Abstract
Obesity is associated with increased risk of cardiovascular disease (CVD), heart failure, diabetes, cancer, and ultimately all-cause mortality. Obesity is causally related to dyslipidemia, hypertension, and diabetes, all strong CVD risk factors, and so causally related to CVD risk. In fact, a substantial part of the risk imparted by obesity on CVD outcomes operates via traditional risk factors. Obese men are almost twice as likely and women almost two and half times as likely to develop hypertension. Obese individuals are around 50% more likely to have a stroke and have around 6–12 times higher risks of developing type 2 diabetes compared to those with a normal BMI.
Obesity is also linked to greater risk for development of heart failure. Yet, there appears to be an obesity paradox in established heart failure such that the risk of death is lower in overweight and mildly obese individuals than in those with normal weight. Such observations are likely partially driven by reverse causality whereby disease-specific issues drive weight loss rather than higher weight per se being protective.
While obesity is most commonly defined by BMI, the importance of body fat distribution and markers such as waist circumference, waist: hip ratio, visceral and ectopic fat volumes are becoming better appreciated. The concept of harmful fat distribution is therefore topical and recent evidence suggest those who can store more fat subcutaneously (and so delay their ectopic depot expansions until much heavier) have lesser diabetes and cardiovascular risks. This paradigm may also largely explain men’s greater risks for both chronic conditions at similar BMI’s to women.
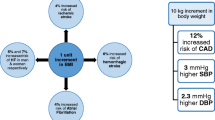
Trials of weight loss add strong support for causal links between adiposity and CVD; for example, the best evidence suggests that losing around 1 kg reduces SBP by around 1 mmHg. Weight loss also improves lipid profiles with reduced total cholesterol, LDL-cholesterol, and in particular triglyceride levels. Weight loss of around 5 kg reduces the risk of obese individuals progressing to impaired glucose tolerance and type 2 diabetes. In those with type 2 diabetes, 5 to <10% intentional weight loss is associated with 3.5 times increased odds of obtaining a 0.5% reduction in HbA1c. Not surprisingly, substantial weight loss has been associated with significantly lower mortality from several causes.
This chapter will show how the best epidemiological evidence, using methods to lessen the impact of reverse causality, supports strong graded links between adiposity and CVD. It will also examine and explain the apparent obesity paradox of heart failure. The chapter will then describe the effect on CVD outcomes of robust lifestyle and surgical intervention studies and trials. Finally, we will also explain how genetics data have helped support causal associations between increasing BMI and CVD, including understanding better the causal links between regional adiposity and CVD.
In conclusion, several lines of evidence, including observational, trial, and genetic, collectively support causal links between obesity, cardiovascular morbidity and mortality, and all-cause mortality.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Obesity
- Cardiovascular disease
- Heart failure
- Obesity paradox
- Weight reduction
- Bariatric surgery
- Regional adiposity
- Ectopic fat
Obesity: Definitions, Epidemiology, Measures, and Associations with All-Cause Mortality
The World Health Organization (WHO) estimates >1.9 billion adults are overweight worldwide, of whom 600 million are obese (World Health Organisation 2016). A recent pooled analysis of 1698 population-based measurement studies with 19.2 million participants across 186 countries showed the global age-standardized mean BMI increased from 21.7 kg/m2 in 1975 to 24.2 kg/m2 in 2014 in men and from 22.1 kg/m2 in 1975 to 24.4 kg/m2 in 2014 in women. The age-standardized prevalence of obesity has increased from 3.2% in 1975 to 10.8% in 2014 in men and from 6.4% to 14.9% in women. 2.3% of the world’s men and 5% of women were severely obese with a BMI ≥35 kg/m2. It is projected by 2025, the global obesity prevalence will be 18% in men and surpass 21% in women; severe obesity will surpass 6% in men and 9% in women (NCD Risk Factor Collaboration 2016).
Obesity is classically defined according to body mass index, calculated by dividing the person’s weight in kilograms by the square of their height in meters. Normal BMI is defined as 18.5–24.9 kg/m2; overweight, a BMI of 25–29.9 kg/m2; and obesity, a BMI ≥ 30 kg/m2. Obesity can be further classified according to severity: obesity grade I: BMI 30–34.9; grade II: BMI 35–39.9; and grade III: BMI ≥40 kg/m2 (World Health Organization 2000). However, these thresholds were largely based on White European populations. Consequently, the WHO recommend defining overweight as a BMI >23 kg/m2 and obese as a BMI >27.5 kg/m2 in Asian populations (World Health Organization Expert Consultation 2004) and the IDF recommend a cut-off waist circumference of 80 cm for Asian women and 90 cm for Asian men (International Diabetes Federation 2006). Analysis of UK Biobank data of around 500,000 individuals confirmed the need for lower BMI cut-offs in nonwhite individuals to reflect the higher diabetes prevalence in these groups. A white participant with a BMI of 30 kg/m2 has the equivalent diabetes prevalence as a South Asian individual with a BMI of 22.0 kg/m2, a black individual with a BMI of 26.0 kg/m2, a Chinese woman with a BMI of 24.0 kg/m2, and Chinese man with a BMI of 26.0 kg/m2 (Ntuk et al. 2014).
Since BMI does not account for adipose distribution and may underestimate cardiovascular risk for certain populations, there are numerous alternative measures of adiposity including waist circumference and waist-to-hip ratio. “Central” obesity is commonly defined as waist circumference >102 cm in men and >88 cm in women, or waist-to-hip ratio > 1.0 in men and >0.85 in women (World Health Organization 2000). Again, these cut-offs vary by ethnicity. Among women, a waist circumference of 88 cm in the white subgroup equated to the following: South Asians, 70 cm; black, 79 cm; and Chinese, 74 cm. Among men, a waist circumference of 102 cm equated to 79, 88, and 88 cm for South Asian, Black, and Chinese participants, respectively (Ntuk et al. 2014).
Obesity has major health implications as it is associated with increased risk of cardiovascular disease (CVD), diabetes mellitus, cancer, and ultimately all-cause mortality (World Health Organisation 2011; The Global BMI Mortality Collaboration 2016). In a large meta-analysis of 239 prospective studies across four continents, the Global BMI Mortality Collaboration investigated the association between BMI and all-cause mortality. Relative to BMI in the healthy range (20–25 kg/m2), all-cause mortality increased throughout the overweight and obese ranges. Those with grade 1 obesity (BMI: 30.0– < 35.0 kg/m2) were 45% as likely to die than those with normal BMI (22·5–<25·0 kg/m2), while those with grade 2 obesity (BMI: 35·0–<40·0 kg/m2) were almost twice as likely to die (HR 1.94). Those with grade 3 obesity (BMI: 40·0–<60·0 kg/m2) had the highest all-cause mortality with a hazard ratio (HR) 2.76 (The Global BMI Mortality Collaboration 2016). Mortality increased log-linearly with increasing BMI >25 kg/m2 across all four continents. This increase was steepest in the young (HR 1.52 aged 35–49 years vs. 1.21 aged 70–89 years for every 5 kg/m2 units higher BMI) and in men (HR 1.51 in men vs. 1.3 in women) (Fig. 1). While attempting to minimize reverse causality through analysis of never-smokers without previous disease surviving at least 5 years, the authors admit full past medical history data was not always available. It is possible that the less pronounced association between increasing BMI and all-cause mortality with age may be due to unidentified chronic illness or subclinical disease. In other words, the stronger associations of BMI with mortality outcomes in the young may be due to less reverse causality.
Association of BMI with all-cause mortality by age category. Mortality increases log-linearly with increasing BMI >25 kg/m2. This increase is steepest in the young. HR 1.52 aged 35–49 years versus 1.21 aged 70–89 years for every 5 kg/m2 units higher BMI. The red arrow shows the reference category BMI 22·5–<25·0 kg/m2 (The Global BMI Mortality Collaboration 2016)
This chapter also noted men had higher risks for dying at similar elevated BMIs to women. This more pronounced increased mortality in men chimes with previous reports that men at equivalent BMIs have increased ectopic fat, for example, liver fat, leading to greater insulin resistance and type 2 diabetes prevalence (Sattar 2013). Unfortunately, this meta-analysis did not include other measures of adiposity to assess fat distribution between subcutaneous, visceral, and ectopic compartments. Interestingly, all-cause mortality showed a U-shaped association with BMI with increased mortality at lower BMIs below the normal range, although once again this U-shape was far less pronounced in the younger group likely due to less reverse causality at this age.
Obesity, CVD Morbidity, and Mortality
Individuals with class I obesity (BMI 30 to <35 kg/m2) have a 75% higher risk of CVD death compared to those with a BMI of 22.5 to <25 kg/m2. This risk appeared to be higher with more severe obesity to over 2.5 fold with grade II obesity (BMI 35 to <40 kg/m2) and a fourfold higher risk in those with grade III obesity (BMI >40 kg/m2). Although less pronounced, those who are overweight are also at an 11–35% higher risk of CVD death. Overall, for every 5 units increase in BMI in those with a BMI >25 kg/m2, the risk of dying from CVD increased 49% (The Global BMI Mortality Collaboration 2016).
Stroke mortality is also higher as BMI increases, with a 42% higher risk for every 5 units increase in BMI. This increase was greatest in Europe (52%), followed by East Asia (50%) and Australia (49%), but failed to reach statistical significance in South Asia. On ascending obesity categories, those with grade I obesity had an overall 49% higher risk of death from stroke and those with grade III obesity an over threefold higher risk. In contrast to CVD, overall there was no statistically significant higher stroke risk in those who were mildly overweight (BMI: 25–27.5), except in East Asia. With a BMI of 27.5 to <30 kg/m2, stroke risk increased by 23%, with most of this driven by a 51% increased stroke mortality risk in East Asia (The Global BMI Mortality Collaboration 2016).
Interestingly, the risk of CVD and stroke mortality was also higher in those who were underweight, with a 33% higher CVD mortality risk and 38% higher stroke mortality risk in those with a BMI of 15 to <18.5 kg/m2. This is despite excluding those with known preexisting chronic disease, the first 5 years of follow-up, and restricting to never-smokers. However, the authors acknowledge not all participants had full data available on coexisting chronic diseases. It is likely some participants had subclinical disease and thus reverse causality likely operated despite several methodological advances included in this study.
Obesity is clearly a CVD risk factor; however, there is debate as to whether this is an independent risk factor per se or whether it is its close association with other risk factors that drives increased risk, namely type 2 diabetes, hypertension, and dyslipidemia. In a meta-analysis by Guh et al., they found a strong association between obesity and type 2 diabetes. The pooled incident rate ratio (IRR) of type 2 diabetes in overweight men was 2.4 and obese men 6.74, while the IRR of diabetes in overweight women was 3.92 and obese women 12.41. Being overweight or obese also increased the likelihood of developing hypertension. The pooled IRR of hypertension in overweight men was 1.28 and for obese men 1.84. The corresponding figures for females were 1.65 and 2.42. The association of obesity with diabetes and hypertension appeared stronger in women (Guh et al. 2009).
In an earlier study, the National Health and Nutrition Examination Survey III, there was a strong association between increasing BMI, hypertension, and dyslipidemia with increased total cholesterol, total cholesterol:HDL-cholesterol ratio, and decreased HDL-cholesterol. Obese men had an average 9 mmHg higher systolic blood pressure (SBP) and obese women an 11 mm Hg higher SBP compared with those with a BMI <25 kg/m2. Mean serum total cholesterol levels were also higher with increasing BMI. Total cholesterol was a mean 18 mg/dL higher in obese men and 22 mg/dL higher in obese women compared to those with a BMI <25 kg/m2. Mean HDL-cholesterol levels were lower in both sexes by around 10 mg/dL as BMI increased from <25 to >30 kg/m2. These relationships were seen across ethnicity and most ages, with the highest relative risk among younger individuals, despite increased prevalence of obesity, hypertension, and lipid abnormalities with increasing age (Brown et al. 2000).
The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration undertook a pooled analysis of 97 prospective cohorts with 1.8 million participants to investigate to what extent the potential causal association of BMI on CHD and stroke were due to adiposity per se versus the associated metabolic risk factors: i.e., higher blood pressure (BP), cholesterol, and glucose. They found for every 5 kg/m2 higher BMI, the risk of CHD increased 27% and that of stroke 18% after adjusting for confounding factors. After additional adjustment for BP, cholesterol, and glucose, this risk fell to 15% for CHD and 4% for stroke. The authors suggested that 46% of excess CHD risk from increased BMI and 76% excess risk for stroke were explained by these three metabolic risk factors. Similar findings were seen between Asian and Western cohorts (North America, Western Europe, Australia, and New Zealand). On individual risk factor analysis, BP was the most important, explaining 31% of excess CHD risk and 65% excess stroke risk (The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration 2014). This is not unexpected as blood pressure is a known risk factor for both, especially stroke.
Compared with those with normal weight, being overweight was associated with a 26% increased CHD risk and 13% increased stroke risk. This was even more apparent in those with a BMI ≥30 kg/m2, where obesity was associated with a 69% increased risk of CHD and 47% increased risk of stroke. This was seen across both Asian and Western cohorts. 50% of this excess CHD risk in the overweight and 44% in those classified as obese was explained collectively by BP, cholesterol, and blood glucose. These factors were even more prominent in stroke, with 98% of excess stroke risk in those overweight and 69% of those with obesity explained by all three metabolic risk factors. Consequently, strategies targeted at lowering blood pressure, cholesterol, and glucose in the overweight and obese could half the excess risk of CHD and cut excess stroke risk by 75%. However, even after accounting for these risk factors, increased BMI is still associated with increased risk, which can only be tackled through effective weight loss (The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration 2014).
The Emerging Risk Factors Collaboration similarly investigated the association of BMI plus additional adiposity measures including waist circumference and waist-to-hip ratio with risk of first-onset CVD. Further, they determined whether the addition of such measures, either alone or in combination, enhanced CVD risk prediction. Analysis of 58 prospective studies including 221,934 people in 17 countries revealed in those with a BMI ≥ 20 kg/m2, for every 1SD (4·56 kg/m2) higher BMI, there was a 23% increased CVD risk. Similar risk was found for a 1SD (12.6 cm) higher waist circumference (27%), and for a 1SD (0·083) higher waist-to-hip ratio (25%), after adjustment for age, sex, and smoking status. Following additional adjustment for systolic blood pressure (SBP), history of diabetes, and total and HDL cholesterol, these risks dropped to 7% with BMI, 10% with waist circumference, and 12% with waist-to-hip ratio. Importantly, on including BMI, waist circumference, or waist-to-hip ratio either individually or together in risk prediction models, this did not improve 10 year CVD risk prediction when conventional risk factors including SBP, diabetes history, and lipid levels were included. The authors argue this does not mean ignoring adiposity markers but rather understanding obesity is a key risk factor for high blood pressure, lipids, and blood glucose, all of which must be targeted to reduce CVD risk. Increased adiposity appears to be a common risk factor for both CVD and stroke (each SD increase in adiposity measure is associated with a 20–25% increased risk of stroke), highlighting the importance of tackling obesity to reduce both heart disease and stroke. Finally, the authors counteract previous claims that waist-to-hip ratio should be the baseline adiposity measure, showing similar risk levels between this and BMI, and that BMI has the best reproducibility. One study limitation was the majority of participants were of European descent (Emerging Risk Factors Collaboration et al. 2011).
Consequently, there is variation in CVD recommendations as to whether to include BMI or other adiposity measures in risk calculators which already incorporate many classic metabolic risk factors. The WHO and the US National Heart, Lung, and Blood Institute recommend assessment of both BMI and waist circumference in those with a BMI of 25–34.9 kg/m2 (World Health Organization 2000; National Institutes of Health 1998). NICE recommends formal CVD risk assessment through the QRISK2–2016 CVD risk calculator which incorporates BMI, as does the JBS3 calculator which is based on QRISK2. However, the ASSIGN risk calculator used in Scotland, as well as many other CVD risk scores, e.g., Framingham and SCORE, do not include adiposity measures.
Thus, there seems to be equipoise on whether or not to include BMI or another measure in CVD risk scoring. Certainly, where lipids are not available, as in low-income countries, researchers have shown that addition of BMI does add considerable value to risk prediction. The biggest single study testing the value of BMI is QRISK2. This prospective open cohort study collected routine general practice data on various cardiovascular risk factors, including BMI, in 2.3million patients aged 35–74, of whom there were 140,000 cardiovascular events. They generated a risk-prediction model, QRISK2, to predict an individual’s risk of cardiovascular disease in the next 10 years. The results showed that for every 5 unit increase in BMI, the risk of CVD was higher by 8% in women and 9% in men independent of other risk factors although there is an age interaction (captured in the risk score) so that with increasing age, BMI associations with outcomes were lower (Hippisley-Cox et al. 2008). BMI is therefore included in the QRISK2 risk calculator recommended by NICE.
Heart Failure and the Obesity Paradox
Obesity is associated with double the risk of developing heart failure. Yet, higher BMIs appear to be associated with a survival “advantage” in those with already established disease, the so-called obesity paradox of heart failure (Oreopoulos et al. 2008).
Early studies by Kenchaiah et al. investigated the relationship between BMI and heart failure incidence among 5881 participants in the Framingham Heart Study (mean age, 55 years; 54% women). After adjusting for established risk factors, for every 1 kg/m2 higher BMI, there was a 5% higher risk of heart failure in men and 7% higher risk in women. Obesity was associated with double the risk of heart failure compared to normal BMI (18.5–24.9), with a hazard ratio (HR) in women of 2.12 and HR 1.9 for men. Heart failure risk increased across BMI categories, with a HR of 1.46 for women and 1.37 in men per increase in BMI category from normal (18.5–24.9) to overweight (25.0–29.9) to obese (≥30.0 kg/m2). There appeared to be no threshold effect (Kenchaiah et al. 2002).
Why does obesity translate into increased heart failure risk? Obesity is associated with numerous well-established heart failure risk factors: hypertension, coronary artery disease, renal disease, diabetes, and obstructive sleep apnea. However, less appreciated is the effect of obesity on cardiac hemodynamics, left ventricular (LV) structure and function, as beautifully outlined by Lavie et al. As body weight increases (including fat-free mass), circulating volume increases, increasing LV stroke volume and cardiac output. This leads to RV hypertrophy, enlargement, and RV heart failure, as well as LV enlargement, increased LV wall stress, LV hypertrophy, and LV systolic or diastolic dysfunction, leading to LV failure. This in turn can cause pulmonary venous hypertension, pulmonary arterial hypertension, and RV failure (Fig. 2). Around 50% of obese individuals have hypertension, with cumulative effects on LV morphology. Hypertension is associated with concentric left ventricular hypertrophy (LVH). When chronic and combined with obesity, a mixed eccentric-concentric LVH occurs, which is now thought to be a form of concentric LVH (Lavie et al. 2013). Thus, many routes can lead from obesity to heart failure.
Pathophysiology of heart failure in obesity. As body weight increases (including fat-free mass), circulating volume increases, increasing left ventricular (LV) stroke volume and cardiac output. This leads to LV enlargement, increased LV wall stress, LV hypertrophy, and LV systolic or diastolic dysfunction, leading to LV failure, which in turn can contribute to RV failure (Lavie et al. 2013)
However, once individuals have heart failure, there appears to exists a paradox whereby being overweight or obese is apparently “protective” compared to being normal weight. This was first reported by Horwich et al. who showed obesity was not associated with increased mortality in heart failure, with higher BMI an independent predictor of improved survival at 2 years, but not 5 years. However, the study consisted only of those with advanced heart failure awaiting transplant (Horwich et al. 2001). Cachexia, associated with poor prognosis, can occur in later stages of heart failure and is characterized by weight loss. It is possible obese patients were not as ill as those with lower BMIs, with survival benefit due to obese patients being at an earlier stage of disease, hence no survival advantage by 5 years. Those who were overweight or obese also used more ACE inhibitors, which are known to improve heart failure prognosis.
Lavie et al. examined the association of obesity with clinical outcomes in those with milder heart failure (New York Heart Association (NYHA) class I to III) using various body composition markers. For every 1% absolute higher percent body fat, this was associated with a > 13% lower risk of major clinical events. However, this study was small (209 patients) with only 28 events and retrospective (Lavie et al. 2003).
A meta-analysis of nine observational trials (total n = 28,209) showed those with chronic heart failure (CHF) who were overweight or obese had lower all-cause and cardiovascular mortality than those without elevated BMI (Oreopoulos et al. 2008). Three of these studies grouped underweight and normal weight individuals together, potentially biasing favorable results to those with higher BMIs; however, their findings were similar even after excluding these studies. There were also biases in patient characteristics, with obese patients being younger, less often smokers, less myocardial infarctions, better ejection fractions, and greater use of beta-blockers but also more comorbidities, e.g., diabetes and hypertension. However, even after adjusting for these factors, the authors argue there was still an apparent “protective” effect of being overweight or obese with CHF. It should be noted these studies were observational and there have been no randomized controlled trials (RCTs) that examined mortality outcomes after intentional weight loss in obese patients with heart failure. While many researchers have tried to look at various ways to explain this paradox, they have been unable to rule out bias and confounding. Thus, randomized trials of weight loss are needed before one can definitively determine whether being obese is genuinely protective in heart failure, or otherwise. For now, researchers should refrain from stating obesity per se is a “protective” factor in patients with heart failure.
Weight Reduction Including the Role of Bariatric Surgery on Cardiovascular Risk Reduction
Intentional weight loss has numerous cardiovascular benefits. Losing around 5 kg has been associated with improved lipid profiles with reduced total cholesterol, LDL-cholesterol, and triglycerides (Poobalan et al. 2004), as well as reduced systolic blood pressure by around 4.4 mmHg (Neter et al. 2003). Weight loss has also been associated with a reduced risk of obese individuals progressing to impaired glucose tolerance and type 2 diabetes. Consequently, lifestyle interventions aimed at a 5–10% (approximately 5–10 kg) weight loss in those with a BMI of 25-35 kg/m2 are recommended to reduce the risk of cardiovascular and metabolic diseases (Scottish Intercollegiate Guidelines Network 2010). In reality, however, individuals attending a lifestyle weight management program lose around 3% body weight (National Institute for Health and Care Excellence 2014).
Weight management programs can provide an effective combination of dietary advice, physical activity, and behavioral therapy. In a comparison of five studies, median weight loss for the combined interventions was 4.6 kg versus 0.48 kg for diet alone (National Institute for Health and Clinical Excellence (NICE) 2006). In the Look AHEAD (Action For Health in Diabetes) study, Wing et al. investigated the effects of an intensive lifestyle intervention versus usual care in improving cardiovascular risk factors in 5145 overweight and obese patients with type 2 diabetes. The intensive lifestyle intervention consisted of reduced calorie intake of 1200–1800 kcal/day depending on baseline body weight and increased physical activity up to 175 min/week of moderate intensity activities with the aim of losing 10% body weight. They found modest weight loss of 5 to <10% was associated with improved glycemic control, lipid profiles (HDL-cholesterol and triglycerides but not LDL-cholesterol) and blood pressure at 1 year, with even greater benefits from further weight loss (Wing et al. 2011).
In a follow-up study, Wing et al. investigated the longer-term effects of intensive lifestyle interventions on CVD risk factors over 4 years by comparing those who maintained various degrees of weight loss versus those who regained weight. They found those who had significant weight loss of 8–20% at 1 year and kept this off at year 4 maintained improvements in systolic blood pressure, lipid profiles (triglycerides, HDL-C), and HbA1c. There were improvements in several of these CVD risk factors even in those who regained some weight (Wing et al. 2016). After a median follow-up of 9.6 years, the intervention group lost more weight than control group (6% vs. 3.5%, respectively) and again benefits in CVD risk factors were seen. However, intensive lifestyle interventions did not reduce cardiovascular morbidity and mortality (HR 0.95, 95% confidence interval 0.83–1.09) (The Look AHEAD Research Group 2013). It may be that greater weight reductions are required to reduce cardiovascular events. The control group also used more statins compared to the intervention group. Notably, given the aforementioned mechanisms of cardiovascular risk driven by obesity via lipids, hypertension, and diabetes, patients in Look AHEAD had generally excellent blood pressure, lipid levels, and glycemic control at baseline. Moreover, most were women and few were smokers so in many ways, Look AHEAD participants had low CVD risks levels even before interventions to lose weight were put in place, making it much harder to show meaningful CVD benefits with significant weight loss.
In those with a BMI ≥40 kg/m2 or ≥35 kg/m2 with comorbidities, bariatric surgery can be considered as this can result in numerous cardiovascular benefits as well as reduced all-cause mortality (Jensen et al. 2014). The Swedish Obesity Study was a prospective controlled study of 4047 obese individuals followed over an average of 10.9 years. Two thousand and ten individuals underwent bariatric surgery, while 2037 matched controls received standard treatment. There were dramatic and sustained improvements in weight loss after 10 years, with weight loss stabilized at 25% after gastric bypass, 16% after vertical-banded gastroplasty, and 14% after banding, compared to an average weight change of <±2% in the control group. Importantly, overall mortality was lower in the bariatric surgery group with a hazard ratio (HR) of 0.76 in the surgery group compared to the control group receiving conventional treatment (95% confidence interval (CI) 0.59–0.99). This lower mortality was clear even after adjusting for sex, age, and additional risk factors (HR 0.71, 95% CI 0.54–0.92) (Sjöström et al. 2007). Due to ethical and pragmatic reasons, this study was not randomized. However, there were no significant interactions between study group and the covariables of sex, presence or absence of diabetes, BMI, age, and previous cardiovascular events. Comparison of baseline characteristics showed no statistically significant difference in history of pre-existing diabetes, previous MI, stroke, cancer, or lipid-lowering therapy between groups.
In a subsequent study of Swedish Obese Subjects, Carlsson et al. showed bariatric surgery was more efficient than usual care in preventing type 2 diabetes in obese individuals. Using a similar, prospectively matched cohort of 1658 patients who underwent bariatric surgery and 1771 obese matched controls, the incidence rates of type 2 diabetes were 28.4 cases per 1000 person-years in the control group and 6.8 cases per 1000 person-years in the surgery group. After multivariable adjustments, the HR for incidence of diabetes was 0.17 in the bariatric surgery group compared to obese matched controls. This was despite the fact that those in the intervention group had higher body weights and more risk factors than the control group (Carlsson et al. 2012).
The association of bariatric surgery, weight loss, and cardiovascular events including myocardial infarction and stroke were analyzed as secondary outcomes (Sjöström et al. 2012). There were fewer cardiovascular deaths in the bariatric surgery group (28 out of 2010 patients) compared to the control group (49 out of 2037 patients), adjusted hazard ratio 0.47. Bariatric surgery was also associated with fewer fatal or nonfatal myocardial infarctions and stroke (199 incident cardiovascular events among 2010 patients in bariatric surgery group versus 234 events among 2037 patients in the control group). Interestingly, there was no interaction between BMI and treatment with regards to cardiovascular events, indicating that among the obese, higher baseline BMI is not necessarily associated with a greater health benefit of bariatric surgery.
Adams and colleagues have reported similar lower mortality rates after bariatric surgery. In a retrospective cohort study, all-cause and cause-specific mortality rates were compared in 7925 surgical patients and 7925 severely obese control subjects matched for age, sex, and BMI. During a mean follow-up of 7 years, adjusted all-cause mortality was lower by 40% in the gastric bypass group compared to the control group (37.6 vs. 57.1 deaths per 10,000 person-years). Coronary artery disease-related deaths were lower by 56% (2.6 vs. 5.9 per 10,000person-years), diabetes-related deaths were lower by 92% (0.4 vs. 3.4 per 10,000 person-years), and cancer deaths lower by 60% (5.5 vs. 13.3 per 10,000 person-years) (Adams et al. 2007).
In a large systematic review and meta-analysis including 29,208 bariatric surgery patients and 166,200 nonsurgical controls, the results suggested a greater than 50% lower mortality risk among the surgery group compared to nonsurgical controls (OR 0.48, 95% CI 0.35–0.64, I2 = 86%). Bariatric surgery was also associated with a 46% lower risk of composite cardiovascular adverse events (OR 0.54, 95% CI 0.41–0.70, I2 = 58%), 54% lower risk of myocardial infarction (OR 0.46, 95% CI 0.30–0.69, I2 = 79%), and 51% lower risk of stroke (OR 0.49, 95% CI 0.32–0.75, I2 = 59%) (Kwok et al. 2014). However, there was significant heterogeneity between the 14 studies analyzed as part of this meta-analysis, and a modest risk of bias could not be discounted in most studies, in line with their observational nature.
Finally, a recent randomized controlled trial compared bariatric surgery versus intensive medical therapy for optimal type 2 diabetes management (Schauer et al. 2017). One hundred and fifty patients with type 2 diabetes and a BMI ranging from 27 to 43 kg/m2 were randomly allocated to either intensive medical therapy alone or intensive medical therapy in addition to Roux-en-Y gastric bypass or sleeve gastrectomy. After 5 years of follow up, 14 of the 49 patients (29%) in the gastric bypass group achieved the primary outcome of a HbA1c level ≤ 6.0%, compared to 11 out of 47 (23%) in the sleeve gastrectomy group, versus 2 of 38 patients (5%) in the medical therapy only group. Those who underwent bariatric surgery had greater reductions in mean HbA1c compared to those on medical therapy only (2.1% versus 0.3% reduction p = 0.003), greater weight loss, less use of insulin, lower triglyceride, higher HDL-cholesterol levels, and improved quality of life after 5 years. There was only one late reoperation in the bariatric surgery group; mild anemia was noted more often in the surgical group. Unfortunately, the study was not powered to examine differences in cardiovascular morbidity and mortality, and larger trials are needed to address these.
What Can Genetic Studies Tell Us About the Impact of Obesity on CVD?
Observational studies have shown an association between obesity and cardiovascular disease. However, they cannot prove causality and are subject to confounding, that is, another variable aside from the exposure of interest contributing to the outcome, and reverse causality, where those with the outcome may be more prone to the exposure. Randomized controlled trials, if well conducted, overcome these issues; however, such trials are not always possible or feasible due to time, ethical, or cost constraints. By using Genome Wide Association Studies (GWAS), a number of Single Nucleotide Polymorphisms (SNPs) in numerous genes associated with BMI have been identified. Using these SNPs, investigators can use Mendelian Randomization to attempt to prove a causal association between increased BMI and adverse cardiovascular outcome. This work, if done well and with SNPs that are pure for the exposure of interest, function as a natural randomized controlled trial (Burgess et al. 2012). The genetic variant is the proxy for the risk factor, that is, the “intervention” group. This is assigned to an individual randomly at conception and thus individuals born with multiple BMI raising genes will have lifelong higher BMI compared to individuals with few or none of these genes.
Nordestgaard et al. used this Mendelian Randomization approach to test a causal association between BMI and ischemic heart disease (IHD). They measured BMI and genotyped 75,627 Danish individuals for three SNPs: FTO(rs9939609), MC4R(rs17782313), and TMEM18(rs6548238). There were 11,056 IHD events. The odds ratio (OR) for BMI and IHD were compared between observational studies and combined allele scores. For every 4 kg/m2 higher BMI, observational studies reported a 26% higher risk of IHD (OR 1.26), whereas an instrumental variable analysis using the allele score suggested a 52% higher IHD risk (OR 1.52) (Nordestgaard et al. 2012). In terms of risk factors, an earlier study by the same group investigated the causal link between BMI and blood pressure with rs9939609 (FTO) and rs17782313 (MC4R) genotypes as surrogates for BMI. Accounting for antihypertensive use and adjusting for numerous variables including age, sex, height, and sociobehavioral variables, for every 10% increase in BMI, they found a 3.85 mmHg increase in systolic blood pressure and 1.79 mmHg increase in diastolic blood pressure, confirming observational associations (Timpson et al. 2009).
A separate Mendelian Randomization analysis by Holmes et al. investigated the causal role of BMI with cardiometabolic disease in 34,538 European-descent individuals (6073 CHD, 3813 stroke cases, and 4407 type 2 diabetes cases). They used a genetic score consisting of 14 BMI-associated SNPs and confirmed causal effects of BMI on type 2 diabetes, systolic blood pressure, HDL-cholesterol, fasting glucose, fasting insulin, and IL-6. However, they did not prove causality for BMI and CHD or stroke, although the analysis may have been underpowered (Holmes et al. 2014).
Hagg et al. confirmed for the first time a causal relationship between BMI and ischemic stroke, with a HR of 1.83 per SD increase in BMI, using a 32 SNP genetic score. They also found a causal role for increased BMI and heart failure (HR 1.93 per SD increase in BMI). However, their initial findings did not show a causal association of BMI with CHD (HR 1.13 per SD increase in BMI, 95% confidence interval 0.7–1.84, p = 0.62) (Hägg et al. 2015).
Overall, genetic studies add some further support for a causal link between obesity, several CVD risk factors and CVD outcomes, and heart failure, although further studies are needed.
Regional Adiposity and Cardiometabolic Disease
It is increasingly recognized that differences in body fat distribution may play an important role in cardiometabolic disease. In particular, increased central obesity has been associated with increased cardiometabolic risk and has been proposed to be a more sensitive marker of risk than BMI. Indeed, a large systematic review of 15,923 subjects with baseline CAD showed a direct relationship between higher waist circumference and WHR (central obesity markers) with higher mortality, with those in the highest central obesity tertile 70% more likely to die compared to the lowest tertile. However, in the same study BMI was inversely associated with mortality (Coutinho et al. 2011). This has led some to question if the “obesity paradox” is actually the “obesity paradox of BMI” in those with established CAD (Després 2011).
However, waist circumference should still be interpreted in relation to the individual’s BMI. It is influenced not only by increased visceral fat but also from increased abdominal subcutaneous fat (Després 2012). Modern imaging techniques, from basic ultrasound to multidetector CT to the gold standard MRI, have allowed us to more accurately assess and quantify subcutaneous and visceral fat volume, as well as ectopic fat volume in the liver, skeletal muscle, pancreas, and heart through 1H Magnetic Resonance Spectroscopy (Britton and Fox 2011).
The ectopic fat hypothesis suggests that as one consumes excess calories/reduces energy expenditure, subcutaneous adipose tissue (SAT) stores become overwhelmed and excess fat “spills over” more rapidly into visceral and ectopic sites including the liver, skeletal muscle, heart, vasculature, and possibly also the pancreas (Fig. 3). These “ectopic” fat stores are not classically associated with substantial tissue fat storage. When free fatty acids are normally deposited in SAT, there is adipocyte hyperplasia. However, when the SAT becomes dysfunctional, the adipocytes hypertrophy and this has been associated with the development of type 2 diabetes (Weyer et al. 2000). Numerous factors including male sex, certain ethnic groups, e.g., South Asians, increasing age, and lifestyle factors, e.g., smoking, may predispose individuals to more rapid filling of visceral and ectopic fat stores at a given BMI but more work is also needed here to expand this body of work (Sattar and Gill 2014; Britton and Fox 2011).
Ectopic fat hypothesis. The ectopic fat hypothesis suggests that as one consumes excess calories/reduces energy expenditure, subcutaneous adipose tissue (SAT) stores become overwhelmed and excess fat “spills over” more rapidly into visceral and ectopic sites including the liver, skeletal muscle, heart, vasculature, and possibly also the pancreas. Certain risk factors such as male sex, ageing, ethnicity, e.g., South Asian origin, and genetics may predispose to more rapid exhaustion of subcutaneous stores into visceral and ectopic stores (Sattar and Gill 2014).
Rather than being causal per se, visceral fat may serve as a marker of more “dangerous” ectopic fat. Rossi et al. (2011) showed a strong correlation between visceral fat volume and liver lipid content and pancreatic lipid content. Nonalcoholic fatty liver disease (NAFLD) is associated with obesity and type 2 diabetes. Excess intrahepatic fat may arise from hyperinsulinemia working in conjunction with excess energy intake to drive de novo lipogenesis in the liver (Stefan et al. 2008). Fatty liver in turn renders the liver more insulin resistant through numerous proposed cellular mechanisms, including through increased hepatic diacylglycerol (DAG). Hepatic gluconeogenesis in such cases is poorly suppressed and so the liver can continue to make glucose when not normally needed resulting in hyperglycemia and type 2 diabetes (Sattar and Gill 2014).
Men have more visceral fat at a given BMI than women which may partially explain the higher prevalence of type 2 diabetes in men compared to women, although other fat depots were not measured in this study (Nordström et al. 2016). There are also ethnic differences in adipose tissue distribution. For example, some studies suggest South Asians are less able to store fat in “safer” superficial subcutaneous adipose stores and fat more easily “spills over” into deeper subcutaneous and visceral fat compartments as well as ectopically into the liver. There may also be differences in subcutaneous abdominal adipocyte morphology between South Asians and Europeans (Sattar and Gill 2014). This may in part account for the observation that South Asian individuals develop type 2 diabetes at lower BMIs than their white European counterparts (Ntuk et al. 2014). However, we await longitudinal studies to gain a better insight into prospective changes in adipose tissue distribution and morphology in response to weight changes.
Ectopic circulatory fat can also accumulate with increased hepatic production of very low density lipoprotein cholesterol (VLDL-C) and increased serum triglyceride levels (Sattar and Gill 2014). It is thought excess ectopic pancreatic fat may also contribute to development of type 2 diabetes through impaired β-cell function (Lim et al. 2011), which is reversible with weight loss.
But does greater central or ectopic fat translate into higher risk for cardiovascular disease? Ding et al. undertook a case-cohort study in 998 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) study and 147 MESA participants who developed incident coronary heart disease, to investigate whether pericardial fat volume assessed by cardiac CT predicted incident CHD. They found that a 1 SD increase in pericardial fat was associated with a 33% higher risk of CHD (relative hazard 1.33) in unadjusted analyses. After adjusting for age, sex, ethnicity, BMI, and other CVD risk factors, this association remained significant, although the confidence interval was broadened (relative hazard 1.26, 95% confidence interval 1.01–1.59). The association did not statistically differ between sexes. After adjustment, those in the highest pericardial fat quartile had over twice the risk of CHD (relative hazard 2.09) relative to those in the lowest quartile (Ding et al. 2009).
Ectopic fat accumulation in the renal sinus may potentially affect renal function through compressing the renal artery and vein (Britton and Fox 2011). Using the Framingham Heart Study, Foster et al. investigated the association of renal sinus fat with CKD and hypertension. Renal sinus fat area was assessed using CT in 2923 individuals. Around 30% of participants had “fatty kidney,” defined using sex-specific cut points of ≥0.710 cm2 in men and ≥0.445 cm2 in women. Those with increased renal sinus fat were over twice as likely to have hypertension (OR 2.12, 95% CI 1.75–2.56, p < 0.0001); this persisted, although was attenuated, after adjusting for BMI (OR 1.49, 95% CI 1.22–1.83, p < 0.0001) or VAT (OR 1.24, 95% CI 1.00–1.53, p = 0.049). The odds of CKD were also doubled in those with “fatty kidney” (OR 2.30, 95% CI 1.28–4.14, p = 0.005). This was only noted with CKD defined by cystatin C and was not significant after multivariable adjustment with creatinine defined eGFR. Cystatin C is produced by adipose tissue and so the prevalence of CKD with this marker may be overestimated in the obese. However, there is still a significant association between CKD estimated from cystatin C and fatty kidney even after adjusting for BMI or abdominal VAT (OR 1.86) (Foster et al. 2011).
Preliminary genetic data has shown 11 “favorable adiposity” variants associated with lower risk of type 2 diabetes, hypertension, and heart disease. These “favorable adiposity” alleles are associated with increased subcutaneous–to–visceral adipose tissue ratio, decreased insulin levels, and higher adipose storage capacity. Using UK Biobank data from over 160,000 participants and data from five other studies, the 50% of participants with the most favorable adiposity alleles had higher BMIs and body fat percentage compared to those with the least number of alleles, yet also had reduced risk of type 2 diabetes, hypertension, heart disease, and lower blood pressures. The key suggestion from these data is that it is not simply an increased BMI which can increase disease risk but rather how and where the excess body fat is stored (Yaghootkar et al. 2016).
Future prospective large-scale studies are now needed to provide sufficient power to associate various ectopic fat depots with adverse cardiovascular outcomes. It is hoped this will be provided through analysis of 100,000 MRI scans of body fat distribution undertaken on UK Biobank participants in the coming years.
Summary
In conclusion, several lines of evidence, including observational, trial, and genetic, collectively support causal links between obesity, cardiovascular morbidity and mortality, and all-cause mortality (Table 1). For prediction, while measures of obesity have clear importance to diabetes risk scores, their inclusion in CVD risk prediction are less frequent, mainly due to far more modest associations and especially once other downstream risk factors (lipids, BP, and diabetes) are included. While numerous studies have identified an apparent obesity paradox in those with heart failure and to a lesser extent in those with existing CVD, these findings are observational and may be subject to reverse causality. Prospective randomized controlled trials of intentional weight loss in obese heart failure patients are needed to properly address the question of whether intentional weight loss in this group is detrimental or not. Finally, more recent evidence, both imaging and novel genetic findings, support differential causal associations between tissue specific fat depositions and cardiometabolic outcomes. Indeed, individuals able to better expand subcutaneous fat stores appear at lower cardiometabolic risks at a given BMI than those less able to do so. The ramifications of current findings to clinical practice and public health agendas are clear – overweight or obese individuals need to be supported to help lose weight or lessen weight gain to reduce their risks of numerous diseases including adverse CVD outcomes.
References
Adams TD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa066603. Accessed 5 Mar 2017.
Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124(24):e837–41. Available at: http://circ.ahajournals.org/cgi/doi/10.1161/CIRCULATIONAHA.111.077602. Accessed 19 Mar 2017.
Brown CD, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–19. Available at: http://doi.wiley.com/10.1038/oby.2000.79. Accessed 7 May 2017.
Burgess S, et al. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ. 2012;345:e7325. Available at: http://www.bmj.com/content/345/bmj.e7325. Accessed 7 May 2017.
Carlsson LMS, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. Available at: http://www.nejm.org/doi/10.1056/NEJMoa1112082. Accessed 16 Dec 2016.
Collaboration, N.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96. Available at: http://linkinghub.elsevier.com/retrieve/pii/S014067361630054X. Accessed 14 Feb 2017.
Coutinho T, et al. Central obesity and survival in subjects with coronary artery disease. J Am Coll Cardiol. 2011;57(19):1877–86. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0735109711007327. Accessed 21 Mar 2017.
Després J-P. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol. 2011;57(19):1887–9. Available at: http://www.onlinejacc.org/content/accj/57/19/1887.full.pdf. Accessed 7 May 2017.
Després J-P. Body fat distribution and risk of cardiovascular disease. Circulation. 2012;126(10):1301–13.
Ding J, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90(3):499–504. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19571212. Accessed 21 Mar 2017.
Emerging Risk Factors Collaboration, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;(9771):377, 1085–1395. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21397319. Accessed 7 May 2017.
Foster MC, et al. Fatty kidney, hypertension, and chronic kidney disease. Hypertension. 2011;58(5):784–90. Available at: http://hyper.ahajournals.org/content/58/5/784.short. Accessed 8 May 2017.
Guh DP, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19320986. Accessed 29 Dec 2016.
Hägg S, et al. Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):578–86. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26016847. Accessed 13 Mar 2017.
Hippisley-Cox J, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336(7659):1475–82. Available at: http://www.bmj.com/content/336/7659/1475. Accessed 6 May 2017.
Holmes MV, et al. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet. 2014;94(2):198–208. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3928659/pdf/main.pdf. Accessed 7 May 2017.
Horwich TB, et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–95.
International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. 2006.
Jensen MD, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129(25 suppl 2):S102–38. Available at: http://circ.ahajournals.org/lookup/doi/10.1161/01.cir.0000437739.71477.ee. Accessed 13 Mar 2017.
Kenchaiah S, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa020245. Accessed 31 Dec 2016.
Kwok CS, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–8. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0167527314003799. Accessed 13 Mar 2017.
Lavie CJ, et al. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891–4.
Lavie CJ, et al. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1(2):93–102.
Lim EL, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–14. Available at: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00125-011-2204-7. Accessed 16 Sept 2016.
National Institute for Health and Care Excellence (NICE). Weight management: lifestyle services for overweight or obese adults. 2014.
National Institute for Health and Clinical Excellence (NICE). Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. 2006. Available at: https://www.nice.org.uk/guidance/cg43/evidence
National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – the evidence report. Obes Res. 1998;6(suppl 2):51–209S.
Neter JE, et al. Influence of weight reduction on blood pressure. Hypertension. 2003;42(5):878–84. Available at: http://hyper.ahajournals.org/content/42/5/878.long. Accessed 7 May 2017.
Nordestgaard BG, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. Minelli C, editor. PLoS Med. 2012;9(5):e1001212. Available at: http://dx.plos.org/10.1371/journal.pmed.1001212. Accessed 7 May 2017.
Nordström A, et al. The higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J Clin Endocrinol Metab. 2016;101(10):3740–6. Available at: http://press.endocrine.org/doi/10.1210/jc.2016-1915. Accessed 4 Sept 2016.
Ntuk UE, et al. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care. 2014;37(9):2500–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24974975. Accessed 4 Sept 2016.
Oreopoulos A, et al. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22.
Poobalan A, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes – a systematic review. Obes Rev. 2004;5(1):43–50. Available at: http://doi.wiley.com/10.1111/j.1467-789X.2004.00127.x. Accessed 7 May 2017.
Rossi AP, et al. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity. 2011;19(9):1747–54. Available at: http://doi.wiley.com/10.1038/oby.2011.114. Accessed 3 Feb 2017.
Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27(4):501–7.
Sattar N, Gill JMR. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25159817. Accessed 24 Aug 2016.
Schauer PR, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med. 2017;376(7):641–51. Available at: http://www.nejm.org/doi/10.1056/NEJMoa1600869. Accessed 10 May 2017.
Scottish Intercollegiate Guidelines Network. Scottish Intercollegiate Guidelines Network SIGN management of obesity. 2010. Available at: http://www.sign.ac.uk/pdf/sign115.pdf. Accessed 7 May 2017.
Sjöström L, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa066254. Accessed 16 Dec 2016.
Sjöström L, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56. Available at: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2011.1914. Accessed 16 Dec 2016.
Stefan N, Kantartzis K, Häring H-U. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29(7):939–60. Available at: http://press.endocrine.org/doi/abs/10.1210/er.2008-0009. Accessed 16 Sept 2016.
The Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0140673616301751. Accessed 27 Dec 2016.
The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383(9921):970–83. Available at: http://linkinghub.elsevier.com/retrieve/pii/S014067361361836X. Accessed 28 Dec 2016.
The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. Available at: http://www.nejm.org/doi/10.1056/NEJMoa1212914. Accessed 7 May 2017.
Timpson, N.J. et al., 2009. Does greater adiposity increase blood pressure and hypertension risk? Hypertension, 54(1):84-90. Available at: http://hyper.ahajournals.org/content/54/1/84.long. Accessed 7 May 2017.
Weyer C, et al. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506. Available at: http://springerlink.bibliotecabuap.elogim.com/10.1007/s001250051560. Accessed 7 May 2017
Wing RR, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes care. 2011;34(7):1481–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21593294. Accessed 13 Mar 2017
Wing RR, et al. Association of weight loss maintenance and weight regain on 4-year changes in CVD Risk Factors: the action for health in diabetes (Look AHEAD) clinical trial. Diabetes Care. 2016;39:1345–55.
World Health Organisation. Global status report on noncommunicable diseases 2010. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44579/1/9789240686458_eng.pdf. Accessed 7 May 2017.
World Health Organisation. WHO | Obesity and overweight. World Health Organization. 2016. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 7 May 2017.
World Health Organization. WHO | Obesity: preventing and managing the global epidemic. WHO. 2000. Available at: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/. Accessed 7 May 2017.
World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Yaghootkar H, et al. Genetic evidence for a link between favorable adiposity and lower risk of type 2 diabetes, hypertension, and heart disease. Diabetes. 2016;65(8):2448–60. Available at: http://diabetes.diabetesjournals.org/content/65/8/2448.long. Accessed 4 May 2017.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Ferguson, L.D., Sattar, N. (2019). Impact of Obesity on Cardiovascular Disease. In: Sbraccia, P., Finer, N. (eds) Obesity. Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-46933-1_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-46933-1_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46932-4
Online ISBN: 978-3-319-46933-1
eBook Packages: MedicineReference Module Medicine