Abstract
The production of n-butanol, as a widely applied solvent and potential fuel, is attracting much attention. The fermentative production of butanol coupled with the production of acetone and ethanol by Clostridium (ABE fermentation) was once one of the oldest biotechnological processes, ranking second in scale behind ethanol fermentation. However, there remain problems with butanol production by Clostridium, especially the difficulty in genetically manipulating clostridial strains. In recent years, many efforts have been made to produce butanol using non-native strains. Until now, the most advanced effort was the engineering of the user-friendly and widely studied Escherichia coli for butanol production. This paper reviews the current progress and problems relating to butanol production by engineered E. coli in terms of prediction using mathematical models, pathway construction, novel enzyme replacement, butanol toxicity, and tolerance engineering strategies.
Graphical Abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords:
1 Introduction
While the infrastructure of the current economy is established on the petrochemical industry, the oil crisis of the 1970s was a warning that humanity’s dependence on oil is not sustainable [23]. It is generally accepted that crude oil will be depleted in the twenty-first century at the speed of current consumption. The shift from a petroleum-based economy to a biomass-based economy has become a global objective. In the drive to find alternatives to fossil products, the production of butanol from renewable resources attracts much attention nowadays [39].
Butanol (butyl alcohol or 1-butanol or n-butanol, C4H9OH, MW 74.12) is a colorless liquid with a distinct odor. It is mainly used to synthesize butyl acrylate and methacrylate esters for latex surface coatings and the production of enamels and lacquers, butyl glycol ether, butyl acetate, and plasticizers. Additionally, butanol can be used directly as the diluent for formulations of brake fluid and as solvent in the production of hormones, vitamins, and antibiotics [23]. Although ethanol has been extensively recognized as a typical biofuel, butanol, as an alternative biofuel, has several important advantages over ethanol, such as higher energy content, lower water absorption, better blending ability with gasoline, and direct use in conventional combustion engines without modification [12].
Butanol is naturally produced via the anaerobic fermentation of biomass substrates by some clostridia species; this is referred to as ABE fermentation because it is coupled with the production of acetone and ethanol. Much progress has been achieved over a century of study on ABE fermentation, such as the development of genetic manipulation tools and omic analyses of the physiology of solventogenic bacteria. However, there are still problems with butanol production by Clostridium: (1) It remains time consuming and difficult to genetically manipulate Clostridium strains although new tools have been developed; (2) it is difficult to improve the butanol yield because of the naturally coupled production of acetone and ethanol; (3) the relatively slow growth and spore-forming life cycle are problems for industrial fermentation; and (4) the relatively unknown genetic system and complex physiology of the microorganism present difficulties in engineering the metabolism for optimal production of butanol. Therefore, construction of the next generation of butanol producers from user-friendly organisms would be an alternative way for producing butanol with lower cost than clostridial strains [20].
However, heterologous production of butanol in non-clostridial microbes is not as simple as simply transferring several known genes. First, the host needs to be genetically manipulated easily to support multiple steps of engineering and many trial-and-error experiments. Second, the butanol pathway needs to be carefully designed and new genes from other organisms need to be tested in establishing an efficient pathway in a new host. Third, the native pathway and carbon flux need to be readjusted through genome engineering. Fourth, the butanol tolerance and use of cheap substrates need to be improved. In this regard, Escherichia coli seems to be an optimal microorganism with well-studied genetic background and rich genetic tools. More importantly, E. coli has been proved to be the successful horses for the microbial cell factories of some products [36]. In recent years, many groups have reported successful butanol production in E. coli [13, 45]. Here, we reviewed the current progress and problems relating to butanol production in non-native microbes, especially in E. coli.
2 Theoretical Prediction to Improve Butanol Production in E. coli Using Computational Models
The first butanol-producing microbe was found by Louis Pasteur in 1861 [23]. The process of natural butanol production is well-known ABE fermentation in which butanol production is coupled with the production of acetone and ethanol. Owing to the demand for large amounts of acetone in the manufacture of cordite in World Wars I and II, ABE fermentation peaked in the 1950s [23]. In the past more than 100 years of ABE fermentation, scientists have learned the butanol synthetic pathway and can now transfer it to many other microbes for the heterologous production of butanol with the help of molecular biology tools [10]. However, the genetic modification of butanol production is not always effective, because engineering of a single gene may lead to unanticipated dramatic changes in the metabolic network. Comprehensive in silico models and highly accurate prediction methods are thus desired to reduce the trial-and-error risk and to improve our understanding of microbial physiology. In recent years, efforts have been made to construct genome-scale metabolic models related to butanol production based on genome annotation and metabolome analysis.
2.1 Flux Balance Analysis (FBA)
Flux balance analysis (FBA) is a mathematical modeling approach often used by metabolic engineers to quantitatively simulate microbial metabolism. FBA assumes that metabolic networks will reach a steady state constrained by the stoichiometry [25]. By performing FBA while maximizing the cell growth and butanol production rate in Clostridium, the relationship between acetate accumulation and butanol production was investigated. It was revealed that the rate of butanol production decreased with a decreasing rate of acetate production [21]. Additionally, by adding reactions involved in butanol production catalyzed by butyryl-CoA dehydrogenase (BCD), butanal dehydrogenase, and butanol dehydrogenase to the metabolic model of E. coli, a genome-scale FBA model was constructed to simulate triple reaction knockouts that contribute to improving butanol production. The model indicated that the knockout of adhE and pta was essential for the high production of butanol. It was confirmed that, by disrupting ethanol and acetate production pathways, 27 % of glucose was converted into butanol. Additionally, it has been evaluated experimentally that the disruption resulted in 1.4-fold butanol yield of the control strain [40].
2.2 Kinetic Simulation Model
Besides FBA analysis, a kinetic model was constructed to simulate the dynamic profiles of microbial metabolism. Shinto et al. designed three kinetic simulation models that describe the dynamic behaviors of metabolites in ABE fermentation by Clostridium saccharoperbutylacetonicum N1-4. The simulation results showed that an increase in kinetic parameters (Vmax1, Km1) at R1 (glucose to fructose-6-P) had the greatest negative impact on butanol production. However, a decrease in acetone production was responsible for butanol production [46]. These results provide targets for further genetic modification of butanol-producing strains.
3 Engineering E. coli for Butanol Production
The paper that James Liao group from University of California, Los Angeles, submitted to the journal Metabolic Engineering on May 18, 2007, is the first work on the production of butanol in a non-native microbe [3]. In the following years, scientists from different countries reported works on the hetero-production of butanol in different hosts and made much progress in strain improvement (Table 1). The best heterologous butanol-producing strains are presently derived from E. coli, which can produce 14–15 g/L butanol with a yield of 31–33 % [13, 45] and thus have industrial advantages over clostridial strains. Here, we mainly summarize the progress made in butanol production by E. coli.
3.1 Establishing a Butanol Synthetic Pathway in E. coli and Selection of Efficient Enzymes
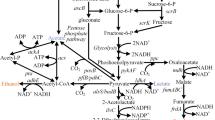
In the initial stage of engineering E. coli for butanol production, it is natural to transfer the whole butanol pathway from Clostridium to E. coli, which includes seven genes thl, hbd, crt, bcd, etfA, etfB, and adhE2, catalyzing two molecules of acetyl-CoA to one molecule of butanol in six steps (Fig. 1). However, when scientists from the USA and Japan firstly transferred the clostridial butanol pathway to E. coli through plasmids in 2007, the engineered strain produced less than 1 g/L butanol (vs. clostridial butanol titer 10–20 g/L) [3, 19], although by-product pathways were disrupted. The results indicate that engineering an efficient butanol-producing E. coli is not as simple as simply expressing several clostridial genes. Determining the rate-limiting step and selecting alternative genes to fit the E. coli host are the key to the heterologous production of butanol (Fig. 1).
Pathway and genes for a heterologous butanol pathway in E. coli. aceEF.lpd: pyruvate dehydrogenase complex from E. coli, pflB: pyruvate–formate lyase, fdh: NAD+-dependent formate dehydrogenase from Candida boidinii, thl: thiolase from Clostridium acetobutylicum, atoB: acetyltransferase from E. coli, phaA: polyhydroxyalkanoate synthase from Ralstonia eutrophus, yqeF: acetyl-CoA C-acetyltransferase from E. coli, fadA: acetyl-CoA acyltransferase from E. coli, hbd: 3-hydroxybutyryl-CoA dehydrogenase from C. acetobutylicum, fadB: fused 3-hydroxybutyryl-CoA epimerase from E. coli, phaB: acetyoacetyl CoA reductase from R. eutrophus,crt: 3-hydroxybutyryl-CoA dehydratase from C. acetobutylicum, phaJ: (R)-specific enoyl-CoA hydratase from R. eutrophus, bcd-etfAB: butyryl-CoA dehydrogenase complex from C. acetobutylicum, ccr: crotonyl-CoA reductase from Streptomyces collinus, ter: NADH-dependent crotonyl-CoA from Treponema denticola, ydiO: acyl-CoA dehydrogenase from E. coli, adhE2: bifunctional acetaldehyde-CoA/alcohol dehydrogenase (CAP0162) from C. acetobutylicum, adhE1: bifunctional acetaldehyde-CoA/alcohol dehydrogenase (CAP0035) from C. acetobutylicum, mhpF: acetaldehyde-CoA dehydrogenase II from E. coli, fucO: L-1,2-propanediol oxidoreductase from E. coli. * phaB and phaJ should be used together
3.1.1 Thiolase
The first step in butanol synthesis is the condensing of two acetyl-CoA moles to one acetoacetyl-CoA mole by a thiolase (encoded by the thl gene). Thiolase is a ubiquitous enzyme that plays key roles in many vital biochemical pathways, including beta oxidation in the degradation of fatty acids and various biosynthetic pathways. E. coli synthesizes two distinct 3-ketoacyl-CoA thiolase enzymes. One is a protein product of the fadA gene; the second is a product of the atoB gene. To date, FadA has not been tested for butanol production in published work. The atoB gene is known to be induced by growth on acetoacetate and exhibits strict substrate specificity for acetoacetyl-CoA. More importantly, AtoB has higher specific activity (1,078 U/mg) than clostridial Thl enzyme (216 U/mg). Hence, when the thl gene was replaced with atoB gene, the titer of butanol increased more than 3-fold [3]. Additionally, E. coli has a yqeF gene that encodes a predicted acetyl-CoA acetyltransferase. Overexpression of the yqeF gene supports a functional reversal of the beta-oxidation cycle in the synthesis of butanol, which has a better effect than the overexpression of atoB [13]. The Chang group at the University of California, Berkeley, constructed a butanol synthetic pathway inspired by the efficient production of polyhydroxyalkanoates in E. coli, which transplanted a three-gene pathway from Ralstonia eutrophus for monomer biosynthesis (phaAB) and polymerization (phaC) to yield a biodegradable plastic that can be produced at 50 % dry cell weight at near-theoretical yields. Overexpression of the phaA gene can support butanol synthesis at 4.65 g/L in laboratory-scale shake-flask experiments [7]. Additionally, the ERG10 gene from Saccharomyces cerevisiae has been shown to be functional in a butanol synthetic pathway in S. cerevisiae [48], but has not been tested in an E. coli host. It should be noted that although several gene candidates encoding acetyl-CoA acetyltransferase for butanol synthesis have been improved, the best effect of one gene should depend on the host context and expression mode.
3.1.2 3-Hydroxybutyryl-CoA Dehydrogenase and 3-Hydroxybutyryl-CoA Dehydratase
Most published work directly uses the hbd gene (3-hydroxybutyryl-CoA dehydrogenase) and crt gene (3-hydroxybutyryl-CoA dehydratase) from C. acetobutylicum for the two reactions of acetoacetyl-CoA to 3-hydroxybutyryl-CoA and 3-hydroxybutyryl-CoA to crotonyl-CoA, respectively, in E. coli [55]. The native bifunctional fadB gene (fused 3-hydroxybutyryl-CoA epimerase) in E. coli was also improved to be able to catalyzing the two reactions [18]. Besides, the phaB (acetyoacetyl CoA reductase) and phaJ ((R)-specific enoyl-CoA hydratase) for the production of polyhydroxyalkanoates from R. eutrophus also could be used for above two reactions [7]. It is worthy to note that phaB and phaJ should be used together, because of the stereoisomerism specificity of these enzymes [7]. However, no evidence indicates which enzyme is the best for the synthetic butanol pathway in E. coli.
3.1.3 Butyryl-CoA Dehydrogenase
The fourth step of butanol synthesis is the reduction of crotonyl-CoA to butyryl-CoA by BCD, which needs EtfAB as an electron carrier. Studies have indicated that BCD catalysis is involved in clostridial ferredoxins, which may not fit the cellular context of E. coli. In practice, all artificial butanol pathways containing BCD in E. coli produced a limited titer of butanol. In the first case of constructing butanol-producing E. coli by the Liao group, BCD-EtfAB was replaced with Ccr (encoding a crotonyl-CoA reductase) from Streptomyces coelicolor. However, the resulting E. coli strain produced less butanol [3]. The Chang group also tested the effects of the ccr gene (from S. collinus) on butanol production. They found that the butanol titer is related to the expression strength of the ccr gene [7], which indicates that this step is rate limiting in the butanol pathway in a non-native E. coli host. Studies have also indicated that the Ccr-catalyzed reduction of crotonyl-CoA to butyryl-CoA is a side reaction of the native reductive carboxylation reaction to form ethylmalonyl-CoA, confirming the Ccr activity is low for butanol production [7]. The biological reduction reaction of enoyl-CoA is ubiquitous in nature, such as in fatty acid synthesis and the beta-oxidation pathway of fatty acid; the reaction requires flavin as factors and is reversible. According to the principles of thermodynamics, the direct hydride transfer from NAD(P)H to the enoyl-CoA that increases the barrier for the reverse oxidation reaction and thus potentially kinetically traps crotonyl-CoA in the synthetic butanol pathway can be achieved by eliminating the less-downhill intermediate state produced in the substrate reduction by the flavin cofactor [7]. Fortunately, a crotonyl-CoA-specific trans-enoyl-CoA reductase (Ter) from Euglena gracilis was improved to catalyze the irreversible oxidation of crotonyl-CoA to butyryl-CoA in the presence of NAD+ or NADP+. The Liao group investigated ter genes from Treponema denticola, Treponema vincentii, Flavobacterium johnsoniae, and Fibrobacter succinogenes and found that the ter gene from T. denticola was the best [45]. The Chang group also selected the ter gene from T. denticola according to enzymatic mechanism analysis for their butanol pathway, which resulted in a butanol titer of 4.65 g/L without removing any by-product pathways [7]. The engineered E. coli containing this ter gene constructed by the Liao group could produce 15 g/L butanol [45].
3.1.4 Aldehyde/Alcohol Dehydrogenase
The final two steps of butanol synthesis are the reduction of butyryl-CoA to butyraldehyde by aldehyde dehydrogenase and the subsequent reduction to butanol by alcohol dehydrogenase, consuming two NADH molecules. In the native butanol-producing model of the bacterium C. acetobutylicum, the two steps can be catalyzed by one enzyme, bifunctional aldehyde/alcohol dehydrogenase, which is encoded by the adhE1 gene (active in the solvent production phase) or adhE2 gene (active in the alcohol production phase). Using the same promoter for the expression of the two genes in E. coli, compared with the adhE1 strain, the adhE2 strain has 8-fold activity for butyrate dehydrogenase but no increase for butanol dehydrogenase activity, leading to 4-fold butanol production [19]. The adhE2 gene was also compared with the adhE gene from E. coli. The results showed that versus adhE, the adhE2 showed 1.5-fold activity when using butyryl-CoA as substrate, and 6-fold selectivity of butyryl-CoA: acetyl-CoA [3]. Although adhE2 was successfully used for butanol production in E. coli, ethanol is still one of the main products (ethanol:butanol ratio exceeding 1:10), thus limiting the butanol yield. Hence, more gene candidates of aldehyde/alcohol dehydrogenase may be screened to reduce the ethanol titer and decrease the ethanol:butanol ratio in future work to improve the strain.
3.2 Optimization of the Gene Expression in the Butanol Pathway
In the initial configuration of a heterologous pathway, the gene expression profile is usually not optimal for maximal carbon flux. Hence, fine tuning of the gene is an essential step in the construction of an efficient microbial cell factory. Different methods of controlling gene expression have been developed, such as the use of a promoter library, the use of an RBS strength prediction algorithm, and the MAGE fine-tuning method. The Yang group from the Institute of Plant Physiology and Ecology at Chinese Academy of Sciences used the strong promoter Alper PLTetO1 or the weak promoter Alper BB to express the thl gene and used the strong promoter Braatsch20 or the weak promoter Braatsch10 to express other genes (one operon) of the butanol pathway in E. coli. The results showed that the combination of Alper PLTetO1-thl and Braatsch10-operon is best and provided a butanol titer that was 3- to 5-fold higher than that of other combinations [49]. There have been few other published works on the systematic fine tuning of genes for butanol production, which should be considered one of the main directions of constructing high-carbon-flux butanol pathways.
3.3 Engineering Reducing Power Balance for Efficient Butanol Production
In the biosynthetic pathway from glucose to butanol, a precise redox balance can be achieved with a maximal theoretical butanol yield of 41.1 % (w/w). However, such balance is difficult to achieve in the practical engineering of E. coli. The main problem relates to the conversion of pyruvate to acetyl-CoA. If the reaction is catalyzed by the PDH complex (encoded by aceEF.lpd genes), two NADH molecules are generated that can provide the redox balance of butanol synthesis. However, the PDH complex is inactive in the anaerobic condition owing to the anaerobic sensitivity of E3 component Lpd (dihydrolipoamide dehydrogenase); an active PDH complex is essential for butanol production. To solve this problem, the Chang group overexpressed aceEF.lpd genes in a plasmid, resulting in a 3-fold increase in PDH activity, a 53 % increase in the NADH concentration, and a 1.6-fold increase in the butanol titer [7]. Another research group from Northern Illinois University employed the same strategy with the aim to engineer a homobutanol fermentation pathway in E. coli; the resulting strain only produced a measurable amount of butanol under anaerobic conditions [17], indicating that other factors should be optimized to couple this strategy. In previous studies, the Ingram group from the University of Florida found an anaerobic active lpd mutant lpd101 (E354K) in the process of the laboratory evolution of E. coli [53], and the Zhang group from the Tianjin Institute of Industrial Biotechnology at the Chinese Academy of Sciences found another anaerobic active lpd mutant lpdA* (C242T, C823T, and C1073T) in an adapted succinate-producing E. coli [59]. However, these lpd mutants have not been used for butanol production in E. coli to date, which should be an efficient strategy for obtaining NADH for butanol production. For the anaerobic growth of E. coli, the cell mainly uses the pyruvate formate-lyase (encoded by the pflB gene) to catalyze pyruvate into acetyl-CoA and formate. The formate is secreted or converted to carbon dioxide and hydrogen by native formate-hydrogen lyase complex. Hence, the reducing power from pyruvate is wasted in the form of formate or hydrogen. It is known that formate can be converted into carbon dioxide and NADH by the specific formate dehydrogenase (encoded by the fdh gene) from yeast. The Liao group successfully used NADH obtained from formate by overexpression of the fdh gene from Candida boidinii as the driving force, to improve the butanol titer and yield, with the reduced formation of by-products [45]. It is worth noting that the Gonzalez group from Rice University obtained a butanol yield of 33 % (vs. max. 41 %) in E. coli with active reversal of the beta-oxidation cycle, without manipulating the reaction of pyruvate to acetyl-CoA [13]. The mechanism of reducing the power supply in the above strain may provide new insights into improving butanol production by E. coli. Although the above cases and strategies improve the capability of butanol production, the best yield of butanol produced by engineered E. coli was only 80 % of the maximal value, indicating that barriers remain to be solved.
3.4 Removing by-product Pathways to Supply Sufficient Precursors for Butanol Production
Butanol production by clostridial strains is naturally coupled with the production of acetone, ethanol, and small amounts of acetate and butyrate, resulting in a low yield of butanol and high feedstock cost. The main purpose of engineering E. coli for butanol production is to improve the butanol yield from sugars, reducing the feedstock cost. According to the well-studied metabolic pathway of E. coli, the key genes for the production of by-products are known, namely frdABCD for succinate, ldhA for lactate, pta-ack for acetate, and adhE for ethanol. In most butanol-producing E. coli strains, these genes were disrupted to provide adequate precursors for butanol production. It is notable that the ldhA gene was not disrupted in the engineered E. coli with active beta-oxidation cycle of the Gonzalez group, which could still produce butanol with 33 % yield [13]. Although the typical by-product pathways were disrupted, known and unknown by-products were still produced by the engineered strains more or less. To solve this problem, more genes of the corresponding by-products need to be disrupted, and the butanol pathway needs to be further optimized to trap more carbon flux from other pathways.
3.5 Using Cheap Substrates for the Low Cost of Butanol Production
Butanol produced from biomass as a bulk chemical or biofuel must have a low production cost to compete with products of crude oil. It is thus important to select cheap feedstocks for butanol production. In constructing butanol-producing E. coli, scientists tested different cheap substrates for butanol production, which included palmitic acid, ionic liquid-treated switchgrass, glycerol, and xylose (Table 1). However, butanol titers from these substrates are lower than 2 g/L. The low titers can be explained that the tested strains were not the best strains, and insufficient effort was made in engineering the substrate utilization. The use of cheap feedstock for butanol production by E. coli should be the key to an economical industrial process and thus needs to be strongly promoted.
4 Butanol Toxicity and Engineering Butanol Tolerance in E. coli
Although E. coli can convert sugars (glucose and xylose) to butanol at a relatively high level, it cannot tolerate 2 % (v/v) butanol [27]. E. coli is unable to produce butanol at a very high level as a result. Considering the relationship between butanol tolerance and butanol production by Clostridial strains [16, 29, 30, 34], butanol toxicity to E. coli is considered a bottleneck for butanol production. It is thus important to develop a butanol-tolerant strain in E. coli for the production of high-titer butanol at levels needed for economic efficiency.
4.1 Butanol Toxicity to Microbes
The toxicity of butanol, as a solvent, to cells begins with the butanol impact on the cell membrane. Cell membranes are composed of a phospholipid bilayer interspersed with proteins. In addition to providing structural integrity and maintaining a barrier to the extracellular environment, they facilitate transport in and out of the cell and are responsible for signal transduction, communication, and energy production [37]. When cells are exposed to butanol, the butanol accumulates in the phospholipid bilayer, the hydroxyl moiety accumulates near the phospholipid polar headgroup, and the aliphatic chains are intercalated between the fatty acyl chains of the phospholipids [54]. The hydroxyl group of the butanol spends more time hydrogen bonded to the phosphate group of the lipid than the more hydrophobic longer-chain n-alkanols, which are more deeply embedded in the bilayer. As a result, butanol generates larger disordering in the phospholipid bilayer than the other n-alkanols [56]. Hereafter, the membrane loses its integrity, and the structural and functional properties of the membranes are affected. An increase in permeability to protons and ions has been observed. Consequently, dissipation of the proton motive force and impairment of intracellular pH homeostasis occur. In addition to the effects of lipophilic compounds on the lipid part of the membrane, proteins embedded in the membrane are affected. The effects on the membrane-embedded proteins probably result to a large extent from changes in the lipid environment [47]. In addition, it has been shown that butanol can affect cells by damaging and denaturing biological molecules, including damage to DNA and lipid damage by oxidative and related mechanisms [37]. These results provide insights into butanol toxicity to E. coli, from which promising strategies for improving the tolerance to butanol can be obtained.
4.2 Mechanisms of Butanol Tolerance
4.2.1 Omic Analyses Revealing Molecular Mechanisms of Butanol Tolerance
Although butanol is toxic to microbes, some species or strains can tolerate butanol to some degree. As shown in Table 2, Pseudomonas putida strains possess a high tolerance to butanol and can grow in 6 % (vol/vol) butanol [43]. Some Lactobacillus and Pediococcus species can tolerate butanol of up to 3 % or more. The tolerance mechanisms are useful in engineering butanol-tolerant strains. In recent years, system biotechnological approaches have been widely used to investigate the molecular mechanism of butanol tolerance.
Comparative proteomic analyses revealed that glycerol metabolism genes (glpA and glpF), numerous stress genes (dnaK, groES, groEL, hsp90, hsp18, clpC, and htrA), the solventogenic operon aad–ctfA–ctfB, and other solventogenic genes were up-regulated in response to butanol stress [1] in the native butanol producer Clostridium acetobutylicum. Most were up-regulated in advance (acidogenic phase) [34]. This suggests that the strain Rh8 may have developed a mechanism to prepare itself for coping with butanol challenges before butanol was produced, leading to increased butanol production [34]. Additionally, the butanol-tolerant mutant strain was shown to have evolved a more stabilized membrane structure and to have developed a cost-efficient energy metabolism strategy, to cope with the butanol challenge [33]. Further, comparative genomic analysis indicated a surprisingly high ratio of rRNA mutations that might contribute to improved butanol tolerance [5]. This suggests that strain Rh8 might mutate some rRNA genes to change the structure and function of the whole ribosome. Engineering the factor involved in the translation process can therefore be considered a new strategy of improving microbial stress tolerance worthy of testing [5]. In addition, it was found that in response to butanol on the membrane, C. acetobutylicum synthesized increased levels of saturated acyl chains [52]. The growth of cells in the stationary phase coincides with a gradual increase in the percentage ratio of saturated to unsaturated fatty acids. An increased synthesis of saturated fatty acids may provide a more stable membrane environment under butanol stress [4].
Besides clostridia, species that tolerate a high concentration of butanol were used to investigate the mechanism of butanol tolerance. The most interesting findings were solvent efflux pumps and the ability to shift from cis isomers to trans isomers. For example, P. putida strains contain mainly palmitoleic acid and vaccenic acid as trans isomers and are directly synthesized from the cis isomer within 1 min of exposure to the solvent with no shift in the position of the double bond. Because organic solvents increase membrane fluidity, P. putida strains shifting their cis-to-trans ratio could counteract this alteration [41]. Efflux pumps are membrane transporters and play an important role in cell survival by exporting a wide range of substrates, including bile salts, antimicrobial drugs, and solvents. The efflux pump srpABC from P. putida S12 has been shown to export hexane, octanol, and several other hydrocarbons. Three efflux pumps (TtgABC, TtgDEF, and TtgGHI) are found in P. putida DOT-T1E and are collectively known as the toluene tolerance genes [14].
These results suggest that the molecular mechanism of butanol tolerance is complex; however, the results suggest candidates to be engineered to improve microbial tolerance to butanol. Some candidates have been confirmed by genetic modification, as summarized below.
4.2.2 Investigation of Candidate Targets Contributing to Butanol Tolerance
-
1.
Glycerol metabolism genes
The expression of the gldA gene that encodes glycerol dehydrogenase can be reduced by antisense ribonucleic acid (RNA). It has been shown that the butanol tolerance of C. beijerinckii is increased by the reduced activity of glycerol dehydrogenase [31].
-
2.
Heat-shock proteins (HSPs)
According to the above studies, many stress-responding proteins, including HSPs, are induced by butanol. The HSP system is a cellular stress response system that works during the folding and degradation of proteins. Overexpression of HSP groESL in C. acetobutylicum ATCC824 resulted in prolonged metabolism and increased butanol production and tolerance [50, 51]. Overexpression of HSPs grpE and htpG improved the butanol tolerance of C. acetobutylicum but did not increase butanol production [32]. Expression of HSP33 from solvent-tolerant Bacillus psychrosaccharolyticus in C. acetobutylicum ATCC824 did not confer increased solvent tolerance during growth, but increased the total solvent titer by 22 % [9]. This suggests that most HSPs contribute to butanol tolerance, which might be applied in engineering a butanol-tolerant E. coli strain.
-
3.
Transcriptional regulator related to solvent production
Spo0A is a multivalent transcription factor regulator. Expression of spo0A in C. acetobutylicum promoted expression of the solvent formation genes in the stationary phase, induced the conversion of acid into solvent, and provided increased tolerance and solvent production under butanol stress [2]. By genomic-library enrichment and DNA microarray analysis, CAC1869 categorized as a singleton transcriptional regulator was found. Overexpression of CAC1869 in C. acetobutylicum ATCC824 increased butanol tolerance by 81 % and prolonged the metabolic activity [8].
-
4.
Other targets contributing to butanol tolerance
Glutathione (GSH) is also involved in protein stabilization, antioxidation, and detoxification; so, a study was conducted by introducing GSH synthetic genes gshAB into C. acetobutylicum DSM1731. The engineered strain DSM1731(pITAB) produced GSH and exhibited improved butanol tolerance and increased butanol production capability [58]. Furthermore, the gene SMB_G1518 in C. acetobutylicum DSM1731 that codes the cysteine-rich zinc-finger domain putatively interacting with alcohol and the close gene SMB_G1519 were shown to be possible negative regulators involved in butanol tolerance [22].
4.3 Engineering E. coli to Improve Butanol Tolerance
On the basis of molecular mechanisms of butanol tolerance and confirmed strategies for Clostridium, efforts were made to improve the butanol tolerance of E. coli (Table 3).
4.3.1 Overexpression or Deletion of Genes to Improve the Butanol Tolerance of E. coli
Butanol is known to affect the membrane by increasing the membrane fluidity. For E. coli, several transcriptional analyses have been performed to clarify the stress caused by butanol. The results indicate an increase in reactive oxygen species during butanol stress. The free radicals directly attack the membrane by lipid peroxidation [44].
To relieve the oxidative stress in the host cell, metallothioneins (MTs), which are known as scavengers of reactive oxygen species (ROS), were engineered in E. coli hosts for both cytosolic and outer-membrane-targeted (osmoregulatory membrane protein OmpC fused) expressions. Cytosolic expression was conducted for the alcohol tolerance measurements of the engineered E. coli strains of MTs from human (HMT), mouse (MMT), and tilapia fish (TMT), while the OmpC-fused MT strains (OmpC-HMT, OmpC-MMT, and OmpC-TMT) were expressed for membrane-targeted MTs. The abilities of these engineered E. coli to scavenge intracellular or extracellular ROS were examined, and TMT was found to perform best among the three MTs, growing in a medium with 1 % (v/v) butanol. Additionally, the membrane-targeted fusion protein, OmpC-TMT, improved host tolerance to 1.5 % butanol, above the tolerance of 1 % for TMT [11].
Efflux pumps play an important role in solvent tolerance. In E. coli, the AcrAB-TolC system acts as an efflux pump, with AcrB being the inner membrane transporter, AcrA being the membrane fusion protein, and TolC being the outer membrane protein. A library of heterologously expressed efflux pumps was examined and none of the pumps were able to increase E. coli tolerance to butanol [15]. Many studies have suggested that efflux pumps are ineffective at exporting short-chain alcohols.
A molecular chaperone is a cellular stress response molecule that works during the folding and degradation of proteins, with HSPs being well-known examples. Overexpression of groESL (a heat-shock gene) in E. coli provided an effective outcome. Cultures of 0.75 % butanol were the only challenged samples in which the strain 10-β(pACYC184) showed a net increase in cell density above the starting point, doubling across the entirety of the experiment, while 10-β(pAC-groESL) doubled more than twice in the same time frame. In 0.75 % butanol, the overexpressed groESL demonstrated a 2.8-fold increase in integrated growth under the curve over the control [60]. In addition, the Hsp33 of B. psychrosaccharolyticus overexpressed in E. coli increased the E. coli’s tolerance to isopropyl alcohol, demonstrating that a psychrophilic protein is functional at higher temperatures and confers a tolerant phenotype [24]. This protein might be functional for improving butanol tolerance in E. coli as well.
An enrichment strategy involving the serial transfer of batch cultures in increasing butanol concentrations (0, 0.9, 1.3, and 1.7 % butanol) along with respective controls was performed recently. The overexpressed genes that conferred the largest increase in butanol tolerance, entC and feoA, were related to iron transport and metabolism and increased the butanol tolerance by 32.86 ± 4.0 % and 49.16 ± 3.3 %, respectively (compared with the initial butanol tolerance of 0.5 %). The gene whose deletion resulted in the largest increase in resistance to butanol was astE, with butanol tolerance being enhanced by 48.76 ± 6.3 % [42].
4.3.2 Transcriptional Engineering of E. coli to Improve Butanol Tolerance
To select a butanol-tolerant E. coli strain, transcriptional engineering of the bacterial RNA polymerase alpha subunit was studied. Results showed a mutant strain with a mutant RNA polymerase alpha subunit grew well in LB medium containing 0.9 % (v/v) butanol [26].
Lee et al. developed a new method of increasing the butanol tolerance of E. coli with artificial transcription factor (ATF) libraries that consist of zinc-finger DNA-binding proteins and an E. coli cyclic AMP receptor protein. Using these ATFs, they selected a butanol-tolerant E. coli that can tolerate butanol up to 1.5 % (v/v), with a concomitant increase in heat resistance [28].
Zhang et al. demonstrated that the butanol tolerance of E. coli can be greatly enhanced through random mutagenesis of global transcription factor cyclic AMP receptor protein. Four mutants (MT1–MT4) with elevated butanol tolerance were isolated from error-prone PCR libraries through enrichment screening. A DNA shuffling library was then constructed using MT1–MT4 as templates, and one mutant (MT5) that exhibited the best tolerance ability among all variants was selected. In the presence of 0.8 % (v/v, 6.5 g/l) butanol, the growth rate of MT5 was found to be 0.28 h−1 while that of wild type was 0.20 h−1. When the butanol concentration increased to 1.2 % (9.7 g/l), the growth rate of MT5 (0.18 h−1) became twice that of the wild type (0.09 h−1) [57].
4.3.3 Evolution Engineering of E. coli Strains to Improve Butanol Tolerance
For E. coli, the ethanol-caused stress is well studied; these results were used for the construction of ethanol-producing strains. Nevertheless, butanol-resistant mutant strains are not so well understood owing to a series of unclear mechanisms. Experimental evolution is an effective method used for chemical tolerance while fermentation is limited by chemical products. However, the phenotype cannot be clearly explained sometimes because of the complex mechanisms.
Researchers isolated three E. coli clones capable of growth in 2 % (w/v) isobutanol in glucose media and two clones capable of growth in 1.75 % isobutanol in xylose media, representing 60 and 40 % improvements in tolerance, respectively, compared with the wild-type strain [35]. On the basis of the similarity of isobutanol and butanol, we suppose this strategy also works for butanol tolerance.
Atsumi et al. employed a method of sequential transfer to the isobutanol production host strain, E. coli JCL260. JCL260 was initially inoculated into LB broth containing 4 g/L isobutanol. After 15 sequential transfers, the isobutanol concentration in the medium had increased to 6 g/L. The isobutanol concentration then reached 8 g/L after the next 15 transfers. After a total of 45 transfers, we isolated the largest single colony, denoted SA481, on an LB agar plate with 8 g/L isobutanol. SA481 showed increased growth compared with JCL260 in the presence of 6 and 8 g/L isobutanol, while maintaining similar growth in the absence of isobutanol. The study demonstrated the isobutanol-tolerant mutants also had increased tolerance to butanol (6 g/L) and 2-methyl-1-butanol (3 g/L).
5 Discussion and Perspectives
E. coli has been improved to be an excellent butanol producer through metabolic engineering of a new synthetic pathway. The butanol yield of 33 % by E. coli is a great advantage over the use of clostridial strains. The maximal butanol titer was 15 g/L, which is lower than the maximal titer of 20 g/L produced by some clostridial strains, and the butanol productivity is lower than that of clostridial strains. Therefore, more effort should be made to improve the performance of E. coli.
Besides E. coli, other species, such as B. subtilis [38], S. cerevisiae [48], P. putida [38], and L. brevis [6], have been used as the host to produce butanol. However, none of these species produce more than 3 g/L of butanol. It is suggested that both the enzymes involved in the butanol synthetic pathway and the matching of the pathway with the host are important in engineering an efficient butanol producer.
Besides the metabolic pathway, the butanol tolerance of host strains is a critical factor affecting butanol production performance. Butanol tolerance is a complex mechanism related to mutagenic changes. Although much progress on the mechanism of butanol toxicity has been achieved and new strategies for improving butanol tolerance developed, such work has not been performed on a butanol-producing strain. The further improvement of the butanol titer may depend on butanol tolerance engineering. Researchers are now using genomics, transcriptomics, proteomics, and metabonomics as tools to analyze the global changes in response to butanol challenge. They hope to understand the tolerance mechanisms clearly and connect the butanol tolerance with yield in E. coli. We suppose the system approach will improve butanol production through metabolic engineering in E. coli.
Finally, cheaper feedstocks such as glycerol and cellulose hydrolysates should be considered, and this will require additional genetic engineering or metabolic evolution of a butanol-producing strain.
References
Alsaker KV, Paredes C, Papoutsakis ET (2010) Metabolite stress and tolerance in the production of biofuels and chemicals: gene-expression-based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol Bioeng 105(6):1131–1147
Alsaker KV, Spitzer TR, Papoutsakis ET (2004) Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell’s response to butanol stress. J Bacteriol 186(7):1959–1971
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Chou KJ, Hanai T, Liao JC (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng 10(6):305–311
Baer SH, Blaschek HP, Smith TL (1987) Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl Environ Microbiol 53(12):2854–2861
Bao G, Dong H, Zhu Y, Mao S, Zhang T, Zhang Y, Chen Z, Li Y (2014) Comparative genomic and proteomic analyses of Clostridium acetobutylicum Rh8 and its parent strain DSM 1731 revealed new understandings on butanol tolerance. Biochem Biophys Res Commun 450(4):1612–1618
Berezina OV, Zakharova NV, Brandt A, Yarotsky SV, Schwarz WH, Zverlov VV (2010) Reconstructing the clostridial n-butanol metabolic pathway in Lactobacillus brevis. Appl Microbiol Biotechnol 87(2):635–646
Bond-Watts BB, Bellerose RJ, Chang MC (2011) Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat Chem Biol 7(4):222–227
Borden JR, Papoutsakis ET (2007) Dynamics of genomic-library enrichment and identification of solvent tolerance genes for Clostridium acetobutylicum. Appl Environ Microbiol 73(9):3061–3068
Bormann S, Baer ZC, Sreekumar S, Kuchenreuther JM, Toste FD, Blanch HW, Clark DS (2014) Engineering Clostridium acetobutylicum for production of kerosene and diesel blendstock precursors. Metab Eng 25:124–130
Branduardi P, de Ferra F, Longo V, Porro D (2014) Microbial n-butanol production from Clostridia to non-Clostridial hosts. Eng Life Sci 14(1):16–26
Chin WC, Lin KH, Chang JJ, Huang CC (2013) Improvement of n-butanol tolerance in Escherichia coli by membrane-targeted tilapia metallothionein. Biotechnol Biofuels 6:130
Dürre P (2007) Biobutanol: an attractive biofuel. Biotech J 2(12):1525–1534
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476(7360):355–359
Dunlop MJ (2011) Engineering microbes for tolerance to next-generation biofuels. Biotechnol Biofuels 4:32
Dunlop MJ, Dossani ZY, Szmidt HL, Chu HC, Lee TS, Keasling JD, Hadi MZ, Mukhopadhyay A (2011) Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol 7:487
Formanek J, Mackie R, Blaschek HP (1997) Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl Environ Microbiol 63(6):2306–2310
Garza E, Zhao J, Wang Y, Wang J, Iverson A, Manow R, Finan C, Zhou S (2012) Engineering a homobutanol fermentation pathway in Escherichia coli EG03. J Ind Microbiol Biotechnol 39(8):1101–1107
Gulevich AY, Skorokhodova AY, Sukhozhenko AV, Shakulov RS, Debabov VG (2012) Metabolic engineering of Escherichia coli for 1-butanol biosynthesis through the inverted aerobic fatty acid beta-oxidation pathway. Biotechnol Lett 34(3):463–469
Inui M, Suda M, Kimura S, Yasuda K, Suzuki H, Toda H, Yamamoto S, Okino S, Suzuki N, Yukawa H (2008) Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl Microbiol Biotechnol 77(6):1305–1316
Jang YS, Lee JM, Malaviya A, Seung DY, Cho JH, Lee SY (2012) Butanol production from renewable biomass: rediscovery of metabolic pathways and metabolic engineering. Biotechnol J 7(2):186–198
Jang YS, Lee JY, Lee JM, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for butanol-ethanol production by complementation of adhE1 and ctfAB genes. New Biotechnol 25:S273–S274
Jia K, Zhang Y, Li Y (2012) Identification and characterization of two functionally unknown genes involved in butanol tolerance of Clostridium acetobutylicum. PloS One 7(6):e38815
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50(4):484–524
Kang HJ, Heo DH, Choi SW, Kim KN, Shim J, Kim CW, Sung HC, Yun CW (2007) Functional characterization of Hsp33 protein from Bacillus psychrosaccharolyticus; additional function of HSP33 on resistance to solvent stress. Biochem Biophys Res Commun 358(3):743–750
Kauffman KJ, Prakash P, Edwards JS (2003) Advances in flux balance analysis. Curr Opin Biotechnol 14(5):491–496
Klein-Marcuschamer D, Santos CN, Yu H, Stephanopoulos G (2009) Mutagenesis of the bacterial RNA polymerase alpha subunit for improvement of complex phenotypes. Appl Environ Microbiol 75(9):2705–2711
Knoshaug EP, Zhang M (2009) Butanol tolerance in a selection of microorganisms. Appl Biochem Biotechnol 153(1–3):13–20
Lee JY, Yang KS, Jang SA, Sung BH, Kim SC (2011) Engineering butanol-tolerance in Escherichia coli with artificial transcription factor libraries. Biotechnol Bioeng 108(4):742–749
Lin YL, Blaschek HP (1983) Butanol production by a butanol-tolerant strain of Clostridium acetobutylicum in extruded corn broth. Appl Environ Microbiol 45(3):966–973
Liu XB, Gu QY, Yu XB (2013) Repetitive domestication to enhance butanol tolerance and production in Clostridium acetobutylicum through artificial simulation of bio-evolution. Bioresour Technol 130:638–643
Liyanage H, Young M, Kashket ER (2000) Butanol tolerance of Clostridium beijerinckii NCIMB 8052 associated with down-regulation of gldA by antisense RNA. J Mol Microbiol Biotechnol 2(1):87–93
Mann MS, Dragovic Z, Schirrmacher G, Lutke-Eversloh T (2012) Over-expression of stress protein-encoding genes helps Clostridium acetobutylicum to rapidly adapt to butanol stress. Biotechnol Lett 34(9):1643–1649
Mao S, Luo Y, Bao G, Zhang Y, Li Y, Ma Y (2011) Comparative analysis on the membrane proteome of Clostridium acetobutylicum wild type strain and its butanol-tolerant mutant. Mol Biosyst 7(5):1660–1677
Mao S, Luo Y, Zhang T, Li J, Bao G, Zhu Y, Chen Z, Zhang Y, Li Y, Ma Y (2010) Proteome reference map and comparative proteomic analysis between a wild type Clostridium acetobutylicum DSM 1731 and its mutant with enhanced butanol tolerance and butanol yield. J Proteome Res 9(6):3046–3061
Minty JJ, Lesnefsky AA, Lin F, Chen Y, Zaroff TA, Veloso AB, Xie B, McConnell CA, Ward RJ, Schwartz DR, Rouillard JM, Gao Y, Gulari E, Lin XN (2011) Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb Cell Fact 10:18
Murphy CD (2012) The microbial cell factory. Org Biomol Chem 10(10):1949–1957
Nicolaou SA, Gaida SM, Papoutsakis ET (2010) A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12(4):307–331
Nielsen DR, Leonard E, Yoon SH, Tseng HC, Yuan C, Prather KLJ (2009) Engineering alternative butanol production platforms in heterologous bacteria. Metab Eng 11(4–5):262–273
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37(1):52–68
Ohno S, Furusawa C, Shimizu H (2013) In silico screening of triple reaction knockout Escherichia coli strains for overproduction of useful metabolites. J Biosci Bioeng 115(2):221–228
Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A, Teran W, Segura A (2002) Mechanisms of solvent tolerance in gram-negative bacteria. Annu Rev Microbiol 56:743–768
Reyes LH, Almario MP, Kao KC (2011) Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PloS One 6(3):e17678
Ruhl J, Schmid A, Blank LM (2009) Selected Pseudomonas putida strains able to grow in the presence of high butanol concentrations. Appl Environ Microbiol 75(13):4653–4656
Rutherford BJ, Dahl RH, Price RE, Szmidt HL, Benke PI, Mukhopadhyay A, Keasling JD (2010) Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl Environ Microbiol 76(6):1935–1945
Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC (2011) Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol 77(9):2905–2915
Shinto H, Tashiro Y, Yamashita M, Kobayashi G, Sekiguchi T, Hanai T, Kuriya Y, Okamoto M, Sonomoto K (2007) Kinetic modeling and sensitivity analysis of acetone-butanol-ethanol production. J Biotechnol 131(1):45–56
Sikkema J, de Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59(2):201–222
Steen EJ, Chan R, Prasad N, Myers S, Petzold CJ, Redding A, Ouellet M, Keasling JD (2008) Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb Cell Fact 7(1):36
Tang W, Li J, Chen J, Yang S (2012) Butanol pathway construction and promoter optimization in Escherichia coli. Chinese J Biotechnol (in Chinese) 28(11):1328–1336
Tomas CA, Beamish J, Papoutsakis ET (2004) Transcriptional analysis of butanol stress and tolerance in Clostridium acetobutylicum. J Bacteriol 186(7):2006–2018
Tomas CA, Welker NE, Papoutsakis ET (2003) Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell’s transcriptional program. Appl Environ Microbiol 69(8):4951–4965
Vollherbst-Schneck K, Sands JA, Montenecourt BS (1984) Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 47(1):193–194
Wang Q, Ou MS, Kim Y, Ingram LO, Shanmugam KT (2010) Metabolic flux control at the pyruvate node in an anaerobic Escherichia coli strain with an active pyruvate dehydrogenase. Appl Environ Microbiol 76(7):2107–2114
Weber FJ, de Bont JA (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta 1286(3):225–245
Wen M, Bond-Watts BB, Chang MC (2013) Production of advanced biofuels in engineered E. coli. Curr Opin Chem Biol 17(3):472–479
Westerman PW, Pope JM, Phonphok N, Doane JW, Dubro DW (1988) The interaction of n-alkanols with lipid bilayer membranes: a 2H-NMR study. Biochim Biophys Acta 939(1):64–78
Zhang H, Chong H, Ching CB, Song H, Jiang R (2012) Engineering global transcription factor cyclic AMP receptor protein of Escherichia coli for improved 1-butanol tolerance. Appl Microbiol Biotechnol 94(4):1107–1117
Zhu L, Dong H, Zhang Y, Li Y (2011) Engineering the robustness of Clostridium acetobutylicum by introducing glutathione biosynthetic capability. Metab Eng 13(4):426–434
Zhu X, Tan Z, Xu H, Chen J, Tang J, Zhang X (2014) Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli. Metab Eng 24:87–96
Zingaro KA, Terry Papoutsakis E (2013) GroESL overexpression imparts Escherichia coli tolerance to i-, n-, and 2-butanol, 1,2,4-butanetriol and ethanol with complex and unpredictable patterns. Metab Eng 15:196–205
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (Grant No. 2011AA02A208 and 2012AA022100) and the National Program on Key Basic Research Project of China (Grant No. 2011CBA00807).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dong, H., Zhao, C., Zhang, T., Lin, Z., Li, Y., Zhang, Y. (2015). Engineering Escherichia coli Cell Factories for n-Butanol Production. In: Ye, Q., Bao, J., Zhong, JJ. (eds) Bioreactor Engineering Research and Industrial Applications I. Advances in Biochemical Engineering/Biotechnology, vol 155. Springer, Berlin, Heidelberg. https://doi.org/10.1007/10_2015_306
Download citation
DOI: https://doi.org/10.1007/10_2015_306
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49159-1
Online ISBN: 978-3-662-49161-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)