Abstract
Cerebellar mutism – a term introduced by Rekate et al. in 1985 – can be considered as the hallmark feature of the posterior fossa syndrome (PFS). This syndrome was first described in detail by Daly and Love in 1958 and consists of specific linguistic, cognitive, behavioral, and affective symptoms following acute posterior fossa damage in children and adults. Although the syndrome has been exceptionally associated with non-tumoral etiologies, it usually develops after a brief period of relatively normal functioning in the immediate postoperative phase following posterior fossa tumor surgery. While the incidence of the PFS in the pediatric population is estimated to range between 8% and 31% after posterior fossa tumor resection, the condition is extremely rare in adults. As such, the PFS is a clinical condition typically affecting children operated for posterior fossa tumors.
Although more than 300 etiologically heterogeneous cases of PFS have been reported in the literature and many hypotheses have been proposed to explain the PFS, the underlying pathophysiological mechanisms remain unclear. This chapter presents a brief overview of the intriguing semiological combination of transient cerebellar mutism and cognitive, behavioral, and affective alterations following acute posterior fossa lesions. Furthermore, the most important pathophysiological hypotheses will be briefly discussed.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
During the last three decades, insights into the non-motor role of the cerebellum in behavioral, cognitive, and affective processes have evolved from a mere afterthought to an exciting area of innovative research across disciplines. Recent advances in the understanding of the neuroanatomy of the cerebellum and its reciprocal connections with the supratentorial brain regions subserving cognition, behavior, and affect, combined with evidence from functional neuroimaging studies, neurophysiological research data, and clinical neuropsychological findings have substantially adjusted the traditional view on the cerebellum as a sole coordinator of autonomic and somatic motor function (Beaton and Mariën 2010). Schmahmann and Sherman (1998) have integrated the evidence from these strands of research in a study of 20 adult patients with cerebellar lesions and concluded in their landmark paper that acute damage to cerebellar structures may induce a typical combination of non-motor cognitive, behavioral, and affective symptoms. This combination of executive, visuospatial, affective, and linguistic symptoms was labeled the “cerebellar cognitive-affective syndrome” (CCAS). Based on a substantial amount of anatomical, experimental, and clinical evidence, Schmahmann and Sherman (1998) related CCAS in patients with focal cerebellar lesions to acute and often transient disruption of the associative cerebro-cerebellar circuitry as reflected by crossed cerebello-cerebral diaschisis. According to this view, cognitive and behavioral dysfunctions within CCAS result from a temporary functional depression of the reciprocal pathways that connect the cerebellum with the limbic circuitry and the prefrontal, temporal, and parietal association cortices. Since its description in the late 1990s, CCAS has been well documented in children and adults with etiologically heterogeneous acquired as well as developmental neurological disorders that primarily affect the cerebellar structures (e.g., Levisohn et al. 2000; Paulus et al. 2002; Mariën et al. 2008, 2010; Baillieux et al. 2010).
The posterior fossa syndrome (PFS), which is also known as “cerebellar mutism syndrome” (CMS) (e.g., Janssen et al. 1998; Robertson et al. 2006) or “mutism and subsequent dysarthria” (MSD) (e.g., Van Dongen et al. 1994; Catsman-Berrevoets et al. 1999), is just like CCAS a syndrome following acute damage to the cerebellum. It is characterized by transient mutism combined with cognitive, behavioral, and affective disturbances. The PFS may occur in the context of non-tumoral etiologies such as traumatic brain injury (Ersahin et al. 1997; Koh et al. 1997; Fujisawa et al. 2005), vascular disorders (Dietze and Mickle 1990; Sinha et al. 1998; Baillieux et al. 2007; Frassanito et al. 2009), and infectious pathologies (Riva 1998; Drost et al. 2000; Papavasiliou et al. 2004; Dimova et al. 2009; Parrish et al. 2010). However, this occurrence of PFS is extremely rare. While the incidence of the PFS after posterior fossa tumor resection in a pediatric population is estimated to range between 8% (Dailey et al. 1995; Pollack et al. 1995; Van Calenbergh et al. 1995) and 31% (Van Mourik et al. 1998; Catsman-Berrevoets et al. 1999, 2003; Robertson et al. 2006), the condition has only very occasionally been documented in adults following etiologically different posterior fossa lesions (e.g., Salvati et al. 1991; Kai et al. 1997; Ildan et al. 2002; Akil et al. 2006) or after focal damage of the brainstem (Messert et al. 1966; Frim and Ogilvy 1995; D’Avanzo et al. 1993). A comprehensive survey of the literature on PFS in adults following cerebellar tumor surgery yielded reports on only 22 adult patients published between 1969 and January 2011 (Mariën et al. 2011). In the same period, only three patients were described who developed the PFS after cerebellar surgery for non-tumoral, vascular etiologies such as arteriovenous malformations (Dunwoody et al. 1997; Idiaquez et al. 2011), and a hematoma (Coplin et al. 1997). The onset of neurobehavioral abnormalities after a symptom-free postoperative interval has attracted substantial attention of the scientific community. Although more than 300 etiologically heterogeneous cases have been reported in the literature, the pathophysiological mechanisms underlying the PFS remain unclear.
This chapter briefly reviews the semiological characteristics of the PFS in adults and children, looks at the attempts to treat the syndrome, and discusses the main pathophysiological explanations that have been proposed.
Semiological Characteristics of the Posterior Fossa Syndrome
Posterior Fossa Syndrome in Children
In 1958, Daly and Love published what is assumed to be the first detailed report of the semiological characteristics of the PFS. In this report, the authors described the postoperative onset and evolution of the various cognitive, behavioral, and affective symptoms in a 14-year-old boy with a posterior fossa grade I astrocytoma. In addition to akinetic mutism, a range of postoperative behavioral and affective abnormalities were recorded as well:
In the early evening following operation the patient was in a deep coma (…). Later that evening there was some lightening of the coma, and the patient began to move his extremities. The next day, the patient slept almost continuously but could be aroused by being spoken to. On command, he would grasp his father’s hand. Although he lay inert, infrequent movements of all extremities suggested that no paralysis existed. He responded to aspiration by coughing and gagging, but he did not speak or utter any sounds. He did not move his eyes (…). By the third postoperative day the patient moved all extremities without signs of weakness. He would grasp objects firmly with both hands and protrude his tongue on request, but he remained mute. When food was placed in his mouth, he would not chew it; nevertheless he readily swallowed liquids put into his mouth. He was incontinent of feces. When undisturbed he was somnolent and inactive. When stimulated he seemingly was aware of, but indifferent to, his environment. (…) Noxious stimuli, such as venipuncture, were disregarded.
In the succeeding days a slow but constant improvement occurred. One week after operation the patient appeared aware of his environment and cooperated slowly with the requests of his Spanish-speaking nurse. Yet he continued mute. (…) Although he seemingly recognized his parents, he showed no emotional response to them. His features were expressionless, and he had no interests in the activities about him. (…)
At the end of two weeks he had yet to utter a sound. Dysphagia, which had been present initially, had virtually disappeared and he could chew and easily swallow food placed in his mouth. He obeyed simple commands and communicated with his parents by sign language. (…) He still slept excessively, although he could be roused readily. He moved more frequently than before and would move his limbs to avoid an awkward position. Because of his slow progress (…) he was given 40 mg. of methylphenidate hydrochloride intravenously; this produced marked increase in alertness and responsiveness. (…) He thenceforth received the drug by mouth in a total daily dose of 100 mg. in divided doses of 20 mg. each. This sharply accelerated the rate of improvement; he slept less, showed more spontaneous movements, and attempted to walk with help. However, he remained mute.
One month after operation the patient was able to follow moderately complicated commands such as, “touch your right index finger to your left ear.” When given several different commands successively, he had increasing difficulty and began to perseverate. Attempts at more accurate testing were difficult to evaluate, since it was not clear whether this failures resulted from lack of comprehension or apathy and indifference. In any event, on doing portions of the Stanford-Binet, Wechsler-Bellevue, and progressive matrices tests, the patient was unable to perform beyond the six-year level. He was able to read a simple written command and execute it properly. He could purse his lips, but could not whistle. He coughed vigorously and swallowed without trouble; yet he had still made no effort to speak. He usually lay quietly in bed, watching the activities around him. He showed no emotion, even with his parents. At his mother’s request he would place his arms about her and kiss her; however, the action was a stiff mechanical gesture which suggested the behavior of a puppet. (…)
On September 5 the patient spoke for the first time when he called his parents by name. Later that day he mentioned his sister’s name, and when his father told him he was to be taken to a neurological conference, he asked: Why? (…) The complexity of his speech rapidly increased the next few days. Within one week he spoke lucidly, but with a scanning measured speech of cerebellar type. He became more interested in his environment and wanted to read magazines. He expressed interest in going home and told his parents that he wished to visit Chicago on the way.
In the following two weeks the content of his speech and his affective behavior returned to normal. Although he was still troubled by his ocular palsies and ataxia, he worked vigorously at rehabilitation in the department of physical medicine. His normal affective response returned, and he would laugh appropriately at jokes and would in turn make jokes. He exhibited considerable interest in learning English and made rapid progress so that it was soon possible to speak to him in simple sentences. His parents felt that his vocabulary and syntax in Spanish had returned to the preoperative level. At the time of dismissal on September 27 he had mild cerebellar speech and moderate ataxia. The ocular palsies persisted but were improved.
(…) On several occasions attempts were made to discontinue its [methylphenidate hydrochloride, 60 mg.] use. Whenever it was stopped for a period of a day, the patient became apathetic and somewhat somnolent. On resuming its use, he would return to a normal state of alertness (p. 238–40).
Although Daly and Love (1958) did not explicitly mention a “symptom-free postoperative interval,” the PFS in patients with tumoral pathologies typically develops in the immediate postoperative phase after a short period of relatively normal functioning. From a review of 283 pediatric cases in De Smet et al. (2007a), it is clear that cerebellar mutism typically develops between 0 and 11 days (mean 1.5 days, SD 1.7) after tumor surgery.
The core symptom of the PFS is total speechlessness or verbal mutism, but it is generally accompanied by (frontal-like) neurobehavioral abnormalities such as apathy, loss of drive or reduced initiative, unconcern, inconsolable crying, and whining. Other symptoms that have been observed are: the inability to initiate voluntary eye opening (eye-lid apraxia), urinary retention or incontinence without any apparent urological or pharmacological reason, autistic behavior, pathological laughing and crying, transient cortical blindness, compulsive pre-sleep behavior, and eating impairments due to the disruption of initiating the mastication and swallowing process in the absence of neurogenic dysphagia (Humphreys 1989; Pollack et al. 1995; 1997; Dimova et al. 2009; Daniels et al. 2005; Baillieux et al. 2007; Catsman-Berrevoets and Aarsen 2010).
Although a transient period of speechlessness is generally considered to be the prototypical feature of the PFS, mutism occasionally does not materialize at all and a wide range of postoperative neurobehavioral deficits have been found instead. Symptoms such as a decreased initiation of voluntary movements (e.g., Kingma et al. 1994; Siffert et al. 2000), eye-lid apraxia (e.g., Sinha et al. 1998; Pollack 2001), executive dysfunction (e.g., Levisohn et al. 2000; De Smet et al. 2009), poor problem solving (e.g., Pollack et al. 1995; Clerico et al. 2002), amnestic disorders (e.g., Humphreys 1989; Kingma et al. 1994), reduced attention span (e.g., Kingma et al. 1994; Aarsen et al. 2004), visual-constructive deficits (e.g., Riva 1998; De Smet et al. 2009) and symptoms consistent with CCAS (e.g., Catsman-Berrevoets and Aarsen 2010) may occur in the absence of mutism as the neurobehavioral consequence of acute surgical damage to the cerebellum.
The majority of patients operated on for a posterior fossa tumor do not develop cerebellar mutism after surgery. However, since formal cognitive assessments are not a standard procedure in the postoperative follow-up, it seems likely that possibly relevant cognitive, behavioral, and affective symptoms remain largely unnoticed. As timely recognition and intervention of neurobehavioral symptoms are of crucial importance, formal investigations are strongly recommended in all patients, both children and adults, who undergo posterior fossa tumor resection.
Duration of the period of postoperative mutism may vary significantly among individuals. In the review of 283 pediatric cases by De Smet et al. (2007a), the duration of the mutism ranged from half a day to 2.5 years (mean 49.7 days, SD 85.5). When excluding the two cases presenting with an extremely long period of mutism, duration of mutism ranged from half a day to 7 months (mean 41.5 days, SD 37.5) (De Smet et al. 2007a). De Smet et al. (2007a) further confirmed that an overwhelming majority of reviewed cases (98.8%) presented with dysarthria after remission of cerebellar mutism. The speech of only 2/167 children (1.2%) was thought to be free from dysarthric symptoms after their mutism had resolved. Post-mutism dysarthric speech symptoms consisted of slow speech rate, monotonous verbal output, and ataxic speech. Catsman-Berrevoets and Aarsen (2010) analyzed the speech characteristics of 40 children either during the PFS in five patients with reduced speech production or after remission of mutism. Slow speech rate affected 33 out of the 40 children and was the most frequently occurring deviant speech symptom. In addition, 13 of these children presented with a severely reduced verbal output, characterized by short but grammatically correct phrases. Surprisingly, excess and equal stress and irregular articulatory breakdown, which represent the two most specific cardinal features of ataxic dysarthria, were only found in one patient. Four other patients presented with only one of these features typical of ataxic dysarthria. A prominent voice tremor additionally occurred in two of these patients. Speech production of one patient was characterized by alternating loudness, which, according to Kluin et al. (1988), represents a specific feature of cerebellar speech pathology.
With regard to recovery of motor speech symptoms, it seems that mutism is always transient (Ersahin et al. 1996) and that dysarthria usually resolves within 1–3 months after onset (Catsman-Berrevoets et al. 1999; Afshar-Oromieh et al. 2010). A less favorable outcome of dysarthric speech symptoms subsequent to mutism documented in earlier reports (Hudson et al. 1989; Dailey et al. 1995; Jones et al. 1996; Doxey et al. 1999) is consistent with more recent studies on the long-term sequelae of the PFS which also identified incomplete recovery of motor speech production (Cornwell et al. 2003; Steinbok et al. 2003; Aarsen et al. 2004; Huber et al. 2006). Steinbok et al. (2003), for instance, described seven patients with postoperative cerebellar mutism of whom one patient remained mute for 2.5 years postsurgery, two patients suffered from residual dysarthria, and three patients had a slow speech rate. These authors were the first to state that the posterior fossa syndrome is not a transient and benign syndrome but a condition that may be associated with disabling residual deficits. Huber et al. (2006) confirmed the presence of long-term motor speech deficits in a follow-up study of more than 5 years and suggested that formal speech and language therapy should be administered.
Apart from motor speech symptoms (dysarthria) which as a rule occur after remission of mutism, a variety of concomitant non-motor language disturbances have recently been identified as well (De Smet et al. 2007b). These include word-finding difficulties (Levisohn et al. 2000; Aarsen et al. 2004), agrammatism (Riva and Giorgi 2000; Siffert et al. 2000), disrupted language dynamics (Siffert et al. 2000; Ozimek et al. 2004), comprehension deficits (Levisohn et al. 2000; Cornwell et al. 2003), and reading (Scott et al. 2001) and writing problems (Aarsen et al. 2004).
At the emotional and affective level, adynamia and symptoms indicating inhibition of frontal lobe functions are often recorded. Many patients present with a lack of initiative, aspontaneity, apathy (Pollack 1997), disinterest (Steinlin et al. 2003), emotional unsteadiness (Catsman-Berrevoets et al. 2003), flattened affect, inadequate emotional coping (Ozimek et al. 2004), diminished eye contact (Liu et al. 1998), and withdrawal (Daniels et al. 2005). Again in sharp contrast to the view that the PFS is a transient phenomenon (Van Dongen et al. 1994), several long-term cognitive consequences of the PFS have been identified such as scholarly underachievement and major cognitive sequelae such as a significant decline of general intelligence, executive dysfunction, disrupted memory, attentional deficits, and distorted spatial cognition (Levisohn et al. 2000; Steinlin et al. 2003; De Smet et al. 2009).
Posterior Fossa Syndrome in Adults
In the limited number of 21 adult cases who had undergone surgery in the cerebellum for a tumor or vascular pathology, the onset of postoperative mutism ranged between 0 and 6 days (mean 2.3 days, SD 3 days) (Mariën et al. 2011). The duration of mutism ranged from 1 to 105 days (mean 38.2 days, SD 35). Although the duration of mutism is substantially longer in children, the difference with adults does not reach significance (Student T-test, p = 0.623) (Mariën et al. 2011).
With respect to motor speech characteristics following the period of mutism, it appears that speech deficits were found to be dysarthric in 14/17 cases (77.8%). They were described as “thick,” cerebellar or ataxic, scanned or staccato, near normal or reduced in voice volume in seven patients (41.2%) (Moore 1969; D’Avanzo et al. 1993; Bhatoe 1997; Coplin et al. 1997; Dunwoody et al. 1997; Sherman et al. 2005). Follow-up information varying between 2 months (Kai et al. 1997) and 4 years post-surgery (Adachi et al. 2005) was provided for 15 of the 20 patients (71.4%) (Moore 1969; Salvati et al. 1991; D’Avanzo et al. 1993; Coplin et al. 1997; Dunwoody et al. 1997; Kai et al. 1997; Caner et al. 1999; Ildan et al. 2002; Sajko et al. 2004; Adachi et al. 2005; Sherman et al. 2005; Akhaddar et al. 2008). Eight of the 15 patients (53.3%) (Moore 1969; Dunwoody et al. 1997; Kai et al. 1997; Caner et al. 1999; Ildan et al. 2002; Adachi et al. 2005; Sherman et al. 2005; Akhaddar et al. 2008) had normal speech at final follow-up. Of the remaining six patients (46.7%), dysarthric symptoms (Salvati et al. 1991; Coplin et al. 1997; Kai et al. 1997; Ildan et al. 2002) and scanning speech (D’Avanzo et al. 1993) persisted during follow-up.
Cognitive, behavioral, and emotional symptoms have only occasionally been documented in adults, and the majority of cases reported in the literature provide no formal cognitive data. Three patients presented with orofacial apraxia (Dailey et al. 1995; Afshar-Oromieh et al. 2010); two patients had memory problems (Moore 1969; Afshar-Oromieh et al. 2010), attention deficits, and dysphagia (Afshar-Oromieh et al. 2010); and one patient had anomia (Afshar-Oromieh et al. 2010). Behavioral-affective characteristics included lethargy and confusion (Moore 1969), depressed mood (Afshar-Oromieh et al. 2010), emotional lability (Coplin et al. 1997; Afshar-Oromieh et al. 2010; Idiaquez et al. 2011), and aspontaneity (Caner et al. 1999; Afshar-Oromieh et al. 2010).
Preoperative Neurocognitive and Neurolinguistic Assessments
Preoperative cognitive, behavioral, and affective symptoms have neither been systematically investigated in the adult patient population nor systematically studied in children. Mariën et al. (2011), for instance, showed that cognitive, behavioral, and affective abnormalities may already exist in the preoperative phase. They conducted cognitive assessments and behavioral observations in an adult patient before posterior fossa tumor surgery took place. A range of discrete but clinically significant deficits were objectified. First, from conversations with the patient’s husband and family, it appeared that a progressive decrease of motivation and initiative, unconcern, social withdrawal, incidences of verbal aggression, and inappropriate verbal remarks had taken place over several months. A general attitude of unconcern and flattened affect were observed during admission, and disinhibited verbal and inappropriate, prefrontal-like behavioral reactions frequently occurred. No indications for a depressive disorder were found. In-depth neurocognitive investigations carried out 2 days before surgery revealed disrupted working memory span, significantly depressed recent memory, impaired attention, and distorted frontal problem-solving and planning skills. These preoperative cognitive, behavioral, and affective symptoms closely resemble the CCAS (Schmahmann and Sherman 1998), reflecting a functional disruption of the cerebello-cerebral network. A quantitative SPECT study conducted in the preoperative phase revealed a pattern of perfusion changes indicating functional disruption of the cerebello-cerebral network crucially involved in the regulation of cognitive, behavioral, and affective processes.
Recently, Di Rocco et al. (2011) found a relation between presurgical language impairment (PLI) and postoperative cerebellar mutism in children diagnosed with posterior fossa tumors. Two groups of children were identified in this prospective study: children with PLI (anomia, reduced verbal fluency, verbal adynamia, apraxia of speech) (n = 11) and children without PLI (n = 23). Seven out of the 34 children (20.6%) developed cerebellar mutism after surgery. All of them belonged to the PLI group (n = 7/11; 63%). Based on these findings, the authors suggested that PLI may represent a subclinical state of cerebellar mutism in some children with posterior fossa tumor. These findings clearly demonstrate the need for thorough pre- and postoperative neurocognitive investigations in children with posterior fossa tumors.
Pathophysiological Hypotheses and Possible Explanations
Several risk factors for the development of the PFS have been identified. Catsman-Berrevoets et al. (1999) found a clear relationship with the type and size of the lesion in that PFS is most likely to occur after resection of a medulloblastoma with a lesion diameter exceeding 5 cm. However, since the PFS may also be associated with other tumor types and in patients with medulloblastomas not exceeding 5 cm, additional risk factors have been considered to play a possible causative role in the genesis of the syndrome in tumoral cases. These include: length of the vermian incision (Dailey et al. 1995), midline tumor location (Pollack et al. 1995; Ozgur et al. 2006; Robertson et al. 2006), tumor location adjacent to the fourth ventricle and postsurgical edema of the pontine tegmentum (Van Dongen et al. 1994), the occurrence of postoperative hydrocephalus or meningitis (Humphreys 1989; Ferrante et al. 1990; Salvati et al. 1996; Robertson et al. 2006), lesions to the deep nuclei of the cerebellum (Pollack et al. 1995; Richter et al. 2005), and multiple bilateral injuries to the proximal dentate-thalamo-cortical pathways (Morris et al. 2009).
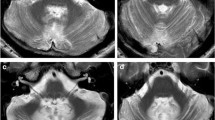
Despite the growing interest in PFS, its pathophysiological substrate is still unclear. Several hypotheses have been put forward to explain the pathophysiological mechanisms subserving the PFS: (1) postoperative vasospasm of the arteries supplying the cerebellum and the brainstem leading to ischemia and subsequent edema (Ferrante et al. 1990; Balasubramaniam et al. 1993; Nagatani et al. 1991; Turgut 1998; Ildan et al. 2002; Maffei et al. 2005), (2) transient dysfunction of the A9 and A10 mesencephalic dopaminergic cell groups and ascending activating reticular system (Catsman-Berrevoets et al. 1992) and, (3) transient dysregulation of neurotransmitter release originating from the tumor removal and the alleviation of long-lasting compression of the brainstem by the tumor (Caner et al. 1999); (4) bilateral surgical damage to the dentate and interpositus nuclei (Dietze and Mickle 1990–91; Pollack et al. 1995; Van Calenbergh et al. 1995; Kusano et al. 2006), or to the afferent and/or efferent pathways passing through these nuclei (Crutchfield et al. 1994; Ersahin et al. 1996; Turgut 1998). The hypothesis that the cognitive and behavioral dysfunctions associated with the PFS may result – as reflected by the phenomenon of cerebello-cerebral diaschisis (CCD) on SPECT – from a temporarily functional depression of the reciprocal pathways that connect the cerebellum with the limbic circuitry and the prefrontal, temporal, and parietal association cortices is currently attracting increased attention (Germano et al. 1998; Sagiuchi et al. 2001; Baillieux et al. 2006; Mariën et al. 2001, 2003, 2008, 2009, 2010; Catsman-Berrevoets and Aarsen 2010; Miller et al. 2010). Mariën et al. (2001, 2003), for instance, reported the preliminary results of a prospective study in which the possible role of CCD in the pathophysiology of PFS was explored. A 5-year-old patient with a posterior fossa medulloblastoma (Fig. 78.1a) was described who already presented with mild dysexecutive symptoms in the preoperative phase as reflected on 99mTc-HMPAO SPECT by perfusional changes in the anatomoclinically suspected but structurally intact prefrontal brain regions (Fig. 78.1c). After surgical resection of the posterior fossa medulloblastoma (Fig. 78.1b), full-blown PFS was associated with a significant aggravation and extension of the preoperative supratentorial perfusional deficits on repeat SPECT (Fig. 78.1d). When akinetic mutism resolved and behavioral and affective symptoms started to ameliorate after a 5-week period, a marked improvement of regional cerebral blood flow was found bilaterally in the prefrontal areas.
(a–d) Preoperative axial FLAIR slice (a) of a 5-year-old right-handed boy shows a large tumoral mass lesion (medulloblastoma) in the posterior fossa. Postoperative follow-up MRI (b) demonstrating a fourth ventricle cystic mass lesion with residual tumor tissue adhering to the wall of the fourth ventricle. Preoperative 99mTc-HMPAO SPECT of the brain (c) reveals a relative hypoperfusion of the right cerebellar hemisphere. Although there is no breach of the cerebral cortex, there is less physiological activation of the left frontal lobe. Postoperative SPECT (d) demonstrates a large scintigraphic defect in the cerebellum slightly off-center to the right. A strikingly increased hypoperfusion deficit in the left frontal lobe is shown
By sharp contrast, no pre- and postoperative supratentorial perfusion alterations were observed in a child who did not develop PFS after posterior fossa astrocytoma resection. Based on a close parallelism between the development and course of neurobehavioral symptoms and perfusional changes on SPECT in the anatomoclinically suspected supratentorial brain regions, Mariën et al. (2001, 2003) concluded that CCD might be intrinsically implicated in the pathophysiology of the PFS. As consistently reflected by CCD, the distant metabolic repercussion of surgical damage to the cerebellum on the supratentorial brain regions crucially involved in language dynamics, and behavioral and affective regulation was later confirmed in an adolescent patient, in an adult patient, and in a larger study of five children with PFS (Baillieux et al. 2006; De Smet et al. 2009; Mariën et al. 2011). Consistent with these findings, Morris et al. (2009) recently concluded from a study of 26 pediatric patients operated for a posterior fossa tumor that (1) multiple bilateral injuries to the proximal dentate-thalamo-cortical pathways may predispose patients to the development of the PFS, (2) functional disruption of the white matter bundles containing efferent axons within the superior cerebellar peduncles is a critical underlying pathophysiological component of PFS, and (3) decreased fractional anisotropy in the fornices and cerebral cortex may be related to the abnormal neurobehavioral symptoms of PFS.
Functional Lateralization of the Cerebellum: Evidence from PFS
In agreement with the hypothesis of a topographically organized and functionally lateralized cerebellum (Schmahmann 2000; Mariën et al. 2001; Stoodley and Schmahmann 2010), Riva and Giorgi (2000) and later Scott et al. (2001) and Siffert et al. (2000) correlated distinct patterns of postoperative neurocognitive symptoms with the lateralization of tumor location in the cerebellum. In the majority of patients, the disruption of linguistic processes resulted from surgical resection of tumors infiltrating the right cerebellar hemisphere, whereas deficits in nonverbal, spatial cognition were consistently found after tumor resection in the left cerebellar hemisphere. Vermal lesions were generally associated with behavioral-affective disturbances (Levisohn et al. 2000; Schmahmann 2004).
Treatment
As yet, no systematic attempts have been undertaken to treat the neurobehavioral symptoms of children and adults with the PFS by means of pharmacological agents or by means of specially developed neurorehabilitation strategies. There are only a few anecdotal reports in which patients received drug treatment to improve their condition. Daly and Love (1958), for instance, administered 40 mg. of methylphenidate hydrochloride intravenously and recorded a beneficial effect of this general central nervous stimulant on the patient’s behavioral condition in terms of alertness and responsiveness. After this improvement, oral administration of the drug in a total daily dose of 100 mg. (divided in five doses of 20 mg. each) was stated to sharply accelerate the rate of recovery. Although the patient remained mute, he slept less, showed more spontaneous movements, and attempted to walk without help. Several attempts to discontinue analeptic drug treatment during follow-up were unsuccessful. Whenever the treatment was stopped for longer than a day, the patient became apathetic and somnolent again. Normal alertness was achieved by resuming treatment.
Since dopamine agonists proved useful to treat akinetic mutism of variable etiology (Messert et al. 1966; Catsman-Berrevoets and van Harskamp 1988; Echiverri et al. 1988; Caner et al. 1999; Adachi et al. 2005; Mateo-Sierra et al. 2005; Shyu et al. 2011), Mariën et al. (2011) started to treat a 38-year-old right-handed woman with postsurgery relapsing-remitting akinetic mutism in the context of PFS with ropinirole hydrochloride. This non-ergoline dopamine agonist which specifically binds with D2 and D3 receptors induced a complete remission of akinetic mutism and substantially improved the patient’s responsiveness and alertness. Although an improvement in mental speed, language dynamics, behavior, and affect was found as well, a wide range of debilitating cognitive, behavioral, and affective abnormalities persisted. In addition, treatment had to be discontinued because the patient developed psychotic symptoms. As demonstrated by quantitative SPECT studies, remission of mutism was associated with restored perfusion in inferolateral frontal regions bilaterally, as well as in the right inferomedial frontal region, and right cingulate gyrus.
The initially favorable neurobehavioral response to treatment seems to corroborate the view that a decrease of dopaminergic input to the prefrontal cortex, as a result of cortical or subcortical lesions affecting the mesocorticolimbic dopaminergic projections, may be an important mechanism involved in akinetic mutism (Cummings 1993).
Concluding Remarks
More than 50 years of clinical research has brought to the fore that “transient cerebellar mutism” does not constitute a uniform syndrome following acute damage to cerebellar structures. Indeed, most studies of children and adults, who developed cerebellar mutism after etiologically heterogeneous neurological disorders predominantly affecting the cerebellum, have shown that cerebellar-induced speechlessness does not occur in isolation but rather as an intrinsic part of a complex of associated neurolinguistic, neuropsychological, and neuropsychiatric phenomena. This complex of symptoms, generally denoted as the PFS, affects children far more often than adults and occurs in the vast majority of reported cases after a short and relatively symptom-free interval following posterior fossa tumor surgery. However, the number of cases with clinically relevant postoperative cognitive, behavioral, and affective abnormalities in which transient cerebellar mutism as the cardinal feature of the PFS syndrome is absent is rapidly growing. Since mounting evidence suggests that there is a considerable variability in the semiological expression of the PFS – ranging on a continuum of different degrees of severity and symptom duration – it may be argued that the PFS is a subtype of the CCAS, comprising full-blown PFS with akinetic mutism to subtle but clinically significant cognitive, behavioral, and affective alterations. Several studies have suggested a possible link between the two cerebellar syndromes (Levisohn et al. 2000; Ronning et al. 2005), and CCAS may be viewed as a long-term consequence of the PFS (De Smet et al. 2009). That CCAS and PFS do not only share overt semiological resemblances (basically differing in extent and severity of symptoms), but possibly also a common pathophysiological substrate is suggested by a consistent parallelism between functional neuroimaging findings and clinical observations. CCAS as well as PFS both seem to reflect functional disruption of the cerebello-cerebral network crucially involved in the regulation of cognitive, behavioral, and affective processes.
Many questions about the intriguing onset and evolution of the semiological characteristics of the PFS, its pathophysiological substrate, its treatment, and its longitudinal outcome remain to be solved. In addition, a systematic lack of in-depth preoperative investigations and the absence of formal neurocognitive and behavioral assessments in patients without postoperative cerebellar mutism make it likely that a number of clinically relevant symptoms have remained unnoticed and still need to be discovered.
References
Aarsen FK, Van Dongen HR, Paquier PF et al (2004) Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology 62:1311–1316
Adachi J, Nishikawa R, Hirose T et al (2005) Mixed neuronal-glial tumor of the fourth ventricle and successful treatment of postoperative mutism with bromocriptine: case report. Surg Neurol 63:375–379
Afshar-Oromieh A, Linhart H, Podlesek D et al (2010) Postoperative cerebellar mutism in adult patients with Lhermitte-Duclos disease. Neurosurg Rev 33:401–408
Akhaddar A, Belhachmi A, Elasri A et al (2008) Cerebellar mutism after removal of a vermian medulloblastoma in an adult. Neurochirurgie 54:548–550
Akil H, Statham PFX, Götz M et al (2006) Adult cerebellar mutism and cognitive-affective syndrome caused by cystic hemangioblastoma. Acta Neurochir 148:597–598
Baillieux H, De Smet HJ, Lesage G et al (2006) Neurobehavioral alterations in an adolescent following posterior fossa tumor resection. Cerebellum 5:289–295
Baillieux H, Weyns F, Paquier P et al (2007) Posterior fossa syndrome after a vermian stroke: a new case and review of the literature. Pediatr Neurosurg 43:386–395
Baillieux H, De Smet HJ, Dobbeleir A et al (2010) Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex 46:869–879
Balasubramaniam C, Subramaniam V, Balasubramaniam V (1993) Mutism following posterior fossa surgery for medulloblastoma. Neurol India 41:173–175
Beaton AA, Mariën P (2010) Language, cognition and the cerebellum: grappling with an enigma. Cortex 46:811–820
Bhatoe HS (1997) Mutism, oropharyngeal apraxia and dysarthria after posterior fossa tumour excision. Br J Neurosurg 11:341–343
Caner H, Altinörs N, Benli S et al (1999) Akinetic mutism after fourth ventricle choroid plexus papilloma: treatment with a dopamine agonist. Surg Neurol 51:181–184
Catsman-Berrevoets CE, Aarsen FK (2010) The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumor surgery. Cortex 46:933–946
Catsman-Berrevoets CE, Van Harskamp F (1988) Compulsive pre-sleep behavior and apathy due to bilateral thalamic stroke: response to bromocriptine. Neurology 38:647–649
Catsman-Berrevoets CE, Van Dongen HR, Zwetsloot CP (1992) Transient loss of speech followed by dysarthria after removal of posterior fossa tumor. Dev Med Child Neurol 32:1102–1109
Catsman-Berrevoets CE, Van Dongen HR, Mulder PG et al (1999) Tumor type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 67:755–757
Catsman-Berrevoets CE, Van Dongen HR, Aarsen FK et al (2003) Transient cerebellar eye closure and mutism after cerebellar tumor surgery: long-term clinical follow-up of neurologic and behavioral disturbances in a 14-year-old girl. Pediatr Neurosurg 38:122–127
Clerico A, Sordi A, Ragni G et al (2002) Transient mutism following posterior fossa surgery studied by single photon emission computed tomography (SPECT). Med Pediatr Oncol 38:445–448
Coplin WM, Kim DK, Kliot M et al (1997) Mutism in an adult following hypertensive cerebellar hemorrhage: nosological discussion and illustrative case. Brain Lang 59:473–493
Cornwell PL, Murdoch BE, Ward EC et al (2003) Dysarthria and dysphagia as long-term sequelae in a child treated for posterior fossa tumor. Pediatr Rehabil 2:67–75
Crutchfield JS, Sawaya R, Meyers CA et al (1994) Postoperative mutism in neurosurgery. Report of two cases. J Neurosurg 81:115–121
Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50:873–880
Dailey AT, McKhann GM, Berger MS (1995) The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg 83:467–475
Daly DD, Love GJ (1958) Akinetic mutism. Neurology 8:238–242
Daniels SR, Moores LE, DiFazio MP (2005) Visual disturbance associated with postoperative cerebellar mutism. Pediatr Neurol 32:127–130
D’Avanzo R, Scuotto A, Natale M et al (1993) Transient “cerebellar” mutism in lesions of the mesencephalic-cerebellar region. Acta Neurol 15:289–296
De Smet HJ, Baillieux H, Catsman-Berrevoets C et al (2007a) Postoperative motor speech production in children with the syndrome of “cerebellar” mutism and subsequent dysarthria: a critical review. Eur J Paediatr Neurol 11:193–207
De Smet HJ, Baillieux H, De Deyn PP et al (2007b) The cerebellum and language: the story so far. Folia Phoniatr Logop 59:165–170
De Smet HJ, Baillieux H, Wackenier P et al (2009) Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology 23:694–704
Di Rocco C, Chieffo D, Frassanito P et al (2011) Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum 10:551–562
Dietze DDJ, Mickle JP (1990/1991) Cerebellar mutism after posterior fossa surgery. Pediatr Neurosurg 16:25–31
Dimova PS, Bojinova VS, Milanov IG (2009) Transient mutism and pathologic laughter in the course of cerebellitis. Pediatr Neurol 41:49–52
Doxey D, Bruce D, Sklar F et al (1999) Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg 31:131–136
Drost G, Verrips A, Thijssen HOM, Gabreëls FJM (2000) Cerebellar involvement as rare complication of pneumococcal meningitis. Neuropediatrics 31:97–99
Dunwoody GW, Alsagoff ZS, Yuan SY (1997) Cerebellar mutism with subsequent dysarthria in an adult: case report. Br J Neurosurg 11:161–163
Echiverri HC, Merens TA, Coker SB (1988) Akinetic mutism: pharmacologic probe of the dopaminergic mesencephalofrontal activating system. Pediatr Neurol 4:228–230
Ersahin Y, Mutluer S, Cagli S et al (1996) Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery 38:60–66
Ersahin Y, Mutluer S, Saydam S et al (1997) Cerebellar mutism: report of two unusual cases and review of the literature. Clin Neurol Neurosurg 99:130–134
Ferrante L, Mastronardi L, Acqui M et al (1990) Mutism after posterior fossa surgery in children. Report of three cases. J Neurosurg 72:959–963
Frassanito P, Massimi L, Caldarelli M et al (2009) Cerebellar mutism after spontaneous intratumoral bleeding involving the upper cerebellar vermis: a contribution to the physiopathogenic interpretation. Childs Nerv Syst 25:7–11
Frim DM, Ogilvy CS (1995) Mutism and cerebellar dysarthria after brainstem surgery: case report. Neurosurgery 36:854–857
Fujisawa H, Yonaha H, Okumoto K et al (2005) Mutism after evacuation of acute subdural hematoma of the posterior fossa. Childs Nerv Syst 21:234–236
Germano A, Baldari S, Caruso G et al (1998) Reversible cerebral perfusion alterations in children with transient mutism after posterior fossa surgery. Childs Nerv Syst 14:114–119
Huber JF, Bradley K, Spiegler BJ et al (2006) Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma resection in childhood. Childs Nerv Syst 22:132–138
Hudson LJ, Murdoch BE, Ozanne AE (1989) Posterior fossa tumors in childhood: associated speech and language disorders post-surgery. Aphasiology 3:1–18
Humphreys RP (1989) Mutism after posterior fossa tumor surgery. In: Marlin AE (ed) Concepts in pediatric neurosurgery, vol 9. Karger, Basel
Idiaquez J, Fadic R, Mathias CJ (2011) Transient orthostatic hypertension after partial cerebellar resection. Clin Auton Res 21:57–59
Ildan F, Tuna M, Erman T et al (2002) The evaluation and comparison of cerebellar mutism in children and adults after posterior fossa surgery: report of two adult cases and review of the literature. Acta Neurochir 144:463–473
Janssen G, Messing-Jünger AM, Engelbrecht V et al (1998) Cerebellar mutism syndrome. Klin Padiatr 210:243–247
Jones S, Kirollos RW, Van Hille PT (1996) Cerebellar mutism following posterior fossa tumor surgery. Br J Neurosurg 10:221–224
Kai Y, Kuratsu J, Suginohara K et al (1997) Cerebellar mutism after posterior fossa surgery – Two case reports. Neurol Med Chir (Tokyo) 38:929–933
Kingma A, Mooij JJA, Metzemaekers JDM et al (1994) Transient mutism and speech disorders after posterior fossa surgery in children with brain tumors. Acta Neurochir 131:74–79
Kluin KJ, Gilman S, Markel DS et al (1988) Speech disorders in olivopontocerebellar atrophy correlate with positron emission tomography findings. Ann Neurol 23:547–554
Koh S, Turkel SB, Baram TZ (1997) Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol 16:218–219
Kusano Y, Tanaka Y, Takasuna H et al (2006) Transient cerebellar mutism caused by bilateral damage to the dentate nuclei. J Neurosurg 104:329–331
Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumor resection in children. Cerebellar cognitive affective syndrome in a paediatric population. Brain 23:1041–1050
Liu GT, Phillips PC, Molloy PT et al (1998) Visual impairment associated with mutism after posterior fossa surgery in children. Neursurgery 42:253–257
Maffei M, Simonetti L, Agati R et al (2005) Cerebellar mutism after medulloblastoma resection: importance of MR features. Riv Neuroradiol 18:201–204
Mariën P, Engelborghs S, Fabbro F et al (2001) The lateralized linguistic cerebellum: a review and new hypothesis. Brain Lang 79:580–600
Mariën P, Engelborghs S, Michiels E et al (2003) Cognitive and linguistic disturbances in the posterior fossa syndrome in children: a diaschisis phenomenon? Brain Lang 87:162
Mariën P, Engelborghs S, Wackenier P et al (2008) Cerebellar cognitive affective syndrome without global mental retardation in two relatives with Gillespie syndrome. Cortex 44:54–67
Mariën P, Baillieux H, De Smet HJ et al (2009) Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex 45:527–536
Mariën P, De Surgeloose D, De Deyn PP et al (2010) Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum 9:405–410
Mariën P, De Smet HJ, Wijgerde E et al (2011) Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex doi:10.1016/j.cortex.2011.06.018
Mateo-Sierra O, Gutiérrez FA, Fernández-Carballal C et al (2005) Akinetic mutism related to hydrocephalus and cerebellar surgery treated with bromocriptine and ephedrine. A pathophysiological review. Neurocirugia (Astur) 16:134–141
Messert B, Henke TK, Langheim W (1966) Syndrome of akinetic mutism associated with obstructive hydrocephalus. Neurology 16:635–649
Miller NG, Reddick WE, Kocak M et al (2010) Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol 31:288–294
Moore MT (1969) Progressive akinetic mutism in cerebellar hemangioblastoma with “normal-pressure hydrocephalus”. Neurology 19:32–36
Morris EB, Philips NS, Laningham FH et al (2009) Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 132:3087–3098
Nagatani K, Waga S, Nakagawa Y (1991) Mutism after removal of a vermian medulloblastoma: cerebellar mutism. Surg Neurol 36:307–309
Ozgur BM, Berberian J, Aryan HE et al (2006) The pathophysiologic mechanism of cerebellar mutism. Surg Neurol 66:18–25
Ozimek A, Richter S, Hein-Kropp C et al (2004) Cerebellar mutism. Report of four cases. J Neurol 251:963–972
Papavasiliou AS, Kotsalis C, Trakadas S (2004) Transient cerebellar mutism in the course of acute cerebellitis. Pediatr Neurol 30:71–74
Parrish JB, Weinstock-Guttman B, Yeh EA (2010) Cerebellar mutism in pediatric acute disseminated encephalomyelitis. Pediatr Neurol 42:259–266
Paulus KS, Magnano I, Satta W et al (2002) Cerebellar cognitive affective syndrome: a nine-month follow-up case report. J Psychophysiol 16:217
Pollack IF (1997) Posterior fossa syndrome. Int Rev Neurobiol 41:412–432
Pollack IF (2001) Neurobehavioral abnormalities after posterior fossa surgery in children. Int Rev Psychiatry 13:302–312
Pollack IF, Polinko P, Albright AL et al (1995) Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 37:885–893
Rekate H, Grubb RL, Aram DM et al (1985) Muteness of cerebellar origin. Arch Neurol 42:697–698
Richter S, Schoch B, Kaiser O et al (2005) Behavioral and affective changes in children and adolescents with chronic cerebellar lesions. Neurosci Lett 381:102–107
Riva D (1998) The cerebellar contribution to language and sequential functions: evidence from a child with cerebellitis. Cortex 24:279–287
Riva D, Giorgi C (2000) The cerebellum contributes to higher functions during development. Evidence from a series of children surgically treated for posterior fossa tumors. Brain 123:1051–1061
Robertson PL, Muraszko KM, Holmes EJ et al (2006) Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg 105:444–451
Ronning C, Sundet K, Due-Tønnessen B et al (2005) Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg 41:15–21
Sagiuchi T, Ishii K, Aoki Y et al (2001) Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med 15:157–160
Sajko T, Talan-Hranilovic J, Al-Qoud H et al (2004) Cerebellar medulloblastoma in an elderly man: an unexpected finding. Acta Clin Croat 43:45–48
Salvati M, Missori P, Lunardi P et al (1991) Transient cerebellar mutism after posterior cranial fossa surgery in an adult. Case report and review of the literature. Clin Neurol Neurosurg 93:313–316
Salvati M, Cervoni L, Santoro A (1996) Cerebellar mutism after posterior cranial fossa surgery. J Neurol Sci 40:59–63
Schmahmann JD (2000) The role of the cerebellum in affect and psychosis. J Neurolinguist 13:189–214
Schmahmann JD (2004) Cognition and the cerebellum. Neurology 63:1991
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Scott RB, Stoodley CJ, Anslow P et al (2001) Lateralized cognitive deficits in children following cerebellar lesions. Dev Med Child Neurol 43:685–691
Sherman JH, Sheenan JP, Elias WJ et al (2005) Cerebellar mutism in adults after posterior fossa surgery: a report of 2 cases. Surg Neurol 63:476–479
Shyu C, Burke K, Souweidane MM et al (2011) Novel use of zolpidem in cerebellar mutism syndrome. J Pediatr Hematol Oncol 33:148–149
Siffert J, Poussaint TY, Goumnerova LC et al (2000) Neurological dysfunction associated with postoperative cerebellar mutism. J Neurooncol 48:75–81
Sinha AK, Rajender Y, Dinahar I (1998) Transient cerebellar mutism after evacuation of a spontaneous vermian haematoma. Childs Nerv Syst 14:460–462
Steinbok P, Cochrane DD, Perrin R et al (2003) Mutism after posterior fossa tumor resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg 39:179–183
Steinlin M, Imfeld S, Zulauf P et al (2003) Neuropsychological long-term sequelae after posterior fossa tumor resection during childhood. Brain 126:1998–2008
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844
Turgut M (1998) Transient “cerebellar” mutism. Childs Nerv Syst 14:161–166
Van Calenbergh F, Van De Laar A, Plets C et al (1995) Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery 37:894–898
Van Dongen HR, Catsman-Berrevoets CE, Van Mourik M (1994) The syndrome of “cerebellar” mutism and subsequent dysarthria. Neurology 44:2040–2046
Van Mourik M, Catsman-Berrevoets CE, Yousef-Bak E et al (1998) Dysarthria in children with cerebellar or brainstem tumors. Pediatr Neurol 18:411–414
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Mariën, P., De Smet, H.J., Paquier, P., De Deyn, P.P., Verhoeven, J. (2013). Cerebellar Mutism. In: Manto, M., Schmahmann, J.D., Rossi, F., Gruol, D.L., Koibuchi, N. (eds) Handbook of the Cerebellum and Cerebellar Disorders. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1333-8_78
Download citation
DOI: https://doi.org/10.1007/978-94-007-1333-8_78
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1332-1
Online ISBN: 978-94-007-1333-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences