. 2017). The development of an integrated and optimized production chain enabling both a positive energy and carbon footprint is thus a seminal objective in order to develop a sustainable microalgae biomass-based economy (De Bhowmick et al. 2019).
1.2 Microalgae as Natural Source of Nutrients and Bioactive Compounds
Despite their potential as platforms for developing environmental-friendly industrial processes is now widely recognized, microalgae are presently on the market only as food/feed additives and for high-added value molecules production in cosmetics (Vanthoor-Koopmans et al. 2013; Gimpel et al. 2015; Sathasivam et al. 2017). There are known examples of microalgae that have been used as food and nutritional supplements for hundreds of years (Gantar and Svirčev 2008) and currently represent one valuable alternative for the sustainable production of protein feedstocks and to the fast-growing demand of consumers for “healthy food” (Kim et al. 2019). Among them, the green microalga Chlorella vulgaris Beijerinck and the cyanobacterium Arthrospira platensis Gomont, also known as Spirulina platensis (Gomont) Geitler, currently represent the most commercialized species, given their high nutrient/vitamin/protein and active polysaccharide content which enables them to provide valuable human diet/feed supplement, finding applications in nutraceutics (Kang et al. 2013; Choi et al. 2013; Yaakob et al. 2014) (Fig. 1b and Table 1). Microalgae biomass has been widely reported to have positive effects on human health, reducing cancer and cardiovascular diseases (Raposo and de Morais 2015; Luo et al. 2015; Wang et al. 2017) by increasing natural killer cell titers and anti-inflammatory response (Kwak et al. 2012).
Among the bioactive molecules microalgae naturally synthesize, the largest market share is currently covered by carotenoids, which had a value of 1.24 billion USD in 2016, and it is projected to reach 1.51 billion by 2021 (Markets and Markets 2016). Such molecules belong to a class of more than 600 natural organic pigments, which play different biological roles (Guedes et al. 2011; Raposo et al. 2015), representing the most abundant natural pigments in nature (Torregrosa-Crespo et al. 2018). Among them, β-carotene is the most abundant antioxidant of microalgae origin currently commercialized (Raposo et al. 2015). Its synthetic analogue consists only of the all-trans isomer, which was reported to be responsible for negative effects on plasma membrane cholesterol levels in mice studies (Harari et al. 2008). β-carotene purified from Dunaliella salina (Dunal) Teodoresco is instead highly enriched in the 9-cis isomer, with no negative effects on health reported so far. A second example is astaxanthin from the green microalgae Haematococcus pluvialis J. Von Flotow, 1844, that has antioxidant and anti-inflammatory activity and also finds application as natural dye for salmon and shrimp pigmentation (Zhang and Wang 2015) (Table 1). Fucoxanthin is the third most commercialized carotenoid for its antioxidant activity, recently proven to prevent epidermal hyperplasia and erythema when administered in creams on mice skin (Rodríguez-Luna et al. 2018). Moreover, microalgae naturally synthesize other pigments with strong bioactive properties, such as chlorophylls, reported to increase liver functionality and cell repair (García et al. 2017).
It is worth noting that the pool of bioactive pigments of microalgae origin we currently have access to is still limited to only the species so far isolated (Guiry 2012; Appeltans et al. 2012). Efforts to identify novel microalgae species will give the chance to isolate new promising candidates for carotenogenesis, possibly bearing broader pigments set to expand further the applications on human health, even if more effective screening methods for pigment accumulation are required (Aburai et al. 2018).
Besides pigments, microalgae biomass is an established sustainable feedstock also for fatty acids. Several marine species are highly enriched in omega-3 and omega-6 long-chain polyunsaturated fatty acids (PUFA), such as docosahexaenoic acid (DHA, C22:6; e.g. from Isochrysis sp. strain T-iso, Pavlova lutheri Butcher, 1952, several species of the Nannochloropsis genus), eicosapentaenoic acid (EPA, C20:5; e.g. Nannochloropsis gaditana L.M.Lubián, Phaeodactylum tricornutum Bohlin) and alpha-linolenic acid [e.g. Rhodomonas salina (Wislouch) D.R.A.Hill & Wetherbee, Tetraselmis suecica (Kylin) Butcher] (Kagan et al. 2014; Tsai et al. 2016) (Table 1), finding potential application as feed in aquaculture (Gimpel et al. 2015; García et al. 2017; Byreddy et al. 2018) and also as food supplement since such molecules have been reported to be beneficial for cardiovascular and inflammatory disorders. PUFAs are currently mainly derived from fish oils, whose production is however rising several concerns for the long-term sustainability. Given the high content of oils in microalgae biomass, they might thus represent a valuable alternative to curb intensive fishing, which is strongly depleting current ocean fish stocks and marine biodiversity (Ryckebosch et al. 2014).
Polysaccharides (EPS) represent another important class of molecules naturally synthesized by some microalgae species, used in the food industry as thickeners and gelling additives (Liu et al. 2016), besides showing several positive effects on human health (Olasehinde et al. 2017) (Table 1). Other bioactive compounds derived from microalgae biomass are triterpenes called sterols (Luo et al. 2015), able to reduce low-density lipoprotein (LDL) abundance, promoting anti-inflammatory and positive cardiovascular effects (Olasehinde et al. 2017) (Table 1). Also complex molecules, such as glyco- and pigment-binding proteins (i.e. phycobiliproteins), are highly abundant in microalgae (Sonani et al. 2016). Among them, phycocyanin and phycoerythrin are currently the main molecules from Spirulina platensis to be commercialized for many different purposes, including natural dyes and nutraceutics with anti-inflammatory, neuroprotective and anticancer properties (Jiang et al. 2017) (Table 1).
Such wide pool of bioactive compounds is naturally synthesized by microalgae, thus enabling a direct administration of the biomass to complement diet. However, to reach a considerable effect, biomass needs to be administered in sufficient quantities, with possible negative consequences on supplemented food texture and flavour (Isleten Hosoglu 2018). A significant drawback to be considered also comes from the high nucleic acid content of some species, which are converted in uric acid with potential adverse health effects (Gantar and Svirčev 2008). In this context, the direct administration of cyanobacteria biomass rises even more concerns. Some species indeed synthesize hepato-/neurotoxins under certain environmental conditions, requiring a better comprehension of the cell physiology before commercialization (Shimizu 2003; Chu 2012). To prevent other biomass components to counteract the positive effect of the bioactive compounds listed in Table 1, the latter can also be purified from the biomass, enabling a more targeted effect on human health but also increasing costs (Kim et al. 2015).
Besides the above described applications, microalgae sustainability advantages have raised a strong interest as potential feedstocks for biofuel production to fulfill part of the global primary energy demand. In fact, several microalgae species are able to accumulate up to 60% dry weight in fatty acids (Simionato et al. 2013b; Jia et al. 2015) as triacylglycerols that are easily convertible in biodiesel through transesterification (Medipally et al. 2015; Park et al. 2015). The oleaginous trait is present in several microalgae, with Nannochloropsis species being the preferable hosts since showing an enrichment in the copy number of genes involved in key steps of lipid biosynthesis (Wang et al. 2014; Zienkiewicz et al. 2017).
1.3 Present Major Limitations of Algae Biotechnology
The great potential of microalgae opens the way for their integration in the current global economy as feedstock for different bio-commodities. However, despite microalgae biomass represents an eco-friendly and energetically sustainable solution to current industrial processes, the theoretical potential is still largely untapped, because implementing microalgae cultivation on a global scale is not commercially feasible yet (Carneiro et al. 2017). Critical issues still to be addressed include the development of efficient and cost-effective cultivation platforms and biomass-harvesting technologies (Acién Fernández et al. 2012; Lam et al. 2018). Moreover, except for fatty acids and other energy-rich storage compounds, the majority of bioactive compounds achieve unsatisfactory final yields (i.e. <1 up to 5% of biomass dry weight, depending on the cultivation conditions and the species) (Markou and Nerantzis 2013), therefore limiting the full realization of their potential.
In order to increase the production of microalgae-derived bio-commodities to allow a better integration in the global economy, intensive research efforts are thus still needed to overcome intrinsic limitations of the current state of the art and concretize such technology into an industrial reality. Such task is not trivial, and research efforts are indeed twofold: channelling more cellular resources towards bioactive molecules accumulation should in fact be complemented with more effective cultivation/harvesting technologies, tailored to large-scale production.
2 Algae Metabolic Engineering to Increase Yield in Bio-commodities
2.1 Challenges in Microalgae Metabolic Engineering
Pushing bioactive compound accumulation in microalgae relies on the ability to manipulate their metabolism, by overexpressing endogenous enzymes or introducing heterologous proteins triggering non-native reactions (Rasala and Mayfield 2015). However, regulatory networks are expected to be species-specific, limiting the development of universal strategies. The development of effective genome-editing approaches based on molecular mechanisms compatible with multiple hosts is currently opening the possibility to apply metabolic engineering to different species. Integrating genetic engineering with the native metabolism of the host is however not a trivial process, and research efforts still need to address multiple constraints. Rewiring the central metabolism might lead to unpredictable secondary effects on cell homeostasis due to the potential accumulation of toxic intermediates.
The impact of overexpression of enzymes depends on the molecular complexity of the target, which the native maturation steps of the host might not be able to support (Spadiut et al. 2014). Prokaryotic microalgae (i.e. cyanobacteria) are not able to efficiently perform post-translational modifications (Spadiut et al. 2014) and are thus likely not suitable hosts for the expression of complex enzymes. The control of post-translational modifications (e.g. disulphide bonds formation and glycosylation) is indeed highly strategic because they are often seminal to support the biological activity of complex enzymes, but if hyperactive they might lead to negative consequences on human health. For example, the yeasts Saccharomyces cerevisiae Hansen, 1883, and Pichia pastoris (Guillermond) Pfaff tend to hyper-glycosylate recombinant proteins, possibly leading to immunogenic effects. Eukaryotic microalgae might thus represent promising solutions to achieve the right balance in this context, because they carry protein maturation processes similar to plants (Ullrich et al. 2015), enabling the correct folding also of complex enzymes and allowing post-translational modifications. Unlike plants, they are however unicellular hosts, allowing an easier transformation process (Böer et al. 2007) and saving time to isolate stable transformants (Peters and Stoger 2011).
Among microalgae, Chlamydomonas reinhardtii Dangeard, 1899, currently presents the most advanced molecular toolkit for genetic manipulation, and all three genomes (nuclear, chloroplast and mitochondrial) have been fully sequenced and are transformable (Remacle et al. 2006; Specht et al. 2010). However, metabolic engineering in such host has so far lagged behind expectations. Gene integration in the nuclear genome of eukaryotic microalgae mainly occurs by non-homologous end joining (NHEJ) (Gumpel et al. 1994), leaving room for positional effects curbing the transcription efficiency of genes of interest (GOI) and eventually leading to silencing (Scranton et al. 2015), particularly frequent for long coding sequences. On the contrary, plastid transformation is not affected by such constraints, enabling the accumulation of target enzymes up to 10% of total soluble proteins (Young and Purton 2015). However plastids, due to their prokaryotic origin, do not carry proteins maturation pathways, limiting the potential of such strategy (Scaife et al. 2015). Research efforts have thus been dedicated to counteract the above-mentioned constraints in C. reinhardtii, and promising strategies have been indeed identified to establish more reliable molecular toolkits in such host (Gimpel et al. 2015). As an example, merging the coding sequence of the enzyme to overexpress with the one of the selection marker, through the foot-and-mouth disease virus 2A (FMDV 2A) self-cleavage peptide, led to a 100-fold increase in the accumulation of the target protein (Rasala et al. 2012). A second strategy involves the insertion of introns in the coding sequence of the GOI, showing positive effect on gene expression (Dong et al. 2017). Introns likely play a seminal role in controlling gene expression through enhancers and mRNA processing activity (Baier et al. 2018). Moreover, in C. reinhardtii native gene expression deals with highly frequent introns also longer than exons. Therefore, mimicking the native gene structure might represent a promising strategy to push non-native genes overexpression in such host, as recently demonstrated (Baier et al. 2018). A similar approach could also be effective in other microalgae species, with a larger industrial production potential than Chlamydomonas. The introduction of introns within the GOI coding sequence increases the level of complexity of such strategy, but it is also expected to minimize the chances of lateral transgene transfer during the cultivation of transgenic microalgae, preventing the GOI to be expressed by prokaryotic organisms (Lauersen 2018).
Increasing subcellular translocation efficiency of the protein of interest is another important target to increase the efficacy of metabolic engineering. In this context, versatile and expandable vector toolkits, enabling an easy replacement of targeting peptides according to operational needs, have been developed, expanding the number of subcellular compartments currently targetable [i.e. ER, chloroplast, nucleus, mitochondria and intracellular microbodies (Lauersen et al. 2013, 2015)].
Most genome-editing tools have been applied to green algae but are rapidly expanding to other species, such as diatoms and Eustigmatophyceae (e.g. Phaeodactylum and Nannochloropsis), which show a larger industrial potential and in some cases also have a lower genetic complexity (Radakovits et al. 2012; Vieler et al. 2012) [e.g. lower intron frequency (Corteggiani Carpinelli et al. 2014)], theoretically enabling easier strategies for metabolic engineering. As an example, recently homologous recombination and CRISPR-based methods have been successfully developed in such species, enabling a more targeted genome editing (Wang et al. 2016; Dolch et al. 2017; Poliner et al. 2018a, b), but still important work is needed to develop efficient tools in multiple species to allow multigene expression.
2.2 Improving the Content of Bioactive Compounds
The synthesis of bioactive compounds in microalgae (Table 1) is often dependent on the exposition to environmental stressful stimuli, from nutrient limitation [nitrogen (N) and phosphorous (P)] and high salinity to saturating light (Chen et al. 2011, 2015; Simionato et al. 2011; Yin-Hu et al. 2012; Menon et al. 2013; Alcántara et al. 2015; Jerez et al. 2015; Alboresi et al. 2016). Such environmental conditions, however, negatively affect growth (Sun et al. 2013; Simionato et al. 2013b), as reported for N limitation which triggers an overall remodelling of cell central metabolism as a consequence of a reduced proteins and nucleic acid biosynthesis. This approach thus requires a two-stage process where first biomass accumulation is maximized, followed by a nutrient limitation phase to induce the accumulation of the desired molecules. This strategy, while very effective at lab scale, increases costs and complexity at the large scale, finally limiting productivity achievable at scale (Rawat et al. 2013).
In order to develop a microalgae-biomass-based economy scalable to fulfill bio-commodities needs on a global scale, bioactive molecule synthesis and biomass accumulation should operate simultaneously. Coupling these two aspects of microalgae cell physiology is however not a trivial process, requiring a deeper understanding of the molecular factors controlling the metabolic channelling of resources. Strategies to push for the accumulation of a specific molecule can act at multiple levels of a metabolic pathway, for instance, increasing its biosynthesis and downregulating the catabolism. Major efforts have however been so far dedicated to the former, with carotenogenesis and lipid biosynthesis being the major targets.
Carotenogenesis takes place in the plastid and is highly integrated with the central metabolism, making efforts for its modification highly challenging. A reasonable strategy to increase carotenoid accumulation is to push the abundance of their precursors (i.e. isoprenoids). Such group of compounds include many molecules of economic interest, representing good targets for metabolic engineering (Lauersen 2018), which even in the case of no success in increasing the accumulation of carotenoids might thus still enhance the commercial value of the microalgal host. Moreover, isoprenoid metabolic pathways are modulatory, making them easy targets to manipulate in multiple hosts. As an example, reliable non-native di-/tri-/sesquiterpenoid production has been recently achieved in P. tricornutum and C. reinhardtii (Lauersen et al. 2016, 2018; Wichmann et al. 2018; D’Adamo et al. 2018), increasing their commercialization potential.
Among isoprenoids, geranylgeranyl pyrophosphate (GGPP) is the precursor of carotenoid synthesis and likely a limiting metabolite for carotenogenesis. Overexpression of geranylgeranyl pyrophosphate synthase (GGPPS) in Chlamydomonas however failed in triggering a remodelling in isoprenoid profile (Fukusaki et al. 2003). Also attempts to increase keto-carotenoid production by overexpressing β-carotene ketolases (BKT) from Dunaliella salina failed to achieve the expected outcomes (León et al. 2007), highlighting the presence of a more complex regulation or additional rate-limiting steps (Fig. 2a).
Simplified biosynthetic pathways for carotenoids (a) and lipids (b) in microalgae. (a) Carotenogenesis. Abbreviations: Metabolites, DMAPP dimethylallyl pyrophosphate, GGPP geranylgeranyl pyrophosphate; enzymes, BKT β-carotene ketolase, CrtR-B β-carotene hydroxylase, LYC-B lycopene β-cyclase, PDS phytoene desaturase, PSY phytoene synthase, ZDS ζ-carotene desaturase. (b) Lipid biosynthesis. Abbreviations: Metabolites, PUFA polyunsaturated fatty acids, TAG triacylglycerols; enzymes, ACCase acetyl-CoA carboxylase, ACP acyl-carrier protein, CoA coenzyme A, FAS fatty acid synthase, LACS long-chain acyl-CoA synthetase, MAT malonyl-CoA/ACP transacylase, ME malic enzyme, PDC pyruvate dehydrogenase, PDK pyruvate carboxylase kinase, TE fatty acyl-ACP thioesterase. Major targets to increase microalgae commercial potential are indicated in red. Scheme adapted from Gimpel et al. (2015)
Phytoene synthase (PSY) and phytoene desaturase (PDS) catalyse the first and second step in carotenogenesis, respectively, and have been identified as important targets to push β-carotene and keto-carotenoids (e.g. astaxanthin) synthesis in several species such as H. pluvialis, D. salina, Chlorella zofingiensis Dönz 1934 and C. reinhardtii (Couso et al. 2011; Cordero et al. 2011; Liu et al. 2013, 2014) (Fig. 2a). However, the biotechnological optimization of carotenoid synthesis is more complex than expected because precursors (i.e. isoprenoids) are shared among different classes of pigments (e.g. carotenoids and chlorophylls) and prioritizing their channelling towards one or the other is not a trivial process, risking to unbalance carotenoid and chlorophyll abundance with detrimental consequences on cell physiology. Merging GGPPS with PSY was recently demonstrated to push the metabolic flux of GGPP towards carotenogenesis in Arabidopsis thaliana (L.) Heynh. (Camagna et al. 2018), leaving room for implementing such technology also in microalgae. In the future, identifying novel candidates (Sugiyama et al. 2017; Lao et al. 2018) and engineering simultaneously multiple enzymatic steps in the pathway might strengthen the metabolic flux redirection towards carotenoid synthesis with no secondary effects on cell physiology; the development of effective targeted genome-editing approaches is currently opening such possibility (Nymark et al. 2016; Banerjee et al. 2018).
Another major target for microalgae metabolic engineering is the increase in lipid accumulation. In the past years, different enzymatic steps of lipid biosynthesis pathway of several species have been targeted to enhance fatty acid accumulation or modify their composition profile, showing different degrees of success (Sun et al. 2018). Acetyl-CoA carboxylase (ACCase) catalyses the first step in fatty acid (FA) biosynthesis, converting acetyl-CoA into malonyl-CoA. However, a higher protein accumulation did not result in an increased lipid content in Cyclotella cryptica Reimann, Lewin & Guillard (Dunahay et al. 1996), while a positive effect was instead observed in P. tricornutum upon chloroplast transformation, highlighting possible other species-specific limiting steps in the biosynthesis pathway (Fig. 2b). Pyruvate dehydrogenase (PDC) catalyses the conversion of pyruvate into acetyl-CoA; therefore it contributes to control the availability of the substrate for FA biosynthesis. Pyruvate carboxylase kinase (PDK) controls PDC activity through phosphorylation, and its downregulation was shown to significatively increase FA content in P. tricornutum (Ma et al. 2014) (Fig. 2b). FA acyl-carrier protein thioesterases (TE) are another important target to remodel lipid biosynthesis, and their overexpression was shown to increase total FA content in P. tricornutum (Gong et al. 2011). Alternatively, the overexpression of Acyl-CoA: diacylglycerol acyltransferase (DGAT) in the same organism was shown to increase neutral lipid accumulation (Niu et al. 2013), pushing also the EPA content of the cell.
FA biosynthesis requires ATP and NADH to operate. Indirect strategies pushing energy/reducing power availability might thus be valuable alternatives to complement direct approaches in remodelling FA biosynthesis. As an example, malic enzyme (ME) converts malate into pyruvate, generating NADH. Its overexpression was shown to increase total lipid accumulation in P. tricornutum under nutrient-replete conditions (Xue et al. 2015), likely as a consequence of the larger availability of reducing power (Fig. 2b).
Strategies affecting lipid biosynthesis might be also complemented with others rewiring their catabolism (Kong et al. 2018). Lipases and TEs involved in acyl-CoA hydrolysis are valuable target to knock-down in this context and in P. tricornutum were proven to increase total lipids (Trentacoste et al. 2013) and TAGs accumulation (Hao et al. 2018). One alternative to “classic” approaches targeting single enzymes is to identify master regulators of FA metabolism whose modification is instead expected to provide a more global remodelling of their accumulation. In such context, transcription factors (TFs) play a critical role and are indeed viable targets for lipid biosynthesis and biotechnological optimization (Hu et al. 2014; Banerjee et al. 2018). Overexpression of different TFs in Nannochloropsis salina D.J.Hibberd was shown to have a strong impact on biomass accumulation as well as on lipid biosynthesis (Kang et al. 2015, 2017; Kwon et al. 2018).
Another possibility is to target the lipid composition rather than the overall content. As example, the insertion of two higher plants TE in P. tricornutum resulted in a larger incorporation of short-chain FA in triacylglycerols (TAGs) (Radakovits et al. 2011). Polyunsaturated fatty acid (PUFA) market share is increasing over time, pushing for the development of reliable engineering strategies to increase their content in microalgae hosts. Desaturases and elongases are here expected to play a major role, driving the research efforts so far (Dolch et al. 2017) (Fig. 2b). The former have been shown to increase PUFA accumulation in C. reinhardtii, P. tricornutum and Nannochloropsis oceanica Suda & Miyashita, highlighting the feasibility of such practice (Zäuner et al. 2012; Peng et al. 2014; Kaye et al. 2015).
The strategies listed above prove the feasibility of genetic engineering to increase/remodel FA content in different microalgae species. Such approaches are however unlikely to be universal because of the huge metabolic diversity of microalgae species, likely to regulate FA metabolism in a species-specific manner. This feature has contributed to lag the achievements in such field behind expectation in the past years, mainly because of the monetary and time costs to elucidate metabolic network regulation and apply genetic engineering, tuned to different species.
It is also worth noting that a global remodelling of lipid metabolism to fulfill multiple bio-commodities productions is challenged by the natural competition for cellular resources between different lipid classes and by the difficulty in simultaneously modifying enzymes belonging to different subcellular compartments. Therefore, the metabolic strategies listed above ought to be tuned not only to the target strain biology but also to the eventual application of the biomass (i.e. human nutrition rather than feed, etc.). A deeper comprehension of both biosynthesis and catabolism of lipids as well as of the metabolic processes involved in length and saturation rate modification is seminal to such purpose, and filling this gap is of extreme importance because the nutritional value of the biomass depends on them (Medipally et al. 2015; Park et al. 2015).
Moreover, pushing bioactive molecule accumulation in microalgae biomass is just the first step to approach a broader impact on economy of microalgae-derived products. Accumulated molecules in some cases must be extracted from the biomass to have a more targeted impact on human health. However, conventional extraction methods have several limitations (i.e. low efficiency and yield and high costs) and need further improvement to be effective but safe for humans and environmentally sustainable (Sosa-Hernández et al. 2018).
3 Strategies to Improve Microalgae Biomass Productivity
3.1 Challenges in Large-Scale Microalgae Cultivation
Microalgae are photosynthetic organisms; therefore their physiology is highly influenced by light availability, which has also been identified as a major parameter affecting growth (Ruiz et al. 2016). Microalgae are generally cultivated in open or closed platforms, namely, ponds or photobioreactors (PBR), respectively, which guarantee a controlled cultivation environment (De Vree et al. 2015). Such systems are exposed to excess irradiance to avoid energy input limitations, and microalgae are here cultivated at high densities to push productivity. In general, conditions experienced during industrial cultivation are different from the natural algae ecological niches, where cells are diluted and often experience nutrient/light limitations which contributed to the evolution of specific traits, often not advantageous in an intensive cultivation (Fig. 3).
Schematic representation of environmental differences microalgae face when switching from natural to intensive cultivation. In nature microalgae are generally diluted and often experience nutrients (here, i.e. NO3 − and PO4 3−), HCO3 − and light limitation. During intensive cultivation, cells experience high cell concentrations and excess nutrients and HCO3 − availability
As example, the general light limitation triggered the evolution of high pigment content per cell, to maximize the chances to prevail on other photosynthetic competitors for the available energy (Kirk 1994). Chlorophyll (Chl) is the main pigment responsible for light harvesting, which consequently is converted into excited forms, i.e. singlet chlorophyll (1Chl*), to ultimate fuel photochemical reactions, leading to the synthesis of ATP and NADPH. Pigments are bound to proteins called light-harvesting complexes (LHC), which accomplish the biological function of harvesting light energy and transfer it to the reaction centres of the two photosystems (PS) (Büchel 2015). An expanded light-harvesting apparatus is seminal in nature where cells are diluted, but in an intensive cultivation environment where cell concentration is high, it is detrimental for the overall population growth. A high efficiency in harvesting light, in fact, causes an inhomogeneous light distribution in the mass culture, with cells exposed to the light source capturing the majority of the incoming light, while inner layers instead experience energy limitation (Simionato et al. 2013a). Therefore, overall microalgae cultivation in PBR is strongly light-limited, compromising the achievable biomass productivity.
A second consequence can be found at the single-cell level. Microalgae generally can efficiently exploit light energy only until other factors (e.g. CO2 availability) become limiting. If irradiance is higher, absorbed energy exceeds cells’ photochemical capacity, causing an excess of 1Chl* that eventually leads to intersystem crossing and formation of Chl triplets (3Chl*). The latter can react with oxygen and generate singlet oxygen (1O2), which is a highly reactive molecule that oxidizes pigments, proteins and lipids, leading to damage and photoinhibition. To counteract the latter scenario, microalgae evolved photoprotection mechanisms [i.e. non-photochemical quenching (NPQ) (Melis 2009)] which are molecular valves to allow a safe de-excitation of excess 1Chl* as heat. This means that in cells exposed to intense illumination, such as those at the PBR surface, a large fraction of the harvested energy [estimated up to 80% (Melis 2009)] is dissipated to avoid possible photodamage and oxidative stress, thus strongly decreasing their PBCE. At the same time, cells in inner layers do not receive enough light to run photochemical reaction. Light energy is here often below the compensation point, sometimes just enough to fulfill cell maintenance demands with therefore negative consequences on CO2 fixation, which operates in suboptimal conditions, limiting biomass accumulation.
The situation is further complicated by the fact that cells in PBR also experience a changing light environment, as a consequence of daily and seasonal variations in the light supply and of the active mixing of the culture which suddenly switches cells from inner light-limited layers to fully exposed ones and vice versa. Microalgae naturally evolved mechanisms to cope with changing light environments to maximize the chances of survival. Among them, microalgae can regulate pigment and photosynthetic component abundance according to their needs, from fast-occurring modifications of pigments content (i.e. between seconds and minutes) to slow-occurring ones (i.e. between minutes to hours), requiring de novo protein synthesis (i.e. antenna-size regulation) (Meneghesso et al. 2016). As an example, full NPQ activation in microalgae requires zeaxanthin (Nilkens et al. 2010; Murchie and Niyogi 2011), a pigment showing a direct activity in scavenging reactive oxygen species (ROS) (Kuczynska et al. 2015), but also essential to trigger conformational changes in specific classes of antenna proteins [light-harvesting complexes stress related (LHCSR and LHCX according to the species) (Büchel 2015)] to allow photoprotection. Such pigment is synthesized from violaxanthin by violaxanthin de-epoxidase (VDE), upon saturating light conditions, and converted back into the latter by zeaxanthin epoxidase (ZE) when cells return to limiting light, to close the so-called xanthophyll cycle. While the first reactions take seconds to occur, the latter can take several minutes (Goss and Jakob 2010). Despite microalgae are equipped with the required molecular mechanism to keep photosynthesis homeostasis in a changing light environment, kinetics of such mechanisms are incompatible with mixing in PBR, which generally operates in a millisecond-to-second timescale (Molina et al. 2001; Carvalho et al. 2011). As a consequence, cells exposed to the PBR surface do not have enough time to relax photoprotection before being exposed to light-limiting conditions in the inner layers, while cells in the latter do not have sufficient time to activate photoprotection mechanisms to counteract saturating irradiances of most exposed regions. Both cell populations therefore spend energy to inefficiently counteract light changes (Eberhard et al. 2008), again subtracting it to photochemical reactions and leading to a further reduction in light-use efficiency, ultimately leading to unsatisfactory PBCE values.
3.2 Metabolic Engineering to Increase Microalgae Light-Use Efficiency
Pushing microalgae PBCE is not a trivial objective, and strategies to optimize the entire cultivation process have been pursued in the past few years. In order to domesticate the photosynthetic metabolism to the artificial cultivation conditions, several issues need to be addressed (see Sect. 3.1) (Perin et al. 2014). The major strategies explored in the past years to pursue this objective were the reduction of the pigment content of the cell/reduction of the size of LHC (i.e. antenna proteins) and remodelling of photoprotection [for a detailed review on the topic, please refer to Perin et al. (2018)] (Fig. 4).
Strategies to increase microalgae light-use efficiency in intensive cultivation. Schematic representation of the major strategies postulated to increase microalgae light-use efficiency in PBR. (a) Lower Chl accumulation and antenna size should increase the light penetration in the mass culture, increasing the amount of light exploitable by cells populating inner culture layers and push the saturation limit of photosynthesis, enabling more exposed cells to channel more light towards photochemical reactions, enhancing CO2 fixation and biomass accumulation. (b) Photoprotection remodelling is expected to provide a valuable contribution to reduce the amount of energy lost. In particular speeding up NPQ relaxation would increase the amount of energy that more exposed cells channel towards CO2 fixation, once reaching inner culture layers. Abbreviation: PS photosystem
The first two strategies respond to the need for increasing available light for cells populating inner layers of the mass culture and pushing single-cell light-use efficiency in most exposed layers, enabling to channel more light energy into photochemistry, pushing CO2 and biomass accumulation (Simionato et al. 2013a) (Fig. 4a). A lower pigment content is in fact expected to reduce the amount of harvestable light by the first layers of a PBR, therefore shifting the saturating limit of photosynthesis towards higher irradiances but also increasing light availability in underneath layers (Oey et al. 2013) (Fig. 4a). Cells in such environment would thus receive an amount of energy exceeding just the one needed for cell maintenance, therefore enabling its channelling towards photochemical reactions, increasing CO2 fixation and biomass productivity (Simionato et al. 2013a). It has indeed been estimated that only a fraction of the Chl molecules of a cell are seminal for the correct assembly of PS reaction centres [i.e. ≈15 and ≈30% for PSII and PSI, respectively, in C. reinhardtii (Melis 1991)], while the others are theoretically dispensable since not necessary for electrons transport reactions. Such strategy has been widely pursued in the past years mainly by applying forward genetic approaches in different microalgae species, with overall positive results in increasing light penetration in a mass culture (Bonente et al. 2011; Oey et al. 2013; Perin et al. 2015). However, pushing light availability in inner culture layers is not always expected to improve biomass productivity, and in fact, if the reduction of Chl molecules affects indispensable photochemistry reactions, the negative consequences on photosynthesis might compensate the advantages of a greater light availability.
The reduction in the size of LHC therefore represents a valuable complementary strategy to alter specific targets (Fig. 4a). Several strategies have been exploited for such purpose in the past, directly targeting LHC encoding genes (Polle et al. 2003; Bonente et al. 2011; Cazzaniga et al. 2014; Perin et al. 2015). Also indirect approaches proved their effectiveness in reducing LHC size, by targeting co-/post-translational molecular factors (Beckmann et al. 2009; Wobbe et al. 2009; Kirst and Melis 2014; Sharon-Gojman et al. 2017; Jeong et al. 2018).
Overall, several strains generated following those strategies indeed showed an improved light-use efficiency and the ability to saturate photosynthesis at higher irradiances with respect to the parental strains (Bonente et al. 2011; Cazzaniga et al. 2014; Perin et al. 2015), validating the above-described approaches to effectively improve the photosynthetic metabolism. However, LHC proteins are not only involved in light harvesting but play a complex biological role, with some classes also involved in regulating photoprotection (see Sect. 3.1) (Horton and Ruban 2005). A reduction in such proteins could therefore trigger unexpected secondary effects, by lowering cell ability to withstand intense illumination and increasing susceptibility to saturating irradiances. Moreover, LHC proteins are bound to both PS, and if LHC reduction is not balanced among them, it might lead to further negative consequences. As an example, the reduction of LHC classes only bound to PSII might result in a reduction in overall light-use efficiency because light energy absorbed by PSI could not be utilized by the linear electron transport, if a consistent adjustment in PSII/PSI ratio would not be introduced (Polle et al. 2000). The above-discussed possible drawbacks of LHC reduction might however be counterbalanced by more targeted approaches, in which only LHC involved in light harvesting will be affected, also potentially balancing their reduction among the two PS. The development of CRISPR-based genome-editing approaches is indeed opening such possibility, by targeting also microalgae species with a larger industrial potential (Banerjee et al. 2018; Verruto et al. 2018).
Metabolic engineering of the photosynthetic metabolism is thus more likely to be successful when balancing light-harvesting and photoprotection efficiencies (Perin et al. 2018). The latter is a seminal feature of microalgae cells to curb photoinhibition and consequent cell damage (Sect. 3.1), and modifications in light-harvesting efficiency should never compromise it. In fact, cells in most exposed PBR layers still need to be able to withstand intense illumination and avoid photodamage, and an overall photoprotection reduction is expected to have serious negative consequences which would compensate for a lower energy loss (Perin et al. 2017a). Rather than modulating NPQ intensity, a preferable strategy could be to remodel photoprotection activation/relaxation kinetics, to enable photosynthesis to respond faster to sudden changes in the light environment (Fig. 4b). As discussed in Sect. 3.1, photoregulation kinetics generally lag behind the timescales of the environmental changes in light intensities experienced in PBR, and therefore a faster response might contribute to respond more promptly to them, increasing light-use efficiency while preserving photoprotection ability. NPQ activation in microalgae is controlled by the xanthophyll cycle; therefore speeding up its kinetics might have an influence on the timescale of NPQ activation/relaxation. Modulating xanthophyll cycle kinetics was indeed recently demonstrated to speed up NPQ relaxation in tobacco plants, pushing biomass accumulation in the field (Kromdijk et al. 2016).
Not only light harvesting and photoprotection contribute to control light-use efficiency, also electron transport reactions in fact play a major role, with alternative electron transport involved in regulating photosynthesis in a changing light environment (Cardol et al. 2011). Strengthening such pathways in microalgae species that lost some of such components during evolution might therefore represent a promising complementary strategy to those listed above. As an example, flavodiiron (Flv) proteins avoid over-reduction in the electron transport chain in fluctuating light conditions, redirecting electrons from NADPH to oxygen and preventing PSI to be photodamaged (Rochaix 2014). Their introduction in Arabidopsis and tobacco plants was indeed proven to speed up the recovery of photochemistry in fluctuating light conditions (Yamamoto et al. 2016; Gómez et al. 2018). Such approaches have not been attempted in microalgae so far, but the elucidation of the regulatory network of photoprotection in different microalgae species (Goss and Lepetit 2015) together with the development of more effective genome-editing approaches might open such possibility.
Overall, metabolic engineering of photosynthesis was proven to be feasible in different microalgae species, leading to significant improvements in PBCE and biomass productivity in some cases. However, such results were observed at the lab scale, and mutant performances in PBR need still to be assessed. The cultivation environment was indeed demonstrated to have a huge impact on genetically engineered microalgae, highlighting how operational conditions may enhance or reduce the performances of strains isolated in the lab (Perin et al. 2017b). Identifying the right genetic traits to target in order to push PBCE in PBR is clearly not a trivial process, since algae performances respond to many environmental parameters. Not only the dynamic environment, but also parameters related to the PBR design and operation such as light path, culture density, mixing rate, temperature and pH might have an influence on photosynthesis, as they control light, CO2 and nutrients availability. It is therefore impossible to extrapolate the impact of a genetic modification in the PBR environment from simple lab-scale evaluations. The elucidation of the impact that different cultivation parameters have on microalgae physiology is therefore seminal to speed up engineering strategies and to direct them towards the biological targets expected to have the greatest impact on PBCE. It should be underlined, however, given the complexity of the final cultivation environment, it is likely that there will be no superior genetically modified strain applicable in all circumstances, but instead the genetic modification ought to be tailored to the local environment and PBR characteristics.
4 Conclusions
The massive environmental impact of current anthropogenic activities calls for the development of alternative sustainable processes to fulfill the growing demand of commodities. Microalgae are able to exploit clean energy/carbon sources to synthesize a wide spectrum of bioactive compounds and biofuel precursors and thus are highly promising in this context. Their metabolic versatility can also enable the production of diverse commodities for multiple markets (i.e. from livestock feed to human healthcare and fuels).
Such potential is however still not realized, and several technological barriers are present, as demonstrated by the relatively small number of operating industrial plants. The cost of microalgae biomass production is still too high and several efforts are being indeed dedicated to the development of cost-effective cultivation platforms and biomass-harvesting technologies. In addition to operational parameters, microalgae metabolism will also need to be optimized to intensive production to achieve maximal productivity. Genetic approaches to improve microalgae metabolism have already demonstrated, at least at the lab scale, their potential in channelling more cellular resources to the synthesis of compounds of interest.
Biomass productivity is the second target requiring optimization to achieve efficient solar-driven production of commodities, and microalgae light-use efficiency is the key limiting parameter which currently curbs the maximum achievable production. Several strategies have been proven effective to tune the photosynthetic metabolism and select beneficial traits to push growth during intensive cultivation. Improved strains have however been so far mainly tested at small scale, and the influence of conditions experienced during large-scale intensive cultivation must be taken in full account for the development of a robust process. Both aspects should be seriously taken into consideration in the future to move from the proof-of-concept stage of current achievements in microalgae biotechnologies to a concrete industrial application.
References
Aburai N, Kazama H, Tsuruoka A, Goto M, Abe K (2018) Development of a whole-cell-based screening method for a carotenoid assay using aerial microalgae. J Biotechnol 268:6–11. https://doi.org/10.1016/j.jbiotec.2017.12.025
Acién Fernández FG, González-López CV, Fernández Sevilla JM, Molina Grima E (2012) Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal? Appl Microbiol Biotechnol 96:577–586. https://doi.org/10.1007/s00253-012-4362-z
Alboresi A, Perin G, Vitulo N, Diretto G, Block M, Jouhet J, Meneghesso A, Valle G, Giuliano G, Maréchal E, Morosinotto T (2016) Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol 171:2468–2482. https://doi.org/10.1104/pp.16.00599
Alcántara C, Fernández C, García-Encina PA, Muñoz R (2015) Mixotrophic metabolism of Chlorella sorokiniana and algal-bacterial consortia under extended dark-light periods and nutrient starvation. Appl Microbiol Biotechnol 99:2393–2404. https://doi.org/10.1007/s00253-014-6125-5
Allison EH, Bassett HR (2015) Climate change in the oceans: human impacts and responses. Science 350:778–782. https://doi.org/10.1126/science.aac8721
Appeltans W, Ahyong STT, Anderson G, Angel MVV, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko CBB, Brandão SNN, Bray RAA, Bruce NLL, Cairns SDD, Chan T-Y, Cheng L, Collins AGG, Cribb T, Curini-Galletti M, Dahdouh-Guebas F, Davie PJFJF, Dawson MNN, De Clerck O, Decock W, De Grave S, de Voogd NJ, Domning DPP, Emig CCC, Erséus C, Eschmeyer W, Fauchald K, Fautin DGG, Feist SWW, Fransen CHJMHJM, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gómez-Daglio L, Gordon DPP, Guiry MDD, Hernandez F, Hoeksema BWW, Hopcroft RRR, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb JBB, Kristensen RMM, Kroh A, Lambert G, Lazarus DBB, Lemaitre R, Longshaw M, Lowry J, Macpherson E, Madin LPP, Mah C, Mapstone G, McLaughlin PAA, Mees J, Meland K, Messing CGG, Mills CEE, Molodtsova TNN, Mooi R, Neuhaus B, Ng PKLKL, Nielsen C, Norenburg J, Opresko DMM, Osawa M, Paulay G, Perrin W, Pilger JFF, Poore GCBCB, Pugh P, Read GBB, Reimer JDD, Rius M, Rocha RMM, Saiz-Salinas JII, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel KEE, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker MLL, Thuesen EVV, Timm T, Todaro MAA, Turon X, Tyler S, Uetz P, van der Land J, Vanhoorne B, van Ofwegen LP, van Soest RWM, Vanaverbeke J, Walker-Smith G, Walter TCC, Warren A, Williams GCC, Wilson SPP, Costello MJJ, De Clerck O, Decock W, De Grave S, de Voogd NJ, Domning DPP, Emig CCC, Erséus C, Eschmeyer W, Fauchald K, Fautin DGG, Feist SWW, Fransen CHJMHJM, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gómez-Daglio L, Gordon DPP, Guiry MDD, Hernandez F, Hoeksema BWW, Hopcroft RRR, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb JBB, Kristensen RMM, Kroh A, Lambert G, Lazarus DBB, Lemaitre R, Longshaw M, Lowry J, Macpherson E, Madin LPP, Mah C, Mapstone G, McLaughlin PAA, Mees J, Meland K, Messing CGG, Mills CEE, Molodtsova TNN, Mooi R, Neuhaus B, Ng PKLKL, Nielsen C, Norenburg J, Opresko DMM, Osawa M, Paulay G, Perrin W, Pilger JFF, Poore GCBCB, Pugh P, Read GBB, Reimer JDD, Rius M, Rocha RMM, Saiz-Salinas JII, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel KEE, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker MLL, Thuesen EVV, Timm T, Todaro MAA, Turon X, Tyler S, Uetz P, van der Land J, Vanhoorne B, van Ofwegen LP, van Soest RWM, Vanaverbeke J, Walker-Smith G, Walter TCC, Warren A, Williams GCC, Wilson SPP, Costello MJJ (2012) The magnitude of global marine species diversity. Curr Biol 22:2189–2202. https://doi.org/10.1016/j.cub.2012.09.036
Baier T, Wichmann J, Kruse O, Lauersen KJ (2018) Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res 46:6909–6919. https://doi.org/10.1093/nar/gky532
Banerjee A, Banerjee C, Negi S, Chang J-S, Shukla P (2018) Improvements in algal lipid production: a systems biology and gene editing approach. Crit Rev Biotechnol 38:369–385. https://doi.org/10.1080/07388551.2017.1356803
Beckmann J, Lehr F, Finazzi G, Hankamer B, Posten C, Wobbe L, Kruse O (2009) Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J Biotechnol 142:70–77. https://doi.org/10.1016/j.jbiotec.2009.02.015
Bilal M, Rasheed T, Ahmed I, Iqbal HMN, Sada EG (2017) High-value compounds from microalgae with industrial exploitability – a review. Front Biosci 9:319–342
Böer E, Steinborn G, Kunze G, Gellissen G (2007) Yeast expression platforms. Appl Microbiol Biotechnol 77:513–523. https://doi.org/10.1007/s00253-007-1209-0
Bonente G, Formighieri C, Mantelli M, Catalanotti C, Giuliano G, Morosinotto T, Bassi R (2011) Mutagenesis and phenotypic selection as a strategy toward domestication of Chlamydomonas reinhardtii strains for improved performance in photobioreactors. Photosynth Res 108:107–120. https://doi.org/10.1007/s11120-011-9660-2
Büchel C (2015) Evolution and function of light harvesting proteins. J Plant Physiol 172:62–75. https://doi.org/10.1016/j.jplph.2014.04.018
Byreddy AR, Yoganantharjah P, Gupta A, Gibert Y, Puri M (2018) Suitability of novel algal biomass as fish feed: accumulation and distribution of omega-3 long-chain polyunsaturated fatty acid in zebrafish. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-018-2906-0
Camagna M, Grundmann A, Bär C, Koschmieder J, Beyer P, Welsch R (2018) Enzyme fusion removes competition for geranylgeranyl diphosphate in carotenogenesis. Plant Physiol 179:1013–1027. https://doi.org/10.1104/pp.18.01026
Cardol P, Forti G, Finazzi G (2011) Regulation of electron transport in microalgae. Biochim Biophys Acta 1807:912–918. https://doi.org/10.1016/j.bbabio.2010.12.004
Carneiro MLNM, Pradelle F, Braga SL, Gomes MSP, Martins ARFA, Turkovics F, Pradelle RNC (2017) Potential of biofuels from algae: comparison with fossil fuels, ethanol and biodiesel in Europe and Brazil through life cycle assessment (LCA). Renew Sust Energ Rev 73:632–653. https://doi.org/10.1016/J.RSER.2017.01.152
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89:1275–1288. https://doi.org/10.1007/s00253-010-3047-8
Cazzaniga S, Dall’Osto L, Szaub J, Scibilia L, Ballottari M, Purton S, Bassi R (2014) Domestication of the green alga Chlorella sorokiniana: reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol Biofuels 7:157. https://doi.org/10.1186/s13068-014-0157-z
Chen M, Tang H, Ma H, Holland TC, Ng KYS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655. https://doi.org/10.1016/j.biortech.2010.09.062
Chen H, Hu J, Qiao Y, Chen W, Rong J, Zhang Y, He C, Wang Q (2015) Ca(2+)-regulated cyclic electron flow supplies ATP for nitrogen starvation-induced lipid biosynthesis in green alga. Sci Rep 5:15117. https://doi.org/10.1038/srep15117
Choi W, Kang D, Lee H (2013) Enhancement of immune activation activities of Spirulina maxima grown in deep-sea water. Int J Mol Sci 14:12205–12221. https://doi.org/10.3390/ijms140612205
Chu W-L (2012) Biotechnological applications of microalgae. IeJSME 6:24–37
Cordero BF, Couso I, León R, Rodríguez H, Vargas MA (2011) Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl Microbiol Biotechnol 91:341–351. https://doi.org/10.1007/s00253-011-3262-y
Corteggiani Carpinelli E, Telatin A, Vitulo N, Forcato C, D’Angelo M, Schiavon R, Vezzi A, Giacometti GM, Morosinotto T, Valle G (2014) Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol Plant 7:323–335. https://doi.org/10.1093/mp/sst120
Couso I, Vila M, Rodriguez H, Vargas MA, León R (2011) Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids. Biotechnol Prog 27:54–60. https://doi.org/10.1002/btpr.527
D’Adamo S, Schiano di Visconte G, Lowe G, Szaub-Newton J, Beacham T, Landels A, Allen MJ, Spicer A, Matthijs M (2018) Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production. Plant Biotechnol J. https://doi.org/10.1111/pbi.12948
De Bhowmick G, Sarmah AK, Sen R (2019) Zero-waste algal biorefinery for bioenergy and biochar: a green leap towards achieving energy and environmental sustainability. Sci Total Environ 650:2467–2482. https://doi.org/10.1016/j.scitotenv.2018.10.002
de Morais MG, Vaz Bda S, de Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res Int 2015:835761. https://doi.org/10.1155/2015/835761
De Vree JH, Bosma R, Janssen M, Barbosa MJ, Wijffels RH (2015) Comparison of four outdoor pilot-scale photobioreactors. Biotechnol Biofuels 8:1–12. https://doi.org/10.1186/s13068-015-0400-2
Dolch L-J, Rak C, Perin G, Tourcier G, Broughton R, Leterrier M, Morosinotto T, Tellier F, Faure J-D, Falconet D, Jouhet J, Sayanova O, Beaudoin F, Maréchal E (2017) A palmitic acid elongase affects eicosapentaenoic acid and plastidial monogalactosyldiacylglycerol levels in Nannochloropsis. Plant Physiol 173:742–759. https://doi.org/10.1104/pp.16.01420
Dong B, Hu H-H, Li Z-F, Cheng R-Q, Meng D-M, Wang J, Fan Z-C (2017) A novel bicistronic expression system composed of the intraflagellar transport protein gene ift25 and FMDV 2A sequence directs robust nuclear gene expression in Chlamydomonas reinhardtii. Appl Microbiol Biotechnol 101:4227–4245. https://doi.org/10.1007/s00253-017-8177-9
Dunahay TG, Jarvis EE, Dais SS, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. Appl Biochem Biotechnol 57–58:223–231. https://doi.org/10.1007/BF02941703
Eberhard S, Finazzi G, Wollman F-A (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515. https://doi.org/10.1146/annurev.genet.42.110807.091452
Ewel JJ, Schreeg LA, Sinclair TR (2018) Resources for crop production: accessing the unavailable. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2018.10.008
Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P (2008) Land clearing and the biofuel carbon debt. Science 319:1235–1238. https://doi.org/10.1126/science.1152747
Fazeli Danesh A, Mooij P, Ebrahimi S, Kleerebezem R, van Loosdrecht M (2018) Effective role of medium supplementation in microalgal lipid accumulation. Biotechnol Bioeng 115:1152–1160. https://doi.org/10.1002/bit.26548
Fukusaki E-I, Nishikawa T, Kato K, Shinmyo A, Hemmi H, Nishino T, Kobayashi A (2003) Introduction of the archaebacterial geranylgeranyl pyrophosphate synthase gene into Chlamydomonas reinhardtii chloroplast. J Biosci Bioeng 95:283–287
Gantar M, Svirčev Z (2008) Microalgae and cyanobacteria: food for thought. J Phycol 44:260–268. https://doi.org/10.1111/j.1529-8817.2008.00469.x
García JL, de Vicente M, Galán B (2017) Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol 10:1017–1024. https://doi.org/10.1111/1751-7915.12800
Gimpel JA, Henríquez V, Mayfield SP (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front Microbiol 6:1376. https://doi.org/10.3389/fmicb.2015.01376
Gómez R, Carrillo N, Morelli MP, Tula S (2018) Faster photosynthetic induction in tobacco by expressing cyanobacterial flavodiiron proteins in chloroplasts. Photosynth Res 136:129–138. https://doi.org/10.1007/s11120-017-0449-9
Gong Y, Guo X, Wan X, Liang Z, Jiang M (2011) Characterization of a novel thioesterase (PtTE) from Phaeodactylum tricornutum. J Basic Microbiol 51:666–672. https://doi.org/10.1002/jobm.201000520
Gonzalez-Fernandez C, Sialve B, Molinuevo-Salces B (2015) Anaerobic digestion of microalgal biomass: challenges, opportunities and research needs. Bioresour Technol 198:896–906. https://doi.org/10.1016/J.BIORTECH.2015.09.095
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122. https://doi.org/10.1007/s11120-010-9536-x
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172C:13–32. https://doi.org/10.1016/j.jplph.2014.03.004
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644. https://doi.org/10.3390/md9040625
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063. https://doi.org/10.1111/j.1529-8817.2012.01222.x
Gumpel NJ, Rochaix JD, Purton S (1994) Studies on homologous recombination in the green alga Chlamydomonas reinhardtii. Curr Genet 26:438–442
Hao X, Luo L, Jouhet J, Rébeillé F, Maréchal E, Hu H, Pan Y, Tan X, Chen Z, You L, Chen H, Wei F, Gong Y (2018) Enhanced triacylglycerol production in the diatom Phaeodactylum tricornutum by inactivation of a Hotdog-fold thioesterase gene using TALEN-based targeted mutagenesis. Biotechnol Biofuels 11:312. https://doi.org/10.1186/s13068-018-1309-3
Harari A, Harats D, Marko D, Cohen H, Barshack I, Kamari Y, Gonen A, Gerber Y, Ben-Amotz A, Shaish A (2008) A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J Nutr 138:1923–1930
Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56:365–373. https://doi.org/10.1093/jxb/eri023
Hu J, Wang D, Li J, Jing G, Ning K, Xu J (2014) Genome-wide identification of transcription factors and transcription-factor binding sites in oleaginous microalgae Nannochloropsis. Sci Rep 4:5454. https://doi.org/10.1038/srep05454
Isleten Hosoglu M (2018) Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem 240:1210–1218. https://doi.org/10.1016/j.foodchem.2017.08.052
Jeong J, Baek K, Yu J, Kirst H, Betterle N, Shin W (2018) Deletion of the chloroplast LTD protein impedes LHCI import and PSI – LHCI assembly in Chlamydomonas reinhardtii. J Exp Bot 69:1147–1158. https://doi.org/10.1093/jxb/erx457
Jerez CG, Malapascua JR, Sergejevová M, Figueroa FL, Masojídek J (2015) Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta). Mar Biotechnol (NY). https://doi.org/10.1007/s10126-015-9664-6
Jia J, Han D, Gerken HG, Li Y, Sommerfeld M, Hu Q, Xu J (2015) Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res 7:66–77. https://doi.org/10.1016/j.algal.2014.11.005
Jiang L, Wang Y, Yin Q, Liu G, Liu H, Huang Y, Li B (2017) Phycocyanin: a potential drug for cancer treatment. J Cancer 8:3416–3429. https://doi.org/10.7150/jca.21058
Kagan ML, Sullivan DW, Gad SC, Ballou CM (2014) Safety assessment of EPA-rich polar lipid oil produced from the microalgae Nannochloropsis oculata. Int J Toxicol 33:459–474. https://doi.org/10.1177/1091581814553453
Kang HK, Salim HM, Akter N, Kim DW, Kim JH, Bang HT, Kim MJ, Na JC, Hwangbo J, Choi HC, Suh OS (2013) Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J Appl Poult Res 22:100–108. https://doi.org/10.3382/japr.2012-00622
Kang NK, Jeon S, Kwon S, Koh HG, Shin S-E, Lee B, Choi G-G, Yang J-W, Jeong B-R, Chang YK (2015) Effects of overexpression of a bHLH transcription factor on biomass and lipid production in Nannochloropsis salina. Biotechnol Biofuels 8:200. https://doi.org/10.1186/s13068-015-0386-9
Kang NK, Kim EK, Kim YU, Lee B, Jeong W-J, Jeong B, Chang YK (2017) Increased lipid production by heterologous expression of AtWRI1 transcription factor in Nannochloropsis salina. Biotechnol Biofuels 10:231. https://doi.org/10.1186/s13068-017-0919-5
Kaye Y, Grundman O, Leu S, Zarka A, Zorin B, Didi-Cohen S, Khozin-Goldberg I, Boussiba S (2015) Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: overexpression of endogenous Δ12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal Res 11:387–398. https://doi.org/10.1016/j.algal.2015.05.003
Khanna M, Wang W, Hudiburg TW, DeLucia EH (2017) The social inefficiency of regulating indirect land use change due to biofuels. Nat Commun 8:15513. https://doi.org/10.1038/ncomms15513
Kim D-Y, Vijayan D, Praveenkumar R, Han J-I, Lee K, Park J-Y, Chang W-S, Lee J-S, Oh Y-K (2015) Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour Technol 199:300–310. https://doi.org/10.1016/j.biortech.2015.08.107
Kim SW, Less JF, Wang L, Yan T, Kiron V, Kaushik SJ, Lei XG (2019) Meeting global feed protein demand: challenge, opportunity, and strategy. Annu Rev Anim Biosci. https://doi.org/10.1146/annurev-animal-030117-014838
Kirk J (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Kirst H, Melis A (2014) The chloroplast signal recognition particle (CpSRP) pathway as a tool to minimize chlorophyll antenna size and maximize photosynthetic productivity. Biotechnol Adv 32:66–72. https://doi.org/10.1016/j.biotechadv.2013.08.018
Kong F, Romero IT, Warakanont J, Li-Beisson Y (2018) Lipid catabolism in microalgae. New Phytol 218:1340–1348. https://doi.org/10.1111/nph.15047
Koutra E, Economou CN, Tsafrakidou P, Kornaros M (2018) Bio-based products from microalgae cultivated in digestates. Trends Biotechnol 36:819–833. https://doi.org/10.1016/j.tibtech.2018.02.015
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354:857–861. https://doi.org/10.1126/science.aai8878
Kuczynska P, Jemiola-Rzeminska M, Strzalka K (2015) Photosynthetic pigments in diatoms. Mar Drugs 13:5847–5881. https://doi.org/10.3390/md13095847
Kwak JH, Baek SH, Woo Y, Han JK, Kim BG, Kim OY, Lee JH (2012) Beneficial immunostimulatory effect of short-term Chlorella supplementation: enhancement of natural killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial). Nutr J 11:53. https://doi.org/10.1186/1475-2891-11-53
Kwon S, Kang NK, Koh HG, Shin S-E, Lee B, Jeong B, Chang YK (2018) Enhancement of biomass and lipid productivity by overexpression of a bZIP transcription factor in Nannochloropsis salina. Biotechnol Bioeng 115:331–340. https://doi.org/10.1002/bit.26465
Lam GP, Vermuë MH, Eppink MHM, Wijffels RH, Van Den Berg C (2018) Multi-product microalgae biorefineries: from concept towards reality. Trends Biotechnol 36:216–227. https://doi.org/10.1016/j.tibtech.2017.10.011
Lao YM, Jin H, Zhou J, Zhang HJ, Zhu XS, Cai ZH (2018) A novel hydrolytic activity of tri-functional geranylgeranyl pyrophosphate synthase in Haematococcus pluvialis. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcy173
Lauersen KJ (2018) Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta. https://doi.org/10.1007/s00425-018-3048-x
Lauersen KJ, Berger H, Mussgnug JH, Kruse O (2013) Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J Biotechnol 167:101–110. https://doi.org/10.1016/j.jbiotec.2012.10.010
Lauersen KJ, Kruse O, Mussgnug JH (2015) Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl Microbiol Biotechnol 99:3491–3503. https://doi.org/10.1007/s00253-014-6354-7
Lauersen KJ, Baier T, Wichmann J, Wördenweber R, Mussgnug JH, Hübner W, Huser T, Kruse O (2016) Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii. Metab Eng 38:331–343. https://doi.org/10.1016/J.YMBEN.2016.07.013
Lauersen KJ, Wichmann J, Baier T, Kampranis SC, Pateraki I, Møller BL, Kruse O (2018) Phototrophic production of heterologous diterpenoids and a hydroxy-functionalized derivative from Chlamydomonas reinhardtii. Metab Eng 49:116–127. https://doi.org/10.1016/J.YMBEN.2018.07.005
León R, Couso I, Fernández E (2007) Metabolic engineering of ketocarotenoids biosynthesis in the unicelullar microalga Chlamydomonas reinhardtii. J Biotechnol 130:143–152. https://doi.org/10.1016/j.jbiotec.2007.03.005
Levy BS, Patz JA (2015) Climate change, human rights, and social justice. Ann Glob Health 81:310–322. https://doi.org/10.1016/j.aogh.2015.08.008
Liu J, Gerken H, Huang J, Chen F (2013) Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Process Biochem 48:788–795. https://doi.org/10.1016/j.procbio.2013.04.020
Liu J, Sun Z, Gerken H, Huang J, Jiang Y, Chen F (2014) Genetic engineering of the green alga Chlorella zofingiensis: a modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl Microbiol Biotechnol 98:5069–5079. https://doi.org/10.1007/s00253-014-5593-y
Liu L, Pohnert G, Wei D (2016) Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs 14:191. https://doi.org/10.3390/md14100191
Luo X, Su P, Zhang W (2015) Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar Drugs 13:4231–4254. https://doi.org/10.3390/md13074231
Ma Y-H, Wang X, Niu Y-F, Yang Z-K, Zhang M-H, Wang Z-M, Yang W-D, Liu J-S, Li H-Y (2014) Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb Cell Factories 13:100. https://doi.org/10.1186/s12934-014-0100-9
Maeda Y, Yoshino T, Matsunaga T, Matsumoto M, Tanaka T (2018) ScienceDirect Marine microalgae for production of biofuels and chemicals. Curr Opin Biotechnol 50:111–120. https://doi.org/10.1016/j.copbio.2017.11.018
Markets and Markets (2016) Carotenoids Market. https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html?gclid=CjwKCAiAlvnfBRA1EiwAVOEgfJMz-lKGAoDHXopaDs3fcTYkbuYM3T7sVCwcZHmvZoGVSp31jHoUZRoCK2wQAvD_BwE. Accessed 28 Nov 2018
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Medipally SR, Yusoff FM, Banerjee S, Shariff M (2015) Microalgae as sustainable renewable energy feedstock for biofuel production. Biomed Res Int 2015:519513. https://doi.org/10.1155/2015/519513
Melis A (1991) Dynamics of photosynthetic membrane composition and function. BBA-Bioenergetics 1058:87–106. https://doi.org/10.1016/S0005-2728(05)80225-7
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280. https://doi.org/10.1016/j.plantsci.2009.06.005
Meneghesso A, Simionato D, Gerotto C, La Rocca N, Finazzi G, Morosinotto T (2016) Photoacclimation of photosynthesis in the Eustigmatophycean Nannochloropsis gaditana. Photosynth Res 129. https://doi.org/10.1007/s11120-016-0297-z
Menon KR, Balan R, Suraishkumar GK (2013) Stress induced lipid production in Chlorella vulgaris: relationship with specific intracellular reactive species levels. Biotechnol Bioeng 110:1627–1636. https://doi.org/10.1002/bit.24835
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197. https://doi.org/10.1016/J.RSER.2015.12.150
Molina E, Ferna J, Acie FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, van Vuuren DP, Carter TR, Emori S, Kainuma M, Kram T, Meehl GA, Mitchell JFB, Nakicenovic N, Riahi K, Smith SJ, Stouffer RJ, Thomson AM, Weyant JP, Wilbanks TJ (2010) The next generation of scenarios for climate change research and assessment. Nature 463:747–756. https://doi.org/10.1038/nature08823
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155:86–92. https://doi.org/10.1104/pp.110.168831
Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, de Souza CO, Vich DV, de Carvalho GC, Nascimento MA (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res 6:1–13. https://doi.org/10.1007/s12155-012-9222-2
Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797:466–475. https://doi.org/10.1016/j.bbabio.2010.01.001
Niu Y-F, Zhang M-H, Li D-W, Yang W-D, Liu J-S, Bai W-B, Li H-Y (2013) Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar Drugs 11:4558–4569. https://doi.org/10.3390/md11114558
Nymark M, Sharma AK, Sparstad T, Bones AM, Winge P (2016) A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci Rep 6:24951. https://doi.org/10.1038/srep24951
Oey M, Ross IL, Stephens E, Steinbeck J, Wolf J, Radzun KA, Kügler J, Ringsmuth AK, Kruse O, Hankamer B (2013) RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS One 8:e61375. https://doi.org/10.1371/journal.pone.0061375
Olasehinde TA, Olaniran AO, Okoh AI (2017) Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 22:480. https://doi.org/10.3390/molecules22030480
Ooms MD, Dinh CT, Sargent EH, Sinton D (2016) Photon management for augmented photosynthesis. Nat Commun 7:12699. https://doi.org/10.1038/ncomms12699
Park J-Y, Park MS, Lee Y-C, Yang J-W (2015) Advances in direct transesterification of algal oils from wet biomass. Bioresour Technol 184:267–275. https://doi.org/10.1016/j.biortech.2014.10.089
Peng K-T, Zheng C-N, Xue J, Chen X-Y, Yang W-D, Liu J-S, Bai W, Li H-Y (2014) Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J Agric Food Chem 62:8773–8776. https://doi.org/10.1021/jf5031086
Perin G, Segalla A, Basso S, Simionato D, Meneghesso A, Sforza E, Bertucco A, Morosinotto T (2014) Biotechnological optimization of light use efficiency in nannochloropsis cultures for biodiesel production. Chem Eng Trans 37:763–768. https://doi.org/10.3303/CET1437128
Perin G, Bellan A, Segalla A, Meneghesso A, Alboresi A, Morosinotto T (2015) Generation of random mutants to improve light-use efficiency of Nannochloropsis gaditana cultures for biofuel production. Biotechnol Biofuels 8:161. https://doi.org/10.1186/s13068-015-0337-5
Perin G, Bernardi A, Bellan A, Bezzo F, Morosinotto T (2017a) A mathematical model to guide genetic engineering of photosynthetic metabolism. Metab Eng 44:337–347. https://doi.org/10.1016/j.ymben.2017.11.002
Perin G, Simionato D, Bellan A, Carone M, Occhipinti A, Maffei ME, Morosinotto T (2017b) Cultivation in industrially relevant conditions has a strong influence on biological properties and performances of Nannochloropsis gaditana genetically modified strains. Algal Res 28:88–99. https://doi.org/10.1016/j.algal.2017.10.013
Perin G, Bellan A, Bernardi A, Bezzo F, Morosinotto T (2018) The potential of quantitative models to improve microalgae photosynthetic efficiency. Physiol Plant. https://doi.org/10.1111/ppl.12915
Peters J, Stoger E (2011) Transgenic crops for the production of recombinant vaccines and anti-microbial antibodies. Hum Vaccin 7:367–374
Poliner E, Farré EM, Benning C, Benning C (2018a) Advanced genetic tools enable synthetic biology in the oleaginous microalgae Nannochloropsis sp. Plant Cell Rep. https://doi.org/10.1007/s00299-018-2270-0
Poliner E, Pulman JA, Zienkiewicz K, Childs K, Benning C, Farré EM (2018b) A toolkit for Nannochloropsis oceanica CCMP1779 enables gene stacking and genetic engineering of the eicosapentaenoic acid pathway for enhanced long-chain polyunsaturated fatty acid production. Plant Biotechnol J 16:298–309. https://doi.org/10.1111/pbi.12772
Polle JEW, Benemann JR, Tanaka A, Melis A (2000) Photosynthetic apparatus organization and function in the wild type and a chlorophyll b-less mutant of Chlamydomonas reinhardtii. Dependence on carbon source. Planta 211:335–344
Polle JEW, Kanakagiri S-DD, Melis A (2003) tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 217:49–59. https://doi.org/10.1007/s00425-002-0968-1
Radakovits R, Eduafo PM, Posewitz MC (2011) Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab Eng 13:89–95. https://doi.org/10.1016/j.ymben.2010.10.003
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun 3:686. https://doi.org/10.1038/ncomms1688
Ramos Tercero EA, Sforza E, Morandini M, Bertucco A (2014) Cultivation of Chlorella protothecoides with urban wastewater in continuous photobioreactor: biomass productivity and nutrient removal. Appl Biochem Biotechnol 172:1470–1485. https://doi.org/10.1007/s12010-013-0629-9
Raposo MF, de Morais AMMB (2015) Microalgae for the prevention of cardiovascular disease and stroke. Life Sci 125:32–41. https://doi.org/10.1016/j.lfs.2014.09.018
Raposo MF, de Morais AMMB, de Morais RMSC (2015) Carotenoids from marine microalgae: a valuable natural source for the prevention of chronic diseases. Mar Drugs 13:5128–5155. https://doi.org/10.3390/md13085128
Rasala BA, Mayfield SP (2015) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123:227–239. https://doi.org/10.1007/s11120-014-9994-7
Rasala BA, Lee PA, Shen Z, Briggs SP, Mendez M, Mayfield SP (2012) Robust expression and secretion of Xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS One 7:e43349. https://doi.org/10.1371/journal.pone.0043349
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. https://doi.org/10.1016/j.apenergy.2012.10.004
Remacle C, Cardol P, Coosemans N, Gaisne M, Bonnefoy N (2006) High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc Natl Acad Sci U S A 103:4771–4776. https://doi.org/10.1073/pnas.0509501103
Renuka N, Guldhe A, Prasanna R, Singh P, Bux F (2018) Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol Adv 36:1255–1273. https://doi.org/10.1016/j.biotechadv.2018.04.004
Robertson GP, Hamilton SK, Barham BL, Dale BE, Izaurralde RC, Jackson RD, Landis DA, Swinton SM, Thelen KD, Tiedje JM (2017) Cellulosic biofuel contributions to a sustainable energy future: choices and outcomes. Science 356:eaal2324. https://doi.org/10.1126/science.aal2324
Rochaix J-D (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65:287–309. https://doi.org/10.1146/annurev-arplant-050213-040226
Rockström J, Gaffney O, Rogelj J, Meinshausen M, Nakicenovic N, Schellnhuber HJ (2017) A roadmap for rapid decarbonization. Science 355:1269–1271. https://doi.org/10.1126/science.aah3443
Rodríguez-Luna A, Ávila-Román J, González-Rodríguez ML, Cózar MJ, Rabasco AM, Motilva V, Talero E (2018) Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar Drugs 16:378. https://doi.org/10.3390/md16100378
Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith JH, Eppink MHM, Kleinegris DMM, Wijffels RH, Barbosa MJ (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043. https://doi.org/10.1039/C6EE01493C
Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160:393–400. https://doi.org/10.1016/j.foodchem.2014.03.087
Salih FM (2011) Microalgae tolerance to high concentrations of carbon dioxide: a review. J Environ Prot 2:648–654. https://doi.org/10.4236/jep.2011.25074
Santos FM, Pires JCM (2018) Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresour Technol 267:725–731. https://doi.org/10.1016/j.biortech.2018.07.119
Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF (2017) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci. https://doi.org/10.1016/J.SJBS.2017.11.003
Scaife MA, Nguyen GTDT, Rico J, Lambert D, Helliwell KE, Smith AG (2015) Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J 82:532–546. https://doi.org/10.1111/tpj.12781
Schellnhuber HJ, Rahmstorf S, Winkelmann R (2016) Why the right climate target was agreed in Paris. Nat Clim Chang 6:649–653. https://doi.org/10.1038/nclimate3013
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry. Elsevier, Waltham
Scranton MA, Ostrand JT, Fields FJ, Mayfield SP (2015) Chlamydomonas as a model for biofuels and bio-products production. Plant J 82:523–531. https://doi.org/10.1111/tpj.12780
Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Tokgoz S, Hayes D, Yu T-H (2008) Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319:1238–1240. https://doi.org/10.1126/science.1151861
Sharon-Gojman R, Leu S, Zarka A (2017) Antenna size reduction and altered division cycles in self-cloned, marker-free genetically modified strains of Haematococcus pluvialis. Algal Res 28:172–183. https://doi.org/10.1016/J.ALGAL.2017.09.015
Shimizu Y (2003) Microalgal metabolites. Curr Opin Microbiol 6:236–243. https://doi.org/10.1016/S1369-5274(03)00064-X
Simionato D, Sforza E, Corteggiani Carpinelli E, Bertucco A, Giacometti GMGM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032. https://doi.org/10.1016/j.biortech.2011.02.100
Simionato D, Basso S, Giacometti GMGM, Morosinotto T (2013a) Optimization of light use efficiency for biofuel production in algae. Biophys Chem 182:71–78. https://doi.org/10.1016/j.bpc.2013.06.017
Simionato D, Block MAMA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013b) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676. https://doi.org/10.1128/EC.00363-12
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. https://doi.org/10.1016/J.BIOMBIOE.2012.12.019
Sonani RR, Rastogi RP, Patel R, Madamwar D (2016) Recent advances in production, purification and applications of phycobiliproteins. World J Biol Chem 7:100–109. https://doi.org/10.4331/wjbc.v7.i1.100
Sosa-Hernández JE, Escobedo-Avellaneda Z, Iqbal HMN, Welti-Chanes J (2018) State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 23:2953. https://doi.org/10.3390/molecules23112953
Spadiut O, Capone S, Krainer F, Glieder A, Herwig C (2014) Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol 32:54–60. https://doi.org/10.1016/j.tibtech.2013.10.002
Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 32:1373–1383. https://doi.org/10.1007/s10529-010-0326-5
Sugiyama K, Ebisawa M, Yamada M, Nagashima Y, Suzuki H, Maoka T, Takaichi S (2017) Functional lycopene cyclase (CruA) in cyanobacterium, Arthrospira platensis NIES-39, and its role in carotenoid synthesis. Plant Cell Physiol 58:831–838. https://doi.org/10.1093/pcp/pcx015
Sun D, Zhu J, Fang L, Zhang X, Chow Y, Liu J (2013) De novo transcriptome profiling uncovers a drastic downregulation of photosynthesis upon nitrogen deprivation in the nonmodel green alga Botryosphaerella sudeticus. BMC Genomics 14:715. https://doi.org/10.1186/1471-2164-14-715
Sun X-M, Ren L-J, Zhao Q-Y, Ji X-J, Huang H (2018) Enhancement of lipid accumulation in microalgae by metabolic engineering. Biochim Biophys Acta Mol Cell Biol Lipids. https://doi.org/10.1016/j.bbalip.2018.10.004
Torregrosa-Crespo J, Montero Z, Fuentes J, Reig García-Galbis M, Garbayo I, Vílchez C, Martínez-Espinosa R (2018) Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar Drugs 16:203. https://doi.org/10.3390/md16060203
Trentacoste EM, Shrestha RP, Smith SR, Gle C, Hartmann AC, Hildebrand M, Gerwick WH (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci 110:19748–19753. https://doi.org/10.1073/pnas.1309299110
Tsai H-P, Chuang L-T, Chen C-NN (2016) Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem 192:682–690. https://doi.org/10.1016/j.foodchem.2015.07.071
Ullrich KK, Hiss M, Rensing SA (2015) Means to optimize protein expression in transgenic plants. Curr Opin Biotechnol 32:61–67. https://doi.org/10.1016/j.copbio.2014.11.011
United Nations (2017) World population prospects: the 2017 revision
Usher PK, Ross AB, Camargo-Valero MA, Tomlin AS, Gale WF (2014) An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 5:331–349. https://doi.org/10.1080/17597269.2014.913925
Vanthoor-Koopmans M, Wijffels RH, Barbosa MJ, Eppink MHM (2013) Biorefinery of microalgae for food and fuel. Bioresour Technol 135:142–149. https://doi.org/10.1016/j.biortech.2012.10.135
Verruto J, Francis K, Wang Y, Low MC, Greiner J, Tacke S, Kuzminov F, Lambert W, McCarren J, Ajjawi I, Bauman N, Kalb R, Hannum G, Moellering ER (2018) Unrestrained markerless trait stacking in Nannochloropsis gaditana through combined genome editing and marker recycling technologies. Proc Natl Acad Sci U S A 115:E7015–E7022. https://doi.org/10.1073/pnas.1718193115
Vieler A, Wu G, Tsai C-HH, Bullard B, Cornish AJ, Harvey C, Reca I-BB, Thornburg C, Achawanantakun R, Buehl CJ, Campbell MS, Cavalier D, Childs KL, Clark TJ, Deshpande R, Erickson E, Armenia Ferguson A, Handee W, Kong Q, Li X, Liu B, Lundback S, Peng C, Roston RL, Sanjaya SJP, TerBush A, Warakanont J, Zäuner S, Farre EM, Hegg EL, Jiang N, Kuo M-HH, Lu Y, Niyogi KK, Ohlrogge J, Osteryoung KW, Shachar-Hill Y, Sears BB, Sun Y, Takahashi H, Yandell M, Shiu S-HH, Benning C (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8:e1003064. https://doi.org/10.1371/journal.pgen.1003064
Walsh BJ, Rydzak F, Palazzo A, Kraxner F, Herrero M, Schenk PM, Ciais P, Janssens IA, Peñuelas J, Niederl-Schmidinger A, Obersteiner M (2015) New feed sources key to ambitious climate targets. Carbon Balance Manag 10:26. https://doi.org/10.1186/s13021-015-0040-7
Wang D, Ning K, Li J, Hu J, Han D, Wang H, Zeng X, Jing X, Zhou Q, Su X, Chang X, Wang A, Wang W, Jia J, Wei L, Xin Y, Qiao Y, Huang R, Chen J, Han B, Yoon K, Hill RT, Zohar Y, Chen F, Hu Q, Xu J (2014) Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLoS Genet 10. https://doi.org/10.1371/journal.pgen.1004094
Wang Q, Lu Y, Xin Y, Wei L, Huang S, Xu J (2016) Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. Plant J 88:1071–1081. https://doi.org/10.1111/tpj.13307
Wang H-MD, Li X-C, Lee D-J, Chang J-S (2017) Potential biomedical applications of marine algae. Bioresour Technol 244:1407–1415. https://doi.org/10.1016/j.biortech.2017.05.198
Whitaker J, Field JL, Bernacchi CJ, Cerri CEP, Ceulemans R, Davies CA, DeLucia EH, Donnison IS, McCalmont JP, Paustian K, Rowe RL, Smith P, Thornley P, McNamara NP (2018) Consensus, uncertainties and challenges for perennial bioenergy crops and land use. Glob Change Biol Bioenergy 10:150–164. https://doi.org/10.1111/gcbb.12488
Wichmann J, Baier T, Wentnagel E, Lauersen KJ, Kruse O (2018) Tailored carbon partitioning for phototrophic production of (E)-α-bisabolene from the green microalga Chlamydomonas reinhardtii. Metab Eng 45:211–222. https://doi.org/10.1016/J.YMBEN.2017.12.010
Wobbe L, Blifernez O, Schwarz C, Mussgnug JH, Nickelsen J, Kruse O (2009) Cysteine modification of a specific repressor protein controls the translational status of nucleus-encoded LHCII mRNAs in Chlamydomonas. Proc Natl Acad Sci U S A 106:13290–13295. https://doi.org/10.1073/pnas.0900670106
Xue J, Niu Y-F, Huang T, Yang W-D, Liu J-S, Li H-Y (2015) Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab Eng 27:1–9. https://doi.org/10.1016/j.ymben.2014.10.002
Yaakob Z, Ali E, Zainal A, Mohamad M, Takriff MS (2014) An overview: biomolecules from microalgae for animal feed and aquaculture. J Biol Res 21:6. https://doi.org/10.1186/2241-5793-21-6
Yamamoto H, Takahashi S, Badger MR, Shikanai T (2016) Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat Plants 2:16012. https://doi.org/10.1038/nplants.2016.12
Yin-Hu W, Yin Y, Xin L, Hong-Ying H, Zhen-Feng S (2012) Biomass production of a Scenedesmus sp. under phosphorous-starvation cultivation condition. Bioresour Technol 112:193–198. https://doi.org/10.1016/j.biortech.2012.02.037
Young REB, Purton S (2015) Codon reassignment to facilitate genetic engineering and biocontainment in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J. https://doi.org/10.1111/pbi.12490
Zäuner S, Jochum W, Bigorowski T, Benning C (2012) A cytochrome b5-containing plastid-located fatty acid desaturase from Chlamydomonas reinhardtii. Eukaryot Cell 11:856–863. https://doi.org/10.1128/EC.00079-12
Zhang L, Wang H (2015) Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar Drugs 13:4310–4330. https://doi.org/10.3390/md13074310
Zienkiewicz K, Zienkiewicz A, Poliner E, Du Z-Y, Vollheyde K, Herrfurth C, Marmon S, Farré EM, Feussner I, Benning C (2017) Nannochloropsis, a rich source of diacylglycerol acyltransferases for engineering of triacylglycerol content in different hosts. Biotechnol Biofuels 10:8. https://doi.org/10.1186/s13068-016-0686-8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Perin, G., Morosinotto, T. (2019). Potential of Microalgae Biomass for the Sustainable Production of Bio-commodities. In: Cánovas, F., Lüttge, U., Leuschner, C., Risueño, MC. (eds) Progress in Botany Vol. 81. Progress in Botany, vol 81. Springer, Cham. https://doi.org/10.1007/124_2019_30
Download citation
DOI: https://doi.org/10.1007/124_2019_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36326-0
Online ISBN: 978-3-030-36327-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)