Abstract
The migration of leukocytes from blood vessels into inflamed tissues is an essential immunity component. Neutrophils are the first leucocytes to arrive at sites of infection or tissue injuries where they exhibit numerous effector functions. Neutrophil recruitment to inflamed vascular endothelium has been described as a multistep process modulated by chemokines, selectins, and integrins that engage in a stepwise manner to initiate intracellular signals and adhesive bond formation. Thus, chemoattractant-triggered inside-out and integrin-initiated outside-in signaling events cooperate concurrently to increase integrin affinity to its ligands and to stabilize and prolong the arrest of circulating neutrophils. This process enables neutrophils to efficiently navigate the journey from the blood stream to inflammatory sites, which is critical for host defense. However, excessive recruitment of activated neutrophils has been observed to sometimes cause local tissue damage and contribute to the development of inflammatory disorders. Therefore, neutrophils have been implicated in the pathogenesis of both acute and chronic vascular inflammatory diseases. Vascular diseases represent major health problems worldwide. Therefore, due to the economic, social, and health impact, early and precise detections of new biomarkers are crucial to identify the exposed population. Accordingly, the present chapter summarizes recent findings in this area. The aims of this review are to focus on new insights of mechanisms that mediate neutrophil transmigration and to evaluate the adhesive properties of neutrophils as potential biomarkers for vascular diseases.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts of Adhesive Properties of Neutrophils
-
Neutrophils comprise of 40–60 % of the leukocyte population in human blood and play a crucial role in defending the organism, digesting microorganisms by phagocytosis.
-
In order to play the defense function in the organism, neutrophils must first receive the information of an existent infection and then migrate to the area infected through the endothelium line.
-
Endothelium is a tissue that recovers the vascular beds internally. Besides coating and delimitation functions, it acts as a semipermeable membrane, regulating the molecules’ traffic, controlling the regulation of blood flow in vascular resistance, and modulating the immune and inflammatory responses.
-
Cytokines are proteins produced by various cells that send many stimulatory, modulatory, or inhibitory signals for the various cells of the immune system.
-
Selectins are proteins present in endothelial and immune cells. They are responsible for the rolling of leukocytes over the vascular endothelium line. This is the beginning of a cascade of events that leads to the extravasation of neutrophils at sites of injury and to the inflammatory process.
-
The interaction of selectins with their ligands results in a dramatic decline in the speed rate of neutrophils rolling, which allows the activation of proteins known as integrins.
-
Integrins are also transmembrane proteins that promote the firm adhesion of neutrophils to the endothelium.
Definitions
Acute ischemic stroke
Occurs when the blood supply to a part of the brain is cut off due to atherosclerosis or to a blood clot which has blocked a blood vessel.
Acute myocardial infarction
Necrosis of myocardial tissue due to ischemia, usually due to the blockage of a coronary artery by a thrombus Acute ischemic stroke.
Biomarkers
Measurable indicators of a certain biological state or condition.
Cytokines
Cytokines are proteins secreted by cells which have a specific effect on the interactions and communications between cells to coordinate appropriate immune responses.
Deep vein thrombosis
Formation of a blood clot (thrombus) within a deep vein.
“Inside-out” signaling
A process in which stimuli received by cell surface receptors for chemokines, cytokines, and foreign antigens initiate intracellular signals that impinge on integrin cytoplasmic domains and alter adhesiveness for extracellular ligands.
Integrins
Transmembrane receptors responsible for cell-cell and cell-extracellular matrix (ECM) interactions.
Neutrophils
Leukocytes responsible by mediating immune responses against infectious microorganisms.
“Outside-in” signaling
A process in which ligand binding transduces signals from the extracellular domain to the cytoplasm in the classical outside-in direction.
Pulmonary embolism
A condition caused by blood clots that travel to the lungs from the legs or (rarely) other parts of the body.
Vascular disease
Circulation disorders that affect blood vessels (arteries and veins).
Venous thromboembolism
Condition that includes both deep vein thrombosis and pulmonary embolism.
Introduction
Adhesive Properties of Neutrophils
Neutrophils are considered short-lived cells (<6 days) and comprise of 40–60 % of the leukocyte population in human blood. These cells are the first leucocytes to arrive at sites of infections or injuries. Neutrophils present an elaborate and complex migrating process out of the vascular lumen, where they execute the first efforts of defense, exhibiting functions such as killing and phagocytosis of invading pathogens. Furthermore, neutrophils are involved in the inflammatory response, recruiting other leukocytes by the release of proinflammatory cytokines and chemokines (Voisin and Nourshargh 2013).
Neutrophil recruitment to inflamed vascular endothelium has been described as a multistep process modulated by chemokines, selectins, and integrins that engage in a stepwise manner to initiate intracellular signals and adhesive bond formation (Ley 2002). Studies have been conducted using new methods to evaluate and understand the physiology of the leukocyte migration process, and a general, widely validated model describing the entire process has been generated (Montresor et al. 2012).
Briefly, the process of leukocytes adhesion and transmigration through the endothelial wall consists of chemoattraction and rolling, followed by firm attachment and migration to extravascular tissues (Ley et al. 2007; Woodfin et al. 2010). For leucocytes-endothelial cells attachment, it is necessary that rolling leukocytes become fully resistant to the flow and stop on the vessel wall, which is known as stable arrest phase and is considered a critical process in leukocytes adhesion mechanisms. A sudden change in integrin avidity mediates this process (Montresor et al. 2012; Takada et al. 2007).
Integrins are cell adhesion receptors expressed on different cell types that participate in cell-cell or cell-matrix interactions. The integrins on the cell membranes exist as heterodimers composed of one α (alpha) and one β (beta) subunit. In humans, at least 24 different heterodimers formed by the combination of 18 α and 8 β subunits have been indentified (Chigaev and Sklar 2012). Each subunit contains a large extracellular domain, a single transmembrane helix, and a short cytoplasmic domain (Hynes 2002).
In the circulation, integrins are normally found in a low-affinity state for the ligands. However, these receptors undergo a structural and topological change to increase its binding efficiency, through spatial rearrangement on the cell plasma membrane. This rearrangement leads to the integrin activation, which is mandatory for the quick arrest of the circulating cells (Montresor et al. 2012).
The step of integrin activation involves bidirectional signals across the cytoplasmic membrane, called inside-out and outside-in signaling pathway (Ginsberg et al. 2005). Wang and Luo (2010) have suggested a model of inside-out activation of integrins that involves the binding of intracellular proteins to the integrin cytoplasmic domains. It has been described that the cellular activator talin mediates the binding of the actin filaments to integrin β subunit cytoplasmic domain, promoting the separation of the integrin α and β helix (Hu and Luo 2013; Tadokoro et al. 2003). After separation, the α subunit helix maintains a similar structure, whereas the β subunit helix is tilted by inserting 5–6 residues into the hydrophobic lipid membrane core. This process of separation of the two transmembrane helices leads to the extension and swing-out of the hybrid domain, resulting in a switchblade-like conformational change of the integrin’s extracellular domains; as a result, integrin exhibits a high-affinity state for ligands (Hogg et al. 2011; Schürpf and Springer 2011).
The outside-in signaling pathway requires the binding of integrins to extracellular ligands, which results in a variety of signal transductions across the plasma membrane, which enhance cell adhesiveness (Arnaout et al. 2005; Montresor et al. 2012). Possibly the most important process of outside-in signaling is the lateral mobility. This process initiates with the contact of integrins with extracellular matrix ligands and integrins clustering, which increases ligand binding valency and avidity. This lateral association of integrin heterodimers transfers extracellular information into corresponding intracellular reactions by the recruitment of effectors to the integrin cytoplasmic tail, as well as defines stable connections to the extracellular matrix (Hu and Luo 2013). The outside-in signaling is responsible for the regulation of cell migration, differentiation, proliferation, and survival (Wang et al. 2011; Wang and Luo 2010; Luo et al. 2007; Hu and Luo 2013) (Fig. 1).
Integrin activation process Integrin activation involves bidirectional signals across the cytoplasmic membrane, called “inside-out” and “outside-in” signaling pathway. Both mechanisms act together to promote integrin conformational changes and activation. Integrin conformational states differ both in their overall extension over the plasma membrane as well as in the arrangement of their headpiece. (a) Resting state (low affinity); (b) First step activation involves switchblade-like conformational change of integrin extracellular domains; (c) Integrin’s high affinity state, with hybrid domain epitope exposed
Neutrophils express a variety of adhesion molecules on their surfaces that are required for transendothelial migration. The l- and P-selectin mediate tethering and rolling on the endothelium, while firm adhesion is mediated by Very Late Antigen-4 (VLA-4, CD49D/CD29) and by β2 integrin-complex, as Macrophage antigen-1 (Mac-1, CD11b/CD18) and Lymphocyte Function-associated Antigen-1 (LFA-1, CD11a/CD18) (Petri and Bixel 2006).
VLA-4 Integrin exhibits a low-affinity state, bent conformation, with a hidden hybrid domain epitope, in the absence of an extracellular ligand. Recent insights based in small fluorescent ligand-mimicking probes have suggested that the activation process of VLA-4 includes multiple complex molecule conformational states that involve extension of integrin and hidden or exposed hybrid domain epitope (Chigaev and Sklar 2012).
The most detailed description during mechanisms of integrin activation in leukocytes comes from studies of LFA-1 (Montresor et al. 2012). LFA-1 adhesion molecule was one of the first integrins described as a participant of the firm cell adhesion process of activated cells (Chigaev and Sklar 2012).
Recently, it has been demonstrated that LFA-1 may assume at least three distinct conformations, distinct either in their complete extension over the cytoplasmic membrane or in the availability of their headpiece (Montresor et al. 2012; Springer and Dustin 2012; Nishida et al. 2006).
It has been suggested that the extended conformation of LFA-1, with high topographical availability of the ligand-binding headpiece, can also present a low-affinity state (Montresor et al. 2012; Salas et al. 2006). This conformation of low/intermediate-affinity state could enhance the ability of LFA-1 in mediating the rolling process on endotheIial cells; in this situation the affinity of LFA-1 for ICAM-1 (Intercellular Adhesion Molecule I) increases upon the selectin triggering (Montresor et al. 2012; Miner et al. 2008). Importantly, low, intermediate, and high affinity integrins possibly constitute discrete and reversible states in a progression of integrin structural rearrangement (Montresor et al. 2012; Shamri et al. 2005).
Therefore, neutrophils transmigration process can be moderated by the same adhesion molecule existing in different conformers, which can be reversibly controlled through these cellular signaling pathways (Chigaev and Sklar 2012). Furthermore, application of a mechanical force can lead to the stabilization of ligand binding or “catch bond” (Kong et al. 2009), once lateral shear force can notably change the activity state of LFA-1 molecule (Hogg et al. 2011) (Fig. 2).
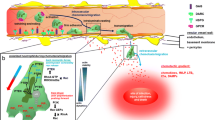
Neutrophils adhesion and transmigration through the endothelium line Process of (1) leukocytes adhesion and transmigration through the endothelial wall consists of chemoattraction and rolling (2), followed by firm adhesion (3) and migration to extravascular tissues (4). Circulating leucocytes attach to the endothelium by the interaction of leucocytes and endothelium selectins and initiate the rolling process. When the rolling leukocytes become fully resistant to the flow and stop on the vessel wall, a sudden change in integrin avidity may occur and promote a firm leucocyte-endothelial adhesion. The leucocyte-endothelial adhesion is mediated mainly by MAC-1 and LFA-1 interactions with their endothelial ligands (ICAM-1). After the firm adhesion process, neutrophils migrate through the endothelium to the site of inflammation. ICAM-1: Intercellular Adhesion Molecule-1, MAC-1: Macrophage antigen-1, LFA-1: Lymphocyte Function-associated Antigen-1
Accordingly, integrin-initiated outside-in and chemoattractant-triggered inside-out signaling cascades simultaneously to collaborate to the enhanced integrin affinity for the ligand and to the maintenance and prolongation of the arrested leukocytes in circulation (Montresor et al. 2012). Thereby, these phenomena modulate the process of neutrophil adhesion and migration to areas of inflammation, which is important for host defense (Askari et al. 2009).
However, researchers have observed that excessive recruitment of activated neutrophils can cause damage to the host and contribute to the development of inflammatory disorders. Therefore, neutrophils have been implicated in the pathogenesis of both acute (e.g., myocardial infarction) and chronic (e.g., atherosclerosis) vascular inflammatory conditions (Voisin and Nourshargh 2013).
Vascular Disease and Adhesive Properties of Neutrophils
Vascular diseases are multifactorial pathological conditions that represent a major health problem worldwide. Therefore, due to the economic, social, and health impact, early and precise detections of new biomarkers are crucial to identify the exposed population.
Acute coronary events are associated with activated leukocytes and an intense inflammatory response. Moreover, inflammatory pathways promote thrombosis, a late and dreaded complication of atherosclerosis responsible for myocardial infarctions and most strokes (Libby 2002).
Studies of necropsies of patients with acute myocardial infarction (AMI) have shown neutrophilic infiltration of necrotic myocardial tissue within the first day of onset of acute myocardial infarction (Swirski 2014). However, the presence of leukocytes in the myocardium requires endothelial transmigration or diapedesis, which is facilitated by increasing the expression of adhesion molecules by endothelial and leukocyte cells (Meisel et al. 1998). Therefore, the increased neutrophil counts during an episode of myocardial infarction could possibly be accompanied by a corresponding increase in the expression of cell-surface adhesion molecules (Meisel et al. 1998).
Meisel and coworkers (1998) evaluated the expression of neutrophil adhesion molecules in patients with AMI, and they observed that the expression of Mac-1 was increased in patients by 133 % (p < 0.001) on day 1 compared with age-matched control subjects. In addition, neutrophils isolated from AMI patients showed elevated neutrophil adhesion to endothelial cells compared to those isolated from controls. The treatment with anti-CD11b antibodies significantly reduced neutrophil adhesion when compared with the untreated control group (Han et al. 2012). The described evidences of changes in the expression of neutrophil cell surface adhesion molecules are important and clinically relevant. Enhanced neutrophil adhesiveness could be involved in the myocardial reperfusion failure after thrombolysis, known as “no-reflow” or “slow-reflow” phenomenon (Gibson et al. 1996), and could also be associated with postinfarction events such as ongoing ischemia and infarction extension. Activated leukocytes exposing adhesion molecules in a high-affinity state could adhere to altered coronary endothelium, culminating in a lesion and alteration in the local vasomotor function, therefore compromising runoff flow (Meisel et al. 1998; Mügge et al. 1991).
The increased neutrophil adhesion in AMI patients can be related to increased inflammation. Peripheral white blood cell (WBC) counts increase significantly after myocardial infarction and are associated with disease severity (Packard and Libby 2008; Chia et al. 2009). In response to inflammatory signals, the adhesion and migration of leukocytes are crucial, and neutrophil adhesion to endothelial cells through adhesion molecules is a central feature of the inflammatory response (Butcher 1991).
Another study showed that ICAM-1-dependent neutrophil adherence plays an important role in reperfusion injury and that neutrophils’ adherence and infiltration contribute significantly to coronary endothelial dysfunction (Ma et al. 1992).
Inflammation also plays an important role in acute ischemic stroke. Much of the damage develops gradually over the course of a few hours. It is believed that leukocytes liberate inflammatory cytokines and other neurotoxins in the ischemic brain. Moreover, previous evidence has demonstrated that microvascular occlusion is started through platelet-leukocyte-endothelium interactions in the ischemic penumbra (Alvaro-González et al. 2002; Chamorro 2004; Tsai et al. 2009).
Patients with ischemic stroke demonstrated changes in β2 integrin expression (Kim et al. 1995). Furthermore, neutrophil adhesion molecules were also evaluated in patients during the stroke onset and on days 7, 30, and 90 post stroke (Tsai et al. 2009). This study highlighted at least three important points. First, the findings showed increased expressions of P-selectin glycoprotein ligand-1 (PSGL-1) of neutrophils in acute stroke patients, and this neutrophil activation persisted for at least 3 months after the onset of cerebral ischemia. The PSGL-1 glycoprotein link to the E-selectin and P-selectin expressed in the surface of endothelium cells; besides, neutrophils can adhere to platelets via PSGL-1/P-selectin interaction. Neutrophil PSGL-1 plays an important role in arterial thrombogenesis by forming stable platelet-leukocyte aggregates (McEver and Cummings 1997). This finding suggests that the persistent activation of circulating neutrophils play a pathophysiological role in the acute and chronic phases following an ischemic stroke. Additionally, this study observed that the expression of Mac-1 on neutrophils is enhanced immediately after the stroke and is normalized during the following months. Thus, it may be hypothesized that circulating neutrophils interact continuously with activated endothelium after acute ischemic stroke. Sustained leukocyte-endothelium interaction after cerebral ischemia may cause substantial inflammatory reaction and lead to secondary injury of potentially salvageable neurons in the penumbra surrounding the infarct. Finally, neutrophil PSGL-1 expression on day 1 was observed to be significantly higher in patients who develop neurological deterioration (END). Increasing evidence indicates that there is an association between increased risk of reinfarction and inhospital death with high WBC counts, especially neutrophil counts (Fisher and Meiselmann 1994). Consequently, early recruitment-adherent neutrophils after ischemic stroke seem to play an important role in patients with a stroke in progression (Tsai et al. 2009).
The adhesive properties of neutrophils have also been discussed in sickle cell disease (SCD) (Canalli et al. 2011). SCD is characterized by red blood cell sickling, hemolysis, and a chronic inflammatory state in which the leukocyte plays an important role. Microvascular occlusion is responsible for much of the pathophysiology that underlies the clinical manifestations of SCD. Although vascular occlusion is mediated at least in part by the inability of poorly deformable, irreversibly sickled red blood cells to traverse microcirculation, other vaso-occlusive processes have also been implicated (Kasschau et al. 1996). Sickle cell crises are often associated with infection and neutrophil counts are higher in individuals with this disease (Okpala 2004).
Reports suggest that initiation and propagation of a vaso-occlusive event occurs by impaired blood flow due to excessive recruitment of adherent leucocytes to the vascular endothelium and their interactions with circulating erythrocytes (Canalli et al. 2008; Chiang and Frenette 2005).
In addition, studies using intravital microscopy techniques during a flowing inflammatory stimulus demonstrated that leukocytes, particularly neutrophils, of mice expressing sickle haemoglobin adhere to the vascular endothelium and interact with sickle red cells initiating a vaso-occlusive process (Turhan et al. 2002). This data strengthens the hypothesis that neutrophils play a direct role in the sickle cell vaso-occlusion and vascular complications.
Studies in vitro have demonstrated that neutrophils from SCD patients have an increased adherence to endothelial layers compared to control neutrophils, and similar studies showed that SCD neutrophils also display augmented adhesion to integrin ligands such as fibronectin (extracellular matrix component) and ICAM-1 (Fadlon et al. 1998; Kasschau et al. 1996; Canalli et al. 2008). β2 integrins, particularly the Mac-1, have been reported as highly expressed on the surface of neutrophils from SCD patients in steady state (Lum et al. 2004). Interestingly, Mac-1 expression is further increased in the presence of interleukin-8 (IL-8) (Assis et al. 2005). IL-8 is a chemokine found in high levels in the circulation of SCD individuals, demonstrating that inflammatory environment may further augment altered SCD neutrophil functions (Gonçalves et al. 2001).
Data provided by in vitro investigation indicated that, in healthy individuals, neutrophil adhesions to endothelial cells are mediated mainly by the Mac-1 integrin with a contribution from the LFA-1 integrin, under inflammatory stimulus. On the other hand, under basal and inflammatory conditions, Mac-1, LFA-1 integrin, as well as VLA-4 integrins apparently mediate the adhesion of SCD neutrophils to the endothelium (Canalli et al. 2011). These results suggest that VLA-4 integrins also play a role in SCD neutrophil adhesion to the vascular endothelium.
However, previous data from the same SCD cohort, under similar experimental conditions, showed that neither Mac-1 nor LFA-1 nor VLA-4 surface expressions were significantly altered on nonstimulated SCD neutrophils (Canalli et al. 2008; Assis et al. 2005). LFA-1 and Mac-1 integrins are believed to mediate adhesive interactions via conformational changes, resulting in increased ligand affinity. Thus, these results consistently indicate that increased integrin affinity, rather than significant changes in surface protein expression, bring about the observed increase in adhesive properties of SCD neutrophils.
Despite the VLA-4 integrin low expression in the SCD neutrophil cell membrane, as described previously, this integrin may be found in several conformational states and affinities (Chigaev and Sklar 2012). The exposure of the hybrid domain epitope can also be used to determine VLA-4 ligand binding affinity for unlabeled ligands (Chigaev et al. 2009; Njus et al. 2009). Furthermore, VLA-4 integrin has been implicated in the recruitment of neutrophils during chronic inflammation (Burns et al. 2001; Issekutz et al. 2003), and possibly the inflammatory state associated with SCD stimulates this adhesion molecule on neutrophils.
A recent study using intravital microscopy, in venous thromboembolism (VTE), a disease which comprises of deep venous thrombosis (DVT) and pulmonary embolism (PE), demonstrated that a reduction in blood flow induces a proinflammatory endothelial phenotype that initiates neutrophil recruitment. Recruited neutrophils start fibrin formation via blood cell-derived tissue factor (TF), which is the decisive trigger to the massive fibrin deposition seen in DVT (Saha et al. 2011).
In addition, it was demonstrated in a recent study that an inflammatory profile, expressed by increased adhesion of neutrophils, was associated with a hypercoagulability state in VTE patients, even after the acute DVT episode (between 1 and 6 years after the thrombotic event) (Zapponi et al. 2014). The results could demonstrate a trend toward an increase in the adhesive properties of neutrophils in VTE patients when compared with healthy individuals. Patients were also analyzed in separate groups, and VTE patients with higher d-dimer plasma levels and residual vein occlusion (RVO) presented the highest neutrophils adhesiveness and also had higher levels of circulating inflammatory markers, such as interleukin-6 (IL-6), IL-8, and TNF-α. Interestingly, increased d-dimer levels is a known marker of hypercoagulability (Verhovsek et al. 2008; Tosetto et al. 2012; Carrier et al. 2011; Cosmi et al. 2005; Cosmi et al. 2010). Furthermore, the increase of neutrophils adhesive properties was positively correlated with IL-6 and d-dimer levels, suggesting a possible relationship between these factors.
The scientific community has been discussing the relationship between inflammation and coagulation in the pathogenesis of vascular disease. Evidence points to extensive cross-talk between these two systems, involving platelet activation, fibrin formation and resolution, as well as anticoagulant pathways (Levi et al. 2004).
Inflammation-induced activation of coagulation is a described mechanism believed to be beneficial for host defense in distinct situations. Procoagulant proteins, particularly the tissue factor, expressed by inflammatory cells mediate activation of coagulation cascade and thrombin generation. Thrombin activates platelets and generates platelet-fibrin thrombi. Proinflammatory cytokines may also affect all these coagulation mechanisms and the natural anticoagulant pathways (Levi and Van der Poll 2010).
Moreover, activated coagulation proteases, anticoagulants, or components of the physiological fibrinolytic system can modulate the inflammatory response through specific cellular receptors on inflammatory and endothelial cells. In addition, the binding of tissue factor-factor VIIa to the protease-activated receptors (PAR-2) results in upregulation of inflammatory responses affecting neutrophil infiltration and proinflammatory cytokine expression (Cunningham et al. 1999).
Therefore, the increased inflammatory markers in VTE patients could enhance the expression of adhesion molecules on endothelial cells (Mihara et al. 2012; Romano et al. 1997; Rincon 2012) and neutrophil adhesion properties, triggering a vicious circle involving inflammation, increased neutrophil adhesion, and activation of coagulation.
In summary, recent studies have supported the hypothesis of an association between inflammation and hypercoagulability, and highlighted the role of neutrophils in this process.
The Applicability of Adhesive Properties of Neutrophils in Clinical Practice
Considerable efforts have been made to achieve the validity and usefulness of diagnostic tests in the interface between clinical medicine and scientific methods. To consider a new method as a potential biomarker, at least four aspects should be evaluated: sensitivity, specificity, predictive value, and prognosis value (Sackett and Haynes 2002).
Sensitivity evaluates whether patients with a target disorder and normal individuals differ regarding test results. As a result, this phase of a diagnostic test evaluation cannot be translated into diagnostic action; however, these studies add biological insights into the mechanisms of disease. As discussed previously, all studies conducted on the adhesive properties of neutrophils in patients with vascular diseases compared with normal individual controls answered the sensitivity issue, highlighting that neutrophil adhesion is higher in patients with vascular diseases.
Specificity of an assay is detected when the test results discriminate between patients and normal individuals. Studies of specificity were not conducted using neutrophil adhesion as a biomarker.
The predictive value of an assay is accessed when specific results predict the disease diagnosis. Studies of predictive value were not conducted using neutrophil adhesion as a biomarker either.
The prognosis value is assessed to evaluate the case where patients who undergo a specific diagnosis fare better (in their ultimate health outcomes) than similar patients who have not been tested (Sackett and Haynes 2002). In this context, neutrophil adhesion may be a marker of poorer prognosis for vascular diseases. At least two studies addressed this question, despite this issue not being their specific goal. A recent study performed by our group, that evaluated the adhesive properties of neutrophils in VTE patients, demonstrated that patients with increased adhesive profile of neutrophils also presented the highest risk of recurrence of the disease (factors known) (Zapponi et al. 2014). Another study that aimed to assess whether the adhesion molecules of leukocytes could be predictive of the clinical outcomes in patients after a stroke showed that neutrophil PSGL-1 expression was significantly higher in patients with early neurological deterioration (Tsai et al. 2009). Therefore, early recruitment-adherent neutrophils after ischemic stroke seem to play an important role in patients with stroke in progression, as well as in VTE patients.
In conclusion, until now, one cannot assume that the adhesive properties of neutrophils can be used as biomarkers of vascular disease. Nevertheless, the adhesive properties of neutrophils can be viewed as potential biomarkers, as they appear to present some sensitivity and prognostic value to evaluate vascular diseases. Therefore, these results should be validated in other independent studies to arrive at a definite conclusion. In addition, this possible diagnostic test can be associated with a multivariate combination of several other clinical signs detected in medical history, physical examination, or other tests.
Methods to Evaluate Adhesive Properties of Neutrophils
Advances in the understanding of neutrophil adhesive properties have been accomplished using in vitro tools. The first step to evaluate adhesive properties of neutrophils is the isolation of neutrophil cells from the peripheral blood. Neutrophils may be isolated from fresh peripheral blood collected in heparin-containing tubes, using two layers of Ficoll-Paque with different densities (Assis et al. 2005; Zapponi et al. 2014).
Flow cytometry has been widely used in biomedical research. Presently, flow cytometry is used as an additional technique to confirm diagnosis carried out by morphological studies and provides valuable prognostic information. As the number of laboratories with flow cytometry increases, greater quality control, standardization of techniques, and interlaboratory programs will be required (Wu et al. 2010).
Flow cytometry is a powerful analytical tool for the analysis of multiple biological parameters of individual cells or multiple heterogeneous cell populations (Wu et al. 2010). The methodology of flow cytometry enables fast, accurate, and quantitative analysis of cells in suspension. Furthermore, as this is an automatized technique, flow cytometry is one of the methods of choice to evaluate the adhesive properties of neutrophils. As a limitation, the method is costly and demands a technical expert to be performed.
Samples are analyzed by immunofluorescence staining with different fluorochromes, which are excited by the laser. The antibodies bound to the fluorochrome react with the specific antigenic determinants or epitopes on the surface or inside the cells. The initial cell separation step is critical, because it can help eliminate some unwanted populations. Light scattering can be utilized to separate these populations. Cells with more intense fluorescence are those which are tagged with antibodies. Currently, there is a range of antibodies that not only evaluate the expression of adhesion molecules but also their activated epitopes, which facilitates the evaluation of the activated adhesive properties of the neutrophils by flow cytometry. However, in the context of adhesive properties of neutrophils, one of the limitations of flow cytometry is that the integrins’ avidity is not taken into account (Tables 1, 2).
Another technique frequently used to evaluate the adhesive properties of neutrophils is the static adhesion assay . The static adhesion assay is a sensitive and versatile in vitro assay, known for mimicking low-shear conditions or interrupted blood flow, which may occur in occlusive vascular events. The main advantages of the static adhesion assay, when compared to other methods of measuring cell adhesion, may be the ability to measure both avidity and affinity of adhesion molecules, examine multiple experimental conditions simultaneously, and it does not require expensive equipment. Another advantage of this method is the ability to detect a small number of cell-adhesion events with accuracy (Bellavite et al. 1992). The intra-assay validation requires that more than 95 % of the cells are viable. In case of significant variability within the same condition, more than three identical replicates should be used. However, this technique is not free of limitations. One potential weakness is the fact that the cell-adhesion events are represented as a percentage, a relative number which may vary from one experiment to another. In order to achieve uniform results, some steps in the protocol must be followed, such as the incubation time and the number of seeded cells which must be uniform among all conditions. Small differences in incubation time may result in inaccurate measurements. It is also important to aliquot exactly the same amount of cells in each well. Another limitation is the fact that this method requires the use of freshly viable cells, and for that the samples should be collected and prepared quickly, which may not be practical in a daily routine. Future improvements of the static adhesion assay may involve automatized cell-aliquot and washing, in order to standardize the process. In addition, an automatized version of the assay would enable the performance of large-scale screening tests (Zapponi et al. 2014).
Conclusions and Perspective
Structural, biochemical, and biophysical studies have greatly contributed to the understanding of the mechanisms of integrin bidirectional signaling across the plasma membrane. Chemoattractant-triggered inside-out and integrin-initiated outside-in signaling events are known to concurrently cooperate to increase integrin affinity for the ligand and to stabilize and prolong the arrest of circulating leukocytes. This process enables neutrophils to efficiently navigate from the blood stream to inflammatory sites, which is critical for host defense. Nevertheless, the excessive recruitment of activated neutrophils can cause damage to the host and contribute to the development of inflammatory disorders. In this context, studies have emphasized the participation of adherent neutrophils in the pathogenesis of both acute and chronic vascular diseases but more importantly have highlighted an association between neutrophils and disease progression. However, until now, it has not been possible to assume that the adhesive properties of neutrophils could be used as biomarkers of vascular disease, mainly because the methodologies carried out so far are not applicable in a clinical routine, and also due to the lack of validation of the results of studies conducted to date. However, adhesive properties of neutrophils are possibly potential biomarkers, as they seem to present some sensitivity and prognostic value to evaluate vascular diseases.
Summary Points
-
This chapter focuses on the adhesive properties of neutrophils as a potential biomarker to evaluate vascular disease.
-
The migration of neutrophils from blood to inflamed tissues is modulated by chemokines, selectins, and integrins that initiate intracellular signals and adhesive bond formation.
-
The excessive recruitment of activated neutrophils can cause local tissue damage and contribute to the development of inflammatory disorders.
-
Neutrophils have been implicated in the pathogenesis of both acute and chronic vascular inflammatory diseases.
-
Vascular diseases are disorders of the vascular system that impair the blood flow. These diseases are caused by the formation of clots in the blood stream or by the inflammation of the endothelial tissue.
-
Vascular diseases represent major health problems worldwide; therefore, due to the economic, social, and health impact, early and precise detections of new biomarkers are crucial to identify the exposed population.
Abbreviations
- AMI:
-
Acute myocardial infarction
- CRP:
-
C-reactive protein
- CRP:
-
C-reactive protein
- DVT:
-
Deep venous thrombosis
- END:
-
Neurological deterioration
- ICAM-1:
-
Intercellular Adhesion Molecule 1
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- LFA-1:
-
Lymphocyte Function-associated Antigen-1 alphaL beta2 integrin
- Mac-1:
-
Macrophage antigen-1 alphaM integrin
- PAR:
-
Protease-activated receptors
- PE:
-
Pulmonary embolism
- PSGL-1:
-
P-selectin glycoprotein ligand-1
- RVO:
-
Residual vein occlusion
- SCD:
-
Sickle cell disease
- TF:
-
Tissue factor
- TNF-α:
-
Tumor necrosis factor- alpha
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VLA-4:
-
Very Late Antigen-4 alpha4 beta1 integrin
- VTE:
-
Venous thromboembolism
- WBC:
-
White blood cell
References
Alvaro-González LC, Freijo-Guerrero MM, Sádaba-Garay F. Inflammatory mechanisms, arteriosclerosis and ischemic stroke: clinical data and perspectives. Rev Neurol. 2002;35(5):452–62.
Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410.
Askari JA, Buckley PA, Mould AP, et al. Linking integrin conformation to function. J Cell Sci. 2009;122(Pt 2):165–70.
Assis A, Conran N, Canalli AA, et al. Effect of cytokines and chemokines on sickle neutrophil adhesion to fibronectin. Acta Haematol. 2005;113(2):130–6.
Bellavite P, Chirumbolo S, Mansoldo C, et al. Simultaneous assay for oxidative metabolism and adhesion of human neutrophils: evidence for correlations and dissociations of the two responses. J Leukoc Biol. 1992;51(4):329–35.
Burns JA, Issekutz TB, Yagita H, et al. The alpha 4 beta 1 (very late antigen (VLA)-4, CD49d/CD29) and alpha 5 beta 1 (VLA-5, CD49e/CD29) integrins mediate beta 2 (CD11/CD18) integrin-independent neutrophil recruitment to endotoxin-induced lung inflammation. J Immunol. 2001;166(7):4644–9.
Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–6.
Canalli AA, Franco-Penteado CF, Saad ST, et al. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica. 2008;93(4):605–9.
Canalli AA, Proença RF, Franco-Penteado CF, et al. Participation of Mac-1, LFA-1 and VLA-4 integrins in the in vitro adhesion of sickle cell disease neutrophils to endothelial layers, and reversal of adhesion by simvastatin. Haematologica. 2011;96(4):526–33.
Carrier M, Rodger MA, Wells PS, et al. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost. 2011;9(6):1119–25.
Chamorro A. Role of inflammation in stroke and atherothrombosis. Cerebrovasc Dis. 2004;17(3):1–5.
Chia S, Nagurney JT, Brown DF, et al. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103(3):333–7.
Chiang EY, Frenette PS. Sickle cell vaso-occlusion. Hematol-Oncol Clin North Am. 2005;19:771–84.
Chigaev A, Sklar LA. Aspects of VLA-4 and LFA-1 regulation that may contribute to rolling and firm adhesion. Front Immunol. 2012;3:242.
Chigaev A, Waller A, Amit O, et al. Real-time analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation. J Biol Chem. 2009;284(21):14337–46.
Cosmi B, Legnani C, Cini M, et al. d-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. 2005;94(5):969–74.
Cosmi B, Legnani C, Iorio A, et al. Residual venous obstruction, alone and in combination with d-dimer, as a risk factor for recurrence after anticoagulation withdrawal following a first idiopathic deep vein thrombosis in the prolong study. Eur J Vasc Endovasc Surg. 2010;39(3):356–65.
Cunningham MA, Romas P, Hutchinson P, et al. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94(10):3413–20.
Fadlon E, Vordermeier S, Pearson TC, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266–74.
Fisher TC, Meiselmann HJ. Polymorphonuclear leukocytes in ischemic vascular disease. Thromb Res. 1994;74(1):S21–34.
Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–88.
Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17(5):509–16.
Gonçalves MS, Queiroz IL, Cardoso AS, et al. Interleukin 8 as a vaso-occlusive marker in Brazilian patients with sickle cell disease. Braz J Med Biol Res. 2001;34(10):1309–13.
Han L, Shen X, Pan L, et al. Aminobenzoic acid hydrazide, a myeloperoxidase inhibitor, alters the adhesive properties of neutrophils isolated from acute myocardial infarction patients. Heart Vessels. 2012;27(5):468–74.
Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–26.
Hu P, Luo BH. Integrin bi-directional signaling across the plasma membrane. J Cell Physiol. 2013;228(2):306–12.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87.
Issekutz AC, Nakazato S, Issekutz TB. Differential roles of VLA-4(CD49d/CD29) and LFA-1(CD11a/CD18) integrins and E- and P-selectin during developing and established active or adoptively transferred adjuvant arthritis in the rat. Immunol Cell Biol. 2003;81(5):397–408.
Kasschau MR, Barabino GA, Bridges KR, et al. Adhesion of sickle neutrophils and erythrocytes to fibronectin. Blood. 1996;87(2):771–80.
Kim JS, Chopp M, Chen H, et al. Adhesive glycoproteins CD11a and CD18 are upregulated in the leukocytes from patients with ischemic stroke and transient ischemic attacks. J Neurol Sci. 1995;128(1):45–50.
Kong F, García AJ, Mould AP, et al. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185(7):1275–84.
Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–704.
Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2):S26–34.
Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89.
Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev. 2002;186:8–18.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74.
Lum AF, Wun T, Staunton D, et al. Inflammatory potential of neutrophils detected in sickle cell disease. Am J Hematol. 2004;76(2):126–33.
Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47.
Ma XL, Lefer DJ, Lefer AM, et al. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992;86(3):937–46.
McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11):S97–103.
Meisel SR, Shapiro H, Radnay J, et al. Increased expression of neutrophil and monocyte adhesion molecules LFA-1 and Mac-1 and their ligand ICAM-1 and VLA-4 throughout the acute phase of myocardial infarction: possible implications for leukocyte aggregation and microvascular plugging. J Am Coll Cardiol. 1998;31(1):120–5.
Mihara M, Hashizume M, Yoshida H, et al. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122(4):143–59.
Miner JJ, Xia L, Yago T, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112(5):2035–45.
Montresor A, Toffali L, Constantin G, et al. Chemokines and the signaling modules regulating integrin affinity. Front Immunol. 2012;3:127.
Mügge A, Heistad DD, Padgett RC, et al. Mechanisms of contraction induced by human leukocytes in normal and atherosclerotic arteries. Circ Res. 1991;69(3):871–80.
Nishida N, Xie C, Shimaoka M, et al. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25(4):583–94.
Njus BH, Chigaev A, Waller A, et al. Conformational mAb as a tool for integrin ligand discovery. Assay Drug Dev Technol. 2009;7(5):507–15.
Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Rev. 2004;18(1):65–73.
Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38.
Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J. 2006;273(19):4399–407.
Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33(11):571–7.
Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–25.
Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ. 2002;324(7336):539–41.
Saha P, Humphries J, Modarai B, et al. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31(3):506–12.
Salas A, Shimaoka M, Phan U, et al. Transition from rolling to firm adhesion can be mimicked by extension of integrin alphaLbeta2 in an intermediate affinity state. J Biol Chem. 2006;281(16):10876–82.
Schürpf T, Springer TA. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30(23):4712–27.
Shamri R, Grabovsky V, Gauguet JM, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6(5):497–506.
Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24(1):107–15.
Swirski FK. Inflammation and repair in the ischaemic myocardium. Hamostaseologie. 2014;35(1):34–6.
Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302(5642):103–6.
Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215.
Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. 2012;10(6):1019–25.
Tsai NW, Chang WN, Shaw CF, et al. The value of leukocyte adhesion molecules in patients after ischemic stroke. J Neurol. 2009;256(8):1296–302.
Turhan A, Weiss LA, Mohandas N, et al. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047–51.
Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: d-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med. 2008;149(7):481–90. W94.
Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5(4):336–47.
Wang W, Luo BH. Structural basis of integrin transmembrane activation. J Cell Biochem. 2010;109(3):447–52.
Wang W, Zhu J, Springer TA, et al. Tests of integrin transmembrane domain homo-oligomerization during integrin ligand binding and signaling. J Biol Chem. 2011;286(3):1860–7.
Woodfin A, Voisin MB, Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr Opin Hematol. 2010;17(1):9–17.
Wu DY, Patti-Diaz L, Hill CG. Development and validation of flow cytometry methods for pharmacodynamic clinical biomarkers. Bioanalysis. 2010;2(9):1617–26.
Zapponi KC, Mazetto BM, Bittar LF, et al. Increased adhesive properties of neutrophils and inflammatory markers in venous thromboembolism patients with residual vein occlusion and high d-dimer levels. Thromb Res. 2014;133(5):736–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Zapponi, K.C.S. et al. (2016). Adhesive Properties of Neutrophils as a Possible Biomarker of Vascular Disease. In: Patel, V., Preedy, V. (eds) Biomarkers in Cardiovascular Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7678-4_24
Download citation
DOI: https://doi.org/10.1007/978-94-007-7678-4_24
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7677-7
Online ISBN: 978-94-007-7678-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences