Abstract
Recent preclinical studies demonstrated the use of engineered cells as a potential way to treat many diseases and disorders. Tailoring the cell’s function and interactions using surface engineering methods is a very promising approach in developing novel cell-based therapeutics. For instance, cell surface modification has been used for the development of universal blood donor cells. In another example, it has been shown that surface modification of stem cells is a doable approach to regulate the fate of cells into specific phenotypes, which is necessary to regain function in specific environment such as different injury sites. Cell surface engineering using macromolecules/polymers could provide desired properties and functions to cells for applications in targeted delivery, biosensing, transfection, imaging techniques, and in the regulation of cell fate. This chapter will review the recent advancements in polymer-based cell surface engineering approaches for various applications. In terms of the cell types, we have chosen to focus, specifically, on red blood cells, lymphocytes, splenocytes, stem cells (multipotent and pluripotent), islet cells, endothelial cells, and hepatocytes as they offer the most promise in generating cell-based therapeutics. In terms of modification approaches, we mainly highlighted the literature associated with the use synthetic polymers via covalent conjugation and non-covalent bonding. We also discuss the future of such cell surface engineering methods for their potential clinical utility.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Background
Cell surface is a highly heterogeneous environment with distinct types of proteins, glycans, and lipids [1,2,3,4,5,6,7,8]. Cell surface has a critical role in governing the fate of cells as it would regulate cell-cell interaction, cell-niche communication, and intracellular signaling pathways [2, 6,7,8,9,10]. Although the cell surface is highly dynamic and complex in nature, several ways have been developed for manipulating the cell surface in order to alter their functionalities/properties for variety of applications (Fig. 1). Controlling the biochemical and cellular functions of cells by resurfacing the cell membrane with biomaterials alongside inhabitant functionalities allows new opportunities in drug delivery, cell-based therapeutics, transfusion, and tissue engineering [2,3,4, 9, 11,12,13,14,15]. Most common cell surface engineering applications include bioimaging, manipulating cell biology, tissue engineering, cell therapies, and targeting cells to the desired sites of the body [2, 8, 9, 15]. For instance, by systemic infusion of engineered hematopoietic stem cells (HSCs) with cell homing ligands onto their surface, it is possible to home such cells to the bone marrow to have a safe and efficient cell targeting for transplantation applications [16]. Although proposed approaches in this field have been promising, many challenges remain. Such issues include the development of cell-friendly modification methods that suit highly heterogeneous and super dynamic nature of the cell surface to have a significant effect on cellular functions such as adhesion, proliferation, and differentiation without compromising the viability.
An illustration of the eukaryotic cell membrane with different functional moieties; amine, thiol, and carbonyls in very small quantities are tethered to either membrane bound proteins or glycoproteins or carbohydrate component of glycolipids. (Adapted from [17] with the kind permission of Royal chemical society)
In this chapter, we will discuss cell surface engineering approaches using synthetic polymers for various applications. It will also address how chemical approaches including covalent and non-covalent methods are used to manipulate the cell surface effectively to enhance their therapeutic potential and other cellular functions. These include covalent conjugation of polymers to primary amine groups on cell surface proteins, incorporation of amphiphilic polymers into lipid membrane of cells via hydrophobic interaction, electrostatic binding between cationic macromolecules and negatively charged cell surface, and grafting from strategies and modifications through nonnative functional groups. In addition, this chapter also highlights a huge body of work on the engineering the surface of cells, including red blood cells (RBCs), white blood cells, multipotent and pluripotent stem cells, islet cells, endothelial cells, and hepatocytes for transplantation and transfusion applications. These cells are selected due to their promising therapeutic potential for various diseases. We review the advantages and challenges associated with these methods and how these approaches can be applied to improve the therapeutic applications of cells. Finally, conclusions about the current state of the field and insight into the future directions are given.
2 Methods for Cell Surface Engineering Using Polymers
Cell surface engineering has a pivotal role in tuning the cell function by controlling their biochemical interactions with their environments [2,3,4, 15, 18]. The availability of different functional groups on the cell surface will be an excellent opportunity for cell surface modification (Fig. 1). However, surface modification is quite challenging due to the fact that cell surface is not static, and also the modification should only have minimal effect on biological function of the cells [5, 7, 19, 20]. In the past two or three decades, researchers have focused on developing various tools for engineering the surface of cells [2, 9, 19, 21, 22]. A variety of functional groups and bioactive substances have been introduced onto cell surface by different biological transformations and physicochemical methods [9, 14, 21, 22]. Here, our intention is to focus on methods that are commonly used and utilize hydrophobic, electrostatic, covalent interactions and enzymatic approaches for cell surface modification.
2.1 Hydrophobic Insertion into the Cell Membrane
Hydrophobic interactions are frequently exploited for cell surface engineering, especially poly(ethylene glycol) (PEG) conjugated phospholipid amphiphilic polymers are known for their ability to attach on the cell membrane [23, 24]. Tatsumi et al. have developed a versatile method to engineer the surface of hepatocytes by immobilizing polymers on the cell membrane [25]. They used a PEG-phospholipids conjugate bearing FITC (FITC-PEG-lipid, in particular, 1,2-dimyristoyl-sn-glycerol-3-phosphatidylethanolamine (DMPE)) to intercalate into the hepatocyte lipid membrane. This modification process did not alter either functional parameters in vitro or engraftment potential of the cell in vivo. Moreover, Iwata and co-workers highlighted the importance of hydrophobic modification of the cell surface using a layer-by-layer method [26]. They used a combination of PEG-conjugated phospholipid derivatives and poly(vinyl alcohol) (PVA) bearing different anchoring units. When PEG-phospholipid conjugates bearing maleimide groups were incubated with islet suspension, a thin layer of PEGs on islet surface formed spontaneously by a hydrophobic insertion mechanism. Then, the first PVA layer was introduced via a maleimide/thiol reaction between maleimide group of the PEG layer and thiol groups on modified PVA. The PVA layer was further enforced using a layer-by-layer method which utilized thiol/disulfide exchange reactions [27, 28]. Figure 2 showed how such approach is used to engineer the surface of islet cells.
A schematic view of surface engineering of the islet cells by a multilayered PVA membrane approach [Adapted from reference [26], with the kind permission of Elsevier Ltd.]
Recently, Temura et al. reported another surface engineering approach using PEG-lipid derivatives and DNA hybridization instead of PVAs for cell-cell interactions [29]. In this approach, a single-stranded ss-DNA (polyA20 and polyT20 with the thiol group) was conjugated with PEG-phospholipid (Mal-PEG-lipid), and the resulting polymeric conjugate was inserted onto the cell membrane [30]. In another study, Won et al. optimized the mesenchymal stem cell (MSC) surface by incorporating recombinant CXCR4 (rCXCR4) protein on the membrane of MSC to improve the homing of MSCs. They incubated a PEG-phospholipid which was conjugated to a rCXCR4 with MSCs at room temperature to bind the stromal-derived factor-1 (SDF-1) on MSC’s surface [21]. Another eloquent study was on the use of lipid-modified hyperbranched polyglycerol (HPG) for generating a coating on stem cell to deliver them to target tissues (Fig. 3) [16]. Bioactive HPGs conjugated with octadecyl chains and vasculature-binding peptides (VBP) were used for directing MSCs to vascular endothelium.
Stem cell modification via hydrophobic insertion of modified hyperbranched polyglycerol (HPG). A bioactive HPG modified with octadecyl chains and vasculature binding peptides (VBPs) was utilized for the modification of stem cells as a novel cell-guidance molecule and guide them to defective vasculature. In vitro studies demonstrated the proof-of-concept. Adapted from reference [16], with the kind permission of American chemical society
2.2 Electrostatic Interactions
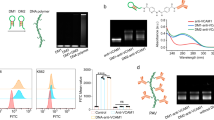
By exploiting the charged nature of cell membrane, various electrostatic methods were explored to engineer the cell surface. Over the two past decades, layer-by-layer assembly of polyelectrolytes to form polyelectrolyte multilayer (PEM) film, which is formed by alternate assembly of polycations and polyanions, represents a renowned approach for engineering the cell surfaces at the molecular level. Ionic polymers, such as chitosan, poly(allylamine hydrochloride), poly(styrene) sulfate, poly-l-lysine (PLL), and poly(ethyleneimine) (PEI), strongly interact with negatively charged mammalian cell surface and have been widely used for this approach [31]. However, the cytotoxicity of the polycations is one of the main limitations of this approach. To circumvent this issue, several groups have used a spacer between a polycation and the cell surface to avoid the direct contact between the cationic polymer and cell surface [32,33,34]. Recently, Wilson and co-workers reported a PEG-modified cationic polymers by taking advantage of PEG-dependent conformational changes and biocompatibility (Fig. 4) [22].
Graphical illustration of coating of a pancreatic islet cell surface by layer-by-layer self-assembly of poly(ethyleneimine) films. Appropriate combination of poly-l-lysine-g-PEG copolymer and poly(alginate) was used. (Adapted from [22] with the kind permission of American chemical society)
In another eloquent work, Brooks and Kizhakkedathu groups reported that neutral polymers can be utilized for cell surface modification. They have decorated hyperbranched polyglycerols (HPGs) with choline phosphate (CP) (neutral zwitterionic polymers) to modify the cell surface by electrostatically interacting CP groups with the phosphatidylcholine end group of the cell surface lipids on cell membrane (Fig. 5) [35]. Various groups adopted this methodology to modify the cell surface for different applications [22, 31, 36]. The encapsulation of live cells in polymeric coatings is a versatile approach to modulate or control the response of cells to their environment. The layer-by-layer assembly of non-immunogenic polyelectrolytes is employed here to attenuate or suppress the binding of antibodies to red blood cells (RBCs) and, consequently, decrease their inherent immunogenicity in vitro. The optimized shell was composed of four bilayers of alginate (AL) and chitosan-graft-phosphorylcholine (CH-PC) surrounded by two bilayers of AL and poly-l-lysine-g-polyethylene glycol (PLL-g-PEG).
An illustration represents the structure of polyvalent choline phosphate molecules contained hyperbranched polyglycerol (HPG-CP) and schematic representation of adsorption of HPG-CP on cell membrane. Adapted from reference [35] with the kind permission of Springer Nature
2.3 Covalent Modification
Although a variety of functional groups are readily available on the cell surface, only a few functional moieties can be used to covalently modify the cell surface proteins due to the extreme complexity and heterogeneity of cell surface. This strategy is involved in a direct chemical reaction of functional groups on the cell surface such as amines, thiols, and carboxylic acids with proteins, polymers, nanoparticles, and other small molecules. The most commonly used functionality for cell surface modification is the amine groups originating from the lysine side chains present on cell surface proteins. The most easily and extensively used strategy is the treatment of amine groups on the cell surface with N-hydroxysuccinimide (NHS)-activated macromolecules at nearly physiological conditions. Although cell surface carbohydrates were used for covalent conjugation of small molecules on cell surface [37a], there is limited information available on such methods used for polymer conjugation.
Wilson et al. masked the pancreatic islet surface covalently with functionalized thrombomodulin in order to reduce donor cell-mediated procoagulant and pro-inflammatory responses [37a]. The bifunctional PEG-based linker, with a triphenylphosphine derivative at one end and an NHS-activated carboxylic acid at the other end, was attached to the amine groups of the pancreatic islet surface by an amide coupling strategy. Subsequently, an azido-functionalized thrombomodulin was attached to PEG linker chemoselectively using Staudinger ligation [22]. The obtained surface modification was very effective in increasing the production of activated protein C with a reduction in islet-mediated thrombogenicity [37a]. Recently, Kizhakkedathu and co-workers have used a similar strategy to modify the RBC surface with HPGs and PEGs in order to camouflage the antigens on the RBC surface. The carboxylic acid functionalized HPG molecules are activated with NHS followed by incubation with RBC in phosphate-buffered saline (PBS) (Fig. 6) [37b]. This RBC surface modification provided significantly higher levels of CD47 self-protein accessibility and greater protection of certain antigens on the RBC surface without changing native properties of RBC. These functional RBCs have greater potential for therapeutic delivery applications. Later on, Hsiao et al. used a bifunctional PEG molecule to couple the mammalian cell surface with biopolymers such ss-DNA [38]. They attached maleimide group on one side of the PEG chain, and the other end group of the PEG chain is modified with NHS ester [38]. The activated carboxyl group reacts with amine groups on the cell surface, whereas maleimide group reacts with thiol group on the ss-DNA. This rapid technique is very effective in the modification of different cell lines, such as RBCs, primary T cells, and cardiac myoblasts. This new protocol greatly expands the applicability of DNA adhesion strategies for different applications [38]. Using a similar strategy, Cheng et al. covalently conjugated peptides, with cysteine C-terminus, on MSC’s surface without affecting cell functions [39]. The engineered MSCs exhibited rolling on E-selectin.
Pictorial representation of covalent modification of cell surface with HPG via NHS ester-amine coupling. Reactions were performed under physiological conditions. (Adapted from [37b] with the kind permission of Elsevier Ltd.)
The covalent conjugation of cell surface amines with cyanuric acid containing polymers is an another commonly used strategy for various cell surface modifications, in particular RBCs and T cells [40,41,42,43,44,45]. Hashemi-Najafabadi et al. developed a cell surface modification technique to mask the RBCs surface via covalent attachment of m-PEG to the cell membrane [41]. They optimized PEGylation conditions in order to achieve the attenuated immunorecognition of RBCs in both organ transplantation and blood transfusion applications. The cyanuric acid derivative of m-PEG-OH was first synthesized under inert conditions; then, the Rh positive RBCs were suspended with cyanuric acid-PEG derivatives for different time intervals at different pH, temperature, and polymer concentrations. PEGylation, with linear PEG of molecular mass 5 kDa, of RBCs through this approach was quite successful at pH 8.7, temperature 14 °C, and reaction time 30 min. The polymer concentration was varied with molecular weight. The morphology of m-PEG-RBCs was intact, and it was further confirmed by light microscopy and scanning electron microscopy. Using similar approach, Scott and co-workers has shown that the grafting of m-PEG on peripheral blood mononuclear cell surface decreased the antibody recognition of different surface receptors involved in essential cell communication [46].
Connecting cell surface amines with aldehyde containing moieties (macromolecules, drugs) through Schiff base formation is another useful strategy. Tucaresol, a molecule bearing an aldehyde group, is an investigational drug as an immunopotentiator in chronic hepatitis B virus and HIV infections. Chen et al. conjugated the Tucaresol with T-cell surface amines via Schiff base formation to understand its immunoresponse mechanism. However, this method is not widely explored [47,48,49].
It is known that mammalian cell surface is abundant with free thiol groups from cysteine residues, at least 15 cell surface proteins have thiol groups in either oxidized (disulfide bridges) or reduced form (free thiols). The balance between oxidized and reduced forms of thiol group is dependent on the redox environment of cells. Various research groups have made use of these thiol groups to engineer the cell surface for various biomedical applications, for instance, controlling immune functionality and cell signaling [27, 50]. A significant advantage of this strategy is the plethora of commercially available reagents and linkers. In addition, the fact that covalent linkage between the reduced form of the disulfide group and targeted moieties can be easily tuned by altering the reaction conditions [51, 52]. Maleimide functionalized probes are one of the best option as they are stable, efficient, light insensitive, and show high chemoselectivity to cysteine thiol groups [53]. The free thiol groups on cell surface can be chemoselectively conjugated with maleimide-containing nanoparticles, biopolymers, and dyes at neutral conditions (pH 6.5–7.5) to form very stable thioether bridges. This thiolation-directed maleimide chemical approach is notable even bigger target molecules, in size of 100–300 nm [54]. Stephan et al. conjugated the maleimide-containing nanoparticles to thiol groups present on the surface of T cells and HSCs to promote them as promising vectors for targeted cell-mediated drug delivery [54] (Fig. 7).
Schematic illustration of covalent attachment of phosphorous lipid maleimide-functionalized nanoparticles with the free thiol group which is linked to the cell membrane proteins at physiological pH. (Adapted from [54] with the kind permission of Springer Nature)
These strategies are very useful to enhance drug loading on the cell surface [55]. In another report, Nacharaju et al. conjugated PEG-maleimides to the RBC surface to camouflage the RBC surface antigens from antibodies. This methodology worked well for different molecular weights of PEG, and also this linkage is stable at in vivo conditions [56].
The process of biotinylation is a covalent attachment of biotin molecule, a water-soluble vitamin B7, to cell surfaces, biomaterials, small molecules, and macromolecules. Biotinylation reagents are commercially available for chemoselective targeting of specific functional groups including amines, thiols, carboxylic acids, carbohydrates, and carbonyl groups. Researchers took advantage of extremely high-affinity binding between biotin and its binding partners such as avidin, streptavidin, and NeutrAvidin to probe the cell surface or for cell surface modification. In addition, this binding is highly resistant to heat, pH, organic solvents, and other denaturing environments. The biotin-avidin interaction (Kd = 10−15 M) is widely explored for different biotechnological and biomedical fields [57,58,59]. Biotinylation of cell surfaces is achieved through a reaction of primary amine groups present on the cell surface with amine-reactive biotin, such as NHS biotin derivatives. Once the cell is labeled with biotin, it can be readily functionalized with a wide range of small molecules, microspehers, polymers, and proteins through a streptavidin bridge [60, 61]. Building on this approach, recently, Dou et al. reported a cell-matrix interaction protocol using diverse types of cells (osteoblastic, human endothelial, and human hepatoma cells) and hydrogels to enhance the cellular adhesion in three-dimensional extracellular matrix which is very important for avoiding cell death. To this end, three-dimensional nanofibrous hydrogel matrix was generated by co-assembly of 1,4-benzyldicarboxamide-based supramolecular gelators and their biotinylated versions [62]. In parallel, the used cells were modified with avidin; the avidin-modified cells were well encapsulated in three-dimensional matrix using the well-known, highly specific avidin-biotin bridge. The adhesive cells showed high cell proliferation rates, and it was confirmed by reverse transcription polymerase chain reactions. The expected cell adhesion was achieved in great amount in comparison with arginylglycylaspartic acid-based adsorption techniques. This approach is appealing more universal and might have potential to expand to other cell lines too (Fig. 8).
(a) Generation of molecular structures of three-dimensional nanofibrous networks from co-assembly of supramolecular gelators and biotinylated gelators. (b) Schematic representation of avidin-modified cells. (c) Cell adhesion was observed in three-dimensional networks through the expected avidin-biotin interaction between and fibrous nanomatrix and avidin-modified cells. (Adapted from [62] with the kind permission of American chemical society)
Although the biotinylation strategy is very versatile and widely used for different applications, it has few limitations, for instance, when natural cell surface functionalities are targeted with highly reactive molecules, the cell surface might have over flooded with biotinylated products; this could generate some toxicity [63]. In addition, the protein component of these techniques is of bacterial origin (e.g., streptavidin) which could generate immune reactions. This will be harmful especially for cell surface engineering for in vivo applications. Although covalent immobilizations/linkages of polymers or macromolecules to cell membranes were expected to be stable for chemical degradation for a long time, in few cases these non-covalent modifications disappeared from the cell surface over the time [14].
2.4 Modifications Through Nonnative Cell Surface Functional Groups
The other cell modification approach involves the generation of cell surface functional groups that are not normally present on cell surface and utilization of these functional groups for covalent grafting. The ligation of nonnative functional groups on cell surface with exogenous materials is used for different applications such as live cell imaging, cell separation process, and cell-based sensors [64,65,66]. In the past few years, various attempts have been employed to generate the different functional groups, in particular, carbonyl, azides, and thiol on the cell surface to chemoselectively modify the cell surface [67,68,69,70]. Carbonyl groups can easily react with amines and alkoxyamines, resulting in stable bonds at ambient conditions. However, the availability of carbonyl groups on the cell surface is very limited. For many years, aldehyde or ketone groups on cell surface are generated with high specificity and turnout by direct chemical modification or enzymatic treatment or by the metabolic incorporation of desired functional moieties [71]. Sialic acid moieties have a crucial role in cell aggregation and recognition and are found on cell surface glycoproteins, linked to either galactose or N-acetylgalactosamine at the nonreducing terminal positions [69, 72]. Gahmberg et al. used galactose oxidase to selectively oxidize the diol units of the sialic acid on the penultimate sugar molecules of the cell membrane glycoproteins in order to generate aldehyde groups (Fig. 9) [73, 74]. Since these diol units are available on the penultimate sugar molecules of the glycoproteins, another selective chemical or enzymatic treatment is necessary. In this case, the enzyme neuraminidase is used to cleave glycoside linkage between the last two terminal sugar units; it provides better access to the diol groups for the subsequent reaction. Sodium periodate-mediated selective oxidation of terminal diol units into aldehydes was known for small molecules, oligosaccharides, and proteins. De Bank et al. demonstrated the generation of aldehyde groups through a mild oxidation of 1,2-diol units of sialic acid residues of living L6 myoblast cells with sodium periodate to induce cell aggregations. This approach showed high cell compatibility and did not show any significant effect on cell morphology [75].
Selective modification of diol units of glycoproteins of cell membrane. (a) A mild sodium periodate oxidation of cell surface proteins generates the aldehyde groups at C-9 positions of the N-acetyl galactose. (b) Neuraminidase enzyme cleaves seductively terminal glyosidic linkages of protein, flowed by galactose oxidase treatment yields aldehydes at C-6 positions of the sugar molecules. (Adapted from [17] with the kind permission of Royal chemical society)
In 2010, Holden et al. also used a similar approach to coat the macrophage surface with polyamidoamine dendrimers through sialic acid modification. The geminal diol units of sialic acid on the cell surface are oxidized with sodium periodate to generate aldehydes, and the cells are dispersed with polymers to form a covalent bond between amine groups of polyamidoamine dendrimers and aldehyde groups on the cell surface. Finally, formed Schiff bases were further converted into stable secondary amine groups using sodium cyanoborohydride [76]. However, the main limitation of this approach is that the reactive groups must be generated prior to the covalent grafting of cells with polymers.
The other prominent way of generating carbonyl groups on the cell surface is metabolic approach. Carbohydrate chains on the cell membrane are very important for most of the cell communications and cell functions [77]. Tagging of specific functionalities on carbohydrates or incorporation of unnatural carbohydrates into live cells surface, followed by surface engineering with polymers would be a viable approach to control the cell functions. Bertozzi research group is pioneered in introducing unnatural functional groups on the live cells membrane, for instance, acetyl, levulinoyl, and azidoacetyl groups, through glycosylation method [78, 79]. Taking advantage of this approach, Akiyoshi and co-workers demonstrated a new cell surface engineering strategy by attaching biomimetic polymer on cell surface via cell surface tag method (Fig. 10) [80]. To achieve proper surface masking, they developed a synthetic library of biofouling 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers, which were modified later with hydrazide functional groups. Metabolic treatment of human promyelocytic leukemia cells with N-levulinoylmannosamine, an unnatural carbohydrate acts as a cell surface tag, generated ketone-containing carbohydrates abundantly on the surface of cells. The N-levulinoylmannosamine-treated human promyelocytic leukemia cells were attached to hydrazide-modified 2-methacryloyloxyethyl phosphorylcholine polymers by selective recognition of a cell surface tag (ketone groups) within 15 min of incubation. The modified cells retained their morphology and showed high cell viability, which is further confirmed by flow cytometry.
(A) Schematic view of cell surface modification with non-fouling MPC polymer surface through cell-surface tags method. (B) Three different biomimetic MPC polymers used for this study. Adapted from reference [80] with the kind permission of American chemical society
Although various enzymatic treatments and metabolic approaches have been also employed to incorporate different functional groups such as biotin, alkyne, azide, thiol, and ketones into live cells surfaces, these technologies might influence cell physiology in the long run [81, 82].
2.5 Grafting from Strategies
Surface-initiated polymerizations such as atom transfer radical polymerization and reversible addition fragmentation chain-transfer polymerization have intensely been studied for different applications including generating non-biofouling surfaces, cell-selective adhesiveness, and stimuli-response materials [83,84,85,86]. Although cell surface with abundance of different functional groups is very attractive for surface-initiated polymerizations, the main challenge is reaction conditions which are very toxic to live cells. In contrast to traditional cell surface modifications, grafting of synthetic polymers on cell surface also offer a few advantages, especially in terms of changing the physical properties of modified surface and increasing the functional groups for secondary interactions, and provide opportunities in the generation of cell-polymer hybrid structures [87]. Recently Niu et al. modified cell surfaces (human Jurkat cells and yeast cells) by a novel “grafting from approach”; they generated the polymers on the cell surface by utilizing covalently linked -NH2 groups on the yeast cell surface proteins as initiators for visible light-mediated photoinduced electron transfer-reversible addition fragmentation chain-transfer polymerization (PET-RAFT) [88]. The cell surface amine groups were conjugated with the dibenzocyclooctyl-based activated ester by an amidation reaction. Then, the highly strained cyclooctyl groups on the cell surface were conjugated with azide-containing chain transfer agent, (2-(butylthiocarbonothioyl) propionic acid), through a copper-free, strain-promoted azide-alkyne cycloaddition. Next, cell surface-initiated PET-RAFT was conducted on yeast cell surface, by adding eosin Y (triethanolamine) as a catalyst and methoxy-PEG acrylamide-1k (PEGA-1k) as a monomer in PBS buffer (pH 7.4) with the aid of a light-emitting diode source (465 nm) to achieve the polymer-grafted yeast cells (Fig. 11).
A scheme showing yeast cell surface modification by “graft from approach.” The chain transfer agents are linked to amine groups on the cell surface followed by polymerization of different monomers by PET-RAFT. (Adapted from [88] with the kind permission of Springer Nature)
Cell viability and proliferation assays were confirmed that high cell viability and compatibility of this modification approach and the density of polymer chains on the cell surface was confirmed by different characterization techniques. From a synthetic prospective, this PET-RAFT process is highly controlled and generated very finely distributed polymers on the cell surface and no polymer formation was observed inside of the cells. However, the similar methodology did not work well for mammalian cell surface since mammalian cells lack a cell wall which protects the cells from unwanted cell stress conditions. Therefore, Niu et al. used a slightly modified chain transfer agent bearing a lipid-type molecule to insert the chain transfer agent non-covalently on the Jurkat cell membrane, and then polymerization process took place as described previously (Fig. 12). Overall, these approaches helped in increasing the number of grafted chains directly on the cell surface when compared with traditional polymer grafting strategies and can also be used to incorporate a wide range of functional groups for post-polymerization functionalization of the cell surface. The chemical reaction is completed in less than 5 min.
A scheme showing non-covalent attachment of chain transfer agents on the cell surface and polymer brushes generation on the mammalian Jurkat cell by PET-RAFT process. (Adapted from [88] with the kind permission of Springer Nature)
Surface-initiated atom transfer radical polymerization, for instance, on solid surfaces, nanoparticles, and metallic surfaces, has been extensively studied. Taking advantage of this, recently Kim et al. reported a yeast cell surface modification with synthetic polymers (“grafting from” approach) using highly cytocompatible surface-initiated activator regenerated by electron transfer, atom transfer radical polymerization (SI-ARGET-ATRP) [89]. They coated the cell surface with polydopamine by dispersing the cells in a solution containing dopamine-bearing ATRP initiator. The polydopamine-coated cell surface was treated with ARGET-ATRP solution for different time intervals to optimize the density of polymer chains on the cell surface. Such polymer-live cells hybrid structures have enormous potential for different applications and might serve as cell-based sensors, biomotors, and diagnostics [83, 90, 91].
3 Cell Surface Modification of Different Cell Types and Applications
The reasoning behind the cell surface engineering is to bring new advances in cell therapies which would eventually lead to control the fate and function of therapeutic cells. Engineered cells will be potentially used for their enhanced survival, proliferation, or differentiated function. In the following section, we will detail various cell surface modification methods applied to different cell types and applications associated with. Our main focus will be on RBCs, stem cells (multipotent and pluripotent), islet cells, endothelial cells, lymphocytes and splenocytes, and hepatocytes. Since cell surface engineering has emerged as a promising method for applications such as tissue replacement, regenerative medicine, transfusion, and transplantation medicine, the aforementioned cell types play key roles in these fields. Such approaches often involve the use of combinations of materials and cells to create functional structures that can be used in place of the original tissue. For instance, stem cells have the unique property of pluripotency, the ability to differentiate into any cell type making them particularly useful in tissue engineering and regenerative medicine. Here, we aim to summarize the benefit of cell surface engineering of these cells and report the current challenges associated with their application.
3.1 Antigen Protected Red Blood Cells
To overcome the challenges in preventing the immunological rejection of donor cells and tissue organs, many biochemical approaches have been designed [39]. Considerable amount of work has been undertaken to generate polymer coating on RBC to mask minor and major antigens on RBC surface for the creation of universal or immunocamouflaged antigen-protected blood donor cells [12, 13, 39, 56, 63, 92,93,94]. In another application, surface-modified RBCs have been used as a natural drug delivery system to carry therapeutic agents in the vasculature [95]. Cell surface engineering approaches would allow introducing different drugs onto the surface of RBCs for such applications. Figure 13 shows the schematic of common methods in engineering the surface of RBCs.
One of the earlier approaches for modification of RBCs is by covalent grafting of PEG on the surface of RBCs to protect or mask the blood group antigens on the surface which was pioneered by Scott and co-workers [46, 92, 96]. The polymer coating on the cell surface acts as a shield to prevent the interaction of antibodies and recognition of cell surface antigens. Scott and co-workers have shown that engineering the surface of RBCs using methoxy-PEG (m-PEG) had decreased anti-blood group antibody binding to human RBCs and the importance of polymer size on the antigen camouflage [46, 92, 96] (Fig. 14). Results from these studies have shown that m-PEG-modified sheep RBC which were transfused into mice resulted in improved survival when compared to the untreated RBC. This immunocamouflaged strategy has shown to drastically reduce the immunogenicity of the foreign cells and tissues. For instance, Bradley et al. have shown that PEG modification significantly could attenuate RBC immunogenicity while maintaining normal morphology and function. Moreover, PEG-modified RBCs have shown normal in vivo survival in murine models [92]. More importantly, Scott’s group has shown that engineering the surface of RBCs with Cm-PEG (cyanuric chloride-activated methoxy-PEG) has the potential to prevent alloimmunization in chronically transfusion diseases such as sickle cell anemia and thalassemia [46b]. In addition to these reports, Nacharaju et al. have introduced PEG onto the surface of RBCs by thiolation-mediated chemistry with maleimidophenyl-PEG (Mal-Phe-PEG) with different molecular weights [56]. Using this approach, they could successfully mask the antigenicity of group A Rh(D)+ and B Rh(D)+ to universal blood donor cells (group O Rh(D)-), confirming the masking of these antigens [56].
Immunocamouflage of membrane antigens is a function of linker chemistry, polymer size, and polymer surface density. (a) Shown is a graphical representation of the RBC membrane and the topical distribution of the Rh (C/c), Kidd (Jka/b), and MNS (S/s) blood group antigens. The PEG exclusion layer is the physical entity which gives rise to the immunocamouflage of the membrane antigens. (b) Influence of polymer size of Rhc antigen camouflage. (c) Influence of polymer size on immunocamouflage of the MNS and Kidd blood group antigens. The Rh antigens are located close to the membrane surface; consequently even relatively short polymers (e.g., 5 kDa) can effectively camouflage these sites, and however, MNS and Kidd blood group antigens extend far from the cell surface needs large molecular weight polymer. (Adapted from [46b] with the kind permission of Elsevier Ltd)
Due to the issues related to the antibody generation associated with PEG [46, 92, 96], our group has extensively explored the RBC surface engineering using a novel branched polymer and hyperbranched polyglycerol (HPG) [12, 13, 37, 63, 93, 97], which has shown similar or better biocompatibility than PEG. Chapanian et al. developed antigen-protected RBCs by grafting succinimidyl succinate (SS) group modified onto the surface of RBCs [63]. HPG modification resulted in significant reduction in binding of blood group antibodies to cell surface of engineered RBCs compared to control RBCs [63]. In another study, Chapanian et al. investigated the in vivo circulation of grafted RBCs with HPG in mice and showed a normal circulation behavior for RBCs modified with HPG of selected molecular weights and graft concentration (Fig. 15) [12]. The molecular weight of HPG and grafting concentrations are two important parameters that influenced both camouflage of red cell antigens and the viability of modified cells [98]. A comparison of grafting PEG and HPG has shown that HPG-modified cells gave promising results compared to PEG-grafted RBCs due to the compact nature of HPG [94, 98]. Recent studies also highlight the fact the HPG modification on RBC surface can be utilized to modulate the innate immune response to surface-modified cells. Leung et al. have shown that the complement activation on cell surface-modified HPG-dependent on the molecular weight and graft density. Recent studies also tested polymers such as polyethyloxazoline as replacement to PEG [97]; however, the immunocamouflage was lower, but this polymer showed improved RBC morphology. Overall, cell surface engineering of RBCs using covalent grafting of polymers has shown promising results in production of antigen-protected RBCs toward universal blood donor cells. In addition, these methods provide a general working principle for cell surface engineering using simple non-nucleated cells that can be adapted for other type of complex cell types.
Blood circulation of HPG-grafted RBCs in mice at different graft concentrations. (a) HPG 20 kDa, (b) HPG 60 kDa. (Adapted from [13] with the kind permission of Elsevier Ltd). Polymer concertation played a significant role in tuning the blood circulation times of grafted RBCs
3.2 Lymphocytes and Splenocytes
Due to the complexity of human immune system and antigenic diversity of the human cells, rejection of biomaterial, foreign tissues, and donor organs is still a great challenge in translational medicine. This questions the long-term compatibility of transplanted tissues and quality of the recipient’s life. Especially, graft-versus-host disease causes significant morbidity and mortality among the transplanted patients. Although few pharmacologic agents, for instance, azathioprine and methotrexate, have been successfully used to inhibit T-cell activation, these drugs are highly toxic to the kidney, liver, and gastrointestinal glands [99,100,101]. Surface engineering of the cells with biomaterials may provide a viable solution to reduce the rejection of allografts. Scott and co-workers examined the covalently m-PEG (5 kDa)-grafted allogeneic lymphocytes in minimizing of allorecognition necessary for T-cell activation and graft-versus-host disease both in vitro and in vivo in murine models. The masking of cell surface dramatically attenuated allorecognition of cells, and it was evident by dramatic differences in T-cell proliferation between unmodified and m-PEG-modified versions in both one- and two-way mixed lymphocyte reactions and flow cytometric analysis. Further, the in vivo murine models, graft-versus-host disease is induced in immunocompetent as well as immunocompromised mice via transfusion of allogeneic splenocytes which are collected from murine major histocompatibility complex disparate mice, further validate the effect of m-PEG derivatization in attenuation of allorecognition and subsequent reduction of the risk of graft-versus-host disease in mice [40].
The grafting density and depth of m-PEG brushes on cell membrane are vital in controlling the efficacy of immunocamouflage of the grafted cells [21]. The density of polymer brush border on the cell membrane is highly dependent on linker chemistry, molecular weight, and the concentration of m-PEG derivatives that are being used. In order to achieve the optimum immunological efficacy of polymer grafting, Chen et al. elaborated their studies to understand the role of linker in m-PEG grafting on murine splenocytes. Three different modifying chemistries and PEG derivatives (cyanuric chloride m-PEG (Cm-PEG), m-PEG-1-benzotriazolyl carbonate (BTCm-PEG) and tresylchloride m-PEG (Tm-PEG)) were used, and the murine splenocytes were modified with the 0–5 mM m-PEGs at pH 8.0 (Table 1) [102]. Flow cytometric analysis of leukocyte markers and mixed lymphocyte reactions demonstrated that both Cm-PEG and BTCm-PEG were highly efficient at camouflaging cell surface markers, while Tm-PEG was ineffective. Furthermore, Cm-PEG and BTCm-PEG significantly blocked mixed lymphocyte reactions allorecognition and cellular proliferation. The length of the polymer chain is highly detrimental in these studies; immunocamouflage of cells with BTCm-PEG-2 (20 kDa) was very effective than other counter parts.
Immunoquiescence, a state of low baseline immune activation, is another parameter to evaluate the efficiency of immunocamouflaged cell surface. Wang et al. studied whether m-PEG-modified allogeneic human peripheral blood mononuclear cells (PBMC) or murine splenocytes can produce immunoquiescence or tolerance in both in vitro and murine in vivo models [103]. Lymphocyte proliferation, differentiation, and cytokine production was verified in mixed lymphocyte reactions and conditioned media experiments. The studies demonstrated that PEG grafting does not have any significant effect on cell viability and immunomodulatory response and cytokine production, whereas the controls demonstrated significant (p < 0.001) effect on pro-proliferative potential and enhancing interleukin-2, tumor necrosis factor alpha, and interferon gamma levels.
m-PEG-grafted donor murine splenocytes showed significant in vivo immunosuppressive effects in H2-disparate mice. In contrast to unmodified to allogeneic splenocytes, PEG-modified allogeneic splenocytes showed significant increment in Tregs and baseline levels of Th17 lymphocytes. And also, this effect was seen in at least 30 days post challenge and was not reversed by unmodified allogeneic cells (Fig. 16). These studies conformed that the PEGylation of allogeneic lymphocytes induced an immunoquiescent state both in vitro and in vivo in murine models [104].
The PEGylated splenocytes showed long-term immunomodulatory effects; inhibited the changes in T regulatory (Treg) and Th17 (pro-inflammatory and allorejection) levels consequent to rechallenge with unmodified allogeneic cells. As shown in this figure, Treg levels persistently elevated, while Th17 levels remain the same or decreased from that of naive mice even after 30 days transfusion of modified allogenic cells. (Adapted from [103] with the kind permission of Elsevier Ltd)
Although various reports provided the detailed understanding of late events in T-cell activation of allografts such as T-cell proliferation and cytokine secretion, a better understanding of initial triggering events/molecular mechanisms is limited. Scott research group, in their subsequent studies, monitored the initial triggering events by examining the effect of PEGylation of cells in initial cell-cell interactions, changes to activation pathways, and apoptosis [101, 104, 105]. The role of these events in minimizing proliferative response is observed in modified cells during mixed lymphocyte reactions. The m-PEG-engineered cells exhibited significant global immunocamouflage of surface proteins of lymphocytes and also minimized interactions with antigen-presenting cells and other intracellular signaling process. And also, the reported PEG approach is nontoxic. Due to the global immunocamouflage of this approach, it overcomes the biological redundancy inherent to surface adhesion, costimulatory, and growth receptors and shows no evidence of systemic toxicity.
3.3 Modification of Stem Cells
Stem cells are currently known as one of the most promising candidates for developing novel and clinically translatable cell therapy [106,107,108,109]. In particular, focuses on multipotent and pluripotent stem cells (PSCs) have been significantly increasing [108, 109]. PSCs are characterized by immortality – the ability to continuously self-renew – and pluripotency, the ability to differentiate into all somatic cell types [108]. PSCs include both embryonic stem cells (ESCs) and induced PSCs (iPSCs). Both pluripotent and multipotent stem cells can generate the necessary quantities of cells required for transplantation due to their ability to continuously divide. These cells can then be differentiated into desired phenotypes for therapeutic applications. HSC’s transplantation is also known as one of the most commonly used cells for clinical trials [18, 39, 110,111,112].
Cell surface engineering approaches have been used to modify the cells to provide them with a desired property. Stephan et al. have proposed the conjugation of drug-loaded liposome nanoparticles onto the surface of HSCs [113]. Not only such approach did compensate normal HSC’s function, but they also increased the self-renewal durability of such cells. In addition to self-renewal and differentiation capability of stem cells, one of the current importance of stem cell therapy is the safe and efficient delivery of such cells into the desired tissue without losing their prominent properties such as proliferation or differentiation. For the delivery of stem cells into their desired tissues, the cell surface can be engineered to enhance the homing properties. In particular for MSCs, due to insufficient expression of surface markers, these cells would not present efficient homing properties. Consequently, cell surface modification approaches can play a key role in enhancing and presenting surface ligands onto the cell surface in order to address such issues. For instance, Sarkar et al. presented a promising modification technique in which they modified MSCs with a nanometer-scale polymer containing SLeX which has been found to be present on the surface of leukocytes and regulate the cell rolling of MSCs [107].
Figure 17 presents the schematic of such engineering strategy for enhancing the rolling of MSCs. It has been reported that the modified MSCs not only showed a great homing in vivo but also the MSC phenotypic properties including multi-lineage differentiation have been conserved as well. In another study, Cheng et al. have used a specific peptide conjugation strategy based on peptide-selectin interaction to improve adhesion of such cells onto blood vessels [39]. Zhao et al. have also shown that engineered P- or L-selectin binding nucleic acid onto MSCs made them engaged to inflamed endothelial cells and leukocytes [6]. Their in vitro results showed that such engineered MSCs can be directly captured from the flow stream by selectin surfaces or selectin-expressing cells under flow conditions [39]. Joeng et al. have used HPG to covalently conjugate oligopeptide containing the VHSPNKK sequence into polymer to create a bioactive HPG [16]. The bioactive HPG can be then associated with the surface of MSCs by hydrophobic insertion to further increase their homing properties. Finally, they have shown that the coating MSCs surface with such bioactive polymer significantly enhanced the cellular affinity for the vascular endothelial adhesion molecule which is overexpressed by inflamed blood vessels (Fig. 18) [16].
Engineering the MSC surface using the conjugation of SLeX by covalent biotinylation and a streptavidin-biotin bridge to improve their rolling interactions in vitro. (Adapted from [107] with the kind permission of American chemical society)
In vitro evaluation of the VHSPNKK-HPG-g-C18 grafted MSCs. (a) Graphical illustration of delivery of MSC to the targeted inflamed endothelium. (b) Microscopic images indicated adhesion of MSCs on endothelial cells; inflamed endothelium was exposed to uncoated MSCs (I) and grafted MSCs (II) for different time intervals. (c) Quantification of MSCs adhered to the inflamed endothelial cells. (Adapted from [16] with the kind permission of American chemical society)
In another study, improving the homing transplantation of MSCs were carried out by using engineered mRNA-transfected MSCs which highly expressed homing ligands, such as P-selectin glycoprotein ligand-1 (PSGL-1) and sialyl-Lewisx (SLeX), leading eventually to enhancing homing of such cells into mouse’ inflamed ear vascular endothelium. Lecy et al. have shown that engineered MSCs with the homing ligands PSGL-1/SLeX via mRNA transfection significantly improved their homing to the mouse bone marrow [110]. Their results confirmed that mRNA-transfected MSCs have enhanced homing to inflamed ear 2 h after injection by 30% compared to native MSCs.
So far, we have explored most studies that investigated the effects of cell membrane modification on regulating the fate of stem cells. Glycans are considered as one of the most important cellular component of stem cells where they are in charge of cell signaling communications to their exterior environment. Therefore, the glycan engineered using different techniques to stimulate the fate of stem cells into their desired properties accordingly. Such modulation would be mainly controlled by the signaling molecule transmission such as fibroblast growth factor 2 (FGF2), Wnt, and Notch and other lineage-specific signatures such as the stage-specific embryonic antigens (Lewis X, stage-specific embryonic antigens-1, stage-specific embryonic antigens 3 and 4). For instance, Huang et al. have shown that by using synthetic neoproteoglycans (neoPGs) they were successful in engineering the surface of mouse ESCs to further enhance their affinity to bind to FGF2 [114]. FGF2 is widely used for culturing many stem cell types including ESCs and PSCs. Using such synthetic approach, these researchers could remodel the glycocalyx of mouse ESCs which eventually lead to enhance the neural differentiation of such cells. In another study on engineering the glycans of stem cells, Pulisipher et al. have focused on how to regulate the fate of ESCs into neural phenotypes [112]. They have used HaloTag proteins (HTPs) to present heparin sulfate glycosaminoglycan (HS-GAG) anchor onto the membrane of mouse ESCs. It has been shown that remodeling the glycocalyx of ESCs with such strategy could accelerate the self-renewal exit and eventually promote neural lineage commitment and their differentiation into mature neuronal cells. Pulisipher et al. showed that, consistent with an accelerated loss of pluripotency, transcription factor NANOG levels in heparin sulfate chloroalkane-treated cells was decreased which then accompanied by a corresponding increase in the neuroectoderm-specific marker SOX1. Overall, bioengineering approaches to regulate the fate of stem cells toward their desired application is becoming very promising for preclinical and clinical researchers in the field.
3.4 Surface Modification of Islets
Pancreatic islets are responsible for regulating the sugar levels in the blood [115,116,117,118]. They are a cluster of pancreatic cells that are made up of different cells. Many research groups have published reports highlighting the importance of islet transplantation as an extremely promising therapy for diabetic patients [14, 26, 115,116,117,118,119]. Despite of their clinical promises, minimizing the risk of immune rejection of such cells is one the challenging problems. To address these challenges, cell surface modification has been adopted. Researchers have used amphiphilic PEG-lipid and the biotin/streptavidin reactions to immobilize human embryonic kidney cells 293 (HEK293) on the surface of islets, as shown in Fig. 19 [14, 26]. In another work, Golab et al. have shown that by using biotin polyethylene glycol-N-hydroxysuccinimide (biotin-PEG-NHS), they could successfully attach T cells onto the surface of islet cells without any loss of islets viability and function [115]. Although this technique is very promising, major limitation is that streptavidin is derived from bacteria and is a potent antigen in humans. Therefore other strategies were investigated. Specific DNA pairing with its complementary sequence can be used to control cell-cell interaction. Inserted single-stranded DNA-polyethylene glycol (PEG)-lipid (poly A) on the cell membrane showed a specific attachment to the complementary DNA-coated cell (poly T) or a glass surface to further improve the function of islet cells.
Encapsulation of islets with live cells. (a) Schematic illustration of the interaction between streptavidin and biotin-PEG-lipid at the lipid bilayer cell membrane. (b) Schematic illustration depicting how to enclose an islet with live cells utilizing the avidin and biotin interaction. (c) Hamster islets modified with biotin-PEG-lipid were immobilized with streptavidin-immobilized HEK293 cells. HEK293 cells were labeled with cell tracker. (Adapted from [28] with the kind permission of Elsevier Ltd.)
Teramura and Iwata have reviewed various cell surface engineering methods which can be applied to provide non-recognizable surfaces against the immune system. Such methods have been challenged for immune evasion: PEG23, multilayered PVA-PEG-lipid24, hyperbranched alginate-poly(amidoamine) dendrimer complex, hyperbranched polyglycerol, complement receptor 1-heparin layer-by-layer assembly, factor H-binding peptide, and apyrase [14, 26].
3.5 Endothelial Cell Engineering
Another important class of cells are endothelial cells which protect the vasculature and provide antithrombotic and anti-inflammatory properties [120]. Surface modification of endothelial cells has been used as a tool to manipulate the properties so that cell adhesion and cell behavior can be altered. One of the earlier studies describing such approaches uses avidin-biotin method (Fig. 20). Studies have shown that high-affinity avidin-biotin binding was successful to bring biotinylated cell surface onto synthetic surfaces and co-adsorbed with avidin and fibronectin [121, 122]. This cell surface modification eventually enhanced the formation of lower affinity integrin-mediated focal adhesions. Engineered biotinylated endothelial cells hold great promise for enhancing the attachment of endothelial surfaces onto the synthetic surfaces especially when the cells are placed in flow conditions. Such methods have been used to enhance the adhesion of intact biochemically viable endothelial cell layer on polymeric vascular grafts [73]. It is reported that the protein-PEG conjugates can be used to prevent the undesirable platelet deposition in the case of damaged arterial tissues. Deglau et al. further expanded this concept by employing PEG-modified human coronary artery endothelial cells for site-specific targeted delivery in ex vivo conditions [123]. First, the efficiency of targeted delivery to damaged arteries was confirmed using NeutrAvidin-coated polystyrene microspheres. Under arterial shear stress flow conditions, solution of PEG-biotin conjugates was delivered to scrape-damaged bovine carotid arteries that were loaded into the tubular perfusion chamber, followed by NeutrAvidin-coated fluorescently tagged microspheres for 10 min. A very high dense layer of styrene microspheres, almost sixfold higher, was found on NHS-PEG-biotin treated bovine carotid arteries, whereas control arteries showed minimal adhesion. To deliver the endothelial cells, the same experiment was repeated with sequential injections of PEG-biotin, fluorescently tagged NeutrAvidin (as a bridging molecule) and PEG-biotin-modified endothelial cells to scrape-damaged bovine carotid arteries. The damaged endothelium surface was well coated with injected PEG-modified endothelial cells and was confirmed by epi-fluorescence microscopy. Although the demonstrated approach has severe limitations and examined only in vitro conditions, this strategy might ultimately find applications in catheter-based or surgical procedures. Such studies are ongoing and have immense potential for clinical translation. It is worth mentioning that even though avidin-biotin was shown to significantly promote initial endothelial cell adhesion, there is no report on the effects of avidin at longer adhesion times [121].
Schematic of the endothelial surface grafting with microspheres through biotin-avidin bridge [124]
3.6 Hepatocytes Modification
Hepatocyte-based therapies have immense potential to be alternatives to liver transplantations in many liver-related diseases including liver failures and other liver disorders. However, the sufficient grafting of hepatocytes and their viability is highly essential for the success of both hepatocyte-based therapies and liver tissue engineering applications. A detailed understanding of the surface modification/interaction of hepatocytes with biomimetic materials is another crucial factor for the success of this therapeutic approach [25, 125, 126]. To better understand the hepatic cell interactions with biomaterial surface, recently, Kojima et al. coated hepatic cells with poly(lactic acid) through avidin-biotin binding system without losing crucial metabolic functions such as serum protein secretion and metabolic capacity [126]. Initially, hepatic cells were attached with sulfo-NHS-biotin, and these modified cells were grafted on an avidin-adsorbed flat poly(l-lactic acid) surface. The adhesion process is completed in less than 10 min. The proliferation of these modified cells was intact and almost comparable with cells cultured in collagen plates.
Hepatic functions of the attached cells including albumin secretion, induction of genes, metabolic capacity, and molecular signaling transfer ability of transmembrane receptor complexes were not compromised. Later, Ohashi and co-workers reported another interesting hepatic cell surface modification approach using PEG-lipid derivatives [25]. Murine primary hepatocyte cell surface was immobilized with different PEG-phospholipid conjugates bearing a fluorophore. All the vital hepatocyte functions such as cell viability, protein secretion ability, gene expression ability, induction of cytochrome P450, and hepatocyte transplantation ability were assessed using different in vitro and in vivo experiments (Fig. 21). This modification process was also tested successfully for engineering of hepatocyte sheets in order to generate ectopic liver tissues for different therapeutic applications. This cell surface modification process might show new avenues to advance hepatocyte-based therapies and drug discovery research.
In vivo studies of PEG-grafted hepatocytes. (A) Surface engineering of transplanted hepatocytes was assessed by measuring recipient serum human alpha-1 antitrypsin (hA1AT) levels at day 3 and 10. Dotted lines and straight lines indicate the recipient groups that were transplanted with cells via portal vein to liver and under kidney capsules, respectively. FITC-PEG-DMPE-modified hepatocytes were resuspended in Dulbecco’s modified Eagle medium and transplanted into the liver (B) and kidney (C). Histochemical staining of hA1AT of livers (D, E) and kidneys (f, g) were harvested for 10 days after transplantation of control hepatocytes (D, F) and FITC-PEG-DMPE-modified hepatocytes (E, G). (Adapted from reference [25] with the kind permission of Elsevier Ltd)
4 Summary and Future Prospective
The attachment of polymers onto the surface of different cell types opens a new and exciting avenue in the field of cell-based therapy which needs to be further explored in clinical studies. In fact, cell surface engineering enhances the therapeutic potential of cells used for transfusion and transplantation applications. Cell surface engineering explores how manipulation of cell fate can present a dominant innovative technology that will likely find wide applications in cell therapy, tissue engineering, drug delivery, and biosensing/bioimaging.
Here, we have described three major types of modification used for cell surface engineering including hydrophobic insertion, electrostatic, and covalent modification, along with the modifications through nonnative cell surface functional groups, and graft from strategies for most clinically important cells such as RBCs, stem cells, islets, lymphocytes/splenocytes, endothelial cells, and hepatocytes. Although we have highlighted the importance of polymer-based methods, advances in enzyme engineering can enhance the efficiency of cell surface engineering approaches by introducing new biomolecules that are designed for specific targets and applications. In addition to the benefits of enzymatic approaches, metabolic strategies to engineer the cell surface glycans are very promising as well, but considerable progress still needs to be achieved using the various pathways. For instance, unlike enzymatic approaches, which, for example, delete entire saccharides from cell surface proteoglycans [127], metabolic strategies can modify these structures in a manner to engineer the cell surface to eventually regulate the function of cells.
Overall, advances in cell surface engineering approaches, especially in the design of new polymers for modifying the cell surface with specific biological functions combined with either enzymatic approach or metabolic modification, will hold great promises in the fields of bioengineering and transplantation medicine.
Abbreviations
- AL:
-
Alginate
- BAEC:
-
Bovine aortic endothelial cells
- BNHS:
-
Biotin N-hydroxysuccinimidyl
- CAM:
-
Cell adhesion molecules
- CH-PC:
-
Chitosan-graft-phosphorylcholine
- CNS:
-
Central nervous system
- CP:
-
Choline phosphate
- DMPE:
-
1,2-Dimyristoyl-sn-glycerol-3-phosphatidylethanolamine
- EC:
-
Endothelial cells
- ECM:
-
Extracellular matrix
- ESCs:
-
Embryonic stem cells
- FGF2:
-
Fibroblast growth factor 2
- GAGs:
-
Glycosaminoglycans
- HPGs:
-
Hyperbranched polyglycerols
- HS:
-
Heparan sulfate
- HSCs:
-
Hematopoietic stem cells
- HTPs:
-
HaloTag proteins
- ICAM-1:
-
Intercellular cell adhesion molecule-1
- IPSC:
-
Induced pluripotent stem cells
- LbL:
-
Layer-by-layer
- Mal-Phe-PEG:
-
Maleimidophenyl-PEG
- m-PEG:
-
Methoxypoly(ethylene glycol)
- MSC:
-
Mesenchymal stem cell
- neoPGs:
-
Neoproteoglycans
- NHS:
-
N-hydroxysuccinimidyl
- NSCs:
-
Neural stem cells
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Poly(ethylene glycol)
- PEI:
-
Poly(ethyleneimine)
- PEM:
-
Polyelectrolyte multilayer film
- PLL:
-
Poly-l-lysine
- PLL-PEG:
-
Poly-l-lysine-graft-polyethylene glycol
- PMNs:
-
Polymorphonuclear leukocytes
- PPG:
-
Palmitated protein G
- PSCs:
-
Pluripotent stem cells
- PVA:
-
Poly(vinyl alcohol)
- RBCs:
-
Red blood cells
- SDF-1:
-
Stromal-derived factor-1
- SLeX:
-
Sialyl-Lewisx
- SS:
-
Succinimidyl succinate
- VCAM:
-
Vascular endothelial adhesion molecule
References
J. Spatz, Molecular engineering of cellular environments: cell adhesion to nano-digital surfaces. Tissue Eng. 13(4), 877–878 (2007)
D. Falconnet, G. Csucs, H.M. Grandin, M. Textor, Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 27(16), 3044–3063 (2006)
L. Lu, J. Gao, Z. Guo, Labeling cell surface gpis and GPI-anchored proteins through metabolic engineering with artificial inositol derivatives. Angew. Chem. Int. Ed. 54(33), 9679–9682 (2015)
M.E. Medof, S. Nagarajan, M.L. Tykocinski, Cell-surface engineering with GPI-anchored proteins. FASEB J. 10(5), 574–586 (1996)
E. Saxon, C.R. Bertozzi, Chemical and biological strategies for engineering cell surface glycosylation. Annu. Rev. Cell Dev. Biol. 17, 1–23 (2001)
W.A. Zhao, G.S.L. Teo, N. Kumar, J.M. Karp, Chemistry and material science at the cell surface. Mater. Today 13(4), 14–21 (2010)
M.D. Mager, V. LaPointe, M.M. Stevens, Exploring and exploiting chemistry at the cell surface. Nat. Chem. 3(8), 582–589 (2011)
M.M. Stevens, J.H. George, Exploring and engineering the cell surface interface. Science 310(5751), 1135–1138 (2005)
B. Wang, P. Liu, R. Tang, Cellular shellization: surface engineering gives cells an exterior. BioEssays 32(8), 698–708 (2010)
Y. Teramura, H. Chen, T. Kawamoto, H. Iwata, Control of cell attachment through polyDNA hybridization. Biomaterials 31(8), 2229–2235 (2010)
B. Wang, J. Song, H. Yuan, C. Nie, F. Lv, L. Liu, S. Wang, Multicellular assembly and light-regulation of cell-cell communication by conjugated polymer materials. Adv. Mater. 26(15), 2371–2375 (2014)
R. Chapanian, I. Constantinescu, D.E. Brooks, M.D. Scott, J.N. Kizhakkedathu, In vivo circulation, clearance, and biodistribution of polyglycerol grafted functional red blood cells. Biomaterials 33(10), 3047–3057 (2012)
R. Chapanian, I. Constantinescu, N. Medvedev, M.D. Scott, D.E. Brooks, J.N. Kizhakkedathu, Therapeutic cells via functional modification: influence of molecular properties of polymer grafts on in vivo circulation, clearance, immunogenicity, and antigen protection. Biomacromolecules 14(6), 2052–2062 (2013)
Y. Teramura, H. Iwata, Cell surface modification with polymers for biomedical studies. Soft Matter 6(6), 1081–1091 (2010)
Y. Teramura, S. Asif, K.N. Ekdahl, B. Nilsson, Cell surface engineering for regulation of immune reactions in cell therapy. Adv. Exp. Med. Biol. 865, 189–209 (2015)
J.H. Jeong, J.J. Schmid, R.E. Kohman, A.T. Zill, R.J. DeVolder, C.E. Smith, M.-H. Lai, A. Shkumatov, T.W. Jensen, L.G. Schook, S.C. Zimmerman, H. Kong, Leukocyte-mimicking stem cell delivery via in situ coating of cells with a bioactive hyperbranched polyglycerol. J. Am. Chem. Soc. 135, 8770–8773 (2013)
B. Kellam, P.A. De Bank, K.M. Shakesheff, Chemical modification of mammalian cell surfaces. Chem. Soc. Rev. 32(6), 327 (2003)
J.C. Kim, G. Tae, Recent advances in cell surface engineering focused on cell therapy. Bull. Kor. Chem. Soc. 36(1), 59–65 (2015)
J. Robertus, W.R. Browne, B.L. Feringa, Dynamic control over cell adhesive properties using molecular-based surface engineering strategies. Chem. Soc. Rev. 39(1), 354–378 (2010)
L.K. Mahal, K.J. Yarema, C.R. Bertozzi, Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276(5315), 1125–1128 (1997)
Y.W. Won, A.N. Patel, D.A. Bull, Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 35(21), 5627–5635 (2014)
J.T. Wilson, W.X. Cui, V. Kozovskaya, E. Kharlampieva, D. Pan, Z. Qu, V.R. Krishnamurthy, J. Mets, V. Kumar, J. Wen, Y.H. Song, V.V. Tsukruk, E.L. Chaikof, Cell surface engineering with polyelectrolyte multilayer thin films. J. Am. Chem. Soc. 133(18), 7054–7064 (2011)
S. Miura, Y. Teramura, H. Iwata, Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials 27(34), 5828–5835 (2006)
Y. Teramura, H. Iwata, Islets surface modification prevents blood-mediated inflammatory responses. Bioconjug. Chem. 19(7), 1389–1395 (2008)
K. Tatsumi, K. Ohashi, Y. Teramura, R. Utoh, K. Kanegae, N. Watanabe, S. Mukobata, M. Nakayama, H. Iwata, T. Okano, The non-invasive cell surface modification of hepatocytes with PEG-lipid derivatives. Biomaterials 33(3), 821–828 (2012)
Y. Teramura, Y. Kaneda, H. Iwata, Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials 28(32), 4818–4825 (2007)
T. Totani, Y. Teramura, H. Iwata, Immobilization of urokinase on the islet surface by amphiphilic poly(vinyl alcohol) that carries alkyl side chains. Biomaterials 29(19), 2878–2883 (2008)
Y. Teramura, H. Iwata, Islet encapsulation with living cells for improvement of biocompatibility. Biomaterials 30(12), 2270–2275 (2009)
Y. Teramura, Cell surface modification with ssDNA-PEG-lipid for analysing intercellular interactions between different cells. Biomaterials 48, 119–128 (2015)
T. Matsui, Y. Arima, N. Takemoto, H. Lwata, Cell patterning on polylactic acid through surface-tethered oligonucleotides. Acta Biomater. 13, 32–41 (2015)
V. Gribova, R. Auzely-Velty, C. Picart, Polyelectrolyte multilayer assemblies on materials surfaces: from cell adhesion to tissue engineering. Chem. Mater. 24(5), 854–869 (2012)
C. Boura, P. Menu, E. Payan, C. Picart, J.C. Voegel, S. Muller, J.F. Stoltz, Endothelial cells grown on thin polyelectrolyte multilayered films: an evaluation of a new versatile surface modification. Biomaterials 24(20), 3521–3530 (2003)
P. Tryoen-Toth, D. Vautier, Y. Haikel, J.C. Voegel, P. Schaaf, J. Chluba, J. Ogier, Viability, adhesion, and bone phenotype of osteoblast-like cells on polyelectrolyte multilayer films. J. Biomed. Mater. Res. 60(4), 657–667 (2002)
L. Richert, F. Boulmedais, P. Lavalle, J. Mutterer, E. Ferreux, G. Decher, P. Schaaf, J.C. Voegel, C. Picart, Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 5(2), 284–294 (2004)
X.F. Yu, Z.H. Liu, J. Janzen, I. Chafeeva, S. Horte, W. Chen, R.K. Kainthan, J.N. Kizhakkedathu, D.E. Brooks, Polyvalent choline phosphate as a universal biomembrane adhesive. Nat. Mater. 11(5), 468–476 (2012)
S. Mansouri, Y. Merhi, F.M. Winnik, M. Tabrizian, Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules 12(3), 585–592 (2011)
a) J.T. Wilson, C.A. Haller, Z. Qu, W. Cui, M.K. Urlam, E.L. Chaikof, Biomolecular surface engineering of pancreatic islets with thrombomodulin. Acta Biomater. 6(6), 1895–1903 (2010). b) R. Chapanian, I. Constantinescu, N.A.A. Rossi, N. Medvedev, D.E. Brooks, M.D. Scott, J.N. Kizhakkedathu, Influence of polymer architecture on antigens camouflage, CD47 protection and complement mediated lysis of surface grafted red blood cells. Biomaterials 33(31), 7871–7883 (2012)
S.C. Hsiao, B.J. Shum, H. Onoe, E.S. Douglas, Z.J. Gartner, R.A. Mathies, C.R. Bertozzi, M.B. Francis, Direct cell surface modification with DNA for the capture of primary cells and the investigation of myotube formation on defined patterns. Langmuir 25(12), 6985–6991 (2009)
H. Cheng, M. Byrska-Bishop, C.T. Zhang, C.J. Kastrup, N.S. Hwang, A.K. Tai, W.W. Lee, X.Y. Xu, M. Nahrendorf, R. Langer, D.G. Anderson, Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials 33(20), 5004–5012 (2012)
A.M. Chen, M.D. Scott, Immunocamouflage: prevention of transfusion-induced graft-versus-host disease via polymer grafting of donor cells. J Biomed Mater Res Part A 67A(2), 626–636 (2003)
S. Hashemi-Najafabadi, E. Vasheghani-Farahani, S.A. Shojaosadati, M.J. Rasaee, J.K. Armstrong, M. Moin, Z. Pourpak, A method to optimize peg-coating of red blood cells. Bioconjug. Chem. 17(5), 1288–1293 (2006)
Y.-M. Wee, D.-G. Lim, Y.-H. Kim, J.-H. Kim, S.-C. Kim, E. Yu, M.-O. Park, M.Y. Choi, Y.-H. Park, H.-J. Jang, E.-Y. Cho, M.-H. Cho, D.-J. Han, Cell surface modification by activated polyethylene glycol prevents allosensitization after islet transplantation. Cell Transplant. 17(10–11), 1257–1269 (2008)
D.P. Blackall, J.K. Armstrong, H.J. Meiselman, T.C. Fisher, Polyethylene glycol–coated red blood cells fail to bind glycophorin A–specific antibodies and are impervious to invasion by the Plasmodium falciparum malaria parasite. Blood 97(2), 551–556 (2001)
A.M. Chen, M.D. Scott, Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs 15, 833–847 (2001)
D. Yun Lee, J. Hee Nam, Y. Byun, Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials 28(11), 1957–1966 (2007)
a) M.D. Scott, K.L. Murad, F. Koumpouras, M. Talbot, J.W. Eaton, Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 94(14), 7566–7571 (1997). b) A.J. Bradleya, K.L. Murada, K.L. Regana, M.D. Scott, Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim. Biophys. Acta 1561(2), 147–158 (2002)
J. Chen, G.H. Altman, V. Karageorgiou, R. Horan, A. Collette, V. Volloch, T. Colabro, D.L. Kaplan, Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J. Biomed. Mat. Res 67A(2), 560–570 (2003)
H. Chen, J. Rhodes, Schiff base forming drugs: mechanisms of immune potentiation and therapeutic potential. J. Mol. Med. 74(9), 497–504 (1996)
J. Rhodes, H. Chen, S.R. Hall, J.E. Beesley, D.C. Jenkins, P. Collins, B. Zheng, Therapeutic potentiation of the immune system by costimulatory Schiff-base-forming drugs. Nature 377(6544), 71–75 (1995)
Y. Yuan, X.J. Wang, B. Mei, D.X. Zhang, A.M. Tang, L.N. An, X.X. He, J. Jiang, G.L. Liang, Labeling thiols on proteins, living cells, and tissues with enhanced emission induced by FRET. Sci. Rep-Uk. 3, 3523 (2013)
A.G. Torres, M.J. Gait, Exploiting cell surface thiols to enhance cellular uptake. Trends Biotechnol. 30(4), 185–190 (2012)
M.M. Fretz, N.A. Penning, S. Al-Taei, S. Futaki, T. Takeuchi, I. Nakase, G. Storm, A.T. Jones, Temperature-, concentration- and cholesterol-dependent translocation of l- and d-octa-arginine across the plasma and nuclear membrane of CD34(+) leukaemia cells. Biochem. J. 403, 335–342 (2007)
K. Tyagarajan, E. Pretzer, J.E. Wiktorowicz, Thiol-reactive dyes for fluorescence labeling of proteomic samples. Electrophoresis 24(14), 2348–2358 (2003)
B. Sahaf, K. Heydari, L.A. Herzenberg, L.A. Herzenberg, Lymphocyte surface thiol levels. Proc. Natl. Acad. Sci. U. S. A. 100(7), 4001–4005 (2003)
M.T. Stephan, J.J. Moon, S.H. Um, A. Bershteyn, D.J. Irvine, Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16(9), 1035–U135 (2010)
P. Nacharaju, F.N. Boctor, B.N. Manjula, S.A. Acharya, Surface decoration of red blood cells with maleimidophenyl-polyethylene glycol facilitated by thiolation with iminothiolane: an approach to mask A, B, and D antigens to generate universal red blood cells. Transfusion 45(3), 374–383 (2005)
B.K. Kay, S. Thai, V.V. Volgina, High-throughput biotinylation of proteins, in High Throughput Protein Expression and Purification: Methods and Protocols, ed. by S.A. Doyle (Humana Press, Totowa, 2009), pp. 185–198
M. González, L.A. Bagatolli, I. Echabe, J.L.R. Arrondo, C.E. Argaraña, C.R. Cantor, G.D. Fidelio, Interaction of biotin with streptavidin: thermostability and conformational changes upon binding. J. Biol. Chem. 272(17), 11288–11294 (1997)
O. Livnah, E.A. Bayer, M. Wilchek, J.L. Sussman, Three-dimensional structures of avidin and the avidin-biotin complex. Proc. Natl. Acad. Sci. U. S. A. 90(11), 5076–5080 (1993)
Z. Ding, R.B. Fong, C.J. Long, P.S. Stayton, A.S. Hoffman, Size-dependent control of the binding of biotinylated proteins to streptavidin using a polymer shield. Nature 411(6833), 59–62 (2001)
S.H. Yang, S.M. Kang, K.-B. Lee, T.D. Chung, H. Lee, I.S. Choi, Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 133(9), 2795–2797 (2011)
X.Q. Dou, J. Zhang, C. Feng, Biotin-avidin based universal cell-matrix interaction for promoting three-dimensional cell adhesion. ACS Appl. Mater. Interfaces 7(37), 20786–20792 (2015)
R. Chapanian, I. Constantinescu, D.E. Brooks, M.D. Scott, J. Kizhakkedathu, Antigens protected functional red blood cells by the membrane grafting of compact hyperbranched polyglycerols. Jove-J. Vis. Exp. (71), e50075. https://doi.org/10.3791/50075 (2013)
I.M. Brockman, K.L.J. Prather, Dynamic metabolic engineering: new strategies for developing responsive cell factories. Biotechnol. J. 10(9), 1360–1369 (2015)
C.M. Cole, J. Yang, J. Šečkutė, N.K. Devaraj, Fluorescent live-cell imaging of metabolically incorporated unnatural cyclopropene-mannosamine derivatives. ChemBioChem 14(2), 205–208 (2013)
B.D. Ratner, The engineering of biomaterials exhibiting recognition and specificity. J. Mol. Recognit. 9(5–6), 617–625 (1996)
S.-G. Sampathkumar, M.B. Jones, K.J. Yarema, Metabolic expression of thiol-derivatized sialic acids on the cell surface and their quantitative estimation by flow cytometry. Nat. Protoc. 1(4), 1840–1851 (2006)
C.T. Campbell, S.-G. Sampathkumar, K.J. Yarema, Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol. Biosyst. 3(3), 187–194 (2007)
D.H. Dube, C.R. Bertozzi, Metabolic oligosaccharide engineering as a tool for glycobiology. Curr. Opin. Chem. Biol. 7(5), 616–625 (2003)
K.J. Yarema, L.K. Mahal, R.E. Bruehl, E.C. Rodriguez, C.R. Bertozzi, Metabolic delivery of ketone groups to sialic acid residues: application to cell surface glycoform engineering. J. Biol. Chem. 273(47), 31168–31179 (1998)
Y. Iwasaki, M. Sakiyama, S. Fujii, S.-I. Yusa, Surface modification of mammalian cells with stimuli-responsive polymers. Chem. Commun. 49(71), 7824–7826 (2013)
B. Wang, J. Brand-Miller, The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 57(11), 1351–1369 (2003)
C.G. Gahmberg, M. Tolvanen, [3] Nonmetabolic radiolabeling and tagging of glycoconjugates. Methods Enzymol. 230, 32–44 (1994)
M. Tolvanen, C.G. Gahmberg, In vitro attachment of mono- and oligosaccharides to surface glycoconjugates of intact cells. J. Biol. Chem. 261(20), 9546–9551 (1986)
P.A. De Bank, B. Kellam, D.A. Kendall, K.M. Shakesheff, Surface engineering of living myoblasts via selective periodate oxidation. Biotechnol. Bioeng. 81(7), 800–808 (2003)
C.A. Holden, Q. Yuan, W.A. Yeudall, D.A. Lebman, H. Yang, Surface engineering of macrophages with nanoparticles to generate a cell–nanoparticle hybrid vehicle for hypoxia-targeted drug delivery. Int. J. Nanomedicine 5, 25–36 (2010)
D.J. Moloney, R.S. Haltiwanger, The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-β1,3-glucosyltransferase activity from Chinese hamster ovary cells. Glycobiology 9(7), 679–687 (1999)
L.K. Mahal, K.J. Yarema, C.R. Bertozzi, Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276(5315), 1125 (1997)
E. Saxon, C.R. Bertozzi, Cell surface engineering by a modified staudinger reaction. Science 287(5460), 2007 (2000)
Y. Iwasaki, E. Tabata, K. Kurita, K. Akiyoshi, Selective cell attachment to a biomimetic polymer surface through the recognition of cell-surface tags. Bioconjug. Chem. 16(3), 567–575 (2005)
M.G. Paulick, M.B. Forstner, J.T. Groves, C.R. Bertozzi, A chemical approach to unraveling the biological function of the glycosylphosphatidylinositol anchor. Proc. Natl. Acad. Sci. 104(51), 20332–20337 (2007)
D. Rabuka, M.B. Forstner, J.T. Groves, C.R. Bertozzi, Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes. J. Am. Chem. Soc. 130(18), 5947–5953 (2008)
S. Bi, S. Yue, S. Zhang, Hybridization chain reaction: a versatile molecular tool for biosensing, bioimaging, and biomedicine. Chem. Soc. Rev. 46, 4281–4298 (2017)
J. Couet, J.D.J.S. Samuel, A. Kopyshev, S. Santer, M. Biesalski, Peptide–polymer hybrid nanotubes. Angew. Chem. Int. Ed. 44(21), 3297–3301 (2005)
J.O. Zoppe, Y. Habibi, O.J. Rojas, R.A. Venditti, L.-S. Johansson, K. Efimenko, M. Österberg, J. Laine, Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 11(10), 2683–2691 (2010)
D. Bontempo, H.D. Maynard, Streptavidin as a macroinitiator for polymerization: in situ protein−polymer conjugate formation. J. Am. Chem. Soc. 127(18), 6508–6509 (2005)
I. Cobo, M. Li, B.S. Sumerlin, S. Perrier, Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. Mater. 14(2), 143–159 (2015)
J. Niu, D.J. Lunn, A. Pusuluri, J.I. Yoo, M.A. O'Malley, S. Mitragotri, H.T. Soh, C.J. Hawker, Engineering live cell surfaces with functional polymers via cytocompatible controlled radical polymerization. Nat. Chem. 9(6), 537–545 (2017)
J.Y. Kim, B.S. Lee, J. Choi, B.J. Kim, J.Y. Choi, S.M. Kang, S.H. Yang, I.S. Choi, Cytocompatible polymer grafting from individual living cells by atom-transfer radical polymerization. Angew. Chem. Int. Ed. 55(49), 15306–15309 (2016)
I. Drachuk, M.K. Gupta, V.V. Tsukruk, Biomimetic coatings to control cellular function through cell surface engineering. Adv FunMater 23(36), 4437–4453 (2013)
R.F. Fakhrullin, Y.M. Lvov, “Face-Lifting” and “make-up” for microorganisms: layer-by-layer polyelectrolyte nanocoating. ACS Nano 6(6), 4557–4564 (2012)
A.J. Bradley, S.T. Test, K.L. Murad, J. Mitsuyoshi, M.D. Scott, Interactions of IgM ABO antibodies and complement with methoxy-PEG-modified human RBCs. Transfusion 41(10), 1225–1233 (2001)
R. Chapanian, D.H. Kwan, I. Constantinescu, F.A. Shaikh, N.A.A. Rossi, S.G. Withers, J.N. Kizhakkedathu, Enhancement of biological reactions on cell surfaces via macromolecular crowding. Nat. Commun. 5, 4683 (2014)
N.A.A. Rossi, I. Constantinescu, D.E. Brooks, M.D. Scott, J.N. Kizhakkedathu, Enhanced cell surface polymer grafting in concentrated and nonreactive aqueous polymer solutions. J. Am. Chem. Soc. 132, 3423–3430 (2010)
V.R. Muzykantov, Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Del 7(4), 403–427 (2010)
M.D. Scott, A.M. Chen, Beyond the red cell: pegylation of other blood cells and tissues. Transfus. Clin. Biol. 11(1), 40–46 (2004)
V.L. Leung, J.N. Kizhakkedathu, The mechanism and modulation of complement activation on polymer grafted cells. Acta Biomater. 31, 252–263 (2016)
N.A.A. Rossi, I. Constantinescu, R.K. Kainthan, D.E. Brooks, M.D. Scott, J.N. Kizhakkedathu, Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials 31, 4167–4178 (2010)
B. Afzali, R.I. Lechler, M.P. Hernandez-Fuentes, Allorecognition and the alloresponse: clinical implications. Tissue Antigens 69(6), 545–556 (2007)
R. Snanoudj, C. Frangié, B. Deroure, H. François, C. Créput, S. Beaudreuil, A. Dürrbach, B. Charpentier, The blockade of T-cell co-stimulation as a therapeutic stratagem for immunosuppression: focus on belatacept. Biol. Targets Ther. 1(3), 203–213 (2007)
K.L. Murad, E.J. Gosselin, J.W. Eaton, M.D. Scott, Stealth cells: prevention of major histocompatibility complex class ii-mediated t-cell activation by cell surface modification. Blood 94(6), 2135 (1999)
A.M. Chen, M.D. Scott, Comparative analysis of polymer and linker chemistries on the efficacy of immunocamouflage of murine leukocytes. Artif. Cells Blood Substit. Biotechnol. 34(3), 305–322 (2006)
D. Wang, W.M. Toyofuku, A.M. Chen, M.D. Scott, Induction of immunotolerance via mPEG grafting to allogeneic leukocytes. Biomaterials 32(35), 9494–9503 (2011)
D.L. Kyluik-Price, M.D. Scott, Effects of methoxypoly (ethylene glycol) mediated immunocamouflage on leukocyte surface marker detection, cell conjugation, activation and alloproliferation. Biomaterials 74, 167–177 (2016)
N. Kang, W.M. Toyofuku, X. Yang, M.D. Scott, Inhibition of allogeneic cytotoxic T cell (CD8+) proliferation via polymer-induced Treg (CD4+) cells. Acta Biomater 57, 146–155 (2017). S1742-7061(17)30263-5
T. Maerz, R. Mu, K.C. Baker, Cell and scaffold surface engineering to enhance cell migration and tissue regeneration. Surf. Innov. 2(1), 17–25 (2014)
D. Sarkar, P.K. Vemula, G.S.L. Teo, D. Spelke, R. Karnik, L.Y. Wee, J.M. Karp, Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug. Chem. 19(11), 2105–2109 (2008)
K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, S. Yamanaka, Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5), 861–872 (2007)
K. Takahashi, S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4), 663–676 (2006)
O. Levy, W. Zhao, L.J. Mortensen, S. Leblanc, K. Tsang, M. Fu, J.A. Phillips, V. Sagar, P. Anandakumaran, J. Ngai, C.H. Cui, P. Eimon, M. Angel, C.P. Lin, M.F. Yanik, J.M. Karp, mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 122(14), e23–e32 (2013)
W.L. Murphy, T.C. McDevitt, A.J. Engler, Materials as stem cell regulators. Nat. Mater. 13(6), 547–557 (2014)
A. Pulsipher, M.E. Griffin, S.E. Stone, L.C. Hsieh-Wilson, Long-lived engineering of glycans to direct stem cell fate. Angew. Chem. Int. Ed. 54(5), 1466–1470 (2015)
M.T. Stephan, J.J. Moon, S.H. Um, A. Bershteyn, D.J. Irvine, Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16(9), 1035–1041 (2010)
M.L. Huang, R.A. Smith, G.W. Trieger, K. Godula, Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J. Am. Chem. Soc. 136(30), 10565–10568 (2014)
K. Golab, S. Kizilel, T. Bal, M. Hara, M. Zielinski, R. Grose, O. Savari, X.J. Wang, L.J. Wang, M. Tibudan, A. Krzystyniak, N. Marek-Trzonkowska, J.M. Millis, P. Trzonkowski, P. Witkowski, Improved coating of pancreatic islets with regulatory t cells to create local immunosuppression by using the biotin-polyethylene glycol-succinimidyl valeric acid ester molecule. Transplant. Proc. 46(6), 1967–1971 (2014)
D. Vats, H. Wang, D. Esterhazy, K. Dikaiou, C. Danzer, M. Honer, F. Stuker, H. Matile, C. Migliorini, E. Fischer, J. Ripoll, R. Keist, W. Krek, R. Schibli, M. Stoffel, M. Rudin, Multimodal imaging of pancreatic beta cells in vivo by targeting transmembrane protein 27 (TMEM27). Diabetologia 55(9), 2407–2416 (2012)
E.S. Yolcu, H. Zhao, L. Bandura-Morgan, C. Lacelle, K.B. Woodward, N. Askenasy, H. Shirwan, Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J. Immunol. 187(11), 5901–5909 (2011)
S. Cabric, T. Eich, J. Sanchez, B. Nilsson, O. Korsgren, R. Larsson, A new method for incorporating functional heparin onto the surface of islets of langerhans. Tissue Eng. Part C Methods 14(2), 141–147 (2008)
N. Takemoto, Y. Teramura, H. Iwata, Immobilization of sertoli cells on islets of langerhans. Biomater. Sci. 1(3), 315–321 (2013)
G.A. Zimmerman, T.M. McIntyre, M. Mehra, S.M. Prescott, Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J. Cell Biol. 110(2), 529–540 (1990)
V.D. Bhat, G.A. Truskey, W.M. Reichert, Using avidin-mediated binding to enhance initial endothelial cell attachment and spreading. J. Biomed. Mater. Res. Part A 40(1), 57–65 (1998)
I.K. Ko, T.J. Kean, J.E. Dennis, Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials 30(22), 3702–3710 (2009)
T.E. Deglau, J.D. Johnson, F.S. Villanueva, W.R. Wagner, Targeting microspheres and cells to polyethylene glycol-modified biological surfaces. J. Biomed. Mater. Res. Part A 81A(3), 578–585 (2007)
T.E. Deglau, T.M. Maul, F.S. Villanueva, W.R. Wagner, In vivo PEG modification of vascular surfaces for targeted delivery. J. Vasc. Surg. 55(4), 1087–1095 (2012)
K.-H. Park, Arg-Gly-Asp (RGD) sequence conjugated in a synthetic copolymer bearing a sugar moiety for improved culture of parenchymal cells (hepatocytes). Biotechnol. Lett. 24(17), 1401–1406 (2002)
N. Kojima, T. Matsuo, Y. Sakai, Rapid hepatic cell attachment onto biodegradable polymer surfaces without toxicity using an avidin–biotin binding system. Biomaterials 27(28), 4904–4910 (2006)
D.H. Kwan, I. Constantinescu, R. Chapanian, M.A. Higgins, M.P. Kotzler, E. Samain, A.B.. Boraston, J.N. Kizhakkedathu, S.G. Withers, Toward efficient enzymes for the generation of universal blood through structure-guided directed evolution. J. Am. Chem. Soc. 137(17), 5695–5705 (2015)
Acknowledgments
The authors acknowledge the funding by the Canadian Institutes of Health Research (CIHR) and from the Natural Sciences and Engineering Research Council (NSERC) of Canada to JNK. JNK holds a Career Investigator Scholar award from the Michael Smith Foundation for Health Research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Abbina, S., Mohtaram, N.K., Kizhakkedathu, J.N. (2019). Cell Surface Engineering. In: Jafar Mazumder, M., Sheardown, H., Al-Ahmed, A. (eds) Functional Biopolymers. Polymers and Polymeric Composites: A Reference Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95990-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-95990-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95989-4
Online ISBN: 978-3-319-95990-0
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics