Abstract
Survival in the circulation, extravasation from vasculature, and colonizing new tissues represent major steps of the metastatic cascade and pose a big challenge for metastasizing tumor cells. Tumor cells circulating in blood and lymph vessels need to overcome anoikis, cope with mechanical stimuli including shear stress, and defeat attacks by the immune system. Once adhered to the vessel wall, a circulating tumor cell (CTC) can trick the endothelial cells into loosening their intercellular junctions so that the endothelium becomes penetrable for the tumor cell. Since tumor cells tend to metastasize to predestinated target organs and tissues, called organotropism, the distribution of metastases is anything but random. The molecular-physiological mechanisms underlying CTC survival, extravasation, and organotropism are very likely to include the presence and activity of ion channels/transporters due to the latter’s key function in cytophysiological processes. To date, a very limited number of studies explicitly show the involvement of ion transport. This review describes the contribution of ion channels and transporters to CTC survival, extravasation, and organotropism where known and possible. In addition, supposed connections between ion transport and CTC behavior are demonstrated and imply the potential to be therapeutically taken advantage of.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The degree of malignancy of a tumor disease is determined by the tumor cells’ propensity to invade surrounding tissue, to spread and metastasize. These steps of the metastatic cascade also include the cells’ long-distance transport by blood and lymph flow as well as their ability to adhere to the vessel wall in order to extravasate at a distant organ site far away from the primary tumor (Valastyan and Weinberg 2011). During the course of these events blood cells play a double-edged role. While natural killer (NK) cells represent serious opponents of circulating tumor cells (CTCs), platelets, neutrophils and monocytes/macrophages may even help them to survive the intravascular milieu, extravasate and colonize a new tissue or organ.
In respect of rolling and adhesion to the vessel wall, CTCs quite often mimic or avail themselves of the mechanisms used by leukocytes (Strell and Entschladen 2008). The receptor-ligand pairs involved in rolling are mostly the same in leukocytes and tumor cells, with E- and P-selectins expressed on endothelia as well as (peritoneal) mesothelia being the major receptors (Gebauer et al. 2013; Köhler et al. 2010). In contrast, the receptor-ligand pairs that mediate tumor cell adhesion to the endothelium are quite different from those involved in leukocyte adhesion (Strell and Entschladen 2008). Specific interactions between structures on the tumor cell surface and tissue-/organ-specifically expressed proteins on the endo-/mesothelium, including locally released chemokines (please see Sect. 5.1), contribute significantly to the organ distribution of metastases which is anything but random (Langley and Fidler 2011; Paget 1989). The preference of tumor cells to metastasize to predestinated target-organs is called “organ-specific metastasis” or “metastatic organotropism.”
The present review article describes the travel route of metastasizing tumor cells from the moment of intravasation through to the colonization of the target-organ, including indispensable survival mechanisms. There is hardly any direct evidence for the contribution of ion transport to these steps of the metastatic cascade. However, due to their pivotal role in regulating cellular functions, ion channels and transporters must be inevitably involved. Their involvement will be described and explained where known and possible. In other cases, presumed links between ion transport and the survival of metastasizing cells are pointed up. Central modulators affecting, and being affected by, ion channels and transporters are pH and cytosolic Ca2+ concentrations together with signaling events.
2 Surviving the Intravascular Milieu
Being swept away by the blood flow represents a major challenge for tumor cells. From thousands up to millions of cells that come off the primary tumor every day (Butler and Gullino 1975; Swartz et al. 1999), less than one out of ten thousand circulating tumor cells (<0.01%) may eventually end up as a metastasis (Fidler 1970, 2003; Strilic and Offermanns 2017). In breast cancer patients, the half-life of circulating tumor cells (CTCs) was found to be 1–2.4 h (Meng et al. 2004). Most of these CTCs die due to hemodynamic shear stress in the circulation (Fan et al. 2016) or anoikis, i.e. the loss of cell–cell or cell–matrix contacts including the absence of extracellular matrix-derived survival signals (Kim et al. 2012). A third obstacle to be overcome by CTCs is the immune surveillance, particularly the clutches of natural killer (NK) cells of the innate immune system (Morvan and Lanier 2016).
To cope with all these challenges, CTCs use a number of (molecular) mechanisms (Strilic and Offermanns 2017).
2.1 Coping with Mechanical Stress
In order to resist mechanical destruction by hemodynamic forces, CTCs activate both the RhoA/actomyosin axis and actin-nucleating formins in response to fluid shear stress which, including the activity of myosin II, protects them from plasma membrane damage (Moose et al. 2020). Accordingly, short-term inhibition of myosin II delays metastasis of circulating prostate cancer cells in a mouse model (Moose et al. 2020). Since the CaM-dependent activity of myosin II needs Ca2+, and the resistance to fluid shear stress requires the presence of extracellular Ca2+ (Barnes et al. 2012), CTC adaptation to mechanical stress definitely involves Ca2+ transport across the plasma membrane. In general, a number of mechanosensitive ion channels have a share in Ca2+ signaling of tumor cells: while direct Ca2+ influx can be mediated by Ca2+ conducting channels such as Piezo or TRP channels, K+ outward currents carried by, inter alia, mechanosensitive members of the two-pore domain K+ channel family keep up the electrochemical gradient essential for Ca2+ influx (Pethö et al. 2019). Albeit there is no study to date explicitly proving the nature of the Ca2+ channels and transport mechanisms that are involved in CTCs’ shear stress resistance, exposure to fluid shear stress does trigger Ca2+ influx accompanied by an increase in cell stiffness. Transformed prostate cancer cells (PC-3) show a graduated increase in stiffness in response to the level of shear stress whereas non-transformed prostate epithelial cells (PrEC LH) do not show a significant change (Chivukula et al. 2015). In addition to channels and transporters mediating Ca2+ influx provoked by fluid shear stress, the Na+/H+ exchanger NHE1 may contribute to the increase in stiffness and thus facilitate CTC survival. Its overexpression, typical of a multitude of tumor entities, leads to a reorganization of the cortical cytoskeleton accompanied by a significant increase in cortical stiffness of human melanoma (MV3) cells (Keurhorst et al. 2019). This effect is based on the mere presence of NHE1 as a structural element independently of its ion transport function.
An additional strategy by which single CTCs can protect themselves from mechanical stress-induced death is the recruitment of thrombocytes (platelets) and monocytes/macrophages in order to form a physical shield (Schlesinger 2018; Stegner et al. 2014). To this end, CTCs express tissue factor at their surface (Bourcy et al. 2016; Hisada and Mackman 2019). The tissue factor triggers the coagulation cascade including the activation of platelets which results in the formation of a protective platelet clot around the tumor cells. The clot then recruits monocytes/macrophages to the CTCs (Gil-Bernabé et al. 2012, 2013), and the accruing clusters or microaggregates not only protect the CTCs from mechanical stress but also help them adhere to the endothelium and extravasate at a distant site (Strilic and Offermanns 2017). According to this, an inhibition of mechanisms underlying tumor cell–platelet interaction causes a significant decrease in metastasis (Labelle and Hynes 2012; Mammadova-Bach et al. 2020; Takagi et al. 2013).
Another survival mechanism has been found in highly metastatic human breast cancer cells expressing significant amounts of a truncated form of the channel protein Pannexin 1 (PANX1) (Furlow et al. 2015). PANX1 is an ATP-permeable channel and, under normal cellular conditions, auto-inhibited because it is plugged by its C-terminal tail. During apoptosis, cleavage of the C-terminus by caspase 3 or 7 activates PANX1 and allows ATP release (Chekeni et al. 2010; Ruan et al. 2020; Sandilos et al. 2012). In highly metastatic breast cancer cells, however, co-expression of a truncated form of PANX1 with full-length wild-type PANX1 protects from apoptosis (Furlow et al. 2015). The presence of truncated PANX1 is accompanied by an elevated ATP release through mechanosensitive full-length PANX1 activated by membrane stretch during deformation in the microvasculature. By autocrine binding to purinergic P2Y receptors the released ATP induces a signaling cascade that suppresses deformation-induced apoptosis of the circulating breast cancer cell. Consequently, therapeutic inhibition of PANX1 by small-molecule inhibitors can reduce breast cancer metastasis (Furlow et al. 2015).
2.2 Resistance to Anoikis
A loss of integrin-mediated cell adhesion to extracellular matrix proteins normally induces anoikis, a special type of apoptosis (Tajbakhsh et al. 2019). CTCs utilize a variety of mechanisms to counteract anoikis (Buchheit et al. 2014). An efficient way to avoid anoikis is the retention of cell–cell or even fragmented cell–matrix adhesions within the circulating tumor cell clusters, also termed circulating microemboli. These circulating microemboli can either originate from collectively migrating tumor cells that enter the blood stream via chaotically structured and leaky tumor vessels typical of highly angiogenic tumors (Hou et al. 2011) or they arise from the disintegration of the primary tumor into the vasculature (Liotta et al. 1976). Although circulating tumor cell clusters are rather rare compared to single CTCs, these clusters have a 23–50-fold increased metastatic potential (Aceto et al. 2014).
Since the focal adhesion kinase (FAK) is a central player in integrin-mediated adhesion signaling, single CTCs establish alternative ways of FAK phosphorylation or even bypass FAK signaling. Thus, FAK phosphorylation and signaling in non-adherent cells may be ensured by endosomes that carry integrin dimers while containing integrin-binding extracellular matrix components such as fibronectin (Alanko et al. 2015). Another way to maintain FAK signaling may be integrin-mediated self-stimulation by self-secreted fibronectin or collagen. Stimulation of β1 integrin by fibronectin or collagen causes activation of Kv11.1 (human ether-a-go-go-related gene potassium channel hERG, KCNH2), which is essential for direct FAK phosphorylation (Cherubini et al. 2005; Jehle et al. 2011). FAK phosphorylation in response to Kv11.1 activation may enable detached cells to resist anoikis. In fact, overexpression of both FAK and Kv11.1 has been shown to enhance dissemination and invasiveness of tumors (Kornberg 1998; Lastraioli et al. 2004).
Moreover, fibronectin can promote cell survival, mediate chemo- and radioresistance, and inhibit apoptosis in breast and lung cancer cells (Aoudjit and Vuori 2012; Naci et al. 2015). In pancreatic cancer cells, an increased Wnt2 expression correlates with a TGFβ-activated kinase 1 (TAK1; MAP 3 K7)-dependent upregulation of fibronectin, suppresses anoikis, and facilitates adhesion-independent sphere formation (Yu et al. 2012).
Aside from fibronectin, CTCs could potentially also make use of serum vitronectin and other serum proteins, e.g. osteopontin, thrombospondin or reelin, as ligands in order to keep up integrin-mediated signaling and thus resist anoikis (Bera et al. 2020; Cooper et al. 2002; Lal et al. 2009; Rouanne et al. 2016).
Beyond that, FAK-mediated anoikis resistance has been found to correlate with the expression of carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6), also known as CD66c (Duxbury et al. 2004; Johnson and Mahadevan 2015; Lee et al. 2018). As a bypass or an alternative to missing FAK signaling, anti-apoptotic, pro-survival pathways are upregulated or tumor suppressors and suppressing pathways are downregulated. For instance, the PI3/Akt signaling pathway, which normally is inducible by FAK as well, or the MAPK/ERK pathway is stimulated by overexpressed receptor tyrosine kinases and downregulation of the tumor suppressor PTEN (Paoli et al. 2013). A moderately increased ROS production is often found in tumor cells (Perillo et al. 2020) and helps to counteract anoikis by modulating the activities of redox-sensitive proteins of the PI3/Akt and MAPK signaling pathways and prominent transcription factors such as p53, NF-κB, HIF, AP-1, and Nrf2 (Groeger et al. 2009).
Finally, although not shown explicitly in CTCs, the detachment from the extracellular matrix could induce autophagic and antioxidant effector pathways whose concerted action might (i) enable increased survival in the bloodstream and (ii) facilitate the formation of metastases (Dey et al. 2015). In more detail, cells react to the loss of substrate adhesion by activating a cytoprotective ER stress response consisting of three pathways that are normally kept inactive by the ER-located chaperone GRP-78 (also known as “binding immunoglobulin protein” (BiP) or “heat shock 70 kDa protein 5” (HSPA5)) (Korennykh and Walter 2012): the ATF6 (transmembrane activating transcription factor 6), the IRE1 (iron responsive element 1), and the PERK (transmembrane protein kinase RNA-like endoplasmic reticulum kinase; located in the ER membrane) pathway (Dey et al. 2015; Wakabayashi and Yoshida 2013). Activated PERK directly activates transcription factor Nrf2 and phosphorylates eIF2α (eukaryotic (translation) initiation factor 2α). peIF2α leads to upregulated translation of the cAMP-dependent transcription factor ATF4. ATF4 then triggers a cytoprotective program by upregulating key genes of autophagy, and, by cooperating with Nrf2, activates the antioxidant protein HO-1 (heme oxygenase 1) in order to antagonize the increasing oxidative stress induced by the loss of cell-matrix adhesion (Dey et al. 2015).
In breast cancer cells of the MCF-7 line, incorporation of the STAT3 (signal transducer and activator of transcription 3)-controlled zinc transporter ZIP6 (SLC39A6) into the plasma membrane induces EMT (epithelial-mesenchymal transition), cell detachment, resistance to anoikis and an ongoing proliferative activity of cells in suspension (Hogstrand et al. 2013). ZIP6-mediated Zn2+ influx inactivates the glycogen synthase kinase 3β (GSK-3β) leading to activation of the transcription factor Snail. Snail then oppresses the transcription of E-cadherin resulting in cell rounding and detachment (Hogstrand et al. 2013). Snail is generally considered to be one of the key players inducing EMT accompanied by resistance to anoikis (Paoli et al. 2013; Peyre et al. 2021; Smit et al. 2009).
The Ca2+ activated Cl− channel regulators 1 and 2 (CLCA1, 2; also called Cl− channel accessory 1, 2) are secretory, self-cleaving, Zn2+-dependent metalloproteases that activate Ca2+-dependent Cl− currents (Liu and Shi 2019; Yurtsever et al. 2012). They are involved also in apoptosis (Hutchings et al. 2019; Winpenny et al. 2009). Their downregulation, however, results in resistance to anoikis (Elble and Pauli 2001). While CLCA2 overexpression leads to increased Cl− currents accompanied by a decrease in intracellular pH, a reduced expression of CLCA2 is associated with increases in proliferation, migration, and invasion, and a higher risk of metastasis (Walia et al. 2009, 2012).
2.3 Defeating Attacks by the Immune System
Once in the circulation, tumor cells encounter a huge number of immunosurveilling cells such as natural killer (NK) cells. NK cells express NKG2D (NK group 2d) receptors on their surface in order to recognize and bind their ligands (NKG2DL) which are primarily the cell surface glycoproteins MICA, MICB (MHC class I chain-related molecules A and B), and ULBPs 1–6 (Duan et al. 2019; Ghadially et al. 2017; Molfetta et al. 2017). Basically, the transcription factor Sp1 mediates an upregulation of NKG2DL-expression during EMT resulting in an increased immunogenicity (Huergo-Zapico et al. 2014). However, NKG2DL-expression decreases as the tumor cells continue to dedifferentiate and is completely absent in poorly differentiated human colorectal cancer samples (López-Soto et al. 2013). For camouflage purposes, i.e. in order to elude immune surveillance, CTCs can either shed their NKG2DLs to (1) remain undetected and (2) misdirect the immune system (Dhar and Wu 2018), or they even avoid surface expression of NKG2DLs (Liu et al. 2019a; Schmiedel and Mandelboim 2018) as shown for leukemic stem cells in patients with acute myeloid leukemia (Paczulla et al. 2019).
At transcriptional level, aberrant methylation of the genes encoding NKG2DLs or low acetylation of histones can lead to NKG2DL silencing in tumor cells of different origin (Li et al. 2011a; Ritter et al. 2016). In glioma cells with mutations of the isocitrate dehydrogenase (IDH), loss-of-function mutations induce 2-hydroyglutaric acid-mediated epigenetic and metabolic reprogramming, eventually silencing ULBPs 1 and 3 (Zhang et al. 2016a). In other malignant glioma cells, TGF-β suppresses the transcription of MICA, ULBP2, and ULBP4 without affecting the mRNA levels of MICB, ULBP1, and ULBP3 (Eisele et al. 2006). MICA mRNA expression can be decreased also by IFN-γ as shown for both solid (cervical) and hematological (erythroleukemia and lymphoma) cell lines (Zhang et al. 2008).
At translational level, miR-10b, miR-20a, mir-34a, miR-93, or miR-106 either destabilize the NKG2DLs’ mRNAs or inhibit their translation in a number of tumor cell lines such as melanoma, breast, prostate, or colorectal cancer (Codo et al. 2014; Heinemann et al. 2012; Stern-Ginossar et al. 2008; Tsukerman et al. 2012; Yang et al. 2018). In contrast, miR-889-overexpression protects hepatocellular carcinoma cells from NK cell-mediated lysis, because it significantly inhibits MICB expression (Xie et al. 2018).
At post-translational level, proteolytic enzymes, shedding and secretion help to reduce NKG2DL surface expression in tumor cells (Duan et al. 2019). Thus, IFN-γ not only regulates MICA expression at the transcriptional level but also promotes its hydrolysis by matrix metalloproteinases (MMPs) (Zhang et al. 2008). “A disintegrin and metalloproteases” (ADAMs) 10 and 17 mediate shedding of MICA and MICB from human mammary, pancreatic, and prostate carcinoma cells (Chitadze et al. 2013). A significant amount of soluble NKG2DL is found in sera of leukemia patients where it impairs antileukemia reactivity of NK cells by downregulating their NKG2D (receptor) expression (Hilpert et al. 2012). Similarly, glioblastoma cells secrete lactate dehydrogenase 5 (LDH5) to trigger NKG2DL expression in myeloid cells including monocytes, which then results in the downregulation of NKG2D in NK cells (Crane et al. 2014).
NK cells’ effective antitumor activity requires direct, physical contact. Consequently, physical shielding does not only protect tumor cells from mechanical stress (please see Sect. 2.1) but also helps them to escape from NK cell attacks as coating with tumor cell-activated platelets impedes lysis of tumor cells by NK cells (Nieswandt et al. 1999) and facilitates metastasis (Palumbo et al. 2005). The formation of stable platelet/tumor cell aggregates needs fibrinogen or fibrin crosslinking factor FXIII. Loss of these coagulation factors causes a strong decrease in metastasis in an NK-cell dependent manner (Palumbo et al. 2005, 2008). The adhesion molecule P-selectin is expressed on platelets and mediates platelet/tumor cell adhesion by binding to sialylated, fucosylated glycans on the tumor cell surface (Borsig et al. 2002; Mannori et al. 1995), mostly in a Ca2+-dependent way (Erpenbeck and Schön 2010). Furthermore, by releasing TGFβ, also the platelets cause a reduction of NKG2D receptors on NK cells (Kopp et al. 2009). Finally, platelets can furnish tumor cells with both platelet-derived GITRL (glucocorticoid-induced TNF-related ligand; TNFSF18) which inhibits NK cells’ antitumor reactivity (Placke et al. 2012b) and with normal MHC class I molecules which help the tumor cells to hide from immunosurveillance (Placke et al. 2012a).
While CTC clusters and CTCs surrounded by platelets or leukocytes can easily travel through the macrovasculature as silent emboli, these virtually conglomerate structures need to regroup before entering microvessels and capillaries with diameters of ≤10 μm, so that the single cells can pass through sequentially (Au et al. 2016). In capillary beds, even single CTCs can be halted within <30 min after entering the blood stream (Aceto et al. 2014; Micalizzi et al. 2017). Hence, it seems plausible that extracellular vesicles, exosomes or microparticles released from platelets/leukocytes rather than the actual, intact cells would confer the above-mentioned ligands/receptors to CTCs and thus enable them to camouflage and remain undetected by the immune system.
3 Adhesion to the Vessel Wall
In addition to simply being physically stuck inside small capillaries at the secondary site, CTCs need to adhere to and interact with the endothelium in order to eventually extravasate (Azevedo et al. 2015; Foss et al. 2020; Osmani et al. 2019). While the attachment of CTCs to endothelial cells can be mediated by a variety of ligands and receptors such as selectins, cadherins, integrins, CD44 and immunoglobulin superfamily receptors (Bendas and Borsig 2012; Reymond et al. 2013), CD44 and β1 integrin have been identified as key mediators of CTC adhesion. They counteract the shear forces that otherwise would cause the detachment of CTCs from the endothelial cell layer (Follain et al. 2018, 2020; Osmani et al. 2019). In addition to mediating CTC adhesion to the endothelial cell layer or being a biomarker for cancer cells with stem-like properties (Mani et al. 2008) CD44 may enhance metastatic potential by effectuating homophilic CTC interactions, possibly resulting in the formation of CTC clusters even post-intravasation (Chaffer and Goetz 2018; Liu et al. 2019b; Rodrigues and Vanharanta 2019).
Melanoma cell adhesion molecule (MCAM; also known as MUC18 or CD146) is expressed on both melanoma and endothelial cells, and it is believed that homophilic interactions promote tumor cell extravasation and metastasis because antibodies against MCAM inhibit human melanoma growth and metastasis (Mills et al. 2002), and B16 wild-type cell metastasis to the lungs is drastically reduced in MCAM knockout mice (Jouve et al. 2015). In human melanoma cells of the MV3 cell line, MCAM expression correlates with the expression of the Na+/H+ exchanger NHE1 (SLC9A1), and MV3 cell–cell adhesion is pH-sensitive and depends on NHE1 expression (Hofschröer et al. 2017). This observation together with the aforementioned homophilic interaction of MCAM expressed on melanoma and endothelial cells (Mills et al. 2002) points to a potential contribution of NHE1 to the adhesion of tumor cells to the vessel wall.
4 Extravasation
Specific ligand-mediated interactions between tumor and endothelial cells do not necessarily result in adhesion but are nonetheless required for extravasation. Thus, the homophilic interaction between junctional adhesion molecules C (JAM-C) expressed on melanoma and endothelial cells does not impact adhesion but clearly abets lung metastasis (Langer et al. 2011). Also soluble ligands secreted by endothelial cells, e.g. CXCL12, mediate tumor extravasation by binding to chemokine receptors such as CXCR4 expressed particularly on gastrointestinal tumor cells which then stimulates the small GTPases Rho, Rac, and Cdc42 required for cell migration (Gassmann et al. 2009). The latter is consistent with the observations that (1) Cdc42 depletion in various tumor cells leads to a significant decrease in both β1 integrin-dependent interaction with endothelial cells and experimental lung metastasis (Reymond et al. 2012), and (2) that transient RhoC depletion in prostate cancer (PC3) cells reduces early PC3 cell retention in the lungs and in vivo metastasis formation (Reymond et al. 2015).
Paracellular diapedesis, i.e. squeezing through the endothelial cell layer by moving between endothelial cells, is the prevalent mode of extravasation and requires loosening of inter-endothelial cell junctions (Leong et al. 2014; Schumacher et al. 2013). Transcellular diapedesis, i.e. crossing the endothelium by penetrating individual cell bodies, has been shown in vitro, but seems rather rare and most likely requires both endothelial myosin II activity and E-selectin mediated activation of ERK and p38 MAPKs in endothelial cells (Khuon et al. 2010; Tremblay et al. 2008; Wettschureck et al. 2019). A recent study confirms that the microvascular endothelium reorganizes its membranes and cytoskeletal structures in order to directly contribute to the extravasation of tumor cells into the brain, and that melanoma cells primarily migrate paracellularly while breast cancer cells are able to migrate transcellularly (Herman et al. 2019). However, it needs to be stressed that up to now transcellular extravasation in vivo has been found only in microvascular endothelia, possibly because they are typically characterized by a lack of smooth muscle cells.
Endothelial reorganization is usually induced by the CTCs themselves. Breast cancer cells secrete angiopoietin-like 4 (ANGPTL4) or its C-terminal fibrinogen-like domain (cANGPTL4). cANGPTL4 weakens endothelial cell–cell contacts by activating an α5β1 integrin-mediated Rac1/PAK/β-catenin pathway. In a subsequent step, cANGPTL4 directly interacts with VE-cadherin and claudin-5 which causes disruption of intercellular adhesion, thus allowing for transendothelial tumor cell migration (Huang et al. 2011; Padua et al. 2008). Melanoma cells secrete osteonectin (SPARC). SPARC binds to VCAM1 which triggers actin remodeling and loosening of endothelial junctions, mediated by a ROS-MKK3/6-p38MAPK-MLC2 signaling pathway and promoting extravasation and metastasis (Tichet et al. 2015). Other soluble factors that are secreted by metastatic cells and increase vascular permeability by modulating endothelial tight and adherens junctions include lipid 12(S)-hydroxyeicosatetranoic acid (12(S)-HETE), angiopoietin 2 (Ang-2), the chemokine CCL2 (C-C motif chemokine ligand 2, monocyte chemotactic protein 1), CXCL12 (stromal cell-derived factor 1α, SDF-1α), fibrinogen, HGF/SF, VEGF, PCB 104 (2,2′,4,6,6′-pentachlorobiphenyl), and a group of heat-stable, trypsin-sensitive, O-glycosylated glycoproteins ranging from 10 to 50 kD (García-Román and Zentella-Dehesa 2013).
Instead of gently loosening endothelial cell–cell junctions, a variety of human and murine tumor cells act more ruthlessly by inducing necroptosis in endothelial cells in order to locally perforate the endothelium and hence facilitate extravasation and metastasis (Strilic et al. 2016). To this end, CTCs express amyloid precursor protein (Pandey et al. 2016; Tsang et al. 2018) which binds to its receptor, death receptor 6 (DR6), on endothelial cells to induce necroptotic signaling pathways (Strilic et al. 2016). Additionally, necroptotic endothelial cells could possibly reinforce the opening of the endothelial barrier by releasing damage-associated molecular patterns (DAMPs) such as high-mobility group protein 1 (HMGB1) or ATP (Kaczmarek et al. 2013; Pilzweger and Holdenrieder 2015; Strilic and Offermanns 2017).
4.1 With the Assistance of Blood Cells
4.1.1 Platelets
Also blood cells contribute to CTCs’ extravasation. For instance, platelets normally assist immune cells with their extravasation (Gros et al. 2015). They – like the metastatic CTCs (see above) – release HGF, fibrinogen, VEGF, and 12(S)-HETE, and, in addition, platelet-derived activating factor (PAF), thrombin, ATP and serotonin in order to increase vascular permeability. Indeed, there is evidence that platelets recruited by CTCs occasionally promote CTC extravasation (Foss et al. 2020; Labelle et al. 2014; Schumacher et al. 2013). Dense granule-derived ATP released from tumor cell-activated platelets acts on endothelial junctions and the cytoskeleton, mediated by P2Y2 receptors and with the objective of opening the endothelial barrier to facilitate transendothelial migration and metastasis (Schumacher et al. 2013). Upon activation by ATP, the G-protein coupled P2Y2 receptor leads to (1) Ca2+ release from intracellular stores via stimulation of phospholipase Cβ including the generation of IP3 (Raqeeb et al. 2011) and (2) activation of the PKC/Src pathway (Bilbao et al. 2010). The activated P2Y2 transiently associates with VEGFR-2 and VE-cadherin at endothelial cell–cell adhesions while Src phosphorylates VEGFR-2, VE-cadherin, VE-cadherin-bound p120-catenin, and probably also β- and γ-catenins in order to ensure a coordinated release of endothelial adherens junctions (Liao et al. 2014; Liu et al. 2004; Seye et al. 2004; Zou et al. 2015). Subsequent binding of p120-catenin to the guanine nucleotide exchange factor Vav2 activates Rac1 (Valls et al. 2012) which may induce cytoskeletal rearrangements to further facilitate the passage of CTCs through the newly formed intercellular space (Liao et al. 2014; Spindler et al. 2010). At the same time, the P2Y2 mediated Ca2+ release from intracellular stores results in the activation of SKCa and IKCa channels. The concomitant membrane hyperpolarization causes additional Ca2+ influx via store-operated channels (SOCs, consisting mainly of TRPC1 & 4 and requiring TRPC4 subunits (Cioffi et al. 2005)) further promoting KCa channel activity (Raqeeb et al. 2011; Sheng and Braun 2007). The elevated cytosolic Ca2+ concentration also stimulates the activities of CaM (calmodulin) and eNOS (endothelial nitric oxide synthase) which considerably contributes to the increase in endothelial permeability (Sheng and Braun 2007; Thibeault et al. 2010). On the whole, CTCs usurp the physiological mechanism by which platelets assist neutrophils in extravasating at inflamed sites. Although a number of ion channels and transporters passing Ca2+ and K+ are involved, they just fulfill their regular functions. In this context, their expression and activity cannot be considered to be pathophysiological so that they are barely usable as therapeutic targets. The actual pathological step is the platelet activation by CTCs via either direct physical interaction between mucin-like glycoprotein podoplanin or galectin on the CTC cell surface and CLEC-2 or glycoprotein VI on the platelet surface, respectively, or via ADP, thromboxane A2 or high-mobility group box 1 (HMGB1) released by the CTC to bind to the toll-like receptor 4 (TLR4) on the platelet (Schlesinger 2018).
In addition, the podoplanin, expressed on tumor cell surfaces, stimulates the release of TGFβ from platelets (Takemoto et al. 2017). The TGFβ then activates Smad and NF-κB signaling pathways in the tumor cells leading to a more mesenchymal and invasive phenotype which may contribute to extravasation (Labelle et al. 2011).
4.1.2 Neutrophils
Although neutrophils are known to play pro-metastatic roles, their short half-life makes it difficult to precisely analyze the underlying mechanisms. Nevertheless, it has been shown that granulocyte-colony stimulating factor (G-CSF) mediates conversion of neutrophils into immunosuppressive cells that block the antitumor functions of CD8+ T (Coffelt et al. 2015, 2016) and NK cells (Spiegel et al. 2016). Furthermore, platelets promote tumor cell extravasation indirectly by recruiting granulocytes specifically to the vicinity of platelet/tumor cell aggregates. To this end, tumor cell-activated platelets release CXCL5 and CXCL7 both of which bind to CXCR2 chemokine receptors on granulocytes co-expressing granulocyte marker Ly6G, integrin α-M (=CD11b), and matrix metalloproteinase 9 (MMP9) (Labelle et al. 2014). Releasing MMPs 8 and 9, neutrophils facilitate extravasation by disintegrating the extracellular matrix such as the basement membrane (Cools-Lartigue et al. 2014; Spiegel et al. 2016). The tumor-activated platelets can also trigger neutrophil degranulation including the formation of neutrophil extracellular traps (NETs) (Cedervall et al. 2018). NETs are netlike structures that (1) consist of expelled neutrophil DNA with associated proteolytic enzymes, (2) function as a pathogen trap, and (3) can also sequester circulating tumor cells and thus promote local adhesion and metastasis (Cools-Lartigue et al. 2013; Demkow 2021; Park et al. 2016). The capture of CTCs in NETs can be mediated by NET-associated β1-integrin or CEACAM1 (carcinoembryonic Ag cell adhesion molecule 1) as shown for lung (A549; Najmeh et al. 2017) and colorectal cancer (HT-29, MC38; Rayes et al. 2020) cells. Accordingly, preventing the formation of NETs or disintegrating them by application of DNase I-coated nanoparticles reduces lung metastases in mice (Park et al. 2016), and impeding NET formation with the peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 can slow down the progression of multiple myeloma in mice and humans (Li et al. 2020).
4.1.3 Monocytes/Macrophages
In addition to neutrophils and platelets, monocytes/macrophages contribute to CTC extravasation as well. Metastatic CTCs recruit monocytes/macrophages to the site of extravasation by releasing the CC-chemokine ligand 2 (CCL2) which attracts circulating monocytes expressing CC-receptor 2 (CCR2) and 6C2 (LY6C; in mice) or CD14highCD16negative (in humans) (Cassetta and Pollard 2018; Qian et al. 2011), or indirectly by inducing local endothelial activation which results in E-selectin expression (Häuselmann et al. 2016). The endothelial E-selectin mediates the adhesion of the attracted monocytes to the endothelium, and the bond between E-selectin and its ligand triggers signaling in both the monocytes and the endothelial cells, eventually leading to (1) a stronger, integrin-mediated adhesion, (2) the retraction of endothelial cells, and (3) a subsequent loosening of the endothelial tight junctions through de-phosphorylation of VE-cadherin (Häuselmann et al. 2016). Beyond that, extravasated monocytes in the underlying tissue can differentiate into metastasis-associated macrophages, which then release VEGF to increase vascular permeability and thus promote tumor cell extravasation (Cassetta and Pollard 2018; Qian et al. 2009, 2011).
5 Organotropism
Already in 1889, Stephen Paget postulated that metastasis formation requires both cancer cell-intrinsic properties (“seed”) and a congenial microenvironment (“soil”) (Paget 1989). Accordingly, different cancers show different preferences with regard to the organs they metastasize to (Gao et al. 2019). Renal, thyroid, and liver cancer cells metastasize preferentially to the lungs; ovarian, colon, and gastric cancer cells to liver and peritoneum; pancreatic cancer cells to lungs and liver; lung cancer cells to bone and brain. Breast and prostate cancer share the same preferences with the highest incidence of metastases in bone and lungs. In addition, breast cancer often metastasizes to liver and brain. Melanoma can be considered an all-rounder because it spreads nearly everywhere with the highest incidences of metastases in lungs, liver, brain, bone, and peritoneum (Gao et al. 2019). CTCs can also colonize the primary tumor, i.e. their tumor or origin. This process, called “tumor self-seeding,” may select for cancer cells that are more aggressive than those originally in the primary tumor, and may – at least partly – explain local recurrence after tumor excision (Kim et al. 2009). However, it needs to be stated that so far there is not sufficient clinical evidence to substantially support this concept.
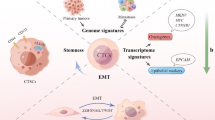
Independently of different cancer types’ preferences including the unique characteristics of each target organ, they share a number of general principles underlying organotropism (Gao et al. 2019). At first, a premetastatic environment is formed. Both soluble factors and exosomes containing (mi)RNA are released from the primary tumor. They interact directly with cells residing at a prospective metastatic site. At the same time, they trigger the release of progenitor cells from bone marrow and conduct their target-oriented travel to a prospective metastatic site. Thus, the soluble factors and exosomes released by the primary tumor in combination with bone marrow-derived cells are significantly involved in arranging the premetastatic niche for later colonization (Fig. 1a; Izraely and Witz 2021; Wang et al. 2021).
Major steps and known factors in organotropism. (A) The primary tumor releases soluble factors and miRNA-containing extracellular vesicles and exosomes that arrive at prospective target organs/tissues and at the same trigger the release of progenitor cells from bone marrow. A premetastatic niche is established by a concerted action of the bone marrow-derived cells, exosomes, and soluble factors from the primary tumor, and, not shown for the sake of clarity, local fibroblasts, mesenchymal stem cells, neutrophils, and macrophages. Chemokines released by local cells decoy the circulating tumor cells into the premetastatic niche, supported by specific, local adhesion events at the endothelial surface often mediated by selectins or integrins. Once in the target tissue, single tumor cells can fall dormant and eventually be woken up by the presence of extracellular matrix components such as laminin emerging from locally induced extracellular matrix digestion events. Metastasizing tumor cells can also repulse local attacks, for instance by releasing serpins to prevent the plasminogen activator (PA) from generating plasmin which otherwise would lead to the release of apoptosis-inducing soluble Fas Ligand. (B) Circulating tumor cells (CTCs) such as metastasizing melanoma, breast or pancreatic cancer cells are directed predominantly to the lungs when their NHE1 activity is reduced, their CAIX expression is high, or when E-cadherin expression is low. Please see text for additional, more detailed information
In a second step, CTCs are then decoyed into this premetastatic niche by inflammatory chemokines released from local cells (Moore 2001; Zlotnik et al. 2011).
5.1 Locally Released Chemokines Lure CTCs
The involved chemokine receptor-ligand pairs include, among others, CXCR1/2-CXCL8 (Ha et al. 2017; Li et al. 2014), CXCR4-CXCL12 (Guo et al. 2016; Iwasa et al. 2009; Müller et al. 2001), CCR6-CCL20 (Ghadjar et al. 2006; Kadomoto et al. 2020), and CCR7-CCL21 (Mashino et al. 2002; Rizeq and Malki 2020). Thus, in patients with axillary node positive primary breast cancer, the expression of chemokine receptors determines the target organ of metastasis. CXCR4 expression increases the risk of metastasis to the liver, CX3CR1 expression favors metastasis to the brain, CCR6 expression causes metastasis to the pleura, and CCR7 expression can be associated with the occurrence of skin metastases (André et al. 2006).
Lung tropism of osteosarcoma is mediated primarily by CXCL8 and IL-6 (Gross et al. 2018). CXCL8 triggers the release of Ca2+ from intracellular stores (Joseph et al. 2010) and causes phosphorylation of Akt and Erk1/2 (Hosono et al. 2017), i.e. two signaling pathways known to drive cell migration and invasion. To date, ion channels and transporters potentially involved in this CXCR1/2-CXCL8-dependent organotropic process, such as K+ or Ca2+ channels, have not been identified.
5.1.1 CXCL12/CXCR4
Another example is CXCL12 (= stromal cell-derived factor 1 (SDF-1)) which is preferentially expressed in lung and liver and thus attracts CXCR4-carrying melanoma, breast cancer (Minn et al. 2005; Müller et al. 2001), and pancreatic cancer cells (Saur et al. 2005). Interestingly, the water/glycerol channel aquaporin-3 (AQP3) is required for CXCL12/CXCR4-dependent, directional breast cancer cell migration, including spontaneous metastasis of orthotopic xenografts to the lungs (Satooka and Hara-Chikuma 2016). CXCL12 induces the membrane NADPH oxidase 2 (Nox2) to generate H2O2. H2O2 then enters the breast cancer cell through AQP3. It oxidizes the phosphatases PTEN (phosphatase and tensin homolog) and PTP1B (protein tyrosine phosphatase 1B), resulting in the activation of the Akt pathway which drives directional cell migration (Satooka and Hara-Chikuma 2016). Similarly, CXCL12-activated Akt and Erk1/2 pathways propel endothelial colony-forming cell (ECFC) migration, homing and incorporation into neovessels, thus re-establishing perfusion in ischemic tissues and promoting tumor vascularization and metastasis (Zuccolo et al. 2018). The activation of the Akt and Erk1/2 pathways requires a CXCL12-induced increase in the intracellular Ca2+ concentration which is initiated by an InsP3-mediated Ca2+ release from the ER and maintained by subsequent store-operated Ca2+ entry across the plasma membrane (SOCE) (Zuccolo et al. 2018).
In invasive glioblastoma, CXCL12 causes the activation of KCa3.1 (IKCa; KCNN4) channels including their long-term functional upregulation. KCa3.1 channel activity mediates glioblastoma cell migration and chemotaxis depending on CXCR4 expression (Sciaccaluga et al. 2010). Accordingly, a combined, simultaneous use of the anti-fungal KCa3.1 blocker clotrimazole, the CXCR4 inhibitor plerixafor (AMD3100), and the histamine 1 (H1) receptor antagonist mirtazapine has been suggested for cytotoxic glioblastoma treatment. The H1 receptor needs to be inhibited because it also can mediate KCa3.1 activation and thus represents a potential bypass of CXCR4 inhibition (Kast 2010).
Kv11.1 (hERG) channels mediate CXCL12/CXCR4-stimulated migration and invasion in leukemia cells (Li et al. 2009). In their plasma membranes, Kv11.1, CXCR4 and β1 integrin assemble to form a multiprotein complex (Pillozzi et al. 2011). Targeting CXCL12 or the CXCL12/CXCR4 axis with peptides and small molecules induces pro-apoptotic effects and may thus help to overcome chemoresistance in leukemia that is induced by CXCL12-releasing bone marrow mesenchymal stromal cells (Pillozzi et al. 2019).
5.1.2 CCL20/CCR6
In general, the CCL20 chemokine/CCR6 chemokine receptor pair contributes to cancer cell motility and metastasis (Korbecki et al. 2020). This has been shown for breast cancer (Muscella et al. 2017), prostate cancer (Ghadjar et al. 2008), ovarian cancer (Liu et al. 2020), lung cancer (Wang et al. 2016), esophageal squamous cell carcinoma (Liu et al. 2017), gastric cancer (Han et al. 2015), pancreatic cancer (Campbell et al. 2005; Kimsey et al. 2004), hepatocellular carcinoma (Huang and Geng 2010), colorectal cancer (Frick et al. 2016), and renal cell carcinoma (Kadomoto et al. 2019).
In patients with primary lung cancer, the production of CCL20 in adrenal glands is most likely to recruit CCR6-expressing lung cancer cells which then leads to the development of adrenal metastases (Raynaud et al. 2010).
Multiple myeloma cells trigger the upregulation of both CCL20 and CCR6 in the bone microenvironment and thus contribute to osteoclast formation and eventually to osteolytic bone lesions (Giuliani et al. 2008).
The expression of CCL20 within the periportal area of the liver is likely to attract CCR6 expressing colorectal cancer (CRC) cells (Dellacasagrande et al. 2003; Frick et al. 2016). Accordingly, liver metastases can be found in approximately 50% of CRC patients (Jemal et al. 2008). Here, too, as described above for the CXCR1/2-CXCL8 and the CXCR4-CXCL12 pairs, Erk1/2 and Akt signaling pathways are activated and promote proliferation and motility of CRC cells (Brand et al. 2006). Furthermore, CCL20 stimulation of CCR6 expressing human colon carcinoma cells causes phosphorylation of BCAR1/p130Cas (Yang et al. 2005), a scaffolding protein overexpressed also in breast, ovarian, prostate, lung, and colorectal cancers as well as in hepatocellular carcinoma, glioma, melanoma, anaplastic large cell lymphoma, and chronic myelogenous leukemia (Barrett et al. 2013). BCAR1/p130Cas is a key component of the pathway by which the focal adhesion kinase (FAK) can drive cell migration (Tikhmyanova et al. 2010). In a monolayer of polarized human colon adenocarcinoma cells, CCR6 stimulation has been associated with cAMP-stimulated electrogenic chloride secretion as CCL20 inhibits forskolin-stimulated cAMP production (Yang et al. 2005). The nature of ion transporters and channels possibly involved has not yet been identified. A potential candidate would be the cAMP-dependent CFTR (cystic fibrosis transmembrane conductance regulator). NKCC1 (Na+, K+, 2Cl− cotransporter 1) could also be involved. NKCC1 activity is sensitive to cytoskeletal dynamics (Hecht and Koutsouris 1999; Matthews et al. 1994), and the BCAR1/p130Cas, phosphorylated in response to CCL20 stimulation, associates with cytoskeletal complexes (Sawada et al. 2006; Defilippi et al. 2006) and could thus be an integrative module linking NKCC1 activity with cytoskeletal dynamics.
5.1.3 CCL19 and 21/CCR7
The CCL21/CCR7 chemokine axis contributes to a metastatic phenotype in a wide variety of cancer types (Rizeq and Malki 2020), including breast (Müller et al. 2001; Weitzenfeld et al. 2016), prostate (Maolake et al. 2018), urinary bladder (Xiong et al. 2017), cervical (Kodama et al. 2007), esophageal (Shi et al. 2015; Goto and Liu 2020), gastric (Ma et al. 2015; Ryu et al. 2018), pancreatic (Hirth et al. 2020; Zhang et al. 2016b), colorectal (Li et al. 2011b), and lung cancer (Zhong et al. 2017), as well as melanoma (Cristiani et al. 2019; Takeuchi et al. 2004), lymphoma (Fleige et al. 2018; Li et al. 2018; Yang et al. 2011), and oral, head, and neck squamous cell carcinoma (Chen et al. 2020; González-Arriagada et al. 2018).
Generally, the binding of CCL19 and CCL21 to the GPCR CCR7 induces the activation of a Gα-subunit and a Giβγ heterodimer which then triggers downstream signaling effectors and signaling cascades. As a result, the activation of ERK1/2, PI3K/Akt, Rho GTPases, MAPK, and JAK/STAT can lead to the transcription and expression of different genes including MMPs and thus promote chemotaxis, cytoskeletal remodeling, extracellular matrix degradation, cell adhesion, migration, invasion, angiogenesis, and proliferation (Rizeq and Malki 2020). To date, it has not been shown explicitly that CCL19, 21/CCR7 mediated changes in tumor cell behavior involve ion channels and transporters. However, the signaling pathways sparked by CCR7 stimulation most likely address ion transport mechanisms as well, also in tumor cells. In CCR7 expressing mature dendritic cells, CCL19 and CCL21 trigger Ca2+ influx from the extracellular space. This Ca2+ influx is accompanied by KCa3.1 mediated K+ efflux and, in presence of a yet undefined Cl− conductance, propels cell migration (Shao et al. 2015).
5.2 Given Factors at the Premetastatic Niche
In addition to being attracted by chemokines CTCs can be retained at the premetastatic niche by specific, local adhesion events. E-selectin, for instance, supports hematogenous metastasis of estrogen-receptor negative (ER−) CD44+ breast cancer cells (Kang et al. 2015). Furthermore, characteristic vascular structures in target organs are associated with special requirements for cancer cell extravasation (Gao et al. 2019; Minami et al. 2019; Nguyen et al. 2009; Weidle et al. 2016), so that the particular architecture of a blood barrier, typical of an organ or a tissue, may select for cancer cells that are able to break down the local endothelial junctions and the appendant basement membrane. This interplay between metastasizing cell and local environment is continued by the cancer cells’ interaction with the unique resident cells and their secretome including the extracellular matrix. However, the initiation of proliferation and growth in the secondary organ appears to be another obstacle for disseminating tumor cells (Chambers et al. 1995).
5.2.1 Falling Asleep and Awakening
Some of the disseminating tumor cells enter a dormant phase, induced by a lack of a sufficient, integrin-mediated adhesion to the extracellular matrix in the secondary organ (Barkan et al. 2010). In order to survive without proper anchorage, detached breast cancer cells autocrinally secrete laminin-5, a component of the basement membrane, which induces their own survival through α6β4-mediated NFκB activation (Zahir et al. 2003). As soon as the biomolecular composition of the surrounding microenvironment changes, for example by the release of membrane receptor-ligands from a locally degrading extracellular matrix or by inflammatory events, dormant cancer cells can be awakened by induction of various signaling pathways leading to the revival of proliferative activity (Park and Nam 2020). Sustained lung inflammation, for instance, can provoke the formation of neutrophil extracellular traps (NETs). Two NET-associated proteases, neutrophil elastase and MMP9, then successively fragment laminin, and the proteolytically remodeled laminin awakens dormant breast cancer cells (Fig. 1a), i.e. induces their proliferation, by activating α3β1 signaling (Albrengues et al. 2018).
5.2.2 Local Nutrient Supply
Furthermore, the nutrient composition in the target organ may differ considerably from that around the primary tumor and thus force the disseminating tumor cells to adapt their metabolic pathways to the new environment (Elia et al. 2018). Accordingly, brain metastases originating from various tissues drive their oxidative TCA cycle utilizing acetate rather than glucose or glutamine (Maher et al. 2012; Mashimo et al. 2014), and breast cancer-derived lung metastases change over to a pyruvate carboxylase-dependent replenishment of the TCA cycle (anaplerosis) due to an elevated bioavailability of pyruvate in the lung environment (Christen et al. 2016).
5.2.3 Defeating the Local Defense System
On the other hand, tumor cells are capable of repulsing attacks by the tissues that they are going to populate. Normally, plasmin from the reactive brain stroma represents a defense against metastatic invasion. Plasmin is generated from plasminogen by plasminogen activator (PA) which in brain is released mainly by astrocytes. Plasmin cleaves off soluble Fas Ligand (sFasL) from the membrane-bound FasL, also expressed on astrocytes. The sFasL then induces apoptosis in metastatic cells and inactivates the axon pathfinding molecule L1CAM, a cell adhesion molecule expressed by metastatic cells for spreading along brain capillaries and for metastatic outgrowth. However, metastasizing breast and lung adenocarcinoma cells express high levels of PA inhibitory serpins (serin-protease inhibitors) to prevent plasmin generation and thus its metastasis-suppressive effects (Valiente et al. 2014).
5.3 Lack of E-Cadherin, Reduced NHE1 Activity, and the Presence of CAIX Each Contribute to Lung Tropism
The epithelial-mesenchymal transition (EMT) does not only confer on epithelial cells the abilities to detach from the cell layer/tissue, migrate, invade the surrounding tissue and degrade components of the extracellular matrix (Lambert et al. 2017), but it can also play a considerable role in metastatic organotropism as shown for pancreatic cancer (Reichert et al. 2018). One characteristic of EMT is a decreased expression of E-cadherin, the main component of adherens junctions. Adherens junction protein p120 (P120CTN) stabilizes E-cadherin at the adherens junctions (Ishiyama et al. 2010; Thoreson et al. 2000). A complete loss of p120ctn in metastatic pancreatic ductal adenocarcinoma (PDAC) cells shifts their organotropic preference from the liver to the lungs. Rescue with a p120ctn isoform restores liver organotropism (Reichert et al. 2018). According to this, and independently of the presence of P120CTN, E-cadherin-expressing PDAC cells prefer to metastasize to the liver while E-cadherin-negative metastases are found predominantly in the lungs (Fig. 1b; Reichert et al. 2018). Analogously, the inhibition of NHE1 by cariporide seems to direct the metastatic spread of murine melanoma (B16V) cells to the lungs (Vahle et al. 2014). NHE1 activity is affected by the NHE regulatory factor (NHERF1), and NHERF1 expression is upregulated in a variety of cancers where its expression level correlates with malignancy (Georgescu et al. 2008; Greco et al. 2019; Ma et al. 2016; Saponaro et al. 2014; Vaquero et al. 2017). The phosphorylation state of NHERF1 on serines S279 and S301 differentially controls NHE1 activity and metastatic organotropism of breast cancer (MDA-MB-231) cells (Greco et al. 2019). Replacing both S279 and S301 by alanine results in a significantly increased NHE1 activity and, in a xenograft mouse model, drives a shift from the predominantly lung colonization to a predominantly bone colonization. This led the authors (Greco et al. 2019) to conclude that NHERF1 phosphorylation can act as a signaling switch in metastatic organotropism.
Also the carbonic anhydrase IX (CAIX) contributes indirectly to organotropism (Fig. 1b). Bone marrow-derived cells (BMDCs), including myeloid-derived suppressor cells (MDSC), macrophages, dendritic cells, and hematopoietic progenitor cells are recruited to potential metastatic sites where they act in concert to establish the premetastatic niche prior to the arrival of metastasizing tumor cells (Gabrilovich et al. 2012; Kaplan et al. 2005; Psaila and Lyden 2009; Quail and Joyce 2013). The production of chemokines and cytokines that mobilize granulocytic MDSCs to a potential (pre)metastatic niche requires the hypoxia-induced expression of CAIX by cancer cells in the (primary) tumor (Chafe et al. 2015). Hypoxic breast cancer cells express significant amounts of CXCL10, CCL5, and the granulocyte colony stimulating factor G-CSF when, and only when, CAIX is expressed. Hypoxia-induced CAIX is needed for the activation of the NF-κB pathway which then results in the generation of G-CSF and eventually promotes breast cancer metastasis to the lungs (Chafe et al. 2015).
6 Conclusion and Outlook
Even though there is hardly any direct evidence proving it, the literature suggests that ion channels and transporters do contribute to both extravasation and organotropism of metastasizing tumor cells. Table 1 summarizes the channels and transporters potentially involved in (1) surviving the intravascular milieu, (2) adhesion to the vessel wall, (3) extravasation, and (4) metastatic organotropism.
NHE1 may be considered as a kind of “all-rounder” due to its dual function. (1) In its role as a structural element contributing to the organization of the cortical actin cytoskeleton and tying it to the plasma membrane, NHE1 possibly protects CTCs from mechanical stress. (2) In its role as H+ extruder, NHE1 may promote both CTC adhesion to the vessel wall and subsequent, organ-specific extravasation by generating pH-nanodomains that modulate not only pH-dependent cell–substrate and MCAM-mediated cell–cell (melanoma-endothelium) adhesions but also the activity of matrix metalloproteases. Finally, there is evidence to suggest that NHE1 activity, regulated by NHERF1, has a hand in organotropism.
Regulation of the intracellular Ca2+ concentration [Ca2+]i is interwoven with the modulation of K+ conductances. K+ channels including mechanosensitive K2P channels stabilize the membrane potential required for Ca2+ influx, e.g. through mechanosensitive channels (TRPs, Piezo), while increases in [Ca2+]i activate Ca2+ sensitive K+ channels (KCas). This interplay, especially the controlled Ca2+ influx, may strengthen the actin cortex of CTCs, accompanied by an increase in cortical stiffness, and thus protect them from shear forces in the blood vessels. In endothelial cells, an elevation of [Ca2+]i (1) can be induced by binding of ATP released from tumor cell-activated platelets to endothelial P2Y2, (2) is mediated by SOC channels, and (3) results in an increased endothelial permeability which facilitates extravasation (Table 1).
In addition to pH and Ca2+ including the affected signaling pathways (e.g., Ca2+/CaM signaling), the FAK signaling and the Akt pathway are major variables being modulated by ion channels/transporters and involved in organotropism and surviving the intravascular milieu. Permanent activation of FAK can prevent anoikis. Some CTCs secrete fibronectin or collagen and thus “autostimulate” their β1 integrin leading to activation of Kv11.1 concomitant with FAK phosphorylation. Another mechanism by which CTCs avoid anoikis is the adoption and perpetuation of mesenchymal features with the help of the Zn2+ transporter ZIP6.
AQP3 in cooperation with the Akt pathway is likely to play a role in organotropism by directing CXCR4 expressing breast cancer cells to the lungs where local CXCL12 stimulates H2O2 production via membrane-bound Nox2. H2O2 crosses the plasma membrane through AQP3 in order to activate the Akt pathway by oxidizing PTEN and PTP1B which eventually stimulates directional cell migration.
Altogether the literature strongly suggests that several ion channels and transporters have a hand in CTC survival, extravasation, and organotropism, which points to their potential usefulness as therapeutic target(s) during and after resection of the primary tumor. Given the great potential to be exploited as therapeutic targets on the one hand, yet the insufficient hitherto existing knowledge and unsatisfying data availability on the other, it becomes apparent that far more efforts need to be made in order to identify and characterize the mechanistic roles of ion channels and transporters in the behavior of CTCs including extravasation and organotropism. Provided that an experimental setting includes chemokines, extracellular matrix (proteins and structure), and preferably also immune cells typically found in the organ of interest, advanced microfluidic models of cancer cell extravasation (Mondadori et al. 2020; Offeddu et al. 2021) may be a suitable tool to validate the involvement of ion channels/transporters in extravasation and organotropism, e.g. by using genetically modified tumor cell lines, and to test their responsiveness to antimetastatic drugs.
References
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122
Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, Goud B, Ivaska J (2015) Integrin endosomal signalling suppresses anoikis. Nat Cell Biol 17:1412–1421
Albrengues J, Shields M, Ng D, Park CG, Ambrico A, Poindexter M, Upadhyay P, Uyeminami D, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton K, Trotman LC, Goldberg MS, Yeh JT-H, Egeblad M (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cells in mice. Science 361:eaao4227
André F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, Soria JC, Cristofanilli M (2006) Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol 17:945–951
Aoudjit F, Vuori K (2012) Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract 2012:283181
Au SH, Storey BD, Moore JC, Tang Q, Chen Y-L, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, Haber DA, Maheswaran S, Langenau DM, Stott SL, Toner M (2016) Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A 113:4947–4952
Azevedo AS, Follain G, Patthabhiraman S, Harlepp S, Goetz JG (2015) Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both? Cell Adhes Migr 9:345–356
Barkan D, Green JE, Chambers AF (2010) Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer 46:1181–1188
Barnes JM, Nauseef JT, Henry MD (2012) Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS One 7:e50973
Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P (2013) p130Cas: a key signaling node in health and disease. Cell Signal 25:766–777
Bendas G, Borsig L (2012) Cancer cell adhesion and metastasis: selectin, integrings and the inhibitory potential of heparins. Int J Cell Biol 2012:676731
Bera A, Subramanian M, Karaian J, Eklund M, Radhakrishnan S, Gana N, Rothwell S, Pollard H, Hu H, Shriver CD, Srivastava M (2020) Functional role of vitronectin in breast cancer. PLoS One 15:e0242141
Bilbao PS, Boland R, Santillán G (2010) ATP modulates transcription factor through P2Y2 and P2Y4 receptors via PKC/MAPKs and PKC/Src pathways in MCF-7 cells. Arch Biochem Biophys 494:7–14
Borsig L, Wong R, Hynes RO, Varki NM, Varki A (2002) Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A 99:2193–2198
Bourcy M, Suarez-Carmona M, Lambert J, Francart M-E, Schroeder H, Delierneux C, Skrypek N, Thompson EW, Jérusalem G, Berx G, Thiry M, Blacher S, Hollier BG, Noël A, Oury C, Polette M, Gilles C (2016) Tissue factor induced by epithelial-mesenchymal transition triggers a procoagulant state that drives metastasis of circulating tumor cells. Cancer Res 76:4270–4282
Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Göke B, Dambacher J (2006) Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem 97:709–723
Buchheit CL, Weigel KJ, Schafer ZT (2014) Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer 14:632–641
Butler TP, Gullino PM (1975) Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res 35:512–516
Campbell AS, Albo D, Kimsey TF, White SL, Wang TN (2005) Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J Surg Res 123:96–101
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17:887–904
Cedervall J, Hamidi A, Olsson A-K (2018) Platelets, NETs and cancer. Thrombos Res 164(Suppl 1):S148–S152
Chafe SC, Lou Y, Sceneay J, Vallejo M, Hamilton MJ, McDonald PC, Bennewith KL, Möller A, Dedhar S (2015) Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res 75:996–1008
Chaffer CL, Goetz JG (2018) CD44 orchestrates metastatic teamwork. Dev Cell 47:691–693
Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, Groom AC (1995) Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev 14:279–301
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Chen Y, Shao Z, Jiang E, Zhou X, Wang L, Wang H, Luo X, Chen Q, Liu K, Shang Z (2020) CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J Cell Physiol 235:5995–6009
Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, Di Stefano P, Degani S, Balzi M, Olivotto M, Wanke E, Becchetti A, Defilippi P, Wymore R, Arcangeli A (2005) Human ether-a-go-go-related gene 1 channels are physically linked to β1 integrins and modulate adhesion-dependent signaling. Mol Biol Cell 16:2972–2983
Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Fürst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH, Kabelitz D (2013) Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer 133:1557–1566
Chivukula VK, Krog BL, Nauseef JT, Henry MD, Vigmostad SC (2015) Alterations in cancer cell mechanical properties after fluid shear stress exposure: a micropipette aspiration study. Cell Health Cytoskelet 7:25–35
Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, Elia I, Buescher JM, Orth MF, Davidson SM, Grünewald TGP, De Bock K, Fendt S-M (2016) Breast cancer-derived lung metastases show increased pyruvate carboxylate-dependent anaplerosis. Cell Rep 17:837–848
Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T (2005) Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res 97:1164–1172
Codo P, Weller M, Meister G, Szabo E, Steinle A, Wolter M, Reifenberger G, Roth P (2014) MicroRNA-mediated down-regulation of NKG2D ligands contributes to glioma immune escape. Oncotarget 5:7651–7662
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, de Visser KE (2015) IL17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522:345–348
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16:431–446
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123:3446–3458
Cools-Lartigue J, Spicer J, Najmeh S, Ferri L (2014) Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci 71:4179–4194
Cooper CR, Chay CH, Pienta KJ (2002) The role of αvβ3 in prostate cancer progression. Neoplasia 4:191–194
Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA, Parsa AT, Lanier LL (2014) Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A 111:12823–12828
Cristiani CM, Turdo A, Ventura V, Apuzzo T, Capone M, Madonna G, Mallardo D, Garofalo C, Giovannone ED, Grimaldi AM, Tallerico R, Marcenaro E, Pesce S, Del Zotto G, Agosti V, Costanzo FS, Gulletta E, Rizzo A, Moretta A, Karre K, Ascierto PA, Todaro M, Carbone E (2019) Accumulation of circulating CCR7(+) natural killer cells marks melanoma evolution and reveals a CCL19-dependent metastatic pathway. Cancer Immunol Res 7:841–852
Defilippi P, Di Stefano P, Cabodi S (2006) p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 16:257–263
Dellacasagrande J, Schreurs OJ, Hofgaard PO, Omholt H, Steinsvoll S, Schenck K, Bogen B, Dembic Z (2003) Liver metastasis of cancer facilitated by chemokine receptor CCR6. Scand J Immunol 57:534–544
Demkow U (2021) Neutrophil extracellular traps (NETs) in cancer invasion, evasion and metastasis. Cancers 13:4495
Dey S, Sayers CM, Verginadis II, Lehman SL, Cheng Y, Cerniglia GJ, Tuttle SW, Feldman MD, Zhang PJ, Fuchs SY, Diehl JA, Koumenis C (2015) ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest 125:2592–2608
Dhar P, Wu JD (2018) NKG2D and its ligands in cancer. Curr Opin Immunol 51:55–61
Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, Wang Y, Xiong F, Guo C, Li Y, Li X, Li G, Zeng Z, Xiong W, Wang F (2019) Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer 18:29
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene 23:465–473
Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA (2006) TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 129:2416–2425
Elble RC, Pauli BU (2001) Tumor suppression by a proapoptotic calcium-activated chloride channel in mammary epithelium. J Biol Chem 276:40510–40517
Elia I, Doglioni G, Fendt S-M (2018) Metabolic hallmarks of metastasis formation. Trends Cell Biol 28:673–684
Erpenbeck L, Schön MP (2010) Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 115:3427–3436
Fan R, Emery T, Zhang Y, Xia Y, Sun J, Wan J (2016) Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci Rep 6:27073
Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5iodo-2′-deoxyuridine. J Natl Cancer Inst 45:773–782
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
Fleige H, Bosnjak B, Permanyer M, Ristenpart J, Bubke A, Willenzon S, Sutter G, Luther SA, Förster R (2018) Manifold roles of CCR7 and its ligands in the induction and maintenance of bronchus-associated lymphoid tissue. Cell Rep 23:783–795
Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA, Solecki G, Garcìa Leòn MJ, Lefebvre O, Fekonja N, Hille C, Chabannes V, Dollé G, Metivet T, Der Hovsepian F, Prudhomme C, Pichot A, Paul N, Carapito R, Bahram S, Ruthensteiner B, Kemmling A, Siemonsen S, Schneider T, Fiehler J, Glatzel M, Winkler F, Schwab Y, Pantel K, Harlepp S, Goetz JG (2018) Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev Cell 45:33–52
Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, Timpson P, Goetz JG (2020) Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer 20:107–124
Foss A, Muῆoz-Sagredo L, Sleeman J, Thiele W (2020) The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin Exp Metastasis 37:47–67
Frick VO, Rubie C, Keilholz U, Ghadjar P (2016) Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: an overview. World J Gastroenterol 22:833–841
Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, Wu YG, Elemento O, Tavazoie SF (2015) Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol 17:943–952
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268
Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH-F (2019) Metastasis organotropism: redefining the congenial soil. Dev Cell 49:375–391
García-Román J, Zentella-Dehesa A (2013) Vascular permeability changes involved in metastasis. Cancer Lett 335:259–269
Gassmann P, Haier J, Schlüter K, Domikowksy B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, Müller A (2009) CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia 11:651–661
Gebauer F, Wicklein D, Stübke K, Nehmann N, Schmidt A, Salamon J, Peldschus K, Nentwich MF, Adam G, Tolstonog G, Bockhorn M, Izbicki JR, Wagener C, Schumacher U (2013) Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp−−/rag2—mice. Gut 62:741–750
Georgescu MM, Morales FC, Molina JR, Hayashi Y (2008) Roles of NHEF1/EBP50 in cancer. Curr Mol Med 8:459–468
Ghadially H, Brown L, Lloyd C, Lewis L, Lewis A, Dillon J, Sainson R, Jovanovic J, Tigue NJ, Bannister D, Bamber L, Valge-Archer V, Wilkinson RW (2017) MHC class I chain-related protein a and B (MICA and MICB) are predominantly expressed intracellularly in tumour and normal tissue. Br J Cancer 116:1208–1217
Ghadjar P, Coupland SE, Na I-K, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C, Keilholz U (2006) Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol 24:1910–1916
Ghadjar P, Loddenkemper C, Coupland SE, Stroux A, Noutsias M, Thiel E, Christoph F, Miller K, Scheibenbogen C, Keilholz U (2008) Chemokine receptor CCR6 expression level and aggressiveness of prostate cancer. J Cancer Res Clin Oncol 134:1181–1189
Gil-Bernabé AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, Pollard JW, Ruf W, Muschel RJ (2012) Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119:3164–3175
Gil-Bernabé AM, Lucotti S, Muschel RJ (2013) Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol 162:433–441
Giuliani N, Lisignoli G, Colla S, Lazzaretti M, Storti P, Mancini C, Bonomini S, Manferdini C, Codeluppi K, Facchini A, Rizzoli V (2008) CC-chemokine ligand 20/macrophage inflammatory protein-3α and cc-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res 68:6840–6850
González-Arriagada WA, Lozano-Burgos C, Zúῆiga-Moreta R, González-Díaz P, Coletta RD (2018) Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J Oral Pathol Med 47:755–763
Goto M, Liu M (2020) Chemokines and their receptors as biomarkers in esophageal cancer. Esophagus 17:113–121
Greco MR, Bon E, Rubino R, Guerra L, Bernabe-Garcia M, Cannone S, Cayuela M-L, Ciaccia L, Marionneau-Lambot S, Oullier T, Fromont G, Guibon R, Roger S, Reshkin SJ, Cardone RA (2019) Phosphorylation of NHERF1 S279 and S301 differentially regulates breast cancer cell phenotype and metastatic organotropism. Biochim Biophys Acta Mol basis Dis 1865:26–37
Groeger G, Quiney C, Cotter TG (2009) Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal 11:2655–2671
Gros A, Ollivier V, Ho-Tin-Noé B (2015) Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol 5:678
Gross AC, Cam H, Phelps DA, Saraf AJ, Bid HK, Cam M, London CA, Winget SA, Arnold MA, Brandolini L, Mo X, Hinckley JM, Houghton PJ, Roberts RD (2018) IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight 3:e99791
Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W (2016) CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35:816–826
Ha H, Debnath B, Neamati N (2017) Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 7:1543–1588
Han G, Wu D, Yang Y, Li Z, Zhang J, Li C (2015) CrkL mediates CCL20/CCR6-induced EMT in gastric cancer. Cytokine 76:163–169
Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, Bauer AT, Schneider SW, Borsig L (2016) Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res 76:5302–5312
Hecht G, Koutsouris A (1999) Myosin regulation of NKCC1: effects on cAMP-mediated cl− secretion in intestinal epithelia. Am J Physiol Cell Physiol 277:C441–C447
Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A (2012) Tumor suppressive microRNAs miR-23a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res 72:460–471
Herman H, Fazakas C, Haskó J, Molnár K, Mészáros Á, Nyúl-Tóth Á, Szabó G, Erdélyi F, Ardelean A, Hermenean A, Krizbai IA, Wilhelm I (2019) Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J Cell Mol Med 23:2619–2631
Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR (2012) Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 189:1360–1371
Hirth M, Gandla J, Höper C, Gaida MM, Agarwal N, Simonetti M, Demir A, Xie Y, Weiss C, Michalski CW, Hackert T, Ebert MP, Kuner R (2020) CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 159:665–681
Hisada Y, Mackman N (2019) Tissue factor and cancer: regulation, tumor growth and metastasis. Semin Thromb Hemost 45:385–395
Hofschröer V, Koch KA, Ludwig FT, Friedl P, Oberleithner H, Stock C, Schwab A (2017) Extracellular protonation modulates cell-cell interaction mechanics and tissue invasion in human melanoma cells. Sci Rep 7:42369
Hogstrand C, Kille P, Ackland ML, Hiscox S, Taylor KM (2013) A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem J 455:229–237
Hosono M, Koma Y-I, Takase N, Urakawa N, Higashino N, Suemune K, Kodaira H, Nishio M, Shigeoka M, Kakeji Y, Yokozaki H (2017) CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget 8:106071–106088
Hou J-M, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C (2011) Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 178:989–996
Huang F, Geng X-P (2010) Chemokines and hepatocellular carcinoma. World J Gastroenterol 16:1832–1836
Huang R-L, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, Kersten S, Ding JL, Li HY, Tan NS (2011) ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118:3990–4002
Huergo-Zapico L, Acebes-Huerta A, López-Soto A, Villa-Álvarez M, Gonzalez-Rodriguez AP, Gonzalez S (2014) Molecular bases for the regulation of NKG2D ligands in cancer. Front Immunol 5:106
Hutchings CJ, Colussi P, Clark TG (2019) Ion channels as therapeutic antibody targets. MAbs 11:265–296
Ishiyama N, Lee S-H, Liu S, Li G-Y, Smith MJ, Reichardt LF, Ikura M (2010) Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 141:117–128
Iwasa S, Yanagawa T, Fan J, Katoh R (2009) Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res 29:4751–4758
Izraely S, Witz IP (2021) Site-specific metastasis: a cooperation between cancer cells and the metastatic microenvironment. Int J Cancer 148:1308–1322
Jehle J, Schweizer PA, Katus HA, Thomas D (2011) Novel roles for hERG K+ channels in cell proliferation and apoptosis. Cell Death Dis 2:e193
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Johnson B, Mahadevan D (2015) Emerging role and targeting of carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) in human malignancies. Clin Cancer Drugs 2:100–111
Joseph PR, Sarmiento JM, Mishra AK, Das ST, Garofalo RP, Navarro J, Rajarathnam K (2010) Probing the role of CXC motif in chemokine CXCL8 for high affinity binding and activation of CXCR1 and CXCR2 receptors. J Biol Chem 285:29262–29269
Jouve N, Bachelier R, Despoix N, Blin MG, Matinzadeh MK, Poitevin S, Aurrand-Lions M, Fallague K, Bardin N, Blot-Chabaud M, Vely F, Dignat-George F, Leroyer AS (2015) CD146 mediates VEGF-induced melanoma cell extravasation through FAK activation. Int J Cancer 137:50–60
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223
Kadomoto S, Izumi K, Hiratsuka K, Nakano T, Naito R, Makino T, Iwamoto H, Yaegashi H, Shigehara K, Kadono Y, Nakata H, Saito Y, Nakagawa-Goto K, Mizokami A (2019) Tumor-associated macrophages induce migration of renal cell carcinoma cells via activation of the CCL20-CCR6 axis. Cancers 12:89
Kadomoto S, Izumi K, Mizokami A (2020) The CCL20-CCR6 axis in cancer progression. Int J Mol Sci 21:5186
Kang S-A, Hasan N, Mann AP, Zheng W, Zhao L, Morris L, Zhu W, Zhao YD, Suh KS, Dooley WC, Volk D, Gorenstein DG, Cristofanilli M, Rui H, Tanaka T (2015) Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Mol Ther 23:1044–1054
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive heamatopoietic bone marrow progenitors initiate the premetastatic niche. Nature 438:820–827
Kast RE (2010) Profound blockage of CXCR4 signaling at multiple points using the synergy between plerixafor, mirtazapine, and clotrimazole as a new glioblastoma treatment adjunct. Turk Neurosurg 20:425–429
Keurhorst D, Liashkovich I, Frontzek F, Nitzlaff S, Hofschröer V, Dreier R, Stock C (2019) MMP3 activity rather than cortical stiffness determines NHE1-dependent invasiveness of melanoma cells. Cancer Cell Int 19:285
Khuon S, Liang L, Dettman RW, Sporn PHS, Wysolmerski RB, Chew T-L (2010) Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci 123:431–440
Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, Massagué J (2009) Tumor self-seeding by circulating cancer cells. Cell 139:1315–1326
Kim Y-N, Koo KH, Sung JY, Yun U-J, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012:306879
Kimsey TF, Campbell AS, Albo D, Wilson M, Wang TN (2004) Co-localization of macrophage inflammatory protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J 10:374–380
Kodama J, Hasengaowa, Kusumoto T, Seki N, Matsuo T, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y (2007) Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol 18:70–76
Köhler S, Ullrich S, Richter U, Schumacher U (2010) E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer 102:602–609
Kopp H-G, Placke T, Salih HR (2009) Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69:7775–7783
Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I (2020) CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci 21:7619
Korennykh A, Walter P (2012) Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol 28:251–277
Kornberg LJ (1998) Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 20:745–752
Labelle M, Hynes RO (2012) The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2:1091–1099
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces and epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20:576–590
Labelle M, Begum S, Hynes RO (2014) Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A 11:E3053–E3061
Lal H, Verma SK, Foster DM, Golden HB, Reneau JC, Watson LE, Singh H, Dostal DE (2009) Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci 14:2307–2334
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168:670–691
Langer HL, Orlova VV, Xie C, Kaul S, Schneider D, Lonsdorf AS, Fahrleitner M, Choi EY, Dutoit V, Pellegrini M, Grossklaus S, Nawroth PP, Baretton G, Santoso S, Hwang ST, Arnold B, Chavakis T (2011) A novel function of junctional adhesion molecule-C in mediating melanoma cell metastasis. Cancer Res 71:4096–4105
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128:2527–2535
Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, Bencini L, Calistri M, Messerini L, Scatizzi M, Moretti R, Wanke E, Olivotto M, Mugnai G, Arcangeli A (2004) herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res 64:606–611
Lee H, Jang Y, Park S, Jang H, Park EJ, Kim HJ, Kim H (2018) Development and evaluation of a CEACAM6-targeting theranostic nanomedicine for photoacoustic-based diagnosis and chemotherapy of metastatic cancer. Theranostics 8:4247–4261
Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, Postenka CO, Turley EA, Courtneidge SA, Chambers AF, Lewis JD (2014) Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 8:1558–1570
Li H, Du YM, Guo L, Jie S, Zhang S, Du W, Chen X, Liu W, Fan L, Zhu J, Zou A, Huang S (2009) The role of hERG1 K+ channels and a functional link between hERG1 K+ channels and SDF-1 in acute leukemic cell migration. Exp Cell Res 315:2256–2264
Li H, Lakshmikanth T, Garofalo C, Enge M, Spinnler C, Anichini A, Szekely L, Kärre K, Carbone E, Selivanova G (2011a) Pharmacological activation of p53 triggers anticancer innate immune response though induction of ULBP2. Cell Cycle 10:3346–3358
Li J, Sun R, Tao K, Wang G (2011b) The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig Liver Dis 43:40–47
Li Z, Wang Y, Dong S, Ge C, Xiao Y, Li R, Ma X, Xue Y, Zhang Q, Lv J, Tan Q, Zhu Z, Song X, Tan J (2014) Association of CXCR1 and 2 expressions with gastric cancer metastasis in ex vivo and tumor cell invasion in vitro. Cytokine 69:6–13
Li W, Xue W, Wang X, Fu X, Sun Z, Li Z, Chang Y, Zhang X, Zhou Z, Chen C, Zhang M (2018) MiR-199a mediated the dissemination of human mantle cell lymphoma by interacting with the CCR7/CCL21 pair. Anti-Cancer Drugs 29:861–870
Li M, Lin C, Deng H, Strnad J, Bernabei L, Vogl ST, Burke JJ, Nefedova Y (2020) A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol Cancer Ther 19:1530–1538
Liao Z, Cao C, Wang J, Huxley VH, Baker O, Weisman GA, Erb L (2014) The P2Y2 receptor interacts with VE-cadherin and VEGF receptor-2 to regulate Rac1 activity in endothelial cells. J Biochem Sci Eng 7:1105–1121
Liotta LA, Saider MG, Kleinerman J (1976) The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 36:889–894
Liu C-L, Shi G-P (2019) Calcium-activated chloride channel regulator 1 (CLCA1): more than a regulator of chloride transport and mucus production. World Allergy Organ J 12:100077
Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L (2004) Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279:8212–8218
Liu J, Zheng X, Deng H, Xu B, Chen L, Wang Q, Zhou Q, Zhang D, Wu C, Jiang J (2017) Expression of CCR6 in esophageal squamous cell carcinoma and its effects on epithelial-to-mesenchymal transition. Oncotarget 8:115244–115253
Liu H, Wang S, Xin J, Wang J, Yao C, Zhang Z (2019a) Role of NKG2D and its ligands in cancer immunotherapy. Am J Cancer Res 9:2064–2078
Liu X, Taftaf R, Kawaguchi M, Chang Y-F, Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel DP, Tsui E, Adorno-Cruz V, Chirieleison SM, Cao Y, Harney AS, Patel S, Patsialou A, Shen Y, Avril S, Gilmore HL, Lathia JD, Abbott DW, Cristofanilli M, Condeelis JS, Liu H (2019b) Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov 9:96–113
Liu W, Wang W, Wang X, Xu C, Zhang N, Di W (2020) Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett 472:59–69
López-Soto A, Huergo-Zapico L, Galván JA, Rodrigo L, García de Herreros A, Astudillo A, Gonzalez S (2013) Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J Immunol 190:4408–4419
Ma H, Gao L, Li S, Qin J, Chen L, Liu X, Xu P, Wang F, Xiao H, Zhou S, Gao Q, Liu B, Sun Y, Liang C (2015) CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 6:24348–24360
Ma Q, Jiao Y, Hao Y, Yan S, Lyu N, Gao H, Li D, Liu Q, Zheng J, Song N (2016) Targeting of NHERF1 through RNA interference inhibits the proliferation and migration of metastatic prostate cancer cells. Oncol Lett 11:1149–1154
Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR, DeBerardinis RJ (2012) Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed 25:1234–1244
Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D, Remer K, Nurden P, Nurden AT, Dandekar T, Nehez L, Dank M, Braun A, Mezzano D, Abrams SI, Nieswandt B (2020) Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 135:1146–1160
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP (1995) Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res 55:4425–4431
Maolake A, Izumi K, Natsagdorj A, Iwamoto H, Kadomoto S, Makino T, Naito R, Shigehara K, Kadono Y, Hiratsuka K, Wufuer G, Nastiuk KL, Mizokami A (2018) Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci 109:1524–1531
Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, DeBerardinis RJ, Tu BP, Maher EA, Bachoo RM (2014) Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159:1603–1614
Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M (2002) Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 62:2937–2941
Matthews JB, Smith JA, Tally KJ, Awtrey CS, Nguyen H, Rich J, Madara JL (1994) Na-K-Cl2 cotransport in intestinal epithelial cells. Influence of chloride efflux and F-actin on regulation of cotransporter activity and bumetanide binding. J Biol Chem 269:15703–15709
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, Haley B, Morrison L, Fleming TP, Herlyn D, Terstappen LWMM, Fehm T, Tocker TF, Lane N, Wang J, Uhr JW (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10:8152–8162
Micalizzi DS, Maheswaran S, Haber DA (2017) A conduit to metastasis: circulating tumor cell biology. Genes Dev 31:1827–1840
Mills L, Tellez C, Huang S, Baker C, McCarty M, Green L, Gudas JM, Feng X, Bar-Eli M (2002) Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res 62:5106–5114
Minami T, Muramatsu M, Kume T (2019) Organ/tissue-specific vascular endothelial cell heterogeneity in health and disease. Biol Pharm Bull 42:1609–1619
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524
Molfetta R, Quatrini L, Santoni A, Paolini R (2017) Regulation of NKG2D-dependent NK cell functions: the yin and the yang of receptor endocytosis. Int J Mol Sci 18:1677
Mondadori C, Crippa M, Moretti M, Candrian C, Lopa S, Arrigoni C (2020) Advanced microfluidic models of cancer and immune cell extravasation: a systematic review of the literature. Front Bioeng Biotechnol 8:907
Moore MA (2001) The role of chemoattraction in cancer metastasis. BioEssays 23:674–676
Moose DL, Krog BL, Kim T-H, Zhao L, Williams-Perez S, Burke G, Rhodes L, Vanneste M, Breheny P, Milhem M, Stipp CS, Rowat AC, Henry MD (2020) Cancer cells resist mechanical destruction in circulation via RhoA/actomyosin-dependent mechano-adaptation. Cell Rep 30:3864–3874
Morvan MG, Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16:7–19
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Muscella A, Vetrugno C, Marsigliante S (2017) CCL20 promotes migration and invasiveness of human cancerous breast epithelial cells in primary culture. Mol Carcinog 56:2461–2473
Naci D, Vuori K, Aoudjit F (2015) Alpha2beta1 integrin in cancer development and chemoresistance. Semin Cancer Biol 35:145–153
Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, Spicer JD (2017) Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer 140:2321–2330
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–284
Nieswandt B, Hafner M, Echtenacher B, Männel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59:1295–1300
Offeddu GS, Hajal C, Foley CR, Wan Z, Ibrahim L, Coughlin MF, Kamm RD (2021) The cancer glycocalyx mediates intravascular adhesion and extravasation during metastatic dissemination. Commun Biol 4:255
Osmani N, Follain G, Garcìa Leòn MJ, Lefebvre O, Busnelli I, Larnicol A, Harlepp S, Goetz JG (2019) Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Rep 28:2491–2500
Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, Tandler C, Mbarga M, Schaefer T, Falcone M, Nievergall E, Dörfel D, Hanns P, Passweg JR, Lutz C, Schwaller J, Zeiser R, Blazar BR, Caligiuri MA, Dirnhofer S, Lundberg P, Kanz L, Quintanilla-Martinez L, Steinle A, Trumpp A, Salih HR, Lengerke C (2019) Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune system. Nature 572:254–259
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J (2008) TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133:66–77
Paget S (1989) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 8:98–101
Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105:178–185
Palumbo JS, Barney KA, Blevins EA, Shaw MA, Mishra A, Flick MJ, Kombrinck KW, Talmage KE, Souri M, Ichinose A, Degen JL (2008) Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. J Thromb Haemost 6:812–819
Pandey P, Sliker B, Peters HL, Tuli A, Herskovitz J, Smits K, Purohit A, Singh RK, Dong J, Batra SK, Coulter DW, Solheim JC (2016) Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 7:19430–19444
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 1833:3481–3498
Park S-Y, Nam J-S (2020) The force awakens: metastatic dormant cancer cells. Exp Mol Med 52:569–581
Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Küttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 8:361ra138
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med 52:192–203
Pethö Z, Najder K, Bulk E, Schwab A (2019) Mechanosensitive ion channels push cancer progression. Cell Calcium 80:79–90
Peyre L, Meyer M, Hofman P, Roux J (2021) TRAIL receptor-induced features of epithelial-to-mesenchymal transition increase tumour phenotypic heterogeneity: potential cell survival mechanisms. Br J Cancer 124:91–101
Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, Amedei A, Veltroni M, D’Amico M, Basso G, Becchetti A, Campana D, Arcangeli A (2011) Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood 117:902–914
Pillozzi S, Bernini A, Spiga O, Lelli B, Petroni G, Bracci L, Niccolai N, Arcangeli A (2019) Peptides and small molecules blocking the CXCR4/CXCL12 axis overcome bone marrow-induced chemoresistance in acute leukemias. Oncol Rep 41:312–324
Pilzweger C, Holdenrieder S (2015) Circulating HMGB1 and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics (Basel) 5:219–253
Placke T, Örgel M, Schaller M, Jung G, Rammensee H-G, Kopp H-G, Salih HR (2012a) Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72:440–448
Placke T, Salih HR, Kopp H-G (2012b) GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol 189:154–160
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9:285–293
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4:e6562
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Raqeeb A, Sheng J, Ao N, Braun AP (2011) Purinergic P2Y2 receptors mediate rapid Ca2+ mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49:240–248
Rayes RF, Vourtzoumis P, Rjeily MB, Seth R, Bourdeau F, Giannias B, Berube J, Huang Y-H, Rousseau S, Camilleri-Broet S, Blumberg RS, Beauchemin N, Najmeh S, Cools-Lartigue J, Spicer JD, Ferri LE (2020) Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastasic progression of colon carcinoma. J Immunol 204:2285–2294
Raynaud CM, Mercier O, Dartevelle P, Commo F, Olaussen KA, de Montpreville V, André F, Sabatier L, Soria J-C (2010) Expression of chemokine receptor CCR6 as a molecular determinant of adrenal metastatic relapse in patients with primary lung cancer. Clin Lung Cancer 11:187–191
Reichert M, Bakir B, Moreira L, Pitarresi JR, Feldmann K, Simon L, Suzuki K, Maddipati R, Rhim AD, Schlitter AM, Kriegsmann M, Weichert W, Wirth M, Schuck K, Schneider G, Saur D, Reynolds AB, Klein-Szanto AJ, Pehlivanoglu B, Memis B, Adsay NV, Rustgi AK (2018) Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Dev Cell 45:696–711
Reymond N, Im JH, Garg R, Vega FM, d’Água BB, Riou P, Cox S, Valderrama F, Muschel RJ, Ridley AJ (2012) Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol 199:653–668
Reymond N, d’Água BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13:858–870
Reymond N, Im JH, Garg R, Cox S, Soyer M, Riou P, Colomba A, Muschel RJ, Ridley AJ (2015) RhoC and ROCKs regulate cancer cell interactions with endothelial cells. Mol Oncol 9:1043–1055
Ritter C, Fan K, Paulson KG, Nghiem P, Schrama D, Becker JC (2016) Reversal of epigenetic silencing of MHC class I chain-related protein a and B improves immune recognition of Merkel cell carcinoma. Sci Rep 6:21678
Rizeq B, Malki MI (2020) The role of CCL21/CCR7 chemokine axis in breast cancer progression. Cancers 12:1036
Rodrigues P, Vanharanta S (2019) Circulating tumor cells: come together, right now, over metastasis. Cancer Discov 9:22–24
Rouanne M, Adam J, Goubar A, Robin A, Ohana C, Louvet E, Cormier J, Mercier O, Dorfmüller P, Fattal S, de Montpreville VT, Lebret T, Dartevelle P, Fadel E, Besse B, Olaussen KA, Auclair C, Soria JC (2016) Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer 16:483
Ruan Z, Orozco IJ, Du J, Lü W (2020) Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 584:646–651
Ryu H, Baek SW, Moon JY, Jo I-S, Kim N, Lee HJ (2018) C-C motif chemokine receptors in gastric cancer. Mol Clin Oncol 8:3–8
Sandilos JK, Chiu Y-H, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA (2012) Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem 287:11303–11311
Saponaro C, Malfettone A, Dell’Endice TS, Brunetti AE, Achimas-Cadariu P, Paradiso A, Mangia A (2014) The prognostic value of the Na+/H+ exchanger regulatory factor (NHERF1) protein in cancer. Cancer Biomark 14:177–184
Satooka H, Hara-Chikuma M (2016) Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol Cell Biol 36:1206–1218
Saur D, Seidler B, Schneider G, Algül H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM (2005) CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 129:1237–1250
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127:1015–1026
Schlesinger M (2018) Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 11:125
Schmiedel D, Mandelboim O (2018) NKG2D ligands - critical targets for cancer immune escape and therapy. Front Immunol 9:2040
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S (2013) Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24:130–137
Sciaccaluga M, Fioretti B, Catacuzzeno L, Pagani F, Bertollini C, Rosito M, Catalano M, D’Alessandro G, Santoro A, Cantore G, Ragozzino D, Castigli E, Franciolini F, Limatola C (2010) CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am J Physiol Cell Physiol 299:C175–C184
Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279:35679–35686
Shao Z, Gaurav R, Agrawal DK (2015) Intermediate-conductance calcium-activated potassium channel KCa3.1 and chloride channel modulate chemokine ligand (CCL19/CCL21)-induced migration of dendritic cells. Transl Res 166:89–102
Sheng J-Z, Braun AP (2007) Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Phys 293:C458–C467
Shi M, Chen D, Yang D, Liu X-Y (2015) CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer Res 34:149
Smit MA, Geiger TR, Song J-Y, Gitelman I, Peeper DS (2009) A twist-snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 29:3722–3737
Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA (2016) Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 6:630–649
Spindler V, Schlegel N, Waschke J (2010) Role of GTPases in control of microvascular permeability. Cardiovasc Res 87:243–253
Stegner D, Dütting S, Nieswandt B (2014) Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res 133(Suppl 2):S149–S157
Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O (2008) Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 9:1065–1073
Strell C, Entschladen F (2008) Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal 6:10
Strilic B, Offermanns S (2017) Intravascular survival and extravasation of tumor. Cancer Cell 32:282–293
Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC, Pasparakis M, Offermanns S (2016) Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536:215–218
Swartz MA, Kristensen CA, Melder RJ, Roberge S, Calautti E, Fukumura D, Jain RK (1999) Cells shed from tumours show reduced clonogenicity, resistance to apoptosis, and in vivo tumorigenicity. Br J Cancer 81:756–759
Tajbakhsh A, Rivandi M, Abedini S, Pasdar A, Sahebkar A (2019) Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): a review. Crit Rev Oncol Hematol 140:17–27
Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N (2013) Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One 8:e73609
Takemoto A, Okitaka M, Takagi S, Takami M, Sato S, Nishio M, Okumura S, Fujita N (2017) A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep 7:42186
Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DSB (2004) CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res 10:2351–2358
Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Deslisle C, Gratton J-P (2010) S-Nitrosylation of β-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell 39:468–476
Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB (2000) Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol 148:189–202
Tichet M, Prod’Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M, Ohanna M, Audebert S, Rocchi S, Giacchero D, Boukari F, Allegra M, Chambard J-C, Lacour J-P, Michiels J-F, Borg J-P, Deckert M, Tartare-Deckert S (2015) Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun 6:6993
Tikhmyanova N, Little JL, Golemis EA (2010) Cas proteins in normal and pathological cell growth control. Cell Mol Life Sci 67:1025–1048
Tremblay P-L, Huot J, Auger FA (2008) Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res 68:5167–5176
Tsang JYS, Lee MA, Ni Y-B, Chan S-K, Cheung S-Y, Chan W-W, Lau K-F, Tse GMK (2018) Amyloid precursor protein is associated with aggressive behavior in nonluminal breast cancers. Oncologist 23:1273–1281
Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, Lankry D, Mandelboim O (2012) MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res 72:5463–5472
Vahle A-K, Domikowksy B, Schwöppe C, Krähling H, Mally S, Schäfers M, Hermann S, Shahin V, Haier J, Schwab A, Stock C (2014) Extracellular matrix composition and interstitial pH modulate NHE1-mediated melanoma cell motility. Int J Oncol 44:78–90
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH-F, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156:1002–1016
Valls G, Codina M, Miller RK, Del Valle-Pérez B, Vinyoles M, Caelles C, McCrea PD, García de Herreros A, Duῆach M (2012) Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J Cell Sci 125:5288–5301
Vaquero J, Nguyen Ho-Bouldoires TH, Clapéron A, Fouassier L (2017) Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance. Oncogene 36:3067–3079
Wakabayashi S, Yoshida H (2013) The essential biology of the endoplasmic reticulum stress response for structural and computational biologists. Comput Struct Biotechnol J 6:e201303010
Walia V, Ding M, Kumar S, Nie D, Premkumar LS, Elble RC (2009) hCLCA2 is a p53-inducible inhibitor of breast cancer cell proliferation. Cancer Res 69:6624–6632
Walia V, Yu Y, Cao D, Sun M, McLean JR, Hollier BG, Cheng J, Mani SA, Rao K, Premkumar L, Elble RC (2012) Loss of breast epithelial marker hCLCA2 promotes epithelial-to-mesenchymal transition and indicates higher risk of metastasis. Oncogene 31:2237–2246
Wang B, Shi L, Sun X, Wang L, Wang X, Chen C (2016) Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J Cell Mol Med 20:920–929
Wang H, Pan J, Barsky L, Jacob JC, Zheng Y, Gao C, Wang S, Zhu W, Sun H, Lu L, Jia H, Zhao Y, Bruns C, Vago R, Dong Q, Qin L (2021) Characteristics of pre-metastatic niche: the landscape of molecular and cellular pathways. Mol Biomed 2:3
Weidle UH, Birzele F, Kollmorgen G, Rüger R (2016) Dissection of the process of brain metastasis reveals targets and mechanisms for molecular-based intervention. Cancer Genom Proteom 13:245–258
Weitzenfeld P, Kossover O, Körner C, Meshel T, Wiemann S, Seliktar D, Legler DF, Ben-Baruch A (2016) Chemokine axes in breast cancer: factors of the tumor microenvironment rehape the CCR7-driven metastatic spread of luminal-a breast tumors. J Leukoc Biol 99:1009–1025
Wettschureck N, Strilic B, Offermanns S (2019) Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev 99:1467–1525
Winpenny JP, Marsey LL, Sexton DW (2009) The CLCA gene family: putative therapeutic target for respiratory diseases. Inflamm allergy – drug. Targets 8:146–160
Xie H, Zhang Q, Zhou H, Zhou J, Zhang J, Jiang Y, Wang J, Meng X, Zeng L, Jiang X (2018) microRNA-889 is downregulated by histone deacetylase inhibitors and confers resistance to natural killer cytotoxicity in hepatocellular carcinoma cells. Cytotechnology 70:513–521
Xiong Y, Huang F, Li X, Chen Z, Feng D, Jiang H, Chen W, Zhang X (2017) CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. Int J Oncol 51:75–90
Yang CC, Ogawa H, Dwinell MB, McCole DF, Eckmann L, Kagnoff MF (2005) Chemokine receptor CCR6 transduces signals that activate p130Cas and alter cAMP-stimulated ion transport in human intestinal epithelial cells. Am J Physiol Cell Physiol 288:C321–C328
Yang J, Wang S, Zhao G, Sun B (2011) Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: clinical and experimental study. J Exp Clin Cancer Res 30:51
Yang X, Kuang S, Wang L, Wei Y (2018) MHC class I chain-related a: polymorphism, regulation and therapeutic value in cancer. Biomed Pharmacother 103:111–117
Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, Ulman MJ, Xega K, Contino G, Alagesan B, Brannigan BW, Milos PM, Ryan DP, Sequist LV, Bardeesy N, Ramaswamy S, Toner M, Maheswaran S, Haber DA (2012) RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 487:510–513
Yurtsever Z, Sala-Rabanal M, Randolph DT, Scheaffer SM, Roswit WT, Alevy YG, Patel AC, Heier RF, Romero AG, Nichols CG, Holtzman MJ, Brett TJ (2012) Self-cleavage of human CLCA1 protein by a novel internal metalloprotease domain controls calcium-activated chloride channel activation. J Biol Chem 287:42138–42149
Zahir N, Lakins JN, Russell A, Ming WY, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM (2003) Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J Cell Biol 163:1397–1407
Zhang C, Niu J, Zhang J, Wang Y, Zhou Z, Zhang J, Tian Z (2008) Opposing effects of interferon-alpha and interferon-gamma on the expression of major histocompatibility complex class I chain-related a in tumors. Cancer Sci 99:1279–1286
Zhang X, Rao A, Sette P, Deibert C, Pomerantz A, Kim WJ, Kohanbash G, Chang Y, Park Y, Engh J, Choi J, Chan T, Okada H, Lotze M, Grandi P, Amankulor N (2016a) IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro-Oncology 18:1402–1412
Zhang L, Wang D, Li Y, Liu Y, Xie X, Wu Y, Zhou Y, Ren J, Zhang J, Zhu H, Su Z (2016b) CCL21/CCR7 axis contributed to CD133+ pancreatic cancer stem-like cell metastasis via EMT and Erk/NF-kappaB pathway. PLoS One 11:e0158529
Zhong G, Chen L, Yin R, Qu Y, Bao Y, Xiao Q, Zhang Z, Shen Y, Li C, Xu Y (2017) Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol Med Rep 15:4100–4108
Zlotnik A, Burkhardt AM, Homey B (2011) Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11:597–606
Zou M, Dong H, Meng X, Cai C, Li C, Cai S, Xue Y (2015) Store-operated Ca2+ entry plays a role in HMGB1-induced vascular endothelial cell hyperpermeability. PLoS One 10:e0123432
Zuccolo E, Di Buduo C, Lodola F, Orecchioni S, Scarpellino G, Kheder DA, Poletto V, Guerra G, Bertolini F, Balduini A, Rosti V, Moccia F (2018) Stromal cell-derived factor-1α promotes endothelial colony-forming cell migration through the Ca2+-dependent activation of the extracellular signal-regulated kinase 1/ 2 and phosphoinositide 3-kinase/AKT pathways. Stem Cells Dev 27:23–34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stock, C. (2021). Circulating Tumor Cells: Does Ion Transport Contribute to Intravascular Survival, Adhesion, Extravasation, and Metastatic Organotropism?. In: Stock, C., Pardo, L.A. (eds) From Malignant Transformation to Metastasis. Reviews of Physiology, Biochemistry and Pharmacology, vol 182. Springer, Cham. https://doi.org/10.1007/112_2021_68
Download citation
DOI: https://doi.org/10.1007/112_2021_68
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99799-1
Online ISBN: 978-3-030-99800-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)