Abstract
Tumor cell metastasis through blood circulation is a complex process and is one of the great challenges in cancer research as metastatic spread is responsible for ∼90% of cancer-related mortality. Tumor cell intravasation into, arrest and adhesion at, and extravasation from the microvessel walls are critical steps in metastatic spread. Understanding these steps may lead to new therapeutic concepts for tumor metastasis. Vascular endothelium forming the microvessel wall and the glycocalyx layer at its surface are the principal barriers to and regulators of the material exchange between circulating blood and body tissues. The cleft between adjacent endothelial cells is the principal pathway for water and solute transport through the microvessel wall in health. Recently, this cleft has been found to be the location for tumor cell adhesion and extravasation. The blood-flow-induced hydrodynamic factors such as shear rates and stresses, shear rate and stress gradients, as well as vorticities, especially at the branches and turns of microvasculatures, also play important roles in tumor cell arrest and adhesion. This chapter therefore reports the current advances from in vivo animal studies and in vitro culture cell studies to demonstrate how the endothelial integrity or microvascular permeability, hydrodynamic factors, microvascular geometry, cell adhesion molecules, and surrounding extracellular matrix affect critical steps of tumor metastasis in the microcirculation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Cancer is the second killer next to the heart disease worldwide (CDC 2015). The cause of death is usually organ failure caused by metastatic tumors that are derived from the primary tumor. Although the metastasis process is low efficiency, only 0.01% disseminated tumor cells would successfully survive and proliferate at new physiology environment (Weber 2007), this process responsible for >90% of cancer-related deaths (Gupta and Massague 2006). Recognized as the detachment and dissemination of the malignant tumor cells from the primary tumor and the development of secondary tumors at distant organs, tumor metastasis is a very complex multistep process; sequentially, it includes local invasion of tissue where tumor first starts, detachment from the primary tumor, intravasation, survival in the circulation and dissemination, arrest and adhesion, extravagation, proliferation, and angiogenesis (Chambers et al. 2002; Fidler 2011; Talmadge and Fidler 2010; Bacac and Stamenkovic 2008). Tumor metastasis can be through blood circulatory system or lymphatic system. The detached tumor cells can directly enter into the circulating blood and go to distant organs (Wyckoff et al. 2000), or they can enter the blood circulatory system indirectly via the lymphatic system (Chambers et al. 2002). The circulating tumor cells could spread from lymph nodes to distant organs via blood vessels associated with the nodes or by entering the venous system via the major lymphatic ducts (e.g., the thoracic duct) (Achen and Stacker 2008; Tobler and Detmar 2006). The preferential metastatic organs for breast cancers are the lungs, liver, brain, and bone (Kang et al. 2003; Minn et al. 2005; Berman et al. 2013). Tumor cells that initiate a metastatic colony must go through the following steps: (1) detach from the primary tumor colony, (2) invade the local host tissue, (3) penetrate into blood vessels or lymphatic vessels, (4) survive within the circulation, (5) arrest or adhere in the microvessels of distant organs, (6) extravasate from the blood stream, (7) adapt to the newly colonized milieu in new foreign environment, and (8) divide to form the new tumor (Bacac and Stamenkovic 2008; Nguyen et al. 2009).
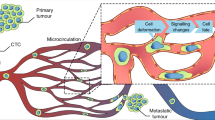
At the microscopic levels, angiogenesis from the primary tumor, tumor cell intravasation through leaky angiogenic microvessels, arrest and adhesion in the microcirculation, interaction with the microvessel walls to increase microvascular permeability, extravasation to the secondary organ, and survival and proliferation in foreign organs are critical steps for tumor growth and metastasis (Fig. 1). Although various anti-angiogenic therapies have been postulated to inhibit tumor growth and intravasation since 1971, strategies targeting at tumor cell arrest and adhesion and targeting at microvessel integrity have not been well developed (Folkman 1971). In addition, while considerable progress has been made in understanding the mechanism of microvascular integrity compromise induced by tumor angiogenic factors, such as vascular endothelial growth factor (VEGF), our understanding of the microvascular integrity and its role in tumor metastasis is poor. Furthermore, previous studies suggested that mechanical factors might interact with the biochemical factors for tumor metastasis (Chambers et al. 2002), but how the localized hydrodynamic factors induced by the microcirculation affect tumor cell arrest, adhesion and accumulation have not been systematically studied. This chapter therefore reports the current advances from in vivo animal studies and in vitro culture cell studies to demonstrate how the endothelial integrity or microvascular permeability, hydrodynamic factors, microvascular geometry, cell adhesion molecules, and surrounding extracellular matrix affect critical steps of tumor metastasis in the microcirculation.
Major steps for tumor metastasis in microvasculature. Tumor cells first get into the microcirculation near the primary tumor by intravasation across the microvessel wall. Then they arrest and adhere to the microvessel walls in a distant organ, where they degrade the microvessel wall integrity such as endothelial surface glycocalyx and junctions between adjacent endothelial cells forming the microvessel wall. Finally, they extravasate into the distant organ through the leaky microvessel wall (courtesy of Dr. Jie Fan)
2 Microvascular Integrity and Tumor Metastasis
Microvascular bed is the primary location where water and nutrients are exchanged between circulating blood and body tissues. Microvessel walls consist mainly of endothelial cells. Under normal conditions, the cleft between endothelial cells (inter-endothelial cleft) is widely believed to be the principal pathway for water and hydrophilic solute (such as glucose, amino acids, vitamins, hormones) transport across the capillary wall (Michel and Curry 1999; Michel and Neal 1999). Electron, confocal, and multiphoton microscopy studies indicate that there are junctional strands with discontinuous leakages (Roberts and Palade 1995; Bundgaard 1984) and fiber matrix components, so-called endothelial surface glycocalyx (ESG) (Luft 1966; Adamson and Clough 1992; Salmon and Satchell 2012; Arkill et al. 2011; van den Berg et al. 2003; Reitsma et al. 2007; Ebong et al. 2011; Yen et al. 2012; Betteridge etal. 2017) at the endothelial surface. The transport of proteins or other macromolecules was thought to be through vesicle shuttle mechanisms (Michel and Curry 1999). Microvascular permeability is a quantitative measure of how permeable the microvessel wall is to all kinds of substances including water and solutes with a variety of sizes. Under healthy conditions, the microvessel wall maintains a normal permeability to water and small solutes for the material exchange during our body’s metabolic processes. While in disease, the integrity of the vessel wall structure can be destroyed, and much larger particles such as proteins, leukocytes, and tumor cells can transfer through the wall. It is the transvascular pathways at the vessel wall and their structural barriers that determine and regulate the microvascular permeability. Therefore, one can quantify the microvascular permeability to determine the microvessel integrity.
2.1 Microvascular Permeability and Permeability Coefficients
The endothelial cells lining microvessel walls provide the rate-limiting barrier to extravasation of plasma components of all sizes from electrolytes to proteins. So far, there are three primary pathways observed in the wall of a continuous microvessel by using electron microscopy: intercellular clefts, transcellular pores, and vesicles. Microvessels of different types and in different tissues may have different primary transvascular pathways. Under different physiological and pathological conditions, the primary pathway can be changed for the same microvessel (Michel and Curry 1999). The cleft between adjacent endothelial cells is widely believed to be the principal pathway for water and hydrophilic solute transport through the microvessel wall under normal physiological conditions. The junction strands with discontinuous leakages in the cleft and ESG maintain the normal microvessel permeability to water and solutes. Changes in permeability are caused by the changes in these structural components. The molecular basis for the passage of molecules at the level of the breaks in tight junctions is more likely to be the localized absence of cell-cell contacts with corresponding loss of a closely regulated molecular sieve as suggested by Fu et al. (1994) and Michel and Curry (1999). Thus the junction break-surface matrix model suggests independent mechanisms to regulate the permeability properties of the microvessel wall. The junction break size and frequency are likely to involve regulation of cell-cell attachment via occludins and other junction proteins including the cadherin-associated junctions (Fu et al. 1994, 2003, 2006; Fu and Shen 2004). On the other hand, the regulation of glycocalyx density and organization is likely to involve interaction of the molecules forming the cell surface with the cytoskeleton and with circulating plasma proteins. Some of the cellular mechanisms underlying these interactions are reviewed in Squire et al. (2001), Tarbell and Pahakis (2006), and Fu and Tarbell (2013). Under physiological and pathological conditions, microvessel permeability can be regulated acutely and chronically by mechanisms that are underway to be understood.
Serial section electron microscopy study on frog and rat mesenteric capillaries by Adamson etal. (2004) demonstrated that the junction strand was interrupted by infrequent breaks that, on average, were 150 nm long, spaced 2–4 μm apart along the strand, and accounted for up to 10% of the length of the strand under control conditions. At these breaks, the space between adjacent endothelial cells (average ∼20 nm) was as wide as that in regions of the cleft between adjacent cells with no strands. The luminal surfaces of endothelial cells (ECs) lining vasculature are coated with a glycocalyx of membrane-bound macromolecules comprised of sulfated proteoglycans, hyaluronic acid, sialic acids, glycoproteins, and plasma proteins that adhere to this surface matrix (Tarbell and Pahakis 2006; Fu and Tarbell 2013). The thickness of this endothelial surface glycocalyx (ESG) was observed from less than 100 nm to ∼1 μm on the microvessels in different tissues and species by using different preparations and observing methods (Luft 1966; Reitsma et al. 2007; Salmon et al. 2009; Salmon and Satchell 2012; Squire et al. 2001; van den Berg et al. 2003; van Hinsbergh and Nieuw Amerongen 2002; Vink and Duling 1996; Yen et al. 2012). Although the ESG thickness varies, its density and organization were hypothesized to be the same among different tissues and species because of its function as a molecular sieve to macromolecules such as serum albumin. The glycocalyx fiber radius is thus proposed to ∼6 nm, and gap spacing between fibers is proposed to be ∼8 nm (Squire et al. 2001). More details about the ESG are presented in Chaps. 1, 2, and 3.
Aforementioned ultrastructural study using electron microscopy and other methods shows that the microvessel wall behaves as a passive membrane for water and hydrophilic solute transport (Michel and Curry 1999). The membrane transport properties are often described by Kedem-Katchalsky equations derived from the theory of irreversible thermodynamics:
where Js and Jv are the solute and total volumetric fluxes; ΔC and Δp are the concentration and pressure difference across the membrane. Lp, the hydraulic conductivity, describes the membrane permeability to water. P, the diffusive permeability, describes the permeability to solutes. σf is the solvent drag or ultrafiltration coefficient that describes the retardation of solutes due to membrane restriction, and σd, the reflection coefficient, describes the selectivity of membrane to solutes. In many transport processes, σf is equal to σd, and thus we often use σ, the reflection coefficient, to represent both of them. R is the universal gas constant and T is the absolute temperature.
2.2 Permeability Measurement
All of the permeability measurements have been interpreted in terms of Lp, P, and σ, which are measured experimentally on intact whole organisms (including human subjects), on perfused tissues and organs, on single perfused microvessels, and on monolayers of cultured microvascular endothelial cells. Different experimental preparations have their advantages and disadvantages. Although measurements made on the intact regional circulation of an animal subject (usually using radioactive isotope-labeled tracers) suffer from uncertainties surrounding the exchange surface area of the microvessel wall and the values of the transvascular differences in pressure and concentration, they usually involve minimal interference with the microvessels themselves. These studies can provide valuable information concerning microvascular exchange under basal conditions. At the other end are measurements on single perfused vessels. The Landis technique has been used to measure the hydraulic conductivity Lp and reflection coefficient σ. Quantitative fluorescence microscope photometry and imaging microscopy are used to measure solute diffusive permeability P. Both of these techniques are described in detail in Michel and Curry (1999), Fu et al. (2003), and Cai et al. (2012). The surface area of the microvessel can be measured directly, as also can the difference in pressure and concentration across the vessel walls. The disadvantages of the single vessel preparation are (1) that they have direct interference with the vessels involved and (2) that they are usually restricted to a small number of convenient vessel types (e.g., mesenteric vessels on a two-dimensional translucent tissue). However, recent development of multiphoton microscopy enables the noninvasive determination of the cerebral microvascular solute permeability in rat brain (Shi et al. 2014).
Although the rapid growth of endothelial cell biology is largely a result of experiments on cultured endothelial cells in vitro (in dishes), there are limitations to the use of monolayers of cultured endothelial cells for gaining direct information about vascular permeability. In general, the in vitro permeability to albumin is 2–10 times larger than that from the in vivo (in live animals) measurement. Although the monolayers of cultured endothelial cells do not completely reflect the permeability characteristics of microvascular endothelium in vivo, they are the most accessible and convenient models for studying the molecular mechanisms by which the microvascular permeability is regulated. The techniques for measuring endothelial monolayer permeability to water and solutes were described in Lee et al. (2003), Cancel et al. (2007), Li et al. (2010), and Fan and Fu (2016).
2.3 Microvascular Hyperpermeability Increases Tumor Cell Adhesion
Microvascular hyperpermeability due to compromised microvessel wall integrity by inflammatory agents and cytokines is one factor that increases tumor cell adhesion to the microvessel endothelium. Vascular endothelial growth factors (VEGFs) are a family of cytokines that act to increase the delivery of nutrients to tissue by three distinct mechanisms: (a) endothelial cell growth, migration, and new blood vessel formation (angiogenesis); (b) increased blood flow (by vasodilatation); and (c) increased vascular permeability to water and solutes (Dvorak et al. 1995; Bates et al. 2001; Feng et al. 1999; Fu and Shen 2004). Combining in vivo permeability measurement and a mathematical model for the inter-endothelial transport, Fu and Shen (2003) predicted that acute effects of VEGF on microvascular integrity are widened gap opening of the inter-endothelial cleft and partial degradation of the ESG. Longer-term effects of VEGF include formation of gaps between adjacent endothelial cells in venular microvessels (Roberts and Palade 1995), vesiculovascuolar organelle pathways (Feng et al. 1999), transcellular pores, and fenestra (Dvorak et al. 1995; Drenckhahn and Ness 1997). Fu et al. (2006) also found that the VEGF-induced microvascular hyperpermeability can be abolished by enhancing intracellular levels of adenosine 3′,5′-cyclic monophosphate (cAMP), which strengthens the microvessel integrity by increasing the number of junction strands in the cleft between endothelial cells forming the microvessel wall.
Previous studies have found that many cancer cells express VEGF to a high degree (Lee et al. 2003), while the microvascular endothelium has abundant VEGF receptors including VEGFR2 (KDR/Flk-1) (Mukhopadhyay et al. 1998). VEGFR2 has been implicated in normal and pathological vascular endothelial cell biology (Olsson et al. 2006). Recently, it has been shown that ectopic administration of VEGF enhances the adhesion and transmigration of human breast cancer MDA-MB-231 cells across a monolayer of human or mouse brain microvascular endothelial cells under a static condition in vitro (Lee et al. 2003; Fan et al. 2011; Fan and Fu 2016). In addition, VEGF enhances the adhesion of malignant MDA-MB-435 s cells and ErbB2-transformed mouse mammary carcinomas to intact rat mesenteric microvessels under flow in vivo (Shen et al. 2010).
2.4 Integrin Signaling, Cell Adhesion Molecules, and Tumor Metastasis
Although the non-specific trapping due to the friction between the tumor cells and the narrow part of microvasculature is found to be responsible for the initial tumor cell arrest (Gassmann et al. 2010; Glinskii et al. 2005; Kienast et al. 2010; Mook et al. 2003), the cell adhesion molecules are required for the adhesion in larger microvessels and transmigration (Brenner et al. 1995; Fan et al. 2011; Gassmann et al. 2010; Giancotti 2007; Giavazzi et al. 1993; Hood and Cheresh 2002; Lee et al. 2003; Liang et al. 2005; Shen et al. 2010; Schluter et al. 2006; Slattery et al. 2005; Steeg and Theodorescu 2008). The integrins are a family of signaling and cell adhesion receptors, which attach cells to the extracellular matrix (ECM) and in some cases to other cells, and cooperate with growth factor and cytokine receptors to regulate cell behavior. Signals elicited by integrins enable tumor cells to survive, proliferate, migrate independently of positional constrains (Guo and Giancotti 2004), and adhere (Fan et al. 2011). The α6β4 integrin is a laminin-5 receptor and was originally described as a “tumor-specific” protein, because of its apparent upregulation in multiple metastatic tumor types (Giancotti 2007). The β4 integrin is unique among integrins because the cytoplasmic portion of the β4 subunit is 1017 amino-acid-long and possesses distinctive adhesive and signaling functions (Giancotti 2007). Upon binding of the ectodomain of β4 to the basement membrane protein laminin-5, the cytoplasmic portion of β4 interacts with the keratin cytoskeleton to promote the assembly of hemidesmosomal adhesions (Litjens et al. 2006). In addition, β4 activates intracellular signaling autonomously as well as by associating with multiple receptor tyrosine kinases (RTKs), including the EGFR, ErbB2, Met, and Ron (Guo and Giancotti 2004; Moasser et al. 2001). Deletion of the β4 signaling domain delayed mammary tumor onset and inhibited primary tumor growth. The tumors arising in mutant mice were significantly more differentiated histologically as compared to control tumors. In addition, primary tumor cells expressing signaling-defective β4 displayed a reduced proliferative rate and invasive ability and underwent apoptosis when deprived of matrix adhesion. Finally, upon injection in the tail vein of nude mice, the mammary tumor cells expressing mutant β4 exhibited reduced ability to metastasize to the lung (Guo et al. 2006).
Recently, Fan et al. (2011) examined the adhesion of ErbB2-transformed mammary tumor cells to mouse brain microvascular endothelial monolayer. They found that integrin β4 signaling does not exert a direct effect on adhesion to the endothelium or the underlying basement membrane. Rather, it enhances ErbB2-dependent expression of VEGF by tumor cells. VEGF in turn partially disrupts the tight and adherens junctions that maintain the adhesion between endothelial cells, enabling tumor cells to intercalate between endothelial cells and extend projections reaching the underlying exposed basement membrane and enabling the adhesion between cell adhesion molecules (e.g., integrins) and ECM proteins (e.g., laminins).
In addition to blocking the cell adhesion molecules at the surface of tumor and endothelial cells or in the ECM, e.g., integrins, ICAM-1, P-selectin, junctional adhesion molecules, and laminins, Shen et al. (2010) measured cancer cell adhesion after pretreatment of tumor cells with the antibody blocking VEGF and pretreatment of the microvessel with VEGF receptor (KDR/Flk-1) inhibitor, SU1498. They found that anti-VEGF and SU1498 almost completely abolished the adhesion of malignant MDA-MB-435 s to vascular endothelium in vivo. In an in vitro experiment, Fan et al. (2011) showed that although VEGF receptor inhibitor, SU1498 did not decrease the basal permeability of a microvascular endothelial monolayer, neither the tumor cell adhesion under the normal permeability conditions, it, however, abolished the microvascular hyperpermeability induced by VEGF as well as the increase in tumor cell adhesion.
2.5 Tumor Cell Adhesion and Transmigration Destroys Microvascular Integrity
Both in vitro and in vivo studies demonstrated that tumor cells prefer to adhere to and transmigrate from the endothelial junctions instead of cell bodies and VEGF or lipopolysaccharide (LPS, an inflammatory agent) increases the adhesion and transmigration (Fan et al. 2011; Fu et al. 2015; Fan and Fu 2016). Fan and Fu (2016) showed that 98% of the breast cancer MDA-MB-231 cells adhere at the endothelial junctions of bEnd3 (mouse brain microvascular endothelial cell) monolayer, of which, 63% at the tri-EC junctions. An in vivo study by Fu et al. (2015) also indicated that ∼90% of cancer cell adhesion occurs at the junctions of ECs in the microvessels under normal physiological flows (Fig. 2). In addition to disrupt the tight and adherens junctions between adjacent endothelial cells in vitro (Fan et al. 2011) and in vivo (Fu et al. 2015), tumor cell adhesion and transmigration degrades endothelial surface glycocalyx (ESG) (Fan and Fu 2016; Cai et al. 2012). A recent study by Cai et al. (2012) showed that tumor cell MDA-MB-231 adhesion to microvessel walls increases microvessel permeability by degrading the ESG (Fig. 3). Degradation of ESG exposed more cell adhesion molecules at the endothelium and underneath basement membrane for the tumor cell adhesion.
In vivo MDA-MB-231 breast cancer cell adhesion to rat mesenteric microvessels under physiological flows. Tumor cells prefer to adhere to the junctions between endothelial cells forming the microvessel wall under control and various treatments scale bar = 30 μm. VEGF increases tumor cell adhesion, while cAMP decreases the adhesion. cAMP can also abolish the increased adhesion by VEGF (from Fu et al. 2015, with permission)
Tumor cell adhesion degrades endothelial surface glycocalyx (ESG). FITC-anti-heparan sulfate-stained ESG (green) at a postcapillary venule under control (left column): midplane view (top) and cross-sectional view (bottom). Adhesion of cell tracker orange-labeled tumor cells (red) in a postcapillary venule at initial adhesion (mid-column) and after 45-min perfusion (right column) (from Cai et al. 2012, with permission)
In contrast, preservation of the ESG by a plasma glycoprotein orosomucoid by enhancing the charge and organization of the ESG decreases the microvessel permeability to albumin and reduces the tumor cell adhesion (Cai et al. 2012). Zhang et al. (2017) also showed that sphingosine-1-phosphate (S1P), a sphingolipid in plasma that plays a critical role in the cardiovascular and immune systems, preserves ESG of the microvessel wall and reduces tumor cell adhesion. Red blood cells (RBCs) are a major source of S1P in plasma, which acts continuously to maintain normal vascular permeability by protecting ESG (Zhang et al. 2016b). Alternatively, reinforcing endothelial junctions, e.g., by enhancing endothelial cAMP levels, can prevent microvessel permeability increase and reduce tumor cell adhesion (Fu et al. 2015) (Fig. 2).
3 Tumor Metastasis under Flow and in Microvasculature
In vitro static adhesion assays have been utilized to investigate tumor cell adhesion to endothelial cells (Earley and Plopper 2006; Lee et al. 2003) and to extracellular matrix (ECM) proteins (Guo et al. 2006). Tumor cell adhesion has also been investigated using flow chambers (Chotard-Ghodsnia et al. 2007; Giavazzi et al. 1993; Slattery et al. 2005) or artificial blood vessels (Brenner et al. 1995) to address flow effects. Direct injection of tumor cells into the circulation has enabled the observation of tumor cell metastasis in target organs after sacrificing the animals (Schluter et al. 2006), while intravital microscopy has been used to observe the interactions between circulating tumor cells and the microvasculature both in vivo and ex vivo (Gassmann et al. 2010; Glinskii et al. 2005; Guo et al. 2014; Kienast et al. 2010; Mook et al. 2003; Shen et al. 2010; Steinbauer et al. 2003; Yan et al. 2012).
3.1 Tumor Cell Adhesion in In Vitro Flow Chambers
Tumor cell extravasation is a dynamic process in which tumor cell adhesion to the vascular endothelium and transendothelial migration occur under flow conditions. Therefore, the geometry of microvasculature and the local hydrodynamic factors, along with the cell adhesion molecules at the tumor cell and endothelial cell should play a crucial role in tumor cell adhesion and extravasation. Tumor cells are exposed to flow while (a) circulating from the primary tumor, (b) arresting on downstream vascular endothelium, and (c) transmigrating into the secondary target organ. Investigations of the role of shear flow in tumor cell adhesion and extravasation should contribute to the understanding of the complex process of tumor metastasis. Tumor cell extravasation would normally occur in the microvasculature where shear forces are relatively low (like in postcapillary venules) although of sufficient magnitudes to activate cell surface receptors and alter vascular cell function. During tumor cell extravasation, there are significant changes in the structure and function of both tumor and endothelial cells. For example, a significant rearrangement of the cell cytoskeleton is required in both the tumor cells during migration (Lauffenburger and Horwitz 1996) and in the endothelial cells as the barrier function is altered (van Hinsbergh and Nieuw Amerongen 2002). The extravasation of tumor cells also induces endothelial cell remodeling (Kienast et al. 2010).
In an in vitro flow chamber study, Slattery et al. (2005) found that the shear rate, rather than the shear stress, plays a more significant role in PMN (polymorphonuclear neutrophils)-facilitated melanoma adhesion and extravasation. β2 integrins/ICAM-1 adhesion mechanisms were examined, and the results indicate LFA (lymphocyte function-associated)-1 and Mac-1 (CD11b/CD18) cooperate to mediate the PMN-EC (endothelial cell)-melanoma interactions under shear conditions. In addition, endogenously produced IL-8 contributes to PMN-facilitated melanoma arrest on the EC through the CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2) on PMN (Liang et al. 2005; Slattery et al. 2005).
3.2 Effects of Hydrodynamic Factors on Tumor Metastasis
In addition to biochemical factors such as cell adhesion molecules (CAMs) and chemical/cytokines, circulating tumor cell adhesion in microvessel walls is affected by hydrodynamic factors, such as blood flow patterns, and flow-induced shear stresses and shear stress gradients (Bacac and Stamenkovic 2008; Wirtz and Searson 2012; Weiss 1992; Strell and Entschladen 2008; Mierke 2008). Numerous studies using in vivo and in vitro models have found that flow-induced hydrodynamic factors activate endothelial cells lining the blood vessel wall to generate reactive oxygen species, nitric oxide, and growth factors (Matsumoto et al. 2007; Bucci et al. 2005; Chiu et al. 2003; Zhang et al. 2016a, b). These substances can up- or downregulate the expression of endothelial CAMs and endothelial nitric oxide synthase (eNOS) depending on the strength of the mechanical factors, the flow patterns (e.g., laminar or turbulent), and the geometry of the vessel (e.g., straight or curved/branched) (Liu et al. 2008; Yan et al. 2010, 2012; Guo et al. 2014).
To investigate tumor cell adhesion in a well-controlled in vivo system, Shen et al. (2010) and Yan et al. (2012) used intravital video microscopy to measure the adhesion rate of malignant MDA-MB-435 s and 231 cells in straight and curved postcapillary venules on rat mesentery. A straight or curved microvessel was cannulated and perfused with tumor cells by a glass micropipette at a velocity of ∼1 mm/s, which is the mean normal blood flow velocity in this type of vessels. At less than 10 min after perfusion, there was a significant difference in cell adhesion to the straight and curved vessel walls. In 60 min, the averaged adhesion rate in the curved vessels was ∼1.5-fold of that in the straight vessels. In 51 curved segments, 45% of cell adhesion was initiated at the inner side, 25% at outer side, and 30% at both sides of the curved vessels. To investigate the mechanical mechanism by which tumor cells prefer adhering at curved sites, Yan et al. (2012) performed a computational study, in which the fluid dynamics was carried out by the lattice Boltzmann method (LBM), and the tumor cell dynamics was governed by the Newton’s law of translation and rotation. The details of this multi-scale modeling are summarized in Chap.~12.
By injecting the tumor cells directly into the systemic circulation via carotid artery, Guo et al. (2014) found that MDA-MB-231 cancer cells prefer to adhere at the branched portion of the microvasculature rather than the non-branched portion. By numerical simulation, they also showed that there are higher shear rates/stresses and higher vorticities at the branching location where there are more arrested tumor cells (Fig. 4). Liu et al. (2008) demonstrated that thrombosis is also initiated at the curved portion of a vessel where there is a higher shear stress/rate and higher shear stress/rate gradient at the vessel wall. Furthermore, Shen et al. (2010) found that tumor cells adhered more in postcapillary venules under a normal blood flow velocity than under a reduced velocity. From these studies, it is likely that tumor cell adhesion sites are associated with localized hydrodynamic factors such as shear stresses and shear stress gradients. A recent study by Yen et al. (2015) showed that blood flow regulates nitric oxide (NO) production in rat mesenteric microvessels. The higher the flow velocity, the higher the endothelial NO production in postcapillary venules.
Vorticity profiles in microvessels at intersections. Seven panels in the left column are arteriole-capillary intersections, and those in the right column are side branch vessel-postcapillary venule intersections. The top two Aa and Av are vorticity contour plots in the midplane of the vessels; Ba-Da and Bv-Dv are enlarged plots near the intersectional regions when branching angle θ is 60°, 90°, and 120°, respectively. Ea-Ga and Ev-Gv are detailed vorticity profiles at walls along dotted lines a–b (red) and c–d (blue) in Ba-Da and Bv-Dv. Arrows are flow directions in the microvessels. It was found that tumor cells prefer to arrest to the inner corners of the turns where there are higher vorticities (from Guo et al. 2014, with permission)
NO is the smallest signaling molecule that regulates a variety of important physiological functions (Forstermann and Sessa 2012). eNOS is responsible for most of the vascular NO production (Cooke et al. 1990). In cancer biology, NO can promote or inhibit tumor growth and metastasis, depending on its concentrations (Ridnour et al. 2006; Xu et al. 2002). Elevated NO and eNOS have been observed in cancer patients in malignancy states (Masri et al. 2005). Although high concentrations of NO are cytotoxic to the circulating tumor cells (Li et al. 1991; Mortensen et al. 2004; Pohl et al. 1991; Qiu et al. 2003; Wang et al. 2000), low levels of NO promote tumor cell arrest and adhesion (Scher 2007; Xu et al. 2002; Yudoh et al. 1997). NO at some optimal levels can inhibit cancer cell adhesion to cytokine-stimulated endothelial cells (Lu et al. 2014; Xu et al. 2002), decrease tumor cell adhesion to naive and lipopolysaccharide (LPS)-treated postcapillary venules (Kong et al. 1996), and reduce invasion ability of cancer cells (Kielbik et al. 2014). Most recently, Zhang et al. (2016a, b) found that under normal physiological flow conditions, tumor cells prefer to adhere to the microvessel locations with a higher NO production such as curved portions. Inhibition of eNOS by NG-monomethyl-L-arginine (L-NMMA) attenuated the flow-induced NO production and reduced tumor cell adhesion. They also found that L-NMMA treatment for ∼40 min reduced microvessel permeability to albumin. Therefore, their results suggest that inhibition of eNOS is a good approach to preventing tumor cell adhesion to intact microvessels under physiological flows.
In addition to the flow-induced hydrodynamic factors in the microvasculature, the mechanical properties such as rigidity of tumor cells determine their metastatic behaviors. Guck et al. (2005) and Koop et al. (1995) observed that tumor cells having a greater deformability are more invasive, malignant, and metastatic. Malignant (MCF-7) breast cells were found to have an apparent Young’s modulus significantly lower (1.4–1.8 times) than that of their nonmalignant (MCF-10A) counterparts (Li et al. 2008). More recently, Swaminathan et al. (2011) found that
cancer cells with the highest migratory and invasive potential are five times less stiff than cells with the lowest migration and invasion potential. However, more rigid tumor cells may also be trapped in narrow capillaries. Most recently, Guo et al. (2014) injected fluorescently labeled human breast carcinoma cells or similarly sized rigid beads into the systemic circulation of a rat. Their arrest patterns in the microvasculature of mesentery were recorded and quantified. They found that ∼93% of rigid beads were arrested either at arteriole-capillary intersections or in capillaries. Only ∼3% were at the capillary-postcapillary venule intersections and in postcapillary venules. In contrast, most of the flexible tumor cells were either entrapped in capillaries or arrested at capillary or postcapillary venule-postcapillary venule intersections and in postcapillary venules. Forty-two percent of the flexible tumor cells were able to escape the capillary trapping.
4 Investigation of Tumor Metastasis by Using Microfluidic Systems
Although above summarized animal models for investigating tumor metastasis in the microcirculation have provided insightful information for the metastasis mechanism, it is necessary to explore the human tumor metastasis in microvessels formed by human endothelial cells. Therefore, the 2D transwell system and 2D and 3D single channel microfluidic system have been developed and used for decades by many researchers in investigating tumor cell and leukocyte adhesion, invasion, and transmigration under static and flow conditions (Cinamon and Alon 2003; Earley and Plopper 2006; Fan et al. 2011; Fan and Fu 2016; Lee et al. 2003; Shea et al. 2017; Slattery et al. 2005; Zhang et al. 2016a, b). Many recent microfluidic systems with complicated patterns have been developed to screen circulating tumor cells for diagnosis (Ferreira et al. 2016; Jiang et al. 2017; Khamenehfar and Li 2016; Khoo et al. 2018; Tadimety et al. 2017), to grow tumor cells with in and out flows for cancer drug screening (Chi et al. 2016; Dereli-Korkut et al. 2014) and to generate chemotaxis conditions for cancer cell migration (Liu et al. 2017; Um et al. 2017). Recently, an all-human 3D ex vivo hepatic microphysiological system was developed to mimic the stiffness of metastatic organ environment but without blood microvasculature (Clark et al. 2016). A 3D microfluidic system with simple blood microvasculature was developed but without real anatomical geometry (Kong et al. 2016). A sophisticated 3D microfluidic device (Fig. 5) with the generated microvasculature from human umbilical vein endothelial cells and human lung fibroblasts has been developed to quantify tumor cell intravasation and extravasation dynamics (Chen et al. 2013, 2017; Jeon et al. 2015; Zervantonakis et al. 2012).
Microfluidic tumor-vascular interface model. (a) Endothelial channel (green), tumor channel (red), and 3D extracellular matrix (ECM, dark gray) between the two channels. Channels are 500 μm wide, 20 mm in length, and 120 μm in height. Black arrow shows the y-junction. (Scale bar, 2 mm.) (b) Phase-contrast image showing the fibrosarcoma cells (HT1080, red) invading through the ECM (gray) toward the endothelium (microvascular endothelial cells, green). A single 3D ECM hydrogel matrix region is outlined with the white dashed square. (Scale bar, 300 μm.) (c) Confocal projection of a representative region of tumor (red) perfused microvascular network (green) at 20×. Representative cross-sectional views of a single transmigrating tumor cell (in dotted white box) and example of extravasation scoring (a, b from Zervantonakis et al. 2012 and c from Chen et al. 2017, with permission)
The recently developed microfluidic systems recreated the tumor-vascular interface in 3D. They not only enabled high-resolution, real-time imaging of tumor cell intravasation, arrest, adhesion, and extravasation and precise quantification of endothelial barrier function but also provided well-controlled tumor microenvironments mimicking various clinical conditions.
5 Summary and Future Study
Although transport across endothelium is a classical problem that has been investigated for more than several decades, the fundamental questions related to the structure-transport function of the microvessel wall and the interaction between the circulating cells and the cells forming the wall still remain unclear. With the help from mathematical models for more accurate interpretations and predictions, new techniques involving transgenic animals with fluorescent protein-expressed endothelial cells and circulating blood and tumor cells, new fluorescent dyes for labeling the structural components of transvascular pathways, new developments in super-resolution optical microscopy and cryo-electron microscopy, and new developments in molecular biology and biochemistry will lead to more fascinating discoveries in this field.
One problem that has not been investigated thoroughly is the spatio-chemical organization and biomechanical properties of the endothelial surface glycocalyx, which is the barrier between circulating cells and endothelial cells forming the microvessel wall in addition to the molecular sieve. Newly developed super-resolution optical microscopy such as STORM (stochastic optical reconstruction microscopy) and STED (stimulated emission depletion) and AFM (atomic force microscopy) can be employed for this purpose. Another problem is to create transvascular models for cells such as leukocytes and cancer cells. The current multi-scale cell adhesion model can be revised to include the mechanical properties and morphological changes of adherent and endothelial cells in developing the cell transmigration models. The transvascular cell transport is crucial in many physiological and pathological processes including inflammatory response and tumor metastasis.
References
Achen MG, Stacker SA (2008) Molecular control of lymphatic metastasis. Ann N Y Acad Sci 1131:225–234
Adamson RH, Clough G (1992) Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol 445:473–486
Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE (2004) Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 557:889–907
Arkill KP, Knupp C, Michel CC, Neal CR, Qvortrup K, Rostgaard J, Squire JM (2011) Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism? Biophys J 101:1046–1056
Bacac M, Stamenkovic I (2008) Metastatic cancer cell. Annu Rev Pathol 3:221–247
Bates DO, Heald RI, Curry FE, Williams B (2001) Vascular endothelial growth factor increases Rana vascular permeability and compliance by different signalling pathways. J Physiol 533(Pt. 1):263–272
Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N (2013) Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer 13:88–94
Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, Bates DO, Salmon AHJ (2017) Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol 595(15):5015–5035
Brenner W, Langer P, Oesch F, Edgell CJ, Wieser RJ (1995) Tumor cell-endothelium adhesion in an artificial venule. Anal Biochem 225:213–219
Bucci M, Roviezzo F, Posadas I, Yu J, Parente L (2005) Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci U S A 102:904–908
Bundgaard M (1984) The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy. J Ultmstruct Res 88:1–17
Cai B, Fan J, Zeng M, Zhang L, Fu BM (2012) Adhesion of malignant mammary tumor cell MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J of Appl Physiol 13(7):1141–1153
Cancel LM, Fitting A, Tarbell JM (2007) In vitro study of LDL transport under pressurized (convective) conditions. Am J Phys 293:H126–H132
CDC Report (2015) Number of deaths for leading causes of death
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Chen MB, Whisler JA, Jeon JS, Kamm RD (2013) Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol (Camb) 5:1262–1271
Chen MB, Whisler JA, Fröse J, Yu C, Shin Y, Kamm RD (2017) On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat Protoc 12(5):865–880
Chi CW, Rezwanuddin Ahmed AH, Dereli-Korkut Z, Wang S (2016) Microfluidic cell chips for high throughput drug screening. Bioanalysis 8(9):921–937
Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW (2003) Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101:2667–2674
Chotard-Ghodsnia R, Haddad O, Leyrat A, Drochon A, Verdier C, Duperray A (2007) Morphological analysis of tumor cell/endothelial cell interactions under shear flow. J Biomech 40:335–344
Cinamon G, Alon R (2003) A real time in vitro assay for studying leukocyte transendothelial migration under physiological flow conditions. J Immunol Methods 273:53–62
Clark AM, Wheeler SE, Young CL, Stockdale L, Shepard Neiman J, Zhao W, Stolz DB, Venkataramanan R, Lauffenburger D, Griffith L, Wells A (2016) A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip 17(1):156–168
Cooke JP, Stamler J, Andon N, Davies PF, McKinley G (1990) Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Phys 259:H804–H812
van den Berg BM, Vink H, Spaan JA (2003) The endothelial glycocalyx protects against myocardial edema. Circ Res 92:592–594
Dereli-Korkut Z, Akaydin D, Ahmed AHR, Jiang X, Wang S (2014) Three dimensional microfluidic cell arrays for ex vivo drug screening with mimicked vascular flow. Anal Chem 86(6):2997–3004
Drenckhahn D, Ness W (1997) The endothelial contractile cytoskeleton. In: Born GVR, Schwartz CJ (eds) Vascular endothelium: physiology, pathology and therapeutic opportunities. Schattauer, Stuttgart, Gennany, pp 1–15
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Earley S, Plopper GE (2006) Disruption of focal adhesion kinase slows transendothelial migration of AU-565 breast cancer cells. Biochem Biophys Res Commun 350:405–412
Ebong EE, Macaluso FP, Spray DC, Tarbell JM (2011) Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31(8): 1908–1915
Fan J, Fu BM (2016) Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Annals of Biomed Eng 44(7):2189–2201
Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM (2011) Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-medicated secretion of VEGF. Ann of Biomed Eng 39(8):2223–2241
Feng D, Nagy JA, Payne K, Hammel I, Dvorak HF, Dvorak AM (1999) Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation 6(1):23–44
Ferreira MM, Ramani VC, Jeffrey SS (2016) Circulating tumor cell technologies. Mol Oncol 10(3):374–394
Fidler IJ (2011) The biology of cancer metastasis. Semin Cancer Biol 21:71
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837
Fu BM, Shen S (2003) Structural mechanisms of vascular endothelial growth factor (VEGF) on microvessel permeability. Am J Phys 284(6):H2124–H2135
Fu BM, Shen S (2004) Acute VEGF effect on solution permeability of mammalian microvessels in vivo. Microvasc Res 68(1):51–62
Fu BM, Tarbell JM (2013) Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med 5:381–390
Fu BM, Weinbaum S, Tsay RY, Curry FE (1994) A junction-orifice-fiber entrance layer model for capillary permeability: application to frog mesenteric capillaries. ASME J Biomech Eng 116:502–513
Fu BM, Chen B, Chen W (2003) An electrodiffusion model for effects of surface glycocalyx layer on microvessel solute permeability. Am J Phys 284: H1240–H1250
Fu BM, Shen S, Chen B (2006) Structural mechanisms in the abolishment of VEGF-induced microvascular hyperpermeability by cAMP. ASME J. Biomech. Eng. 128(3):313–328
Fu BM, Yang J, Shen S, Cai B, Fan J, Zhang L, Yen WY, Zeng M (2015) Reinforcing endothelial junctions prevents microvessel permeability increase and tumor cell adhesion in microvessels in vivo. Scientific Reports Oct 28
Gassmann P, Kang ML, Mees ST, Haier J (2010) In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell-endothelial cell interaction. BMC Cancer 10(177)
Giancotti FG (2007) Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci 28:506–511
Giavazzi R, Foppolo M, Dossi R, Remuzzi A (1993) Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest 92:3038–3044
Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV (2005) Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 7(5):522–527
Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kas J, Ulvick S, Bilby C (2005) Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88(5):3689–3698
Guo W, Giancotti FG (2004) Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5:816–826
Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG (2006) Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126:489–502
Guo P, Cai B, Lei M, Liu Y, Fu BM (2014) Differential arrest and adhesion of tumor cells and microbeads in the microvasculature. Biomech Model Mechanobiol 13:537–550
Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127:679–695
van Hinsbergh VW, Nieuw Amerongen GP (2002) Intracellular signalling involved in modulating human endothelial barrier function. J Anat 200:549–560
Hood JD, Cheresh DA (2002) Role of integrins in cell invasion and migration. Nat Rev Cancer 2:91–100
Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD (2015) Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A 112(1):214–219
Jiang X, Wong KHK, Khankhel AH, Zeinali M, Reategui E, Phillips MJ, Luo X, Aceto N, Fachin F, Hoang AN, Kim W, Jensen AE, Sequist LV, Maheswaran S, Haber DA, Stott SL, Toner M (2017) Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 17(20):3498–3503
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549
Khamenehfar A, Li PC (2016) Microfluidic devices for circulating tumor cells isolation and subsequent analysis. Curr Pharm Biotechnol 17(9):810–821
Khoo BL, Grenci G, Lim YB, Lee SC, Han J, Lim CT (2018) Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat Protoc 13(1):34–58
Kielbik M, Szulc I, Brzezinska M, Bednarska K, Przygodzka P (2014) Nitric oxide donors reduce the invasion ability of ovarian cancer cells in vitro. Anti-Cancer Drugs 25:1141–1151
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkle F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nature Med 16(1):116–122
Kong L, Dunn GD, Keefer LK, Korthuis RJ (1996) Nitric oxide reduces tumor cell adhesion to isolated rat postcapillary venules. Clin Exp Metastasis 14: 335–343
Kong J, Luo Y, Jin D, An F, Zhang W, Liu L, Li J, Fang S, Li X, Yang X, Lin B, Liu T (2016) A novel microfluidic model can mimic organ-specific metastasis of circulating tumor cells. Oncotarget 7(48):78421–78432
Koop S, MacDonald IC, Luzzi K, Schmidt EE, Morris VL, Grattan M, Khokha R, Chambers AF, Groom AC (1995) Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res 55(12):2520–2523
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84: 359–369
Lee TH, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278: 5277–5284
Li LM, Kilbourn RG, Adams J, Fidler IJ (1991) Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res 51:2531–2535
Li QS, Lee GY, Ong CN, Lim CT (2008) AFM indentation study of breast cancer cells. Biochem Biophys Res Commun 374(4):13–609
Li G, Simon M, Shi Z, Cancel L, Tarbell JM, Morrison B, Fu BM (2010) Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery. Ann of Biomed Eng 38(8):2499–2511
Liang S, Slattery MJ, Dong C (2005) Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp Cell Res 310(2):282–292
Litjens SH, de Pereda JM, Sonnenberg A (2006) Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 16:376–383
Liu Q, Mirc D, Fu BM (2008) Mechanical mechanisms of thrombosis in intact bent microvessels of rat mesentery. J Biomech 41:2726–2734
Liu Z, Han X, Zhou Q, Chen R, Fruge S, Jo MC, Ma Y, Li Z, Yokoi K, Qin L (2017) Integrated microfluidic system for gene silencing and cell migration. Adv Biosyst 1(6)
Lu Y, Yu T, Liang H, Wang J, Xie J (2014) Nitric oxide inhibits hetero-adhesion of cancer cells to endothelial cells: restraining circulating tumor cells from initiating metastatic cascade. Sci Rep 4:4344
Luft JH (1966) Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 25(6):1773–1783
Masri FA, Comhair SA, Koeck T, Xu W, Janocha A (2005) Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med 172:597–605
Matsumoto K, Nishi K, Kikuchi M, Kadowaki D, Tokutomi Y (2007) Alpha1-acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol Pharm Bull 30:1226–1230
Michel CC, Curry FE (1999) Microvascular permeability. Physiol Reviews 79(3):703–761
Michel CC, Neal CR (1999) Openings through endothelial cells associated with increased microvascular permeability. Microcirculation 6(1):45–62
Mierke CT (2008) Role of the endothelium during tumor cell metastasis: is the endothelium a barrier or a promoter for cell invasion and metastasis. J Biophys 2008:183516
Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD et al (2005) Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 115:44–55
Moasser MM, Basso A, Averbuch SD, Rosen N (2001) The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res 61:7184–7188
Mook ORF, Marle J, Vreeling-Sindelarova H, Jongens R, Frederiks WM, Noorden CJK (2003) Visualisation of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology 38:295–304
Mortensen K, Christensen IJ, Nielsen HJ, Hansen U, Larsson LI (2004) High expression of endothelial cell nitric oxide synthase in peritumoral microvessels predicts increased disease-free survival in colorectal cancer. Cancer Lett 216:109–114
Mukhopadhyay D, Nagy JA, Manseau EJ, Dvorak HF (1998) Vascular permeability factor/vascular endothelial growth factor-mediated signaling in mouse mesentery vascular endothelium. Cancer Res 58(6): 1278–1284
Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–284
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7(5):359–371
Pohl U, Herlan K, Huang A, Bassenge E (1991) EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol 261:H2016–2023
Qiu H, Orr FW, Jensen D, Wang HH, McIntosh AR et al (2003) Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol 162:403–412
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflug Arch: Eur J Physiol 454:345–359
Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD (2006) The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal 8:1329–1337
Roberts WG, Palade GE (1995) Increased microvascular permeability and endothelial enestration induced by vascular endothelial growth factor. J Cell Sci 108:2369–2379
Salmon AH, Satchell SC (2012) Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 226(4):562–574
Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO (2009) Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res 83(1):24–33
Scher RL (2007) Role of nitric oxide in the development of distant metastasis from squamous cell carcinoma. Laryngoscope 117:199–209
Schluter K, Gassmann P, Enns A, Korb T, Hemping-Bovenkerk A, Holzen J, Haier J (2006) Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol 169:1064–1073
Shea DJ, Li YW, Stebe KJ, Konstantopoulos K (2017) E-selectin-mediated rolling facilitates pancreatic cancer cell adhesion to hyaluronic acid. FASEB J 31(11):5078–5086
Shen S, Fan J, Cai B, Lv Y, Zeng M, Hao Y, Giancotti F, Fu BM (2010) Vascular endothelial growth factor enhances mammary cancer cell adhesion to endothelium in vivo. J of Exp Physiology 95:369–379
Shi L, Zeng M, Sun Y, Fu BM (2014) Quantification of blood-brain barrier solute permeability and brain transport by multiphoton microscopy. J Biomech Eng 136:031005
Slattery MJ, Liang S, Dong C (2005) Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Phys 288:C831–C839
Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel CC (2001) Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol 136:239–255
Steeg PS, Theodorescu D (2008) Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol 5(4):206–219
Steinbauer M, Guba M, Cernaianu G, Köhl G, Cetto M, Kunz-Schugart LA, Gcissler EK, Falk W, Jauch KW (2003) GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term development studies in immunocompetent mice. Clin Exp Metastasis 20: 135–141
Strell C, Entschladen F (2008) Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal 6:10
Swaminathan V, Mythreye K, O’Brien ET, Berchuck A, Blobe GC, Superfine R (2011) Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res 71(15):5075–5080
Tadimety A, Syed A, Nie Y, Long CR, Kready KM, Zhang JX (2017) Liquid biopsy on chip: a paradigm shift towards the understanding of cancer metastasis. Integr Biol (Camb) 23 9(1):22–49
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Tarbell JM, Pahakis MY (2006) Mechanotransduction and the glycocalyx. J Intern Med 259:339–350
Tobler NE, Detmar M (2006) Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol 80:691–696
Um E, Oh JM, Granick S, Cho YK (2017) Cell migration in microengineered tumor environments. Lab Chip 17(24):4171–4185
Vink H, Duling BR (1996) Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79:581–589
Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N et al (2000) B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 60:5862–5869
Weber GF (2007) Molecular mechanisms of cancer. Springer, Netherlands
Weiss L (1992) Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 10:191–199
Wirtz DKK, Searson PC (2012) The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11:512
Wyckoff JB, Jones JG, Condeelis JS, Segall JE (2000) A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 60:2504–2511
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG (2002) The role of nitric oxide in cancer. Cell Res 12:311–320
Yan WW, Liu Y, Fu BM (2010) Effects of curvature and cell-cell interaction on cell adhesion in microvessels. Biomech Model Mechanobiol 9:629–640
Yan WW, Cai B, Liu Y, Fu BM (2012) Effects of wall shear stress and its gradient on tumor cell adhesion in curved microvessels. Biomech Model Mechanobiol 11(5):641–653. https://doi.org/10.1007/s10237-011-0339-6
Yen WY, Cai B, Zeng M, Tarbell JM, Fu BM (2012) Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res
Yen WY, Cai B, Yang J, Zhang L, Zeng M, Tarbell JM, Fu BM (2015) Endothelial surface glycocalyx can regulate flow-induced endothelial NO production in microvessels in vivo. PLoS One 10(1):e0117133
Yudoh K, Matsui H, Tsuji H (1997) Nitric oxide induced by tumor cells activates tumor cell adhesion to endothelial cells and permeability of the endothelium in vitro. Clin Exp Metastasis 15:557–567
Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD (2012) Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A 109(34):13515–13520
Zhang L, Zeng M, Fu BM (2016a) Inhibition of endothelial nitric oxide synthase decreases breast cancer cell MDA-MB-231 adhesion to intact microvessels under physiological flows. Am J Physiol Heart Circ Physiol 310(11):H1735–H1747
Zhang L, Fan J, Zeng M, Curry F-RE, John MT, Fu BM (2016b) Sphingosine-1-phosphate (S1P) maintains normal microvascular permeability by preserving endothelial surface glycocalyx (ESG) in intact microvessels. Microcirculation 23(4):301–310
Zhang L, Zeng M, Fu BM (2017) Sphingosine-1-phosphate reduces adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel walls by protecting endothelialsurface glycocalyx. Cell Mol Biol (Noisy-le-Grand) 63(4):16–22
Acknowledgments
This work was supported by the NSF CBET 0754158, NIH CA153325-01, CA137788-01, and 1UG3TR002151-01.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Fu, B.M. (2018). Tumor Metastasis in the Microcirculation. In: Fu, B., Wright, N. (eds) Molecular, Cellular, and Tissue Engineering of the Vascular System. Advances in Experimental Medicine and Biology, vol 1097. Springer, Cham. https://doi.org/10.1007/978-3-319-96445-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-96445-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96444-7
Online ISBN: 978-3-319-96445-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)