Abstract
We review the importance of developmental mechanisms in animals in constraining evolutionary changes. We first discuss the importance of time scales at which such constraints are relevant and after that focus on near absolute constraints that act on macroevolutionary scales. We could find only a few well-underpinned examples of such near absolute constraints. We discuss three outstanding cases, the ancient metazoan constraint that differentiated cells cannot divide, constraints against changes of phylotypic stages in vertebrates and other higher taxa, and constraints against the evolution of parthenogenesis. These constraints all have major consequences, including many secondary constraints, and they have in common that they are caused by high levels of global developmental interactivity.
The global developmental interactivity almost inevitably causes mutations to have many harmful pleiotropic effects, and thus will be strongly selected against, leading to long-term evolutionary conservation. The discussed developmental constraints have major consequences for evolution and critically restrict regeneration capacity, life-history evolution, and body plan evolution.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Body plan evolution
- Evolutionary constraint
- Parthenogenesis
- Phylotypic stages
- Early organogenesis
- Regeneration capacity
- Cilia
- Centrosome
- Mitosis
- Meiosis
Introduction
We speak of developmental constraints when there is a bias on the production of variant phenotypes or a limitation on phenotypic variability caused by developmental mechanisms (Maynard Smith et al. 1985). Earlier more intuitive mechanistically oriented arguments were put forward by among others Gould and Lewontin (1979), who argued that developmental constraints must be important, based on the apparent conservation of early developmental stages, the required integration of these stages, and the accumulating effects of early errors. Even earlier, Whyte (1964) argued that these constraints relate to internal selection, i.e., the necessity for the machinery of the body, in particular development, to be well-concerted.
The importance of developmental constraints in evolution is still subject to controversy: In which ways and to what extent do developmental mechanisms restrict the range of possible phenotypes? The answer depends largely on the time scale at which the constraints are supposed to act. In evolutionary biology it pays to make at least the following gross distinctions: microevolution (changes in gene frequencies on a population dynamical time scale), meso-evolution (the evolution of quantitative traits through repeated mutation substitutions), and macroevolution (large-scale changes, such as innovations). Quantitative genetics and adaptive dynamics provide the main frameworks for dealing with trait evolution on the micro- and meso-evolutionary scales, respectively, while macroevolutionary discourse is dominated by arguments from functional morphology and evo-devo. We will first discuss why genetic constraints are expected not to lead to constraints acting at macroevolutionary scales and follow-up with a discussion of three well-documented examples of near absolute macroevolutionary developmental constraints, their causes, and their vast evolutionary impact.
Genetic Constraints Do Not Constrain Evolution on Macroevolutionary Scales
In the past, discussions on developmental constraints, in particular among geneticists, have often focused on so-called genetic constraints, especially the potential of genetic covariation to steer evolution (e.g., Conner 2012). Note that genetic covariation is to a large extent caused by the genotype-phenotype map, i.e., by developmental mechanisms. In quantitative genetics, the term genetic constraint is used for the differential responsiveness to selection in the directions of the principal components of the genetic covariance matrix in proportion to their size. Principle components correspond to the direction in trait space supporting the largest variation, the direction orthogonal to that first direction supporting the largest variation, etc. In particular, any zero principal component corresponds to an absolute, i.e., dictionary style, constraint. Although little is known yet about the prevalence of such zero principal components, the general tendency in high-dimensional biological data is that principal components peter out roughly exponentially, suggesting that absolute genetic constraints will be extremely rare, except when directly caused by a physical conservation law. This unlikeliness becomes even greater since the effects of new mutations on phenotypes, as captured by their mutational covariance matrices, and thus their principal components, generally change with progressive evolution. Note that both types of covariances are but phenomenological representations of the phenotypic effects of mutational possibilities combined with development and in the case of the genetic covariances also linkage disequilibrium.
For smooth genotype to phenotype maps the effect of small changes in gene expression is bound to be locally additive. In that case, we can treat the microevolutionary process as governed by additive genetics, leading to a seamless transition from the arguments about microevolution, in terms of shifts in standing variation to those about meso-evolution based on mutant substitutions. However, the genotype-to-phenotype map is invariably nonlinear in the large. Moreover, phenotypic change on that scale necessarily influences the fitness landscape through its effect on the ecology. Adaptive dynamics (e.g., Metz (2012)) focuses on the effects of the latter changes.
Meso-evolution presumably is largely driven by mutations of small effect. This expectation has both a mechanistic and a Darwinian reason. Most trait evolution appears to be regulatory. Most mutational changes in the regulatory mechanisms may be expected to result in small changes in the quantities of the relevant proteins at different points and times in the body and thus to small changes in development, physiology, and behavior. In addition, mutations with large effect tend to bring an otherwise harmoniously operating system into disarray and will therefore contribute little to meso-evolutionary change. The ecology-mediated changes in invasion fitnesses that drive meso-evolution tend to be minor relative to the fitness effects deriving from the need for a well-concerted organismal development and functioning. The latter effects presumably also underlie most macroevolutionary regularities. However, on macroevolutionary scales, trait spaces themselves change through the breaking of hard meso-evolutionary constraints, permitting innovations (e.g., Peterson and Müller 2016). Together the above considerations support the metaphor of meso-evolution as a smooth uphill movement along the crests of a high-dimensional fitness landscape. Selection pushes in the steepest direction with the realized uphill movement determined by the interplay between this push and the current mutational covariances. The relatively featureless landscape on top of the crests continually changes thanks to the ecologically mediated feedback from traits to fitnesses. The gross landscape structure, on the other hand, stays roughly constant as it is dominated by internal selection, i.e., the need for organisms to stay well-concerted. In contrast, macroevolution is guided by large-scale landscape features with key innovations providing wormholes to higher dimensions. This allows little chance for the directional effects of the mutational covariances to leave a visible trace. Thus, genetic covariation undoubtedly steers evolution on micro- and meso-evolutionary scales yet is unlikely to constrain on macroevolutionary scales, except for the effects of rampant pleiotropy discussed below.
Macroevolutionary Constraints
Absolute developmental constraints on the evolution of specific adaptive phenotypes are extremely rare, and Vermeij (2015) even argues that they are absent, given sufficient time. Yet, we argue that there exists one exceptional category of near absolute developmental constraints: when development is highly interactive, the many cascading pleiotropic effects caused by development (relational pleiotropy, sensu Hadorn (1961)) result in high-dimensional variation that, combined with stabilizing selection in most directions, will strongly constrain evolution (Galis et al. 2018). As a result, developmental changes that are initiated in highly interactive developmental stages tend to be constrained even on macroevolutionary scales. We shall discuss the three best established examples of such near absolute developmental constraints in animals: the metazoan constraint that differentiated cells cannot divide, the constraints against changes of phylotypic stages in vertebrates and other higher taxa, and constraints against the evolution of parthenogenesis.
Metazoan Cells Cannot Divide While Differentiated
In 1898, Henneguy and Lenhossek independently proposed a universal developmental constraint for metazoans: ciliated cells cannot divide (Henneguy 1898; Lenhossék 1898). They had observed that the basal bodies of cilia were transformed centrosomes and had never observed ciliated cells divide. They proposed that when a centrosome becomes involved in cilium formation, it cannot form a spindle for cell division. The centrosome, in its entirety with two centrioles, forms a spindle pole. It is duplicated shortly before cell division, and each centrosome forms one of the two poles of the spindle that segregate the duplicated chromosomes. When a cilium is formed, one of the two centrioles of the single centrosome converts to a basal body and migrates to the cell surface, anchors to the cell membrane, and organizes the assembly of the cilium that protrudes from the cell membrane. Hence, the proposed incompatibility of functioning of the centrosome in cilium formation and mitosis, a hypothesis that for metazoans thus far remains uncontested.
Buss (1987) extended the hypothesis from ciliated cells to all differentiated cells of metazoans. In his thought-provoking book, The Evolution of Individuality , he proposed that cell division by mitosis and differentiation are mutually exclusive. He assumed that the cilium is usually involved in the differentiation process of a cell and, additionally, that the centrosome is the only microtubule-organizing center (MTOC) in a cell and that any commitment of the single MTOC to a cilium or to another microtubule-based structure would preclude commitment to the poles of the mitotic spindle, thus inhibiting mitosis. Buss further assumed that there cannot be more than one centrosome in a cell and that this is a phylogenetic constraint inherited from unicellular protist ancestors, whereas other unicellular taxa, such as Euglenophytes, Cryptophytes, and Chlorophytes possess multiple MTOCs, and therefore can simultaneously achieve cell movement with cilia or flagella and cell division. Bell (1989) challenged the phylogenetic constraint hypothesis, arguing that the duplication of centrosomes in cells shortly before mitosis suggested that there cannot exist a constraint preventing the production of more than one centrosome. Indeed, it is now known that, exceptionally, extra centrosomes are formed in cells (e.g., Gönczy 2015). The explosively expanding knowledge of cellular processes has revealed more challenges to Buss’s hypothesis, e.g., experimental removal of centrosomes has indicated that they are not essential for the formation of radial spindles and mitotic cell division (Wu and Akhmanova 2017). However, as we shall discuss, new knowledge indicates that in metazoans it is important that normally there is precisely one centrosome in a cell and primary cilia perform crucial functions in virtually all cells, which again explains that ciliated and differentiated cells cannot divide in metazoans.
No or More than One Centrosome in a Cell
Centrosomes are not absolutely required for mitotic spindle formation and division in many cells, but mitosis in the absence of centrosomes is slower, which increases the risk of chromosomes lagging during the separation and thus causing aneuploidy. Centrosomes thus are necessary for rapid, robust, and error-free separation of the chromosomes during mitosis (Gönczy 2015; Wu and Akhmanova 2017). Very rarely in a cell extra centrosomes are generated de novo. The presence of multiple centrosomes poses grave risks as it can lead to the formation of multiple spindle poles, aneuploidy, genomic instability, abnormal cell migration, cell cycle arrest, and cancer (e.g., Gönczy 2015). This is not surprising given the many key functions of centrosomes in cell cycle control and cell differentiation. They play an important role, among others, in establishing cell fate determination, cell polarity, transmission of polarity to daughter cells, the positioning of cell organelles, de-epithelialization, DNA damage repair, adhesion , migration of cells, and functioning as signaling hubs (Wu and Akhmanova 2017).
More than One MTOC in a Cell
In addition to the centrosome, there are other cell organelles that organize microtubule, like the Golgi apparatus, the nuclear envelope, the cell cortex, and pre-existing microtubules. During differentiation, the microtubule-organizing capacity of the centrosome is partially or fully transferred to such non-centrosomal MTOCs (e.g., Wu and Akhmanova 2017). The division of tasks between the centrosomal and non-centrosomal MTOCs appears to be tightly regulated during cell cycle progression and differentiation, probably in a competitive way, which presumably limits the possibility of centrosomes in differentiated cells to function as spindle poles.
Importance of Primary Cilia
Primary cilia were long thought to be vestigial organelles, which if true, would complicate Buss’ argument that the presence of primary cilia constrains the functioning of centrosomes as mitotic spindles. However, in the last few decades, it was first shown that they function as antennae on almost all metazoan cells and as such play a crucial role in intercellular signaling (e.g., Shh signaling in vertebrates, Walz 2017). Cilium signaling is involved in the organization of most, if not all, developmental processes, including left-right patterning, cell migration, proliferation, cell size and shape, apoptosis, and cell fate decisions. The many diseases caused by malfunctioning cilia, so-called ciliopathies, emphasize the primordial role of cilia in development and tissue homeostasis (among many others, diabetes, polycystic kidney disease, and retinal degeneration (Walz 2017)). As virtually all differentiated cells appear to have a primary cilium, this essentially equates the hypothesis of Henneguy and Lenhossek to the one of Buss that there is a constraint on mitotic divisions of differentiated cells.
Cilia and Centrosomes Regulate Cell Cycle Progression
At the exit of mitosis , cells typically form a primary cilium, unless they continue proliferating, when ciliogenesis appears to be actively suppressed (Walz 2017). In all other cases, also in quiescent stem cells, the mother centriole converts to a basal body and assembles the primary cilium. Upon cell cycle reentry, ciliary resorption begins, the basal body is detached from the cell surface, and the centrosome migrates to near the nucleus. Recent studies have shown that the cell cycle is not so much regulating centrosome and cilium dynamics, but instead, the dynamics of the centrosome and primary cilium actively regulate cell cycle progression and arrest or exit followed by differentiation (Walz 2017). For instance, the physical presence of the primary cilium appears to block cell division, while primary ciliary resorption is thought to unblock it and the length of the cilium influences cell cycle duration, which in turn influences cell fate decisions.
Pleiotropic and Developmental Constraint
The centrosome and primary cilium play a key role in the complex organization of almost all cellular processes in multicellular metazoans. Abnormal numbers of centrosomes and primary cilia disrupt the highly controlled interactivity during mitosis and cell cycle progression. A further contribution to the complexity comes from the competitive interactions between the centrosome and non-centrosomal sites that organize microtubuli. As a result, mutations affecting centrosomal and ciliary functions will have a multitude of deleterious pleiotropic effects that will be strongly selected against, such as apoptosis, genomic instability, aneuploidy, cell cycle progression, and cancer (e.g., Gönczy 2015; Walz 2017). Hence, there is a strong constraint against changes in the number of centrosomes (and primary cilia). As the interactivity is part of the intracellular development, the constraint should be considered developmental, as opposed to genetic. The constraint causes several other fundamental developmental constraints, of which the one that differentiated cells cannot divide is the hardest, i.e., impacting the conservation of phylotypic stages and, thereby, the evolution of body plans.
Evolutionary and Developmental Consequences of the Constraint
Low Fidelity of Meiotic Divisions of Oocytes
Meiotic divisions of animal oocytes occur without centrioles. The centrioles degenerate beforehand, presumably to avoid the problematic presence of a second centrosome in the zygote. The centriole(s) in the zygote are contributed by the sperm. It is thought that the centrioles of the egg cells rather than of the sperm cells degenerate, because of the necessity for the motile sperm cell to have a centriole organizing its flagellum (Manandhar et al. 2005). The absence of a centrosome during the meiotic divisions of the oocyte is associated with a cost of lower fidelity of the divisions, with increased rates of aneuploidy and genetic instability, presumably playing a role in the high rates of miscarriages in humans (Manandhar et al. 2005).
In an unfertilized ovum, the paternal contribution of centrioles is missing, and this forms a constraint against parthenogenesis, as a centrosome is generally necessary to initiate mitotic divisions (e.g., Eisman and Kaufman 2007; see “Constraints against the evolution of parthenogenesis”).
Limited Capacity for Wound Healing and Regeneration
Wound healing and regeneration would presumably be much more effective if all differentiated cells could divide to replace damaged cells of the same type. Regeneration now typically proceeds from a blastema of undifferentiated cells that are either dedifferentiated cells or already locally present tissue-specific progenitor cells (Tanaka and Reddien 2011). Subsequently, complex interactions are required, often similar to those that occurred during development of the part to be regenerated. This requirement of developmental interactions after dedifferentiation seriously limits the possibility of regeneration in more complex metazoans. This is problematic in particular when interactions with transient organs of early embryogenesis are involved, such as the somites and neural tube in vertebrates (Galis et al. 2018).
Constraints on Development and the Evolution of Body Plans
The conflict between differentiation and mitosis has, without doubt, crucially shaped the development and evolution of metazoan body plans. The body plan is mostly defined during embryonic development, when there are still zones that produce pluripotent stem cell colonies that subsequently migrate to other places in the embryo, to initiate their paths of differentiation. The absence of pluripotent cells later in life restricts the building of organ primordia to an early embryonic stage, generally known as the phylotypic stage. This stage, which includes the production of organ primordia, is highly conserved (see the section “Conservation of Phylotypic Stages”). Adults generally lack pluripotent stem cells and only have multipotent, tissue-specific, stem cells that function in cell renewal, wound healing, and regeneration (Tanaka and Reddien 2011). In contrast, plants, that do not have this incompatibility of cell division and differentiation, can generate complete organs throughout their life (Heidstra and Sabatini 2014). Even cells outside the stem cell niches are able to return to a proliferative pluripotent state, whereas in animals the capacity for pluripotency is limited to embryonic stem cells. The largest postembryonic flexibility in animals is provided by changes in the number of segments or the vegetative production of modules that are morphological repeats of the body plan (e.g., cnidarians and bryozoans).
Conservation of Phylotypic Stages
The abovementioned early specification of most organ primordia in animals leads to further constraints on the evolution of body plans due to the intense global interactivity in the embryo during the phylotypic stage. Embryologists have long noticed that the early organogenesis stage is less variable morphologically than both earlier and later stages. Recently, a large number of studies have shown that during that period there is also strong conservation of gene activity and of epigenetic mechanisms (e.g., Cridge et al. 2016; Hu et al. 2017).
Support for the Pleiotropy Hypothesis
Sander (1983) was the first to propose the interactivity of early organogenesis in the embryo as the root cause of the conservation of the phylotypic stage. His implicit hypothesis is that strong global interactivity causes disturbances to cascade into deleterious pleiotropic effects in other parts of the embryo that become amplified as development proceeds. As a result, mutants with a change during such a highly interactive stage will be strongly selected against (Galis and Metz 2001; Galis et al. 2018). We have called this suite of ideas the pleiotropy hypothesis.
Teratological data on rodents strongly support the pleiotropy hypothesis: disturbances of early organogenesis lead to many deleterious pleiotropic effects, and mortality is considerably higher than during earlier or later stages (Galis and Metz 2001). The interdependent pattern of abnormalities shows that the vulnerability of the stage is not due to one specifically vulnerable process (e.g., neural tube closure) but to the high interactivity of the stage. This implies that a particular, potentially useful, change of this stage, e.g., the induction of a change in the number of cervical vertebrae, kidneys, digits, or even arms, almost always will induce other abnormalities and lethality even before the organism is exposed to ecological selection. Indeed, in humans ca. 90% of individuals with polydactyly or a changed number of cervical vertebrae are dead at birth, while these changes are generally associated with a multitude of deleterious pleiotropic effects (Galis et al. 2018). As organ primordia typically originate during the phylotypic stage, this implies that the conservation of the stage leads to conservation of the number and earliest development of most organs (e.g., lungs, kidneys, limbs, long bones, eyes, ears), which thus can be viewed as due to secondary constraints. Further support for the pleiotropy hypothesis comes from transcriptomics studies on vertebrates and insects that show that during phylotypic stages, there is not only stronger conservation of gene expression than during earlier and later stages but also that in particular regulatory genes and genes with pleiotropic activity in other parts of the embryo are involved (e.g., Hu et al. 2017).
Why So Much Pleiotropy?
During the phylotypic stage, the trunk can be considered to be one large developmental field. The global interactivity and consequent low effective modularity are probably to an important extent due to the interactivity of the patterning of the three body axes and the interactivity of axial patterning with the other simultaneously occurring morphogenetic processes that are simultaneously occurring in the trunk (e.g., Diez del Corral et al. 2003; Galis et al. 2018). In vertebrates, for instance, the opposing and antagonistic gradients of Fgf/Wnt and retinoic acid (RA) in interaction with the segmentation clock play a major role in their coordinated organization. Not only genetic interactions are important: often the crucial importance of self-organizing chemical and physical interactions steering these highly dynamic processes is overlooked, including extensive migration, epithelialization, de-epithelialization, cell division, and cell shape changes (see chapters “Mechanisms of Pattern Formation, Morphogenesis, and Evolution” and “Inherency”). A large study on deceased human fetuses and infants provides strong support for the coupling of axial patterning and morphogenetic processes as a cause of the vulnerability of the stage, indicating among others a particularly strong coupling between segmentation (somitogenesis) and A-P patterning of the vertebral column (Galis et al. 2018).
During the early phylotypic stage, there are only a few large developmental fields. Other than the trunk field, there is the large neural crest-related cardio-craniofacial field and the heart primordium, while at the end, the tail bud increases in importance, and more organ primordia appear. The scarcity and large size of the developmental fields and the intense signaling within, but also between them, can explain a major part of the pleiotropy and low effective modularity characterizing the stage. This holds in particular for the earliest, most strongly conserved part of the stage.
Modularity and Evolvability of Later Stages
As development proceeds, it becomes more compartmentalized, with the appearance of progressively more and more signaling centers, organizing more and more localized developmental fields. Concurrently, the expression patterns of key signaling molecules become more restricted. For example, the signaling in the neural crest-related cardio-craniofacial field and in somites becomes increasingly compartmentalized (Galis et al. 2018). Within these smaller developmental fields, intense signaling between tissues continues to occur, but the interactivity within modules is more intense than the signaling between modules. The higher effective modularity probably underlies the reduced pleiotropy and increased developmental stability of the later developmental stages. This decreased pleiotropy allows for greater evolvability and more evolutionary divergence.
Challenges to the Pleiotropy Hypothesis
A challenge to the pleiotropy hypothesis is that, notwithstanding the vulnerability of early cleavage processes to radiation and toxicants (Jacquet 2004), evolutionary changes in the earliest developmental stages are not uncommon. However, in contrast to early organogenesis, the vulnerability is that of a single process, cell division. As the dividing cells are highly similar and capable of self-renewal, either too many cells are killed and the embryo dies or the damage is reversible and development resumes. As a result, nonlethal mutants with a changed cleavage have a chance to get established. In addition, mutations have a larger chance to be successful, since simple patterns have a lesser chance to be fatally disrupted than complicated ones (Galis et al. 2018). The greater simplicity and associated robustness of early forms may thus be expected to allow greater diversification.
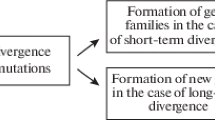
Another challenge to the pleiotropy hypothesis is that cleavage and gastrulation are sometimes remarkably similar, even more so than the phylotypic stages within metazoan phyla and classes. However, this similarity is largely inevitable, given the complete reset of development at the initial single-celled stage (Galis and Sinervo 2002). Only a limited number of permutations is possible when there are only a few undifferentiated cells present, due to the conflict between cell division and differentiation limiting possible developmental pathways. Convergent nutritional and locomotory adaptations cause further similarity, as well as maternal efforts to influence early development (Buss 1987). Gastrulation processes are more diverse than cleavage and, importantly, are far more diverse than their end product, phylotypic stages. Gastrulation almost always results in three germ layers, and the organ systems originating from these germ layers are highly conserved. Furthermore, a fundamental outcome of gastrulation is that sheets of cells come into contact in a precise way, allowing the conserved embryonic inductions that are required for the organization of the body plan during the phylotypic stage. These inductions between adjacent cell populations appear to form a strict spatiotemporal constraint on the outcome of gastrulation , the starting point of the conserved phylotypic stage (Galis and Sinervo 2002).
Consequent Constraints on Body Plan Evolution
Most organ primordia originate during the phylotypic stage, and, as mentioned above, the strong conservation of this stage implies strong conservation of the number and early development of organs. Mutations for duplications of organs occur, but they co-occur with many often fatal pleiotropic effects (Galis et al. 2018). Similarly, the evolutionary loss of organs is constrained, as early developmental interactions cannot easily be done away with. For this reason, loss of organs typically occurs via the slow evolution of earlier and earlier developmental arrest, followed by degeneration. A good example is the many times that cave fishes and salamanders have evolved blindness: the lens always develops and then starts to degenerate. As a result of the slow accumulation of mutations, re-evolution of lost complex organs is virtually impossible, in agreement with Dollo’s law. In contrast, when organ primordia appear during more compartmentalized later developmental stages, organ numbers are considerably more evolvable. Good examples are the number of segments in insects, phalanges and carpal and tarsal elements in tetrapods, nipples in mammals, and teeth in vertebrates. The variability of the number of cervical vertebrae in long-necked nonmammalian amniotes is a case in point. The neck-trunk boundary is determined late in these cases, and, in agreement with this, the more vertebrae in a neck, the more variable the number is (swans have 22–25). In contrast, not only in mammals, but in all amniotes with necks of eight or fewer vertebrae, the number is conserved, e.g., crocodiles, turtles, geckos, and many other limbed lizards. A further arthropod example is the number of segments of centipedes, which is variable, but always odd (Arthur and Farrow 1999). This constraint on even numbers appears to be caused by the conserved oscillatory pattern which generates two segments per cycle; hence variation is generated by the number of cycles, with the oscillatory mechanism set up during the phylotypic stage (see chapter “The Evolution and Development of Segmented Body Plans”).
Breaking of Constraints: Relaxed Stabilizing Selection
Relaxed stabilizing selection occasionally allows the breaking of constraints. Such a relaxation can result from environmental changes like the opening up of new food niches or the disappearance of competitors and predators. Such changes are often associated with the start of adaptive radiations and the emergence of key innovations. Arguably relaxed selection allows novelties to persist for some time, permitting selection against some of the most deleterious pleiotropic effects, such that when stabilizing selection subsequently increases, the chance for persistence of the novelties is increased. Domestic dogs show a useful parallel, as human care allows dogs with extra digits to persist and only selection against some of the more deleterious pleiotropic effects (congenital abnormalities) occurs.
Internal factors can also cause relaxed selection, with slow behavior and low metabolic rates as good examples. Sloths and manatees are exceptional mammals with a changed number of cervical vertebrae. The breaking of the strong constraint on the number of cervical vertebrae in mammals appears to be due to a large tolerance for associated pleiotropic effects, due to their extremely slow activity rates. Adult sloths and manatees frequently have skeletal abnormalities that in deceased human fetuses and infants are commonly associated with cervical ribs (e.g., fused cervical vertebrae and serious ossification defects), and this apparently poses no major problem. Furthermore, in manatees and sloths, another pleiotropic effect of cervical ribs (documented for humans), embryonal tumors, may be less problematic due to the extremely low metabolic rates and presumably low cancer rates (c.f. Galis et al. 2018). Whales and dolphins are also exceptional in frequently having ribs on the seventh vertebra. This is probably similarly due to relaxed selection against skeletal abnormalities, caused by the supporting effect of water. Whales and dolphins also have low cancer rates. Thus, the difficulty of breaking specific constraints varies among taxa, due to differences in the experienced selection regimes and to differences in the specific pleiotropic effects associated with traits.
Developmental Constraints Against the Evolution of Parthenogenesis
In parthenogenesis, development generally starts with an unfertilized ovum and, thus, without contribution from a father. The most important missing paternal contributions are the centrosome and chromosomes. As mentioned above, the centrosome is presumably missing in the ovum as a consequence of the universal metazoan constraint against having more than one centrosome in a cell. If both the ovum and the sperm cell would contribute a centrosome, the zygote would end up with two centrosomes. The paternal chromosomes normally restore the ploidy of the ovum after meiosis, and as the development of haploid ova almost always fails in diploid animals, this forms another strong developmental constraint against the evolution of parthenogenesis. Although obligatory parthenogenesis has the disadvantage of missing genetic recombination, facultative parthenogenesis should be the most advantageous reproductive type, as females can choose between the production of asexual and sexual offspring and, thus, can combine the advantages of both modes of reproduction.
Replacing the Missing Paternal Centrosome
As mentioned earlier, centrosomes are required for rapid, error-free cell divisions. In sporadic parthenogenesis, centrosomes are assembled de novo. However, this occurs in an inefficient way, and the centrosomes are often malfunctioning, diminishing the success of ploidy restoration and mitosis (Eisman and Kaufman 2007). This is probably one of the reasons for the generally extremely low reproductive success of sporadic parthenogenesis and emphasizes the importance of the developmental constraint (Eisman and Kaufman 2007). In regularly parthenogenetic animals, the lack of the paternal centrosome is usually remedied by an efficient de novo production of a centrosome in the ovum, for which a variety of mechanisms has evolved in different taxa (Schön et al. 2009). In stick insects of the genus Bacillus, the centrosome is always assembled de novo in the fertilized ovum, thus removing the constraint (Schön et al. 2009, ch.16). An alternative remedy for the lack of a centrosome is sperm-dependent parthenogenesis, where a sperm cell of a related sexual species or a conspecific is used to initiate mitosis of the ovum, without the sperm-contributing genes (Schön et al. 2009, ch. 19). It is generally assumed that mitosis can only be initiated thanks to the sperm’s centriole. In fishes and amphibians, but also in many other taxa, parthenogenesis is always sperm-dependent.
Replacing the Missing Paternal Chromosomes
In parthenogenetic organisms, the problem of the missing paternal chromosomes is usually solved by one of several mechanisms of ploidy restoration. In sporadic parthenogenesis ploidy is generally restored with no or minimal change of meiosis (Schön et al. 2009, ch. 4). In one of the two most common mechanisms, gamete duplication, the unfertilized ovum starts a cleavage division, and this is followed by fusion of the two daughter nuclei, without affecting meiosis. These nuclei have the same half of the genome of the mother, and hence all loci will be homozygous. In the other common mechanism, terminal fusion, also only half of the maternal genome is transmitted. Here ploidy is restored at the end of meiosis by the fusion of the generally large oocyte with the closest polar body (second, in the row of four meiotic products). This polar body shares the same half of the maternal genome. Heterozygosity is lost, except for some caused by crossing over. Sporadic parthenogenesis is, thus, generally characterized by ploidy restoration with no or minimal change of the meiosis, resulting in heterozygosity loss, which together with the abovementioned poor centrosome function in the ovum results in extremely low viability and fertility rates.
In contrast, in most regularly parthenogenetic animals, ploidy restoration occurs via a drastic change of the normally tightly controlled global interactivity in the cell during meiotic divisions. This is remarkable, as even minor disruptions usually lead to strongly deleterious effects, such as aneuploidy, embryonic death, and sterility. The most common mechanism in obligatory parthenogenesis is the almost complete suppression of meiosis, followed by a mitosis-like division (apomixis), resulting in the transmission of the entire genome of the mother (Schön et al. 2009, ch. 4). Another frequent mechanism involves the duplication of the genome at the onset of meiosis, such that meiosis restores ploidy and the complete genome of the mother is transmitted (so-called premeiotic doubling). Yet another common mechanism is so-called central fusion. Central fusion differs from terminal fusion in that the oocyte is not fusing with the second polar body, but instead there is a fusion of the two inner polar bodies, that replace the oocyte and apparently receive sufficient protoplasm. The inner polar bodies each have a different half of the maternal genome. The entire genome of the mother is thus transmitted, provided crossing over is repressed, for instance, by large inversions, as found in the only obligate parthenogenetic Drosophila species, D. mangabeirai. D. mangabeirai studies show that central fusion involves drastic modifications of meiosis, leading to different relative positions of the polar bodies (Schön et al. 2009 ch).
In summary, in contrast with sporadic parthenogenesis, regular parthenogenesis is generally characterized by ploidy restoration that involves drastic disruptions of meiosis, usually resulting in the transmission of the entire genome of the mother and good centrosome function, and the outcome is good viability and fertility.
Evolutionary Mechanisms That Facilitate the Alteration of Meiosis: Hybridization and Endosymbionts
There is a close association of regular and obligatory parthenogenesis with hybridization or endosymbiont infections. It is probable that the radical alterations of meiosis usually observed in regular and obligatory parthenogenesis have their origin in sudden large cytological events, for instance, due to interspecific hybridization or paternal genome loss due to endosymbionts, such as Wolbachia (Schön et al. 2009). It is counterintuitive that gradual changes of meiosis would be selectively advantageous, given the strongly deleterious effects of even minor changes. All well-investigated unisexual vertebrates are interspecific hybrids, and this has been found for a large expanding pool of invertebrate asexuals as well (Schön et al. 2009). Both successful lab experiments and recent detailed and large-scale genomic analyses have shown that on (extremely) rare occasions, interspecific hybridization followed by backcrossing events can result in parthenogenetic reproduction with a restoration of ploidy that retains heterozygosity. Paternal genome loss due to infection with maternally inherited endosymbionts also radically disrupts meiosis and is found to be associated with the origin of parthenogenesis (Schön et al. 2009). For instance, the initial event in the evolutionary path to sperm-dependent parthenogenesis of males (so-called pseudo-arrhenotoky) is most likely infection with symbionts that inactivate the paternal genome, via cytoplasmic incompatibility or via male-killing (e.g., Engelstädter and Hurst 2006).
The two causes of developmental constraints against parthenogenesis, absence of paternal chromosomes and centrioles, probably combine to provide a near absolute constraint against the gradual evolution of regular parthenogenesis. The genetic constraint of enforced homozygosity associated with sporadic parthenogenesis will further impede this gradual evolution. This probably explains why the advantageous mode of facultative parthenogenesis is not more widespread and is even entirely absent in vertebrates. In contrast, stick insects of the genus Bacillus miss the developmental constraint caused by the missing centrosome, which perhaps has allowed the, otherwise exceptional, gradual evolution of parthenogenesis.
Conclusions
We found three well-underpinned examples of wide-ranging near absolute developmental constraints in animals on the macroevolutionary time scale, which in turn induce many secondary constraints. All three are caused by rampant pleiotropy, itself caused by the complex and highly controlled global interactivity associated with mitosis and meiosis in the cell and with organogenesis in the early embryo (Galis et al. 2018). A strong control adds to the interactivity in leading to a stronger developmental conservation. Such a strong control presumably evolved because of the complexity of the processes and the grave consequences of their disruption (aneuploidy, apoptosis, cancer, sterility, death). As a result, mutations that affect such highly interactive developmental processes almost unavoidably have many deleterious pleiotropic effects that drastically diminish their chance to be successful. Conservation is, thus, caused by consistently strong selection against mutational change thanks to developmentally caused pleiotropic effects. As the interactivity is an intrinsic property of the developmental processes, the constraints are developmental.
The strongest and most universal of the constraints is the incompatibility of ciliated and differentiated cells to undergo mitosis. This results from pleiotropic effects combined with selection against having more than one or no centrosome, since this dramatically affects the interactivity associated with mitosis. As a consequence, in almost all adult metazoans, there are no pluripotent stem cells, only tissue-specific ones, such in strong contrast to plants. For differentiated metazoan cells, the only way around the constraint is to first dedifferentiate and then divide, as happens in wound healing and regeneration (as well as cancer), severely restricting the potential for these processes and protecting us against cancer. Furthermore, the constraint strongly biases the order and timing of proliferation and differentiation during development, the more so the larger the developmental complexity. A further important consequence of the constraint is the early determination of almost all organ primordia, when development is still highly interactive (phylotypic stage). This, among others, leads to the hard constraint against changes of phylotypic stages in many higher taxa, impacting the evolution of body plans. Since most organ primordia originate during this stage, there is strong conservation of the number and earliest development of most organs, like cervical vertebrae, eyes, and digits. The universal constraint against having more than one centrosome in a cell further leads to a hard developmental constraint against parthenogenesis, as zygotes only receive a centrosome from the father and not from the mother. Two centrosomes can be expected to be strongly deleterious. Hence, the missing paternal contribution of a centrosome in an unfertilized ovum hinders reliable cleavage divisions. We also discussed another hard developmental constraint, this time against parthenogenesis, which involves the missing paternal chromosomes. Successful ploidy restoration also appears to be limited due to a constraint, caused by the strong interactivity of meiosis. The combination of the two developmental constraints against parthenogenesis arguably forms a near absolute constraint against the gradual evolution of parthenogenesis from sporadic to regular or obligatory.
References
Arthur W, Farrow M (1999) The pattern of variation in centipede segment number as an example of developmental constraint in evolution. J Theor Biol 200:183–191
Bell G (1989) Darwin and biology. The evolution of individuality. Leo W. Buss, Princeton University Press Princeton, New Jersey 1988.197 pp. J Hered 80:417–421
Buss LW (1987) The evolution of individuality. Princeton University Press, Princeton
Conner JK (2012) Quantitative genetic approaches to evolutionary constraint: how useful? Evolution 66:3313–3320
Cridge AG, Dearden PK, Brownfield LR (2016) The mid-developmental transition and the evolution of animal body plans. Ann Bot 117:833–843
Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K (2003) Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40:65–79
Eisman RC, Kaufman TC (2007) Cytological investigation of the mechanism of parthenogenesis in Drosophila mercatorum. Fly 1:317–329
Engelstädter J, Hurst GDD (2006) Can maternally transmitted endosymbionts facilitate the evolution of haplodiploidy? J Evol Biol 19:194–202
Galis F, Metz JAJ (2001) Testing the vulnerability of the phylotypic stage: on modularity and evolutionary conservation. J Exp Zool B Mol Dev Evol 291:195–204
Galis F, Sinervo B (2002) Divergence and convergence in early embryonic stages of metazoans. Contr Zool 71:101–113
Galis F, Metz JAJ, van Alphen JJM (2018) Development and evolutionary constraints in animals. Ann Rev Ecol Evol Syst 49:499–522
Gönczy P (2015) Centrosomes and cancer: revisiting a long-standing relationship. Nature Rev 15:639–652
Gould SJ, Lewontin RC (1979) The spandrels of the San Marco and the Panglossian paradigm: a critique of the adaptationist program. Proc Royal Soc London B 205:581–598
Hadorn E (1961) Developmental genetics and lethal factors. Methuen, London
Heidstra R, Sabatini S (2014) Plant and animal stem cells: similar yet different. Nature Rev Mol Cell Biol 15:301–312
Henneguy LF (1898) Sur les rapports des cils vibratiles avec les centrosomes. Arch d’Anat Micr 1:481–496
Hu H, Uesaka M, Guo S, Shimai K, Lu S-M, Li F, Fujimoto S, Ishikawa M, Liu S, Sasagawa Y, Zhang G, Kuratani S, Yu J-K, Kusakabe TG, Khaitovich P, Irie N (2017) Constrained vertebrate evolution by pleiotropic genes. Nature Ecol Evol 1:1722–1730
Jacquet P (2004) Sensitivity of germ cells and embryos to ionizing radiation. J Biol Regul Homeost Agenets 18:106–114
Lenhossék M (1898) Ueber Flimmerzellen. Verhandl Der Anat Gesel Kiel 12:106–128
Manandhar G, Schatten H, Sutovsky P (2005) Centrosome reduction during gametogenesis and its significance. Biol Reprod 72:2–13
Maynard Smith J, Burian R, Kauffman S, Alberch P, Campbell J, Goodwin B, Lande R, Raup D, Wolpert L (1985) Developmental constraints and evolution. Q Rev Biol 60:265–287
Metz JAJ (2012) Adaptive dynamics. In: Hastings A, Gross LJ (eds) Encyclopedia of theoretical ecology. University of California Press, Berkeley, pp 7–17
Peterson T, Müller GB (2016) Phenotypic novelty in EvoDevo: the distinction between continuous and discontinuous variation and its importance in evolutionary theory. Evol Biol 43:314–335
Sander K (1983) The evolution of patterning mechanisms: Gleanings from insect embryogenesis and spermatogenesis. In: Goodwin BC, Holder N, Wylie CC (eds) Development and Evolution. Cambridge Univ. Press, Cambridge, UK, p 137
Schön I, Martens K, van Dijk P (eds) (2009) Lost sex. The evolutionary biology of parthenogenesis. Springer, Dordrecht
Tanaka EM, Reddien PW (2011) The cellular basis for animal regeneration. Dev Cell 21:172–185
Vermeij GJ (2015) Forbidden phenotypes and the limits of evolution. Interface Focus 5:20150028
Walz G (2017) Role of primary cilia in non-dividing post-mitotic cells. Cell Tissue Res 369:11–25
Whyte L (1964) Internal factors in evolution. Acta Biotheor 16:33–48
Wu J, Akhmanova A (2017) Microtubule-organizing centers. Ann Rev Cell Dev Biol 33:4.1–4.25
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Galis, F., Metz, J.A.J. (2021). A Macroevolutionary Perspective on Developmental Constraints in Animals. In: Nuño de la Rosa, L., Müller, G.B. (eds) Evolutionary Developmental Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-32979-6_69
Download citation
DOI: https://doi.org/10.1007/978-3-319-32979-6_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32977-2
Online ISBN: 978-3-319-32979-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences