Abstract
Electrofuel produced by microbes utilizing CO2 and electricity as carbon and energy sources, respectively, has received much attention as an alternative to fossil fuels. Based on the inherent capabilities of microorganisms, extracellular electron transfer (EET) was demonstrated with various modes of cathodic electron transfer. With extensive studies on Geobacter sulfurreducens and Shewanella oneidensis, it was confirmed that cytochromes located in the outer membrane are essential for direct EET. Although a few electroactive bacteria are cytochrome independent, key compounds potentially involved in EET can be determined based on their redox functions, which were successfully demonstrated in electroactive acetogens and Ralstonia eutropha. Electroactive acetogens reduce CO2 with electric power at the cathode and direct sunlight with a self-photosensitized nanoparticle for the production of organic compounds. Furthermore, a hybrid water splitting-biosynthetic system, which consists of advanced catalysts and genetically modified R. eutropha, exhibited production of diverse electrofuels with high CO2 reduction efficiency. To improve the production of electrofuels, basic research and engineering of microorganisms and modification of electrodes is essential.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
In the last few decades, much of the concerns over the depletion of fossil fuel, importance of alternative energy , and increase in atmospheric CO2 levels have promoted the development of several biological approaches for conversion of CO2 into fuels or chemical products. Not surprisingly, many microorganisms can utilize various forms of reducing power to fix CO2 into biomass (i.e., autotrophic growth) (Fig. 1) (Nybo et al. 2015). For example, photoautotrophic bacteria capture photon from light to obtain high-energy electrons, which are further used to reduce CO2. Additionally, microorganisms can incorporate electrons extracted from reduced chemicals (such as NH4 +, H2S, or Fe2+) into their metabolism. Several bacteria are of particular interest because of their potential for direct or indirect electron transfer from a cathode to obtain reducing power, which is referred to as microbial electrosynthesis (MES) (Rabaey and Rozendal 2010). MES is a highly attractive method to convert CO2 into fuels, known as electrofuels , by utilizing only the electrons obtained from an electrode. Additionally, a unique system is needed to catalyze the biochemical reactions between an electrode (i.e., the cathode) and bacterial cells, which is called a bioelectrochemical system (BES) . Although the conversion of chemical bond energy into electrical energy at the anode, called microbial fuel cell (MFC) technology, has been extensively studied (Nevin et al. 2010; Rabaey and Rozendal 2010), reversing the reaction at the cathode is thermodynamically unfavorable, where bacteria produce reduced products, usually methane, ethanol, or butanol.
Electrofuel production is an alternative avenue in biofuel production owing to several advantages (Rabaey and Rozendal 2010). Photon-to-fuel efficiency using a solar panel is higher than the efficiency of natural photosynthesis (Zhang 2015). Owing to the early stage of technology development, current CO2 reduction rate and product diversity are limited (Tremblay and Zhang 2015). However, metabolic engineering and synthetic biology approaches will accelerate the efficiency of electrofuel production in various ways. In this chapter, we highlight the latest findings on the electrofuel production and describe how electricity and CO2 facilitate electrofuel production in the most extensively studied microorganisms.
2 Bioelectrochemical System
Electron transfer reactions are essential metabolic processes in living organisms. The electrons are transferred from an electron donor with lower potential to an electron acceptor with higher potential in microorganisms, and this process allows the microbes to generate energy and drive their metabolism. Most microorganisms have different electron transport chains (ETC) (Hernandez and Newman 2001). Electrons are transported from an electron donor to a final electron acceptor via a series of redox reactions of membrane-associated electron carriers, which are involved in the establishment of an electrochemical gradient across the membrane. The net energy gain (Gibbs free energy, ΔG) in the ETC is governed by the difference in redox potential (ΔE) between electron donor and acceptor. Among microbes, some unique bacteria called exoelectrogens, which transfer electrons from the outer membrane to the external environment, or vice versa, have been studied (Potter 1911).
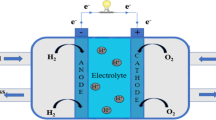
A decade ago, a special phenomenon was observed in some bacteria, wherein they were able to produce or receive electric currents because of the oxidation of organic compounds (Gregory et al. 2004). These unique microbes have been used to develop a novel electro-bioreactor system, which is also called as BES (Fig. 2a). BES contains two electrodes (anode and cathode), membrane between the electrodes, and extracellular electron transfer (EET) microbes. In MFC, microbes oxidize a supplied organic carbon or electron donor to generate a flow of electrons from the anode surface to the cathode surface (Rabaey and Rozendal 2010), where compounds like microbes or oxygen are reduced. Additionally, electricity can be supplied to the BES from renewable sources to permit the thermodynamically unfavorable metabolic process.
Based on the BES system, MES, potentially, is a carbon fixation process that produces organic molecules such as acetate (Nevin et al. 2010, 2011) or methane (Cheng et al. 2009) by using electricity as an energy source and electrochemically active microbes as biocatalysts to reduce CO2, a carbon source (Rabaey and Rozendal 2010). Therefore, production of electrofuels largely depends on the interaction between microbes and surfaces of electrodes.
3 Electron Transfer from a Cathode to Microbes
To transfer the electrons received from cathode directly or indirectly to microorganisms, membrane-associated electron carriers are required. For this process, redox reactions are catalyzed spontaneously by different membrane-associated electron carriers. In general, based on the inherent capabilities of microorganisms, two methods of cathodic electron transfers are proposed (Rabaey and Rozendal 2010): (1) the microbe physically interacts with the cathode by pili or pilus-like appendages (called as nanowires) or (2) the microbe indirectly interacts through certain mediators or hydrogen shuttles (Fig. 2b).
3.1 Direct Electron Transfer
A physical interaction between bacteria and cathode, called direct electron transfer, is an attractive process without a requirement for the diffusion of an electron carrier. Geobacter sulfurreducens and Shewanella oneidensis MR-1 are metal-reducing bacteria that have been extensively studied. Several studies have reported that direct EET typically involves at least a series of outer membrane complexes, and outer-membrane c-type cytochromes are essential for the direct EET for both bacteria (Shi et al. 2009; Mehta et al. 2005)
G. sulfurreducens utilizes the branched OMCs system, which consists of several multiheme c-type cytochromes. The system enables the transfer of electrons between the extracellular metals and the cellular menaquinone (MQ) pool to establish an electrochemical gradient (Lovley et al. 2011). In addition, the electrons can be directly transferred to the electrode surface through conductive pili or pilus-like appendages (Reguera et al. 2005). Similar to G. sulfurreducens, S. oneidensis MR-1 uses the Mtr pathway, which consists of decaheme c-type cytochromes, to transfer electrons beyond the cell envelope (Bretschger et al. 2007). Additionally, both strains have membrane-bound NADH-hydrogenase, soluble electron carriers and H+ ATPase. Additionally, it was reported that both bacteria can take up electrons from the cathode. For electron uptake by S. oneidensis MR-1, the reversed Mtr pathway is used (Ross et al. 2011), but a different pathway is used in G. sulfurreducens (Strycharz et al. 2011). In addition, the mechanisms of the cathodic reaction have not been studied in detail compared to those of the anodic reaction in BES.
3.2 Indirect Electron Transfer
Alternatively, electrons can be transported indirectly via the reduction of mediators (Fig. 2b). Previous studies reported that G. sulfurreducens secretes cytochrome (OmcZ) as a redox mediator within the biofilm matrix (Richter et al. 2009). For S. oneidensis, electrons can be transported indirectly via self-produced flavins as an electron shuttle (Marsili et al. 2008). Although, indirect electron transfer is estimated to be inefficient on a small scale, the carrier-mediated transfer can be sustainable with minimal cost on a large scale. Under these circumstances, hydrogen and formic acid can be used for this purpose (described below).
3.3 Electron Transfer Without Cytochrome
Several microbes have been reported to exchange electrons via the electrode (direct/indirect EET), but their EET mechanisms have not been well understood. Interestingly, a few organisms, which show the absence of the membrane-bound cytochrome (e.g., Clostridium ljungdahlii ), have shown a possibility of being capable of direct EET (Nevin et al. 2011; Köpke et al. 2010). According to their function, the possible mechanisms of electron transfer carriers have been postulated as follows:
-
1.
NADH dehydrogenase: NADH dehydrogenase catalyzes the transfer of electrons from NADH to the quinone pool in the ETC.
-
2.
Ferredoxin-dependent proteins: Iron-sulfur is the prosthetic group of ferredoxins, which mediate electron transfer in diverse metabolic reactions. The Rnf complex is a membrane-associated NADH:ferredoxin oxidoreductase contributing to proton (or sodium) motive force for ATP synthesis in acetogens such as C. ljungdahlii or Acetobacterium woodii (Tremblay et al. 2012). Additionally, the Ech complex (energy-conserving hydrogenase) catalyzes the reduction of ferredoxin with an H+ gradient generated in acetogens such as Moorella thermoacetica (Pierce et al. 2008). Membrane-bound Rnf and Ecf complexes were hypothesized to be key components in electron transport.
-
3.
Cytochromes: Heme is the prosthetic group of cytochromes and participates in electron transfer. Especially in metal-reducing bacteria, such as Shewanella spp., iron is used as the electron carrier and is oxidized or reduced during electron transport processes. Outer membrane cytochromes are key components for extracellular electron transfer, which was demonstrated in Geobacter and Shewanella spp. (Shi et al. 2009).
-
4.
Flavoproteins: Flavin mononucleotide (FMN) is the prosthetic group of flavoproteins. There are many different flavoproteins that participate in either one- or two-electron transfers in the ETC.
-
5.
Quinone: The compound contains lipophilic and catalytic cofactors with soluble electrons and protons, which are located inside the membrane. Quinone plays a crucial role in coupling electron flow to proton movement.
-
6.
Hydrogenase: The protein catalyzes the reduction and oxidation of hydrogen. Recently, the hydrogenase of an electromethanogenic microorganism showed properties of direct electron transfer from a cathode (Deutzmann et al. 2015).
3.4 Electron Transfer via Hydrogen
Hydrogen and formic acid can be used as soluble electron carriers for lithoautotrophic bacteria. Several lithoautotrophic bacteria , including acetogenic bacteria, utilize hydrogen as the energy source; among these, Ralstonia eutropha has been extensively studied. Hydrogen can be produced from the cathode electrochemically in a hybrid microbial-water-splitting catalyst system, leading to the reduction of CO2 to electrofuels (Fig. 2b) (Torella et al. 2015). Current electrolytic hydrogen generation is 50% more efficient and sustainable than direct electron transfer on a large scale (Torella et al. 2015). Because of this advantage, water splitting is a promising mechanism for large-scale conversion using solar energy or other renewable sources of energy (Reece et al. 2011; Hou et al. 2011). Alternatively, formic acid could also be used as a soluble electron carrier. In contrast to hydrogen, formic acid is highly soluble in culture medium and a safe chemical compound, and is produced using electrons, water, and CO2 (Ikeda et al. 1987).
4 Microorganisms for the Production of Electrofuels
For the electricity-driven synthesis of electrofuels, microbes should be able to convert CO2 to intermediate chemicals or fuels by using electrical energy. Some methanogens, acetogens, and oxygen-reducing bacteria have been reported that have the ability for reductive processes at the cathode, resulting in the reduction of CO2 to methane or organic compounds by electric power. In recent years, sunlight has been reported to be directly used by acetogens (M. thermoacetica) for the production of organic compounds, mimicking natural photosynthesis by using self-photosensitized nanoparticles (Sakimoto et al. 2015, 2016).
4.1 Acetogenic Bacteria
Acetogenic bacteria are a physiologically defined group of bacteria that can synthesize acetyl-CoA as central metabolic intermediate from CO2 or CO via the Wood-Ljungdahl pathway (Drake 1994). The unique carbon respiration process of acetogens offers the possibility for the development of a novel approach for the conversion of CO2 into substitute fuels or valuable chemical products. Previous reports indicate that a number of acetogens, including several Clostridium and Sporomusa spp. and M. thermoacetica, accepted electrons from the cathode, reducing CO2 primarily to acetate (Nevin et al. 2010, 2011).
4.2 Energy Conservation and EET in Acetogens
The enzymatic reactions associated with the Wood-Ljungdahl pathway and coupled energy conservation system have been well characterized, especially in M. thermoacetica as a model organism (Huang et al. 2012; Mock et al. 2014; Schuchmann and Müller 2014). However, the mechanism of electron transfer to acetogens from a cathode is largely unknown. In the system, membrane-associated cytochrome enzymes and other electron transfer carriers can be suggested as key components involved in the EET (Schuchmann and Müller 2014; Kracke et al. 2015).
M. thermoacetica has b- and d-type cytochromes (Gottwald et al. 1975; Pierce et al. 2008), which are hypothesized to be involved in direct EET at high coulombic efficiency (Nevin et al. 2011) with mechanism similar to that determined in Shewanella and Geobacter species. Additionally, other membrane-associated electron carriers, which have integrated electron transport involved in the generation of a proton gradient across the membrane, such as NADH dehydrogenase, hydrogenase, menaquinone, energy-converting hydrogenase (Ech complex), could be possible candidates for the electron transfer machinery of M. thermoacetica (Fig. 3a).
(a) Schematic representation of the energy conservation system and applied bioelectrochemical system in Moorella thermoacetica. Abbreviations: MQ menaquinone, Nfn electron-bifurcating transhydrogenase (Nfn complex), Hyd electron-bifurcating hydrogenase, THF tetrahydrofolate, MR methylene-THF reductase, Ech energy-conserving hydrogenase (Ech complex), Fd ferredoxin, Fd2− reduced ferredoxin, Pi inorganic phosphate. (b) The Wood-Ljungdahl pathway in acetogenic bacteria with the fuel production pathway. Abbreviations: THF tetrahydrofolate, CoFeS-P corrinoid [Fe-S] protein, [H] reducing equivalent
Furthermore, the soluble NfnAB and Hyd complexes of M. thermoacetica catalyze the reduction of two NADP+ with one NADH and one Fdred 2− (electron bifurcation) and the reduction of Fd and NAD+ with hydrogen (Fig. 3a), respectively (Wang et al. 2013). Another acetogenic species, Sporomusa ovata is phylogenetically close to M. thermoacetica and contains membrane-associated cytochromes (b- and c-types) and quinones that can directly utilize electrons from a cathode. S. ovata also received electrons with the reduction of CO2 to acetate and 2-oxobutyrate (Nevin et al. 2010).
However, C. ljungdahlii is able to transfer electrons directly from a cathode despite the absence of cytochromes and quinones. Alternatively, the membrane-bound Rnf complex (ferredoxin:NAD+-oxidoreductase) was observed in C. ljungdahlii, which translocates protons across the membrane to synthesize ATP, during autotrophic and heterotrophic growth (Tremblay et al. 2012). Outer membrane redox components, as well as soluble intracellular complexes, are potential part of the electron transfer machinery.
With acetogenic bacteria, MES has been performed using diverse cathode materials ranging from a graphite stick (Nevin et al. 2010) to nickel nanowire (Nie et al. 2013), with cathode potentials from −0.4 V (vs. SHE) to −0.6 V (vs. Ag/AgCl). More recently, novel approaches (Sakimoto et al. 2015, 2016) have been reported. First, cadmium sulfide (CdS) nanoparticles were used to study the production of acetate by precipitation on the membrane of M. thermoacetica (Fig. 3a). Bacteria consumed the electron from the CdS nanoparticle and the photooxidative catalyst (TiO2), which are used as light harvesters to eliminate the need for solid-state cathodes. This approach eliminates the need for hydrogen, which is difficult to store and transport, and solid cathodes, which are difficult to store, transport, and scale up.
4.3 Carbon Fixation and Extended Electrofuel Pathway in Acetogens
With the energy generated from the electron source, acetogens reduce CO2 via the Wood-Ljungdahl pathway by using several electron carriers and enzymes (Ragsdale 2008; Drake et al. 2008). The pathway is coupled with energy conservation systems to permit the occurrence of the thermodynamically unfavorable reaction and is reported to be the most efficient CO2-fixation pathway, among pathways including the Calvin-Benson-Bassham (CBB) cycle , 3-hydroxypropionate cycle, and reverse TCA cycle (Fast and Papoutsakis 2012).
The first reaction of the pathway is the reduction of CO2 to formate by the action of formate dehydrogenase with a ferredoxin- or NADH-dependent reaction. A series of enzymatic reactions involving the methyl and carbonyl branch of the Wood-Ljungdahl pathway results in the conversion of formate to acetyl-CoA (Fig. 3b). Subsequently, the main product, acetate, is produced as a core feature through phosphotransacetylase and acetate kinase in most acetogens (Drake 1994; Ragsdale 2008; Schuchmann and Müller 2014). Additionally, synthesis of targeted products has been demonstrated by introducing biosynthetic pathways for ethanol, 2,3-butanediol, and butanol into C. ljungdahlii, which is known to be a genetically tractable strain for the production of value-added chemicals (Köpke et al. 2010, 2011; Leang et al. 2013). In the near future, under the right circumstances, a rationally designed and genetically modified strain will be able to produce a target product, increase the reaction rate, or tolerate various environmental conditions.
4.4 Ralstonia eutropha
R. eutropha is a gram-negative beta-proteobacterium that is isolated from soil and fresh water environments. R. eutropha proliferates with high cell densities (> 200 g/L) and takes up CO2/H2 autotrophically under aerobic conditions (Lu et al. 2016). The microorganism is considered to be a great platform for the production of electrofuels owing to its ability to route most reduced carbons generated from the CBB cycle to the accumulation of polyhydroxybutyrate (PHB) with CO2/H2 (Brigham et al. 2012). Using available genetic tools, numerous knockout studies involving PHB synthetic pathway have demonstrated that the carbon flux can be redirected from stored PHB into other compounds. Because of these advantages and its genetic tractability, R. eutropha, as a model industrial organism, has been extensively engineered to produce other value-added compounds like alcohols, fatty acids, or esters (Brigham et al. 2012).
4.5 Lithoautotrophic Metabolism of R. eutropha
The central carbon metabolism of R. eutropha completely relies on CO2 fixation, which requires a series of high energy-intensive reactions. R. eutropha produces two [NiFe]-hydrogenases – membrane-bound hydrogenase (MBH) and a cytoplasmic soluble hydrogenase (SH) –to conserve the energy supply through the oxidation of H2 (Cramm 2009; Bowien and Kusian 2002). H2 oxidation is catalyzed by oxygen/carbon monoxide-tolerant hydrogenases. MBH is linked to ETC and SH, which is a bidirectional hydrogenase and produces energy by reducing NAD+ with the oxidation of hydrogen (Fig. 4a).
(a) Schematic representation of lithoautotrophic metabolism of Ralstonia eutropha. Abbreviations: CA carbonic anhydrase, MBH membrane-bound hydrogenase, ETC electron transport chain, SH soluble hydrogenase, Pi inorganic phosphate, Q coenzyme Q. (b) A modified Calvin-Benson-Bassham pathway for the production of biofuels in Ralstonia eutropha. Abbreviations: G3P glyceraldehyde 3-phosphate, PEP phosphoenolpyruvate, P(3HB) poly(3-hydroxybutyrate)
Carbonic anhydrase (CA) and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) are the key enzymes in the CBB cycle (Bowien and Kusian 2002). CA can catalyze the interconversion between CO2 and bicarbonate and can control intracellular pH homeostasis more efficiently by trapping CO2 inside the cell (Fig. 4a). RuBisCo is the second key enzyme used in the carbon fixation step (Fig. 4b), and it (in Form 1C) is more suitable for O2-containing environments (Badger and Bek 2008).
4.6 Hybrid Water Splitting-Biosynthetic System
For the production of electrofuels, a hybrid water splitting-biosynthetic system has been proposed, which consists of water-splitting catalysts and engineered R. eutropha (Torella et al. 2015; Liu et al. 2016). The system depends on the key catalysts (e.g., cobalt-phosphorus electrode) that split water and produce hydrogen to generate biomass and isopropyl alcohol. R. eutropha, the biocatalyst, thrives autotrophically on CO2 and H2 as the carbon and energy sources, respectively. In this system, R. eutropha converts CO2 to multicarbon compounds via the CBB cycle (Fig. 4b).
When R. eutropha faces carbon abundance and nutrient deprivation, PHB was accumulated via the CBB cycle as a form of intracellular carbon storage (Pohlmann et al. 2006). Recently, the carbon flux was reported to be rerouted to prevent the accumulation of PHB, which causes the strain to secrete pyruvate and NADH abundantly and produce biofuel (Lu et al. 2012). By redirecting the rescued pyruvate and NADH into biosynthetic biofuel pathway, the production of isopropanol (Li et al. 2012; Torella et al. 2015; Liu et al. 2016), butanol, isobutanol (Liu et al. 2016), and 3-methyt-l-butanol (Kracke et al. 2015; Liu et al. 2016; Lu et al. 2012) was demonstrated (Fig. 4b). Although butanol production has not been reported yet in R. eutropha, it can be achieved by metabolically engineered R. eutropha.
To generate and transfer hydrogen efficiently from water and electricity to microbes via water splitting, several electrodes such as Pt, stainless steel (SS), cobalt phosphate (CoPi), and NiMoZn alloy have been used (Torella et al. 2015; Li et al. 2012; Schuster and Schlegel 1967). However, these systems are lethal to bacteria because of the generation of reactive oxygen species (ROS) or leaching of Ni particles. The results of a very recent study on catalyst design showed that the combination of an ROS-resistant cobalt-phosphorus alloy cathode and a cobalt phosphate (CoPi) anode increased the high CO2 reduction efficiency (~10%) without ROS production and Ni leaching.
5 Conclusions and Future Research
In summary, electrofuel utilizes CO2 and H2 as carbon and energy sources, respectively. With extensive studies, several microorganisms were demonstrated and modified successfully for the conversion of CO2 into various alternative fuels by using electricity. However, the underlying mechanism of extracellular electron transfer remains unclear. In addition, the performance of MES systems including final product titer and the CO2 consumption rate is still limited. To improve the efficiency of the MES system, elucidation and modification of the EET and the involved metabolic networks are required to develop novel and efficient electrofuel production via systems and synthetic biology approaches. Furthermore, advanced cathode modifications will resolve the remaining technical challenges, such as ohmic voltage loss or the scale-up issue.
6 Research Needs
Electrofuel technology is an attractive and paid by great attentions in recent years, because carbon dioxide can be sequestered and stored as alternative fuels through the microbial conversion of carbon dioxide with renewable energy. Thus, the importance for the economic feasibility and environment problems will continue to grow. With extensive studies, several microorganisms were demonstrated and modified successfully for the conversion of CO2 into various alternative fuels by using electricity. However, the underlying mechanism of extracellular electron transfer remains unclear. In addition, the performance of MES systems including final product titer and the CO2 consumption rate is still limited. To improve the efficiency of the MES system, elucidation and modification of the EET and the involved metabolic networks are required to develop novel and efficient electrofuel production via systems and synthetic biology approaches. Furthermore, advanced cathode modifications will resolve the remaining technical challenges, such as ohmic voltage loss or the scale-up issue
References
Badger MR, Bek EJ (2008) Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot 59(7):1525–1541
Bowien B, Kusian B (2002) Genetics and control of CO(2) assimilation in the chemoautotroph Ralstonia eutropha. Arch Microbiol 178(2):85–93
Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, Culley DE, Reardon CL, Barua S, Romine MF, Zhou J, Beliaev AS, Bouhenni R, Saffarini D, Mansfeld F, Kim B-H, Fredrickson JK, Nealson KH (2007) Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol 73(21):7003–7012
Brigham CJ, Gai CS, Lu J, Speth DR, Worden RM, Sinskey AJ (2012) Chapter 39: Engineering Ralstonia eutropha for production of isobutanol from CO2, H2, and O2. In: Advanced biofuels and bioproducts. Springer, New York
Cheng S, Xing D, Call DF, Logan BE (2009) Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol 43(10):3953–3958
Cramm R (2009) Genomic view of energy metabolism in Ralstonia eutropha H16. J Mol Microbiol Biotechnol 16(1–2):38–52
Deutzmann JS, Sahin M, Spormann AM (2015) Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. MBio 6(2):1
Drake HL (1994) Chapter 1: Acetogenesis, acetogenic bacteria, and the acetyl-CoA “Wood/Ljungdahl” pathway: past and current perspectives. In: Acetogenesis. One Penn Plaza, New York, NY 10119: Springer US
Drake HL, Gößner AS, Daniel SL (2008) Old acetogens, new light. Ann N Y Acad Sci 1125:100–128
Fast AG, Papoutsakis ET (2012) Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr Opin Chem Eng 1(4):380–395
Gottwald M, Andreesen JR, LeGall J, Ljungdahl LG (1975) Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol 122(1):325–328
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6 (6):596–604
Hernandez ME, Newman DK (2001) Extracellular electron transfer. Cell Mol Life Sci 58(11):1562–1571
Hou Y, Abrams BL, Vesborg PCK, Björketun ME, Herbst K, Bech L, Setti AM, Damsgaard CD, Pedersen T, Hansen O, Rossmeisl J, Dahl S, Nørskov JK, Chorkendorff I (2011) Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat Mater 10(6):434–438
Huang H, Wang S, Moll J, Thauer RK (2012) Electron bifurcation involved in the energy metabolism of the acetogenic bacterium Moorella thermoacetica growing on glucose or H2 plus CO2. J Bacteriol 194(14):3689–3699
Ikeda S, Takagi T, Ito K (1987) Selective formation of formic acid, oxalic acid, and carbon monoxide by electrochemical reduction of carbon dioxide. BCSJ 60(7):2517–2522
Köpke M, Held C, Hujer S, Liesegang H, Wiezer A, Wollherr A, Ehrenreich A, Liebl W, Gottschalk G, Dürre P (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc Natl Acad Sci U S A 107(29):13087–13092
Köpke M, Mihalcea C, Liew F, Tizard JH, Ali MS, Conolly JJ, Al-Sinawi B, Simpson SD (2011) 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl Environ Microbiol 77(15):5467–5475
Kracke F, Vassilev I, Krömer JO (2015) Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems. Front Microbiol 6:575
Leang C, Ueki T, Nevin KP, Lovley DR (2013) A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen. Appl Environ Microbiol 79(4):1102–1109
Li H, Opgenorth PH, Wernick DG, Rogers S, Wu T-Y, Higashide W, Malati P, Huo Y-X, Cho KM, Liao JC (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335(6076):1596–1596
Liu C, Colón BC, Ziesack M, Silver PA, Nocera DG (2016) Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352(6290):1210–1213
Lovley DR, Ueki T, Zhang T, Malvankar NS, Shrestha PM, Flanagan KA, Aklujkar M, Butler JE, Giloteaux L, Rotaru A-E, Holmes DE, Franks AE, Orellana R, Risso C, Nevin KP (2011) Geobacter: the microbe electric’s physiology, ecology, and practical applications. Adv Microb Physiol 59:1–100
Lu J, Brigham CJ, Gai CS, Sinskey AJ (2012) Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha. Appl Microbiol Biotechnol 96(1):283–297
Lu J, Brigham CJ, Li S, Sinskey AJ (2016) Ralstonia eutropha H16 as a platform for the production of biofuels, biodegradable plastics, and fine chemicals from diverse carbon resources. In: Biotechnology for biofuel production and optimization. Elsevier, Amsterdam, pp 325–351
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105(10):3968–3973
Mehta T, Coppi MV, Childers SE, Lovley DR (2005) Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol 71(12):8634–8641
Mock J, Wang S, Huang H, Kahnt J, Thauer RK (2014) Evidence for a hexaheteromeric methylenetetrahydrofolate reductase in Moorella thermoacetica. J Bacteriol 196(18):3303–3314
Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyenbos-West OL, Lovley DR (2011) Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol 77(9):2882–2886
Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1(2):e00103–10
Nie H, Zhang T, Cui M, Lu H, Lovley DR, Russell TP (2013) Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys Chem Chem Phys 15(34):14290–14294
Nybo SE, Khan NE, Woolston BM, Curtis WR (2015) Metabolic engineering in chemolithoautotrophic hosts for the production of fuels and chemicals. Metab Eng 30:105–120
Pierce E, Xie G, Barabote RD, Saunders E, Han CS, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG, Ragsdale SW (2008) The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ Microbiol 10(10):2550–2573
Pohlmann A, Fricke WF, Reinecke F, Kusian B, Liesegang H, Cramm R, Eitinger T, Ewering C, Pötter M, Schwartz E, Strittmatter A, Voss I, Gottschalk G, Steinbüchel A, Friedrich B, Bowien B (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24(10):1257–1262
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc Lond B Biol Sci 84(571):260–276
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat Rev Microbiol 8(10):706–716
Ragsdale SW (2008) Enzymology of the wood-Ljungdahl pathway of acetogenesis. Ann N Y Acad Sci 1125:129–136
Reece SY, Hamel JA, Sung K, Jarvi TD, Esswein AJ, Pijpers JJH, Nocera DG (2011) Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334(6056):645–648
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435(7045):1098–1101
Richter H, Nevin KP, Jia H, Lowy DA, Lovley DR, Tender LM (2009) Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energ Environ Sci 2(5):506
Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR (2011) Towards electrosynthesis in shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS One 6(2):e16649
Sakimoto KK, Wong AB, Yang P (2015) Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351(6268):74–77
Sakimoto KK, Zhang SJ, Yang P (2016) Cysteine-cystine photoregeneration for oxygenic photosynthesis of acetic acid from CO2 by a tandem inorganic-biological hybrid system. Nano Lett. https://doi.org/10.1021/acs.nanolett.6b02740
Schuchmann K, Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12(12):809–821
Schuster E, Schlegel HG (1967) Chemolithotrophic growth of hydrogenomonas H16 using electrolytic production of hydrogen and oxygen in a chemostat. Arch Microbiol 58(4):380–409
Shi L, Richardson DJ, Wang Z, Kerisit SN, Rosso KM, Zachara JM, Fredrickson JK (2009) The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Environ Microbiol Rep 1(4):220–227
Strycharz SM, Glaven RH, Coppi MV, Gannon SM, Perpetua LA, Liu A, Nevin KP, Lovley DR (2011) Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80(2):142–150
Torella JP, Gagliardi CJ, Chen JS, Bediako DK, Colón B, Way JC, Silver PA, Nocera DG (2015) Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system. Proc Natl Acad Sci U S A 112(8):2337–2342
Tremblay P-L, Zhang T (2015) Electrifying microbes for the production of chemicals. Front Microbiol 6:201
Tremblay P-L, Zhang T, Dar SA, Leang C, Lovley DR (2012) The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4(1):e00406–e00412
Wang S, Huang H, Kahnt J, Thauer RK (2013) A reversible electron-bifurcating ferredoxin- and NAD-dependent [FeFe]-hydrogenase (HydABC) in Moorella thermoacetica. J Bacteriol 195(6):1267–1275
Zhang T (2015) More efficient together. Science 350(6262):738–739
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this entry
Cite this entry
Shin, J., Song, Y., Jin, S., Cho, S., Cho, BK. (2017). Microbial Conversion of Carbon Dioxide to Electrofuels. In: Lee, S. (eds) Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals. Handbook of Hydrocarbon and Lipid Microbiology . Springer, Cham. https://doi.org/10.1007/978-3-319-50436-0_366
Download citation
DOI: https://doi.org/10.1007/978-3-319-50436-0_366
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50435-3
Online ISBN: 978-3-319-50436-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences