Abstract
Broccoli is a rich source of health-promoting antioxidants and anticarcinogenic glucosinolates, which has long been recognized for their outstanding benefits to human nutrition and plant defense. The composition and content of glucosinolate are closely associated with the flavor and anticancer activity of broccoli. Up to now, broccoli is among a few Brassica vegetables, in which the biosynthetic pathway of glucosinolate has been widely studied and has attracted extensive attention. Recent studies in glucosinolate research have also identified the genetic variations, as well as the functions of individual glucosinolate profiles and their degradation products in broccoli, which provide the basic aims and powerful strategies for breeding of broccoli varieties with optimal glucosinolate composition and content. To fully exploit the potentially beneficial effects of broccoli, it is important to investigate the glucosinolate variation and metabolism across the whole food chain, from preharvest production to post-harvest storage, processing, and cooking. This chapter provides a general overview of glucosinolate biosynthetic pathway, as well as the genetic variation and function of individual glucosinolate profiles in broccoli, highlights the recent advances in glucosinolate accumulation of broccoli upon different preharvest and post-harvest handlings, and discusses their potential application in broccoli breeding, production, storage, processing, and consumption.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
Broccoli (Brassica oleracea L. var. italica) is an economically important vegetable crop in many countries and has been highly valued by consumers due to its flavor as well as its nutritional components like minerals, vitamins, polyphenols, and other beneficial phytochemicals, particularly potent anticarcinogenic glucosinolates (GSs) [1–4]. Epidemiological studies have suggested that the consumption of broccoli could lower the risk for the development of certain forms of cancers, which have been attributed to glucosinolates and their degradation products [5, 6].
Glucosinolates , a group of sulfur- and nitrogen-containing secondary metabolites , mainly belong to the Brassicaceae family. The general structure of glucosinolate consists of a β-d-thioglucose group, a sulfonated oxime group, and a side chain derived from amino acids [7]. More than 200 glucosinolates have been identified so far [8] and can be divided into three classes on the basis of their derived amino acid precursors: aliphatic glucosinolates, indole glucosinolates, and aromatic glucosinolates. Usually, glucosinolates with different structures can be degraded into various biologically active breakdown products in broken tissues and living cells of plant, as well as gastrointestinal tract of mammalian [9–14], which contribute to the flavor, anticarcinogenic activity, and resistance of broccoli [15]. As maintaining and enhancing good health through dietary habits have been proposed to deal with increased lifestyle diseases in current society, optimal glucosinolate profile and content in broccoli are expected. The purpose of this chapter is to review the research findings related to glucosinolate biosynthetic pathway and variation among different broccoli genotypes, as well as the regulation of glucosinolate accumulation along the whole food chain for better retention of glucosinolates in broccoli.
2 Biosynthetic Pathway of Glucosinolates in Broccoli and Brassica Plants
So far, the biosynthetic pathway of glucosinolates has been successfully elucidated in Arabidopsis and summarized in several review papers [7, 16]. Generally, aliphatic glucosinolate biosynthesis consists of three separate steps: (i) chain elongation of precursor amino acid, (ii) development of the core glucosinolate structure, and (iii) secondary modifications of the amino acid side chains [7, 16, 17]. There are five reactions required for precursor amino acid elongation: an initial and final transamination, acetyl-CoA condensation, isomerization, and oxidative decarboxylation [18, 19]. In this process, branched-chain amino acid aminotransferase (BCAT), bile acid transporter 5 (BAT5), methylthioalkylmalate synthase (MAM), isopropylmalate isomerase (IPMI), and isopropylmalate dehydrogenase (IPMDH) are involved [20–26]. The core glucosinolate structure formation begins with oxidation of precursor amino acids by cytochrome P450 monooxygenases (cytochrome P450s) of the CYP79 family and then CYP83 family, followed by further metabolic process catalyzed by C-S lyase, glucosyltransferase, and sulfotransferase [27–35]. The secondary modifications are attributed to several loci, including GS-OX which catalyzes methylthioalkyl to methylsulfinylalkyl glucosinolates, GS-ALK/AOP2 which controls the conversion to alkenyl glucosinolates, GS-OHP/AOP3 which is responsible for the production of 3-hydroxypropyl glucosinolates, and GS-OH which participates in the production of 2-hydroxy-3-butenyl glucosinolate in aliphatic glucosinolate biosynthesis [36–38], as well as cytochrome P450 CYP81Fs and indole glucosinolate methyltransferase (IGMT) in indole glucosinolate biosynthesis [39–42]. Furthermore, several regulators of glucosinolate biosynthesis have also been identified, such as nuclear-localized calmodulin-binding protein IQD1, DOF transcription factor AtDof1.1, sulfur limitation1 (SLIM1), TU8, and six R2R3-MYB transcription factors as well as three basic helix-loop-helix (bHLH) transcription factors [43–54].
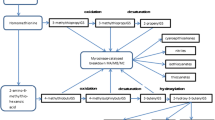
As the genome sequence of Brassica crops including B. oleracea has been reported [55], genes related to glucosinolate biosynthesis in Brassica crops were identified gradually with the assistance of bioinformatics. The aliphatic glucosinolate biosynthesis is described in Fig. 1 based on previous studies and genome sequence. BoGSL-ELONG, BoGSL-ELONGL, BoGSL-PRO, and BoGSL-PROL, which control chain elongation of precursor amino acid in B. oleracea, have been cloned or inferred [56–59]. Li and Quiros [56] have conducted the genetic analysis, expression, and molecular characterization of BoGS-ELONG, which was responsible for the synthesis of four-carbon glucosinolates in B. oleracea. However, BoGS-ELONGL, which corresponds to MAM-L in Arabidopsis, was probably nonfunctional, at least in broccoli variety ‘Early Big’ [58]. In addition, genetic analysis suggested that BoGS-PRO was associated with the biosynthesis of three-carbon side-chain glucosinolates [57], while the function of BoGS-PROL has not been assessed yet [59]. BoGS-ALK was also cloned and demonstrated to be responsible for alkenylation in side-chain modification in glucosinolate pathway [57]. Furthermore, BoCYP79F1, BoCS-lyase, BoS-GT (S-glucosyltransferase), BoGS-OH, and the candidate gene for BoGS-OXID have also been inferred [60, 61].
Although Brassica crops share the similar pathway of glucosinolate biosynthesis with Arabidopsis, the genes involved are different in sequence identities with their counterparts in Arabidopsis as well as with other Brassica crops. For instance, MAM family contains high copies in B. rapa and B. oleracea, however, with different expression patterns, resulting in the fact that the major aliphatic glucosinolates are 3C and 4C glucosinolates in B. oleracea while 4C and 5C glucosinolates in B. rapa [55].
3 Genetic Variation and Function of Glucosinolate Profiles in Broccoli
3.1 Genetic Variation of Glucosinolate Composition and Content
According to previous reports, there are up to 17 different kinds of glucosinolates identified in broccoli (Table 1) with a genetic variation in their composition and content [62–65]. Nevertheless, glucoraphanin is the predominant methionine-derived aliphatic glucosinolate in most varieties. In an early report, Carlson et al. [62] determined the variation of nine glucosinolate profiles in six commercial cultivars and found that glucoraphanin contents were from 29.2 to 88.3 μmol 100 g−1 fresh weight (FW). In 1999, Kushad et al. [64] identified 14 glucosinolates in 50 broccoli accessions, and glucoraphanin levels were in a scope from 0.8 to 21.7 μmol g−1 dry weight (DW). Similarly, Farnham et al. [66] demonstrated that the amount of glucoraphanin varied from 0.04 to 2.94 μmol g−1 FW in 71 pure lines and 5 hybrid checks. The results from Chinese broccoli germplasms indicated that the glucoraphanin concentrations ranged from 0.06 to 24.17 μmol g−1 DW in 143 pure lines and from 1.57 to 5.95 μmol g−1 DW in 5 commercial varieties [65], while progoitrin levels in 5 commercial cultivars differed from 1.77 to 6.07 μmol g−1 with a mean of 3.20 μmol g−1 DW. However, reports about high levels of progoitrin or epiprogoitrin were few in primary broccoli cultivars from America [62, 64, 67], England [68], Germany [69], Portugal [70], and Spain [71]. Likewise, relatively higher concentration and variation in indole glucosinolate were reported by most surveys [65, 68–71] except for Kushad et al. [64]. Generally, the content of aromatic glucosinolates, mainly gluconasturtiin, is much lower than aliphatic or indole group. Moreover, some specific lines with altered glucosinolate profiles were observed in broccoli germplasms [65]. Former studies have demonstrated that the accumulation of aliphatic glucosinolates in broccoli head is mainly regulated by genetic factors, while the content of indole glucosinolates is greatly affected by environment and environment × genotype interaction [63, 85, 86]. Recently, the identification of two glucosinolate transporters, GTR1 and GTR2, which are response for the transportation of glucosinolates in Arabidopsis, provided us a useful means to control the allocation of glucosinolates in different tissues [87].
The breakdown pathway as well as products of glucosinolates is a leading area in glucosinolate research in recent years. The classical breakdown pathway in plant depends on classic myrosinase (β-thioglucoside glucohydrolase, TGG) [17], which is localized in separate plant cells or in separate intracellular compartments with glucosinolates in intact plant tissue while tissue damage brings them together and initiates TGG-catalyzed glucosinolate hydrolysis [88, 89]. In addition, an atypical myrosinase, PENETRATION2 (PEN2), is thought to be involved in a new indole glucosinolate catabolic pathway in living plant cell [39, 40, 90]. Furthermore, intact glucosinolates could also been degraded by the resident microflora of the gastrointestinal tract to form ITCs, amines, or nitriles depending on the type of bacterial myrosinase-like activity involved [10–14].
In Arabidopsis, there are six myrosinase genes with different gene sequences and expression patterns [9]. Besides, some specific proteins , such as epithiospecifier protein (ESP) and nitrile-specifier protein (NSP), are also involved in the hydrolysis of glucosinolates, affecting the final breakdown products. Mithen et al. [91] compared the ability to induce phase II detoxification enzymes in two broccoli lines at similar glucosinolate level with ‘Marathon’, a standard commercial broccoli cultivar. They found one line showed 80 times the ability to induce quinone reductase compared to ‘Marathon’, while the other showed little induction. The detection of breakdown products showed that plenty of ITCs (95%) were degraded from glucoiberin and glucoraphanin, the predominant glucosinolates of broccoli, in the former line, while high levels of nitriles, and only 1% ITCs, were formed from glucoiberin and glucoraphanin in the later line. The results of sequences for B. oleracea sp. capitata indicated that the B. oleracea species also had a complex glucosinolate-myrosinase system [55]. In vitro, a recombinant broccoli ESP from cv. Packman directed myrosinase-dependent degradation of epiprogoitrin toward the formation of epithionitrile and glucoraphanin to sulforaphane nitrile instead of sulforaphane [92]. In addition, the variety of breakdown products from aliphatic glucosinolates, mainly glucoraphanin, in floret and sprout among different broccoli varieties was observed in some studies [91–93]. These researches indicated that the typical glucosinolate-myrosinase was much complex upon tissue damage, and genetic effect played an important role in hydrolysis of glucosinolates, especially aliphatic glucosinolates.
3.2 Biological Functions of Individual Glucosinolate Profiles
Glucosinolates and their hydrolysis products in broccoli have been considered to be bioactive, while some of them may have adverse effects for human and animal. There are in vitro and in vivo evidences that ITCs play multiple roles in cancer prevention. For example, some ITCs from methionine- and aromatic-derived glucosinolates such as sulforaphane, allyl isothiocyanate, and phenethyl isothiocyanate can strongly inhibit phase I enzymes and induce phase II enzymes, as well as promote cell cycle arrest and apoptosis in various cancer cell lines [94–100]. Indole-3-carbinol (I3C), a breakdown product from indole glucosinolate, has been found to exert chemoprotective activity through altering estrogen metabolism [101, 102]. Moreover, ITCs, notably sulforaphane, make contributions to cancer protection via inhibition of tumor invasion and angiogenesis [103], anti-inflammatory [104], as well as immunomodulatory activities [98]. In addition to these cancer chemopreventive properties, ITCs also play important roles in protection of the central nervous system [105], preventing against cardiovascular disease [106, 107] and bacterial infection [76].
As we have mentioned above, glucosinolates could be degraded to not only ITCs but also nitriles due to different hydrolytic environments. Previous research verified that quinone reductase and glutathione S-transferase activities in hepatic, colonic mucosal, and pancreatic were induced by high doses of sulforaphane but not by sulforaphane nitrile. Quinone reductase activity of sulforaphane and sulforaphane nitrile treated Hepa 1c1c7 cells also suggested that production of sulforaphane rather than sulforaphane nitrile could increase the potential chemoprotective effects of broccoli [108].
Glucosinolates and their breakdown products also show some adverse effects besides their healthy benefit functions. Indole derivatives (e.g., I3C) have been classified as bifunctional inducers [109, 110]. It has been reported that I3C inhibited the development of cancer in animals when given before or with a carcinogen, whereas enhanced development of cancer was observed when administered after a carcinogen in some cases [5]. Certain glucosinolates, such as sinigrin, progoitrin, and gluconapoleiferin, are responsible for bitter taste [111], which may decrease consumer acceptance. Furthermore, progoitrin has been considered as natural toxicants for its derivative has goitrogenic effects on mammals [112]. However, the amount of progoitrin in broccoli is quite low, and the occurrence of these substances is dependent on many cofactors. In addition, this malnutritional effect can also be avoided by normal iodine intake [113].
Glucosinolates and their hydrolytic products have been demonstrated to be crucial in plant defense response . They can stimulate feeding or oviposition by crucifer-specialist herbivores [16, 114–116]. Aliphatic glucosinolates were reported to be important for resistance of plants to pests [117], and sulforaphane functioned in nonhost resistance in Arabidopsis-Pseudomonas pathosystem [118]. Moreover, PEN2-dependent potential hydrolysis products of 4-methoxy glucobrassicin were found to activate innate immunity, leading to protection against fungal penetration [39, 40], while TGG-catalyzed degradation of indole glucosinolates attenuated mycotoxin fumonisin B1-induced programmed cell death in Arabidopsis [119].
4 Breeding of Broccoli Varieties with Optimal Glucosinolate Composition and Content
A high-glucoraphanin commercial hybrid, Beneforté®, was developed by Mithen’s group through genome introgression from the wild species Brassica villosa [120]. In former surveys of this group, a major quantitative trait locus (QTL) on linkage group 2 that determined the concentrations of methionine-derived glucosinolates in the high-glucoraphanin hybrids was found, and a microsatellite marker OI12-F02 originated from a wild species Brassica villosa was developed [68, 91]. Recently, researchers of this group cloned the major dominant QTL and identified an MYB28 allele resulted in higher expression of MYB28 in the leaves of high-glucoraphanin hybrids compared to the standard broccoli cultivars. There are three SNPs identified in the MYB28 allele: two located in the intronic region upstream of exon 3 and one at the 5’-end of exon 3 [120]. Sotelo et al. [121] used a DH mapping population of F1 from a Chinese kale and a DH broccoli line to detect the significant QTLs in leaves, flower buds, and seeds. They identified three loci controlling the content of aliphatic glucosinolates and four loci manipulating the accumulation of indole glucosinolates. Therefore, besides MYB28 allele from Brassica villosa, there might be other gene/locus variation determining the variety of aliphatic glucosinolate level in broccoli. In addition, significant general combining ability was observed between glucoraphanin concentration and total head content in 2-year assessment of 36 combinations by crossing nine pure parents’ lines [122]. Based on putative high-glucoraphanin materials, Gu et al. [86] also obtained sixteen high-glucoraphanin hybrids with acceptable agronomic traits by direct crossbreeding and screened two high-glucoraphanin parental lines.
The basic aims of breeding broccoli varieties with optimal glucosinolate composition and content include the following: (1) to reduce the content of anti-nutritional component, notably 2-hydroxy-3-butenyl glucosinolate; (2) to improve the content of beneficial compositions (glucoraphanin, glucoiberin, and glucoerucin) for better nutritional qualtiy, and to regulate the biosynthetic and hydrolytic pathways of glucosinolate profiles involved in interaction with pathegens and pests for enhanced resistance; (3) to promote the formation of ITCs rather than nitriles; (4) to preserve acceptable agronomic traits for head and considerable seed yield for sprout; and (5) to preserve other beneficial compounds, such as vitamins, minerals, and flavonoids. The related principles/strategies to reach above aims are as follows:
-
1.
To select the parents without alkenyl glucosinolates through direct detection or markers (e.g., BoGS-ALK and BoGS-OH) selection.
-
2.
To detect glucosinolates directly or analyze the critical genes or markers, which could regulate the aliphatic glucosinolate accumulation in edible parts. For example, the microsatellite marker OI12-F02 or other primers designed for the BoMYB28 allele, which are located in chromosome 2.
-
3.
Due to no available markers for breakdown products variation, direct detection of breakdown products is required and enough for determination of the ability to deliver aliphatic glucosinolates to correspondent ITCs.
-
4.
Other nutrients are not affected in the final hybrid. Furthermore, glucosinolate metabolism is widely involved in various defense reactions, especially against insects and pathogens. It is needed to consider the adaptability and resistance of glucosinolate hybrid in complex environments.
In addition to genotype, glucosinolate contents in broccoli are also influenced by many factors along the whole food chain from farm to table. Preharvest factors are pivotal to enhancement of glucosinolate accumulation, and suitable post-harvest handlings are essential for maintaining the glucosinolate content of broccoli products during the storage after harvest, while processing and cooking are other effective ways for glucosinolate retention before consumption.
5 Factors Influencing Glucosinolate Accumulation in Broccoli
5.1 Preharvest Factors
Plenty of investigations have been conducted to search useful tools for modulation of glucosinolate accumulation in broccoli before harvest. Regulation of glucosinolate by light as an environmental factor, as well as by phytohormones, sugars, salinity, and fertilization as chemical regulation , has been widely studied, and many valuable results were obtained.
5.1.1 Light
Light is a crucial environmental factor in plant life, which regulates seed germination, phototropism, and flowering [123, 124]. Recent studies have revealed that light influenced the accumulation of phytochemicals such as vitamin C, phenolic compounds and carotenoids, as well as glucosinolates [125–128]. It has been reported that UV-B radiation caused an induction of glucosinolate accumulation, especially of glucoraphanin and 4-methoxy glucobrassicin in broccoli sprouts and florets [129, 130]. In contrast, preharvest supplemental far-red light resulted in reduction of glucosinolate content, whereas supplemental red and blue light did not show a significant effect in broccoli florets [131].
5.1.2 Phytohormones
According to previous studies, glucosinolate accumulation in broccoli can be manipulated through treatment with several kinds of plant hormones, such as jasmonates , auxin, and brassinosteroids (BRs).
It has been shown that jasmonic acid (JA) and methyl jasmonate (MeJA) enhanced glucosinolate accumulation in broccoli. Liuann et al. [132] reported that significantly increased levels of indole glucosinolates including glucobrassicin, neoglucobrassicin, and gluconasturtiin were observed in 250 μM MeJA-treated broccoli (B. oleracea var. Green Magic), while the contents of aliphatic glucosinolates remained unchanged. Similar results were also observed in another individual survey, which demonstrated that the content of indole glucosinolates was increased by 3- to 20-fold upon JA treatment when compared to the control [133].
Since the indole acetaldoxime serves as a common precursor for indole glucosinolate and indole-3-acid (IAA) biosynthesis, close attention has been paid to the cross talk between these two kinds of indole compounds [134]. In broccoli, exogenous auxin treatment generally resulted in substantially higher levels of glucosinolates, especially indole glucosinolate. However, the effects of auxin varied with the types and concentrations used. IAA application at low concentration (0.1 mg L−1) led to the highest content of glucosinolates, followed by 0.1 mg L−1 indole-3-butyric acid (IBA), while 1 mg L−1 naphthalene acetic acid (NAA) treatment resulted in the lowest accumulation of glucosinolates [135].
Our previous survey has found that BR treatment downregulates glucosinolate accumulation in Arabidopsis [136], but a dose effect of BR existed in broccoli. Our other survey showed that the content of total glucosinolates and glucoraphanin in broccoli sprouts treated with 2 nM epibrassinolide (EBR) plus 40 mmol/L NaCl was increased by 86% and 85%, respectively. However, the reduction in glucosinolate content was observed due to the enhanced activity of myrosinase upon 20 nM EBR treatment alone [137].
5.1.3 Sugars
Sugar is not only an important source of carbon and energy but also an effective signal molecule modulating many developmental and metabolic processes in all phases of plant life cycle [138–143]. Our former studies have shown that glucosinolate accumulation in broccoli sprouts was enhanced by various kinds of sugars including sucrose, glucose, fructose, and mannitol [144, 145], with sucrose being the most effective one. Though mannitol treatment also led to an increase in glucosinolate content, the effect was not as strong as that resulted from sucrose treatment, which suggested that sucrose might function as a signal instead of osmotic stress in inducing glucosinolate accumulation [145]. Moreover, different concentrations of sugars have been demonstrated to have distinct influences on glucosinolate accumulation in broccoli sprouts. A relatively higher concentration of 176 mM sucrose and mannitol treatments dramatically increased glucosinolate content, whereas no significant difference was found after 88 mM sucrose or mannitol treatments [145]. As the glucosinolate level in broccoli is a reflection of two opposing physiological processes, glucosinolate biosynthesis and hydrolysis by myrosinase [146], two reasons may account for increased glucosinolates by sugar treatment. For one thing, the biosynthesis of glucosinolate might be induced by sugar treatment. It has been reported that Bo-ELONG, an important biosynthetic gene of aliphatic glucosinolate biosynthesis, was upregulated in broccoli sprouts by sucrose application [144]. For another, the activity of myrosinase was not changed after sucrose treatment, indicating that the hydrolysis of glucosinolate might not be affected by sugar treatment [144].
5.1.4 Salinity
Plants exhibit morphological or physiological alterations when subjected to salt stress. Besides, several reports indicated that salt stress was an important abiotic factor regulating glucosinolate accumulation in broccoli. The research by Carmen et al. [147] demonstrated that the total glucosinolate content in broccoli leaves was significantly increased after salt treatment and application of 80 mM NaCl displayed a more obvious effect when compared with 40 mM NaCl. This result was consistent with the data obtained from another research, which indicated a tendency of increase for the total glucosinolates in broccoli leaves upon NaCl treatment at concentrations of 60 mM and 90 mM [148]. However, studies in broccoli sprouts presented a contradictory result. We found that application of 40 mM and 80 mM NaCl did not notably enhance the accumulation of glucoraphanin in 4-day-old broccoli sprouts [149]. Similarly, our another study based on 7-day-old broccoli sprouts showed that total glucosinolate level was markedly decreased after treatment with NaCl at concentrations of 20, 40, and 60 mM [150]. These different responses might be due to rapid growth of sprouts in which Na+ and C1− ions become utilizable or different broccoli organs used for analysis [150].
5.1.5 Fertilization
The content of glucosinolate, a category of nitrogen- and sulfur- containing secondary metabolites, could be remarkably affected by nitrogen (N) and sulfur (S) fertilization. It has been demonstrated that levels of glucosinolates in broccoli were decreased by N fertilization but increased by N deficiency [151–153]. Schonhof et al. [152] reported that the elevation of glucosinolate content by N deficiency was mainly attributed to the presence of the alkyl glucosinolates, glucoraphanin and glucoiberin. However, in other Brassica crops, such as oilseed rape and Indian mustard, the levels of glucosinolates increased upon N application [154, 155]. This distinct response might be due to the difference of species used. In contrast to N fertilization, S fertilization usually led to an increase of glucosinolate content in broccoli in many cases [156]. However, conflicting result has also been demonstrated by another report which showed that broccoli sprouts did not benefit from S fertilization [157].
In addition to N and S fertilization, other fertilizer applications have also been indicated to influence glucosinolate accumulation. It has been reported that total glucosinolate contents in two broccoli cultivars (‘Calabrese’ and ‘Southern star’) were notably increased by organic and bioorganic fertilizers supply, respectively [158]. This is probably because organic and bioorganic manure can serve as alternative mineral fertilizers to improve soil structure [159] and microbial biomass [160].
Selenium (Se), an essential trace element for humans and mammals, often substitutes for S in physiological and metabolic processes in plants due to their chemical and physical resemblance [161]. Selenite and selenate salts, which are taken from the soil by plants through the sulfate absorption pathway, have been shown to improve the antioxidant status of plants. In addition, selenoglucosinolates displayed a higher anticarcinogenic activity than thioglucosinolates [162]. Se fertilization has been reported to exert no significant influence on glucosinolate levels in both broccoli sprouts [163–165] and florets [166]. Hence, Se fertilization is a good approach to accumulating selenium while maintaining glucosinolate content.
5.2 Post-Harvest Handlings
Broccoli is a highly perishable product, whose shelf life and visual quality strongly rely on storag e conditions, such as temperature, atmosphere composition, and relative humidity (RH) [146, 167, 168]. Generally, the declines of their visual quality are accompanied by the loss of nutrients including glucosinolate. Cooling, controlled atmosphere (CA), and modified atmosphere packaging (MAP) are widely used methods to extend the shelf life and reduce the nutrient loss. In addition, inhibiting the action of ethylene by 1-methylcyclopropene (1-MCP) treatment is also an effective way to improve the shelf life and quality of horticultural crops. The effects of these post-harvest handlings on glucosinolates of broccoli are discussed here.
5.2.1 Temperature
Glucosinolate contents generally decrease during post-harvest storage, which is coincided with the loss in visual quality of broccoli, and low temperatures clearly delay the quality decline [167]. Rangkadilok et al. [169] reported a more than 50% decrease in glucoraphanin concentration in ‘Marathon’ broccoli heads after 7-day storage at 20 °C. In contrast, no considerable decline was found after 7-day storage at 4 °C [169]. These results were consistent with the finding of Rodrigues and Rosa [170] that glucoraphanin levels declined to 82% when principal inflorescence of broccoli was left at 20 °C for 5 days but only 31% at 4 °C. The possible explanation is that plant cells are most likely to rapidly become damaged due to the loss of cellular integrity under high temperature and thus allowed the mixing of myrosinase and glucosinolates, resulting in the degradation and rapid decrease of glucosinolates. However, contradictory results were found in several other surveys. The study of Howard et al. [171] showed that sulforaphane decreased by approximately 50% after 21-day storage at 4 °C, and the severe decrease occurred within the first 7 days after harvest. Interestingly, the content of 4-methoxy glucobrassicin in broccoli florets stored at 10 °C increased from 0.4 μmol · g−1 DW to 1.8 μmol · g−1 DW after 9-day storage [172]. Likewise, the contents of 4-hydroxy glucobrassicin and 4-methoxy glucobrassicin in broccoli increased notably after chopping and 48 h storage at room temperature [173]. Moreover, the elevated level of 4-methoxy glucobrassicin in broccoli florets during the first day of storage at 20 °C was also observed by Yuan et al. [174]. It seems that the post-harvest enhancement of some indole glucosinolates counteracted the myrosinase-mediated degradation. Verkerk et al. [173] also proposed a stress-induced increase of glucosinolates by yet unknown mechanism, which plays a vital role in maintaining glucosinolate content of broccoli during post-harvest storage.
In addition to refrigeration, freezing is widely used in food industry. Usually, products are blanched before freezing, which can inactivate myrosinase as well as other enzymes causing deterioration. Rodrigues and Rosa [170] regarded freezing as the best method for preserving the glucosinolates in broccoli. However, blanching before freezing led to severe loss of glucosinolates by leaching them into water [175, 176]. Considering this, refrigeration might be the better procedure for storage of broccoli in comparison with freezing.
Although “cool as soon as possible” is the general recommendation for broccoli handling, delays of several hours before cooling may be encountered in post-harvest handling, especially when forced air or hydrocooling is employed instead of immediate liquid ice cooling in the field. Nevertheless, our former study showed that keeping broccoli florets at 20 °C for 6 h before cooling at 5 °C resulted in little influence on glucoraphanin content as well as shelf life, while keeping them at 20 °C for 24 h led to remarkable decline [168].
5.2.2 Controlled Atmosphere Storage
CA has been widely used to extend the storage period and maintain the quality of horticultural products. Broccoli is one of the commodities that benefit from CA storage [169, 177]. In regard to the changes of glucosinolate contents in broccoli upon CA treatment, discrepant results were obtained in various broccoli cultivars under different storage temperatures combined with diverse O2 and CO2 concentrations. Hansen et al. [172] investigated the changes of glucosinolates in ‘Marathon’ broccoli florets stored under low O2 and high CO2. Results showed that the total glucosinolate content increased 42% under air and 21% under 0.5% O2 + 20% CO2 during 7-day storage at 10 °C when compared to freshly harvested broccoli. However, the study of Fernández-León et al. [178] demonstrated that CA storage (10% O2, 5% CO2) was effective in lowering the decrease of glucosinolates in broccoli heads during cooling as well as room temperature (20 C) storage. Similarly, glucoraphanin concentration in broccoli heads was maintained to a higher level under CA (1.5% O2 + 6% CO2) storage at 4 °C than that under air condition [169]. These were consistent with the results of our former survey, which showed that CA conditions with relative higher CO2 concentrations (10%) and normal O2 concentrations (21%) facilitated the retention of glucoraphanin content at 5 °C [168]. These results suggested that the elevated CO2 concentration might favor the induction of glucoraphanin biosynthesis and/or reduction of its degradation by endogenous myrosinases. Dunford and Temelli [179] have proposed that elevated CO2 concentrations caused myrosinase inactivation, which might explain the decreased glucoraphanin degradation in CA conditions with elevated CO2 concentrations. Contrarily, marked decline in glucoraphanin content was found under 1% O2 or 1% O2 + 10% CO2 treatments compared to air treatment during 20-day storage at 5 °C [168], and 20% CO2 treatment led to a 15% decline of total glucosinolate content in ‘Marathon’ florets during 7-day storage at 10 °C [172]. It is known that CYP79F1, a cytochrome P450 monooxygenase , is responsible for aldoxime production, a key step in biosynthesis of glucoraphanin [16, 30, 180]. Therefore, the oxygen dependence of CYP79F1 action might explain above findings that low concentrations or the absence of O2 might result in reduced biosynthesis and content of glucoraphanin. Moreover, treatment with 20% CO2 in the absence of O2 brought about severe off-odors, injury, and water soaking of the tissue [172]. Thus, normal level of O2 and enhanced level of CO2 are better for CA treatment of broccoli.
5.2.3 Modified Atmosphere Packaging
Cooling and CA are effective in storage of broccoli [181]. However, cooling and CA facilities are not always available in developing countries, where high temperatures are often encountered throughout the post-harvest storage, distribution, and marketing phases of broccoli products [167]. In this case, MAP might be a better choice, which is simple, economical, and also effective in delaying the post-harvest deterioration and maintaining the visual quality of broccoli at either low or high temperature [146, 167, 169, 182]. The investigation of Rangkadilok et al. [169] showed that glucoraphanin content in broccoli heads descended significantly in air packaging at 20 °C with 48% loss at day 3 and 64% loss at day 10, whereas there were no significant changes in glucoraphanin levels in MAP with no holes at 4 °C and two microholes at 20 °C for up to 10 days. In this research, products were wrapped using low-density polyethylene (LDPE) bags (40 μm thick), and the atmospheres of approximately 0% O2 and 21% CO2 were reached in the MAP with microholes after 10 days at 20 °C, while the atmospheres inside the MAP (with no holes) were modified to 0.2% O2 and 15% CO2 after 10 days at 4 °C [169]. However, the glucoraphanin content dropped 48% in ‘Marathon’ heads after 7-day storage at 1 °C under MAP using 11 μm LDPE bags, in which the atmospheres reached 17% O2 and 2% CO2 [183].
A survey by Schreiner et al. [184] demonstrated that modified atmosphere, 8% O2 and 14% CO2, could maintain aliphatic and indole glucosinolates in mini broccoli heads for 7 days after an initial decrease in 4 days in mixed packaging of mini broccoli and mini cauliflower in polypropylene food trays. Similarly, the decreases of aliphatic, indole, and aromatic glucosinolates in ‘Parthenon’ broccoli florets were more severe when stored in air than that in modified atmospheres under microperforated polypropylene plastic after 12 days at 5 °C [182]. We also investigated the effects of MAP treatments on glucosinolate contents in ‘Youxiu’ broccoli heads by comparing with non-wrapped florets [146]. The broccoli florets were packaged by using polyethylene bags (40 μm thick) with no holes (M0), two microholes (M1), and four macroholes (M2) and then stored at 4 °C or 20 °C. The results showed that all three MAP treatments slowed the decline of glucosinolates in broccoli florets when compared to those in the control, with M0 being the most significant, followed by M1 and M2 during 23-day storage at 4 °C or 5-day storage at 20 °C.
In conclusion, MAP has potential in maintaining glucosinolates in broccoli at both low and high temperatures, while the differences in types of polyethylene film used, atmosphere reached in MAP, and genotypes of broccoli had distinct effects in preserving glucosinolates. In addition, it was worth noting that not only atmosphere but also RH was modulated by CA and MAP. Glucosinolate retention could benefit from these two factors as they could prevent cell membrane degradation and subsequent mixing of glucosinolates with myrosinase [167], though the precise mechanism involved remains to be further investigated.
5.2.4 1-Methylcyclopropene
1-MCP has been commercialized and widely utilized in post-harvest handling of fruits and vegetables. Ku et al. [185] indicated that application of 1-MCP could increase the storage life of broccoli. 1-MCP has also been found to maintain phytochemicals including glucosinolates in broccoli. We found that 1-MCP treatment at the concentration of 2.5 μl l−1 reduced the decreasing rate of total glucosinolates in broccoli florets stored at 20 °C [174]. Similar result was also observed by Fernández-León et al. [178] in broccoli heads under 0.6 μl l−1 1-MCP treatment. Moreover, the content of total glucosinolate was remarkably enhanced by application of 1-MCP (25 μl l−1) in broccoli florets stored at 15 °C for 5 days [186]. We found that 1-MCP treatment could inhibit the increase of malondialdehyde (MDA) when compared with the control [174]. It is known that MDA is the product of membrane peroxidation, which could damage the structure and integrity of membrane during the senescence of broccoli florets. Thus, lower MDA was beneficial for preventing the mixing of glucosinolates with myrosinase, namely, reducing glucosinolates hydrolysis. Moreover, the biosynthesis of glucosinolate is proved to participate in plant defensive response, and ethylene might play a role in this process [187]. Finally, it is also possible that the blockage of ethylene action by 1-MCP treatment favored glucosinolate biosynthesis or inhibited some ethylene-related degradative pathways. However, more studies are needed to elucidate the regulation mechanism of 1-MCP in glucosinolate metabolism.
In summary, application of 1-MCP is effective in delaying the decrease in glucosinolate contents during storage at low or relatively high temperature. Furthermore, 1-MCP has been proved nontoxic for humans and environment [188], and it is active at very low concentrations with a negligible residue. With no doubt, commercialization of 1-MCP will be a new tool to maintain glucosinolates in broccoli during post-harvest storage.
In addition, light and 6-benzylaminopurine (6-BA) treatments were also used in glucosinolate retention in post-harvest broccoli. Jin et al. [189] reported that the content of total glucosinolates in post-harvest broccoli florets was profoundly boosted by light-emitting diode (LED) green light . The levels of glucosinolate and sulforaphane in broccoli florets were markedly increased after treatment with 6-BA [190]. It is known that LED lighting sources are durable with small size and cool emitting surfaces, which is suitable for plant growth. 6-BA has been considered nontoxic for human and environment by the US Environment Protection Agency (EPA). Therefore, their applications in post-harvest handling of broccoli are potential.
5.3 Processing and Cooking
Broccoli is mostly consumed as a processed food, and various processings before consumption might cause the degradation or transformation of the health-promoting compounds including glucosinolates. Conventional processing and cooking methods generally affect the content of glucosinolate in broccoli by several aspects: (I) glucosinolate leakage into cooking water, (II) enzymatic hydrolysis by myrosinase, and (III) glucosinolate breakdown in thermal conditions [191]. Here, we try to summarize the effects of processing and cooking conditions on glucosinolate and its derivative in broccoli.
5.3.1 Blanch-Freezing
Broccoli is almost exclusively available to the consumer in two forms, fresh broccoli heads and frozen broccoli florets. Freezing is widely used in broccoli processing as it provides cheaper product with longer shelf life. In commercial industrial freezing , broccoli usually undergoes blanching prior to freezing, a processing method utilizing hot water or steam to inactivate enzymes that may cause degradative changes and thus limit shelf life severely [192]. Unfortunately, blanching also destroyed myrosinase, resulting in the disability to form sulforaphane in pre- and post-cooking in frozen broccoli, and substantially reduced sulforaphane bioavailability [193]. Because of the disnatured myrosinase, blanch-frozen broccoli can retain the same levels of glucosinolates after 90 days of frozen (−20 °C) storage [194]. Furthermore, Alanís-Garza et al. [195] reported that glucosinolate content increased in three tested broccoli cultivars while remained constant in only one cultivar after blanching and freezing. In order to maximize the production of cancer preventative sulforaphane in broccoli florets, blanching step is optimized to maintain myrosinase activity while destroying ESP activity, for a high percentage of glucoraphanin can be converted to a nitrile with ESP as mentioned above. Perez et al. [196] proposed that the optimal blanching conditions to maximize sulforaphane content in broccoli florets were immersion in water at 57 °C for 13 min, coinciding with the minimum glucosinolates and maximum myrosinase activity.
5.3.2 Cooking
Broccoli is always cooked before eating. Domestic cooking methods include boiling, steaming, microwaving, stir-frying, and stir-frying followed by boiling (stir-frying/boiling). All these methods could influence the levels of glucosinolates and their hydrolysis products in broccoli and thus affect its heath protective capacity. Slicing is the common step in food preparation prior to further cooking, which disrupted tissues and facilitated the release of myrosinase, leading to glucosinolate hydrolysis at high degree [146]. General heating methods in cooking such as boiling, microwaving, and steaming would cause decrease of glucosinolate content in broccoli, and the rate of decrease was higher along with increased cooking time [197]. Sones et al. [198] found that the total glucosinolate content of broccoli boiled for 10 min was approximately 40% less than that of fresh broccoli. Moreover, the total glucosinolate content dropped by 62.0% and 67.7% after 5 and 10 min of blanching, respectively, with the greatest decline in glucoraphanin (71.58%) being observed after 1 min of boiling [197]. Boiling with cold start and hot start also shows different glucosinolate retention. A glucosinolate decrease of 50% was observed in boiling-cold start while 41% loss in boiling-hot start, which provide a suggestion for consumers in preferring boiling way [199]. The microwave heating process led to distinct results due to the differences in conditions such as cooking time, power, and the volume of added water [200]. Vallejo et al. [201] reported a reduction of 74% in total glucosinolate concentration after microwaving broccoli at 1000 W for 5 min, which is the result of glucosinolate leaching into the evaporated water. Likewise, a significant loss of glucosinolates (62% reduction in glucoraphanin) was also observed in microwaved broccoli in our former study [202]. As for steaming, our survey showed that it led to a 36.8% loss of total indole glucosinolates and no significant change in the content of total aliphatic glucosinolates [202]. Similarly, steaming caused a decrease of 17% in total glucosinolate content after cooking for 22 min [199]. Interestingly, Gliszczynska-Swiglo et al. [203] found that steaming elevated glucosinolate content in broccoli when compared with the fresh broccoli. Bongoni et al. [199] also reported that steaming increased total glucosinolates’ level by 17% at the end of cooking, which may be attributed to an increase in the extractability of glucosinolates by processing rather than a real increase in their total content. In addition, the contents of phytochemicals including glucosinolate were evaluated when fresh broccoli florets were subjected to stir-frying treatments in various edible oils, and the results indicated that glucosinolate in broccoli stir-fried with extra-virgin olive, soybean, peanut, or sunflower oil was similar to that in the uncooked sample [204]. We compared the effect of all five domestic cooking methods on the retention of glucosinolates in broccoli and found that stir-frying and stir-frying/boiling presented the highest loss of glucosinolates while steaming resulted in the lowest loss [202]. Therefore, steaming method appeared to be the best way to retain glucosinolates in cooking broccoli.
The loss of glucosinolate in broccoli upon cooking is mainly caused by leaching, for numerous glucosinolates were found in cooking media [205, 206]. Furthermore, heating also leads to thermal degradation of glucosinolate. In general, aliphatic glucosinolates are more stable than indole glucosinolates during cooking [197, 201, 202]. The relative thermostability of individual glucosinolates was shown to vary with heating temperature, duo to their respective chemical structures [207, 208]. However, thermal degradation might not be the main cause of glucosinolate loss as the content of glucosinolates dropped considerably within a very short cooking time.
In addition, significant change in sulforaphane content was detected in cooked broccoli. Matusheski et al. [209] showed that sulforaphane production increased when broccoli was cooked at 60 °C for 5 or 10 min, as temperature s higher than 50 °C inactivated ESP. Considering that myrosinase could be inactivated when subjected to 100 °C for 5–15 min, thus cooking at temperature between 50 °C and 90 °C or at 100 °C within 5 min would favor the formation of sulforaphane [209]. This conclusion was also supported by another survey, which found that steaming for 1–3 min produced less nitrile and more sulforaphane yield from a broccoli meal [65]. It has also been reported that microwave heating (900 W) for 0.5 and 0.75 min increased sulforaphane in production while reduced sulforaphane nitrile content. Furthermore, Ghawi et al. [210] found that adding mustard seeds was proved to intensify sulforaphane formation in cooked broccoli, as these seeds contained a more resilient isoform of myrosinase.
5.3.3 Other Processing Methods
An early publication by Rosa et al. [211] reported the effect of dehydration processes on glucosinolates in broccoli. They found that 50–65 °C drying of intact broccoli maintained the original glucosinolate content as well as the myrosinase activity. However, the rehydration process caused the hydrolysis of glucosinolates. Moreover, as ESP is disnatured during drying (50–65 °C), sulforaphane synthesis should be favored in dehydration processes. A recent study suggested that pulsed electric field (PEF) treatment could increase glucosinolate content in both floret and stalk of broccoli, and the optimal condition was 4 kV cm−1 for 525 and 1000 μs [212]. Other processing conditions, such as pH, also had a significant effect on sulforaphane and sulforaphane nitrile production. A neutral or alkaline pH resulted in predominate sulforaphane production, whereas an acidic pH (3.5, typical of salad dressings) led to more sulforaphane nitrile [108].
6 Conclusions
In recent years, a great progress has been achieved in elucidating glucosinolate biosynthetic pathway, as well as its regulation and degradation in broccoli by a combination of molecular, genomic, and bioinformatic approaches, which provides a solid basis for breeding of broccoli for optimal glucosinolate composition and content. However, plant secondary metabolites usually have adverse effects besides beneficial properties, and several glucosinolate breakdown products have anti-nutritional effects. Therefore, more studies are needed to identify the biological activities of the glucosinolate degradation products in greater detail, so that the balance of benefit, risk, and consumer preference can be properly defined.
Currently, metabolic engineering of glucosinolate in Brassica crops is underway, providing a feasible method for improving glucosinolate composition and content in these crops [213–217]. A thorough understanding of glucosinolate biosynthetic pathway and its regulatory network is necessary for regulation of glucosinolate in broccoli by molecular breeding and metabolic engineering, which is promising for better nutrition value and resistance.
In addition, a large number of preharvest factors and post-harvest handlings have been clarified to modulate glucosinolate accumulation in broccoli, and attentions are paid to glucosinolate metabolism in broccoli across the whole food chain, from the production to consumption. Suitable environmental conditions such as light, as well as chemical regulations, help to enhance glucosinolate accumulation during production before harvest. Post-harvest handlings including cooling, CA, MAP, and 1-MCP treatments, as well as freezing processing, are effective in attenuating glucosinolate loss during post-harvest package, storage, and processing period, and steaming is the recommended method for best retention of glucosinolate during cooking before consumption in broccoli. However, the underlying mechanism behind regulation of glucosinolate metabolism by different preharvest and post-harvest handlings remains to be further elucidated.
Abbreviations
- 6-BA:
-
6-Benzylaminopurine
- CA:
-
Controlled atmosphere
- EBR:
-
Epibrassinolide
- ESP:
-
Epithiospecifier protein
- GS:
-
Glucosinolate
- I3C:
-
Indole-3-carbinol
- ITC:
-
Isothiocyanate
- JA:
-
Jasmonic acid
- LDPE:
-
Low-density polyethylene
- M0 :
-
No holes
- M1 :
-
Two microholes
- M2 :
-
Four macroholes
- MAP:
-
Modified atmosphere packaging
- MDA:
-
Malondialdehyde
- MeJA:
-
Methyl jasmonate
- NSP:
-
Nitrile-specifier protein
- PEF:
-
Pulsed electric field
- RH:
-
Relative humidity
References
Kmiecik W, Lisiewska Z, Korus A (2007) Retention of mineral constituents in frozen brassicas depending on the method of preliminary processing of the raw material and preparation of frozen products for consumption. Eur Food Res Technol 224:573–579
Bhandari S, Kwak J-H (2015) Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules 20:1228–1243
Dominguez-Perles R, Mena P, Garcia-Viguera C, Moreno DA (2014) Brassica foods as a dietary source of vitamin C: a review. Crit Rev Food Sci 54:1076–1091
Mahn A, Reyes A (2012) An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Sci Technol Int 18:503–514
Higdon J, Delage B, Williams D, Dashwood R (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55:224–236
Jeffery EH, Araya M (2009) Physiological effects of broccoli consumption. Phytochem Rev 8:283–298
Sønderby IE, Geu-Flores F, Halkier BA (2010) Biosynthesis of glucosinolates – gene discovery and beyond. Trends Plant Sci 15:283–290
Clarke DB (2010) Glucosinolates, structures and analysis in food. Anal Methods-UK 2:310–325
Wittstock U, Burow M (2010) Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. Arabidopsis Book 8, e0134
Slominski BA, Campbell LD, Stanger NE (1987) Influence of cecectomy and dietary antibiotics on the fate of ingested intact glucosinolates in poultry. Can J Anim Sci 67:1117–1124
Elfoul L, Rabot S, Khelifa N, Quinsac A, Duguay A, Rimbault A (2001) Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol Lett 197:99–103
Maskell I, Smithard R (1994) Degradation of glucosinolates during in vitro incubations of rapeseed meal with myrosinase (EC 3.2.3.1) and with pepsin (EC 3.4.23.1)-hydrochloric acid, and contents of porcine small intestine and caecum. Brit J Nutr 72:455–466
Mullaney JA, Kelly WJ, McGhie TK, Ansell J, Heyes JA (2013) Lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from enterobacteriaceae. J Agr Food Chem 61:3039–3046
Rabot S, Nugonbaudon L, Raibaud P, Szylit O (1993) Rapeseed meal toxicity in gnotobiotic rats: influence of a whole human faecal flora or single human strains of Escherichia coli and Bacteroides vulgatus. Brit J Nutr 70:323–331
Abdull Razis AF, Noor NM (2013) Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer P 14:1565–1570
Grubb CD, Abel S (2006) Glucosinolate metabolism and its control. Trends Plant Sci 11:89–100
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Wittstock U, Halkier BA (2002) Glucosinolate research in the Arabidopsis era. Trends Plant Sci 7:263–270
Mikkelsen MD, Petersen BL, Olsen CE, Halkier BA (2002) Biosynthesis and metabolic engineering of glucosinolates. Amino Acids 22:279–295
Schuster J, Knill T, Reichelt M, Gershenzon J, Binder S (2006) Branched-chain aminotransferase4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 18:2664–2679
Gigolashvili T, Yatusevich R, Rollwitz I, Humphry M, Gershenzon J, Flügge U-I (2009) The plastidic bile acid transporter 5 is required for the biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana. Plant Cell 21:1813–1829
Kroymann J, Textor S, Tokuhisa JG, Falk KL, Bartram S, Gershenzon J, Mitchell-Olds T (2001) A gene controlling variation in arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol 127:1077–1088
Textor S, de Kraker J-W, Hause B, Gershenzon J, Tokuhisa JG (2007) MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol 144:60–71
Knill T, Reichelt M, Paetz C, Gershenzon J, Binder S (2009) Arabidopsis thaliana encodes a bacterial-type heterodimeric isopropylmalate isomerase involved in both Leu biosynthesis and the Met chain elongation pathway of glucosinolate formation. Plant Mol Biol 71:227–239
Knill T, Schuster J, Reichelt M, Gershenzon J, Binder S (2008) Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis. Plant Physiol 146:1028–1039
He Y, Mawhinney TP, Preuss ML, Schroeder AC, Chen B, Abraham L, Jez JM, Chen S (2009) A redox‐active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J 60:679–690
Hull AK, Vij R, Celenza JL (2000) Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci U S A 97:2379–2384
Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275:33712–33717
Chen S, Glawischnig E, Jørgensen K et al (2003) CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J 33:923–937
Hansen CH, Wittstock U, Olsen CE, Hick AJ, Pickett JA, Halkier BA (2001) Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J Biol Chem 276:11078–11085
Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of l-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J Biol Chem 275:14659–14666
Naur P, Petersen BL, Mikkelsen MD, Bak S, Rasmussen H, Olsen CE, Halkier BA (2003) CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol 133:63–72
Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R (2001) CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:101–111
Douglas Grubb C, Zipp BJ, Ludwig‐Müller J, Masuno MN, Molinski TF, Abel S (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40:893–908
Piotrowski M, Schemenewitz A, Lopukhina A, Müller A, Janowitz T, Weiler EW, Oecking C (2004) Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J Biol Chem 279:50717–50725
Hansen BG, Kliebenstein DJ, Halkier BA (2007) Identification of a flavin‐monooxygenase as the S‐oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J 50:902–910
Kliebenstein DJ, Lambrix VM, Reichelt M, Gershenzon J, Mitchell-Olds T (2001) Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:681–693
Hansen BG, Kerwin RE, Ober JA, Lambrix VM, Mitchell-Olds T, Gershenzon J, Halkier BA, Kliebenstein DJ (2008) A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol 148:2096–2108
Bednarek P, Piślewska-Bednarek M, Svatoš A et al (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323:95–101
Pfalz M, Mikkelsen MD, Bednarek P, Olsen CE, Halkier BA, Kroymann J (2011) Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 23:716–729
Pfalz M, Vogel H, Kroymann J (2009) The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 21:985–999
Levy M, Wang Q, Kaspi R, Parrella MP, Abel S (2005) Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J 43:79–96
Skirycz A, Reichelt M, Burow M et al (2006) DOF transcription factor AtDof1. 1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47:10–24
Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18:3235–3251
Kim JH, Durrett TP, Last RL, Jander G (2004) Characterization of the Arabidopsis TU8 glucosinolate mutation, an allele of TERMINAL FLOWER2. Plant Mol Biol 54:671–682
Celenza JL, Quiel JA, Smolen GA, Merrikh H, Silvestro AR, Normanly J, Bender J (2005) The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137:253–262
Frerigmann H, Gigolashvili T (2014) MYB34, MYB51 and MYB122 Distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant 7:814–828. doi:10.1093/mp/ssu004
Gigolashvili T, Berger B, Fluegge U-I (2009) Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem Rev 8:3–13
Gigolashvili T, Berger B, Mock HP, Müller C, Weisshaar B, Flügge UI (2007) The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 50:886–901
Gigolashvili T, Engqvist M, Yatusevich R, Müller C, Flügge UI (2008) HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol 177:627–642
Gigolashvili T, Yatusevich R, Berger B, Müller C, Flügge UI (2007) The R2R3‐MYB transcription factor HAG1/MYB28 is a regulator of methionine‐derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 51:247–261
Schweizer F, Fernández-Calvo P, Zander M et al (2013) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25:3117–3132
Frerigmann H, Berger B, Gigolashvili T (2014) bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiol 166:349–369
Liu SY, Liu YM, Yang XH et al (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5
Li GY, Quiros CF (2002) Genetic analysis, expression and molecular characterization of BoGSL-ELONG, a major gene involved in the aliphatic glucosinolate pathway of Brassica species. Genetics 162:1937–1943
Li G, Quiros CF (2003) In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSL-ALK. Theor Appl Genet 106:1116–1121
Gao MQ, Li GY, McCombie WR, Quiros CF (2005) Comparative analysis of a transposon-rich Brassica oleracea BAC clone with its corresponding sequence in A-thaliana. Theor Appl Genet 111:949–955
Gao M, Li G, Potter D, McCombie WR, Quiros CF (2006) Comparative analysis of methylthioalkylmalate synthase (MAM) gene family and flanking DNA sequences in Brassica oleracea and Arabidopsis thaliana. Plant Cell Rep 25:592–598
Giamoustaris A, Mithen R (1996) Genetics of aliphatic glucosinolates.4. Side-chain modification in Brassica oleracea. Theor Appl Genet 93:1006–1010
Gao M, Li G, Yang B, Qiu D, Farnham M, Quiros C (2007) High-density Brassica oleracea linkage map: identification of useful new linkages. Theor Appl Genet 115:277–287
Carlson DG, Daxenbichler ME, Vanetten CH, Kwolek WF, Williams PH (1987) Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens, and kohlrabi. J Am Soc Hortic Sci 112:173–178
Brown AF, Yousef GG, Jeffrey EH, Klein BP, Wallig MA, Kushad MM, Juvik JA (2002) Glucosinolate profiles in broccoli: variation in levels and implications in breeding for cancer chemoprotection. J Am Soc Hortic Sci 127:807–813
Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH (1999) Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agr Food Chem 47:1541–1548
Wang J, Gu H, Yu H, Zhao Z, Sheng X, Zhang X (2012) Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem 133:735–741
Farnham MW, Stephenson KK, Fahey JW (2000) Capacity of broccoli to induce a mammalian chemoprotective enzyme varies among inbred lines. J Am Soc Hortic Sci 125:482–488
Baik HY, Juvik J, Jeffery EH, Wallig MA, Kushad M, Klein BP (2003) Relating glucosinolate content and flavor of broccoli cultivars. J Food Sci 68:1043–1050
Sarikamis G, Marquez J, MacCormack R, Bennett RN, Roberts J, Mithen R (2006) High glucosinolate broccoli: a delivery system for sulforaphane. Mol Breed 18:219–228
Schonhof I, Krumbein A, Bruckner B (2004) Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Nahrung 48:25–33
Rosa EAS, Rodrigues AS (2001) Total and individual glucosinolate content in 11 broccoli cultivars grown in early and late seasons. Hortscience 36:56–59
Vallejo F, Tomas-Barberan FA, Benavente-Garcia AG, Garcia-Viguera C (2003) Total and individual glucosinolate contents in inflorescences of eight broccoli cultivars grown under various climatic and fertilisation conditions. J Sci Food Agr 83:307–313
Ernst IMA, Palani K, Esatbeyoglu T, Schwarz K, Rimbach G (2013) Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol Res 70:155–162
Jakubikova J, Bao Y, Bodd J, Sedlak J (2006) Isothiocyanate iberin modulates phase II enzymes, posttranslational modification of histones and inhibits growth of Caco-2 cells by inducing apoptosis. Neoplasma 53:463–470
Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT (2004) Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis 25:1409–1415
Barillari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, Valgimigli L (2005) Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agr Food Chem 53:2475–2482
Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A (2002) Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo a pyrene-induced stomach tumors. Proc Natl Acad Sci U S A 99:7610–7615
Jang M, Hong E, Kim G-H (2010) Evaluation of antibacterial activity of 3-Butenyl, 4-Pentenyl, 2-Phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J Food Sci 75:M412–M416
van Doorn HE, van der Kruk GC, van Holst GJ, Raaijmakers-Ruijs N, Postma E, Groeneweg B, Jongen WHF (1998) The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of brussels sprouts. J Sci Food Agr 78:30–38
ElSayed G, Louveaux A, Mavratzotis M, Rollin P, Quinsac A (1996) Effects of glucobrassicin, epiprogoitrin and related breakdown products on locusts feeding: schouwia purpurea and desert locust relationships. Entomol Exp Appl 78:231–236
Yamada-Kato T, Nagai M, Ohnishi M, Yoshida K (2012) Inhibitory effects of wasabi isothiocyanates on chemical mediator release in RBL-2H3 rat basophilic leukemia cells. J Nutr Sci Vitaminol 58:303–307
Xiao D, Singh SV (2007) Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res 67:2239–2246
Chinni SR, Li YW, Upadhyay S, Koppolu PK, Sarkar FH (2001) Indole3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 20:2927–2936
Stephensen PU, Bonnesen C, Schaldach C, Andersen O, Bjeldanes LF, Vang O (2000) N-methoxyindole-3-carbinol is a more efficient inducer of cytochrome P-450 1A1 in cultured cells than indol-3-carbinol. Nutr Cancer 36:112–121
Kronbak R, Duus F, Vang O (2010) Effect of 4-Methoxyindole-3-carbinol on the proliferation of colon cancer cells in vitro, when treated alone or in combination with Indole-3-carbinol. J Agric Food Chem 58:8453–8459
Farnham MW, Wilson PE, Stephenson KK, Fahey JW (2004) Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed 123:60–65
Gu HH, Wang JS, Yu HF, Zhao ZQ, Sheng XG, Chen JS, Xu YJ (2014) Development and validation of high-glucoraphanin broccoli F-1 hybrids and parental lines. J Am Soc Hortic Sci 139:460–468
Nour-Eldin HH, Andersen TG, Burow M et al (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488:531–534
Kelly PJ, Bones A, Rossiter JT (1998) Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta 206:370–377
Andreasson E, Jorgensen LB, Hoglund AS, Rask L, Meijer J (2001) Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol 127:1750–1763
Lipka V, Dittgen J, Bednarek P et al (2005) Pre-and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Mithen R, Faulkner K, Magrath R, Rose P, Williamson G, Marquez J (2003) Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor Appl Genet 106:727–734
Matusheski NV, Swarup R, Juvik JA, Mithen R, Bennett M, Jeffery EH (2006) Epithiospecifier protein from broccoli (Brassica oleracea L. ssp italica) inhibits formation of the anticancer agent sulforaphane. J Agric Food Chem 54:2069–2076
Williams DJ, Critchley C, Pun S, Nottingham S, O'Hare TJ (2008) Epithiospecifier protein activity in broccoli: the link between terminal alkenyl glucosinolates and sulphoraphane nitrile. Phytochemistry 69:2765–2773
Mahéo K, Morel F, Langouët S, Kramer H, Ferrec EL, Ketterer B, Guillouzo A (1997) Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res 57:3649–3652
Conaway CC, Yang YM, Chung FL (2002) Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab 3:233–255
Hecht SS (2000) Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev 32:395–411
Nishikawa A, Lee IS, Uneyama C, Furukawa F, Kim HC, Kasahara K, Huh N, Takahashi M (1997) Mechanistic insights into chemopreventive effects of phenethyl isothiocyanate in N-nitrosobis(2-oxopropyl)amine-treated hamsters. Jpn J Cancer Res 88:1137–1142
Dinkova-Kostova AT, Kostov RV (2012) Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 18:337–347
Guo ZY, Smith TJ, Wang E, Sadrieh N, Ma Q, Thomas PE, Yang CS (1992) Effects of phenethyl isothiocyanate, a carcinogenesis inhibitor, on xenobiotic-metabolizing enzymes and nitrosamine metabolism in rats. Carcinogenesis 13:2205–2210
Riedl MA, Saxon A, Diaz-Sanchez D (2009) Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol 130:244–251
Ashok BT, Chen YG, Liu XY, Bradlow HL, Mittelman A, Tiwari RK (2001) Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer 41:180–187
Michnovicz J (1998) Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int J Obes 22:227–229
Meng QH, Goldberg ID, Rosen EM, Fan SJ (2000) Inhibitory effects of Indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat 63:147–152
Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C (2001) Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 276:32008–32015
Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J (2005) Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci 46:979–987
Wu LY, Ashraf MHN, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BHJ (2004) Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci U S A 101:7094–7099
Senanayake GVK, Banigesh A, Wu LY, Lee P, Juurlink BHJ (2012) The dietary phase 2 protein inducer sulforaphane can normalize the kidney epigenome and improve blood pressure in hypertensive rats. Am J Hypertens 25:229–235
Matusheski NV, Jeffery EH (2001) Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J Agric Food Chem 49:5743–5749
Latte KP, Appel KE, Lampen A (2011) Health benefits and possible risks of broccoli – an overview. Food Chem Toxicol 49:3287–3309
Bonnesen C, Eggleston IM, Hayes JD (2001) Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res 61:6120–6130
Drewnowski A, Gomez-Carneros C (2000) Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72:1424–1435
Mithen RF, Dekker M, Verkerk R, Rabot S, Johnson IT (2000) The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J Sci Food Agric 80:967–984
Tripathi MK, Mishra AS (2007) Glucosinolates in animal nutrition: a review. Anim Feed Sci Technol 132:1–27
Stadler E, Renwick JAA, Radke CD, Sachdevgupta K (1995) Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris-rapae. Physiol Entomol 20:175–187
Kim JH, Lee BW, Schroeder FC, Jander G (2008) Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J 54:1015–1026
Kos M, Houshyani B, Wietsma R, Kabouw P, Vet LEM, van Loon JJA, Dicke M (2012) Effects of glucosinolates on a generalist and specialist leaf-chewing herbivore and an associated parasitoid. Phytochemistry 77:162–170
Beekwilder J, van Leeuwen W, van Dam NM et al (2008) The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. Plos One 3, e2068
Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C (2011) Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331:1185
Zhao Y, Wang J, Liu Y et al (2015) Classic myrosinase-dependent degradation of indole glucosinolate attenuates fumonisin B1-induced programmed cell death in Arabidopsis. Plant J 81:920–933
Traka MH, Saha S, Huseby S et al (2013) Genetic regulation of glucoraphanin accumulation in beneforté® broccoli. New Phytol 198:1085–1095
Sotelo T, Soengas P, Velasco P, Rodriguez VM, Cartea ME (2014) Identification of metabolic QTLs and candidate genes for glucosinolate synthesis in Brassica oleracea leaves, seeds and flower buds. Plos One 9, e91428
Abercrombie JM, Farnham MW, Rushing JW (2005) Genetic combining ability of glucoraphanin level and other horticultural traits of broccoli. Euphytica 143:145–151
Bae G, Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Am J Clin Nutr 59:281–311
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61:11–24
Steindal ALH, Mølmann J, Bengtsson GB, Johansen TJ (2013) Influence of day length and temperature on the content of health-related compounds in broccoli (Brassica oleracea L. var. italica). J Agric Food Chem 61:10779–10786
Simioni C, Schmidt EC, Felix MR et al (2014) Effects of ultraviolet radiation (UVA + UVB) on young gametophytes of Gelidium floridanum: growth rate, photosynthetic pigments, carotenoids, photosynthetic performance, and ultrastructure. Photochem Photobiol 90:1050–1060
Brazaitytė A, Sakalauskienė S, Samuolienė G et al (2015) The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem 173:600–606
Seo JM, Arasu MV, Kim YB, Sang UP, Kim SJ (2015) Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem 177:204–213
Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R (2012) UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol 53:1546–1560
Topcu Y, Dogan A, Kasimoglu Z, Sahin-Nadeem H, Polat E, Erkan M (2015) The effects of UV radiation during the vegetative period on antioxidant compounds and postharvest quality of broccoli (Brassica oleracea L.). Plant Physiol Biochem 93:56–65
Steindal ALH, Johansen TJ, Bengtsson GB, Hagen SF, Mølmann JAB (2015) Impact of pre-harvest light spectral properties on health- and sensory-related compounds in broccoli florets. J Sci Food Agric. doi:10.1002/jsfa.7307
Liuann G, Juvikjohn A, Jefferyelizabeth H, Bermanbootylisa D, Clintonsteven K, Jr EJW (2014) Enhancement of broccoli indole glucosinolates by methyl jasmonate treatment and effects on prostate carcinogenesis. J Med Food 17:1177–1182
Van DNM, Leontien W, Aleš S (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161:801–810
Yan X, Chen S (2007) Regulation of plant glucosinolate metabolism. Planta 226:1343–1352
Kim HH, Kwon DY, Uddin MR, Bae H, Kim SJ, Kim YB, Sang UP (2013) Influence of auxins on glucosinolate biosynthesis in hairy root cultures of broccoli (Brassica oleracea var. italica). Asian J Chem 25:6099–6101
Guo R, Qian H, Shen W, Liu L, Zhang M, Cai C, Zhao Y, Qiao J, Wang Q (2013) BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in Arabidopsis. J Exp Bot 64:2401–2412
Guo R, Hou Q, Yuan G, Zhao Y, Wang Q (2014) Effect of 2, 4-epibrassinolide on main health-promoting compounds in broccoli sprouts. LWT Food Sci Technol 58:287–292
Dekkers BJW, Schuurmans JAMJ, Smeekens SCM (2004) Glucose delays seed germination in Arabidopsis thaliana. Planta 218:579–588
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol Am J Clin Nutr 57:675–709
Ramon M, Rolland F, Sheen J (2008) Sugar sensing and signaling. Arabidopsis Book 6, e0117
Wingler A, Masclauxdaubresse C, Fischer AM (2009) Sugars, senescence, and ageing in plants and heterotrophic organisms. J Exp Bot 60:1063–1066
Bolouri-Moghaddam MR, Roy KL, Li X, Rolland F, Ende WVD (2010) Sugar signalling and antioxidant network connections in plant cells. Febs J 277:2022–2037
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Guo R, Yuan G, Wang Q (2011) Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem 129:1080–1087
Guo R, Yuan G, Wang Q (2011) Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci Hortic-Amsterdam 128:159–165
Jia CG, Xu CJ, Wei J, Yuan J, Yuan GF, Wang BL, Wang QM (2009) Effect of modified atmosphere packaging on visual quality and glucosinolates of broccoli florets. Food Chem 114:28–37
Carmen LB, Moreno DA, Micaela C, Cristina GV (2009) Growing hardier crops for better health: salinity tolerance and the nutritional value of broccoli. J Agric Food Chem 57:572–578
Zaghdoud C, Alcaraz-López C, Mota-Cadenas C, Martínez-Ballesta MC, Moreno DA, Ferchichi A, Carvajal M (2012) Differential responses of two broccoli (Brassica oleracea L. var Italica) cultivars to salinity and nutritional quality improvement. Sci World J 2012:291435
Guo L, Yang R, Wang Z, Guo Q, Gu Z (2013) Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int J Food Sci Nutr 65:476–481
Guo RF, Yuan GF, Wang QM (2013) Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J Zhejiang Univ Sci B 14:124–131
Krumbein A, Schonhof I, Rühlmann J, Widell S (2001) Influence of sulphur and nitrogen supply on flavour and health-affecting compounds in Brassicaceae. Dev Plant Soil Sci 92:294–295
Schonhof I, Blankenburg D, Müller S, Krumbein A (2007) Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J Plant Nutr Soil Sci 170:65–72
Xu CJ, Guo RF, Yan HZ, Yuan J, Sun B, Yuan GF, Wang QM (2010) Effect of nitrogen fertilization on ascorbic acid, glucoraphanin content and quinone reductase activity in broccoli floret and stem. J Food Agric Environ 88:179–184
Zhao F, Evans EJ, Bilsborrow PE, Syers JK (1993) Influence of sulphur and nitrogen on seed yield and quality of low glucosinolate oilseed rape (Brassica napus L). J Sci Food Agric 63:29–37
Gerendas J, Podestat J, Stahl T (2009) Interactive effects of sulfur and nitrogen supply on the concentration of sinigrin and allyl isothiocyanate in Indian mustard (Brassica juncea L.). J Agric Food Chem 57:3837–3844
Falk KL, Tokuhisa JG, Gershenzon J (2007) The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biol 9:573–581
Aires A, Rosa E, Carvalho R (2006) Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts Brassica oleracea var. italica. J Sci Food Agr 86:1512–1516
Naguib EMM, El-Baz FK, Salama ZA, Hanaa HAEB, Ali HF, Gaafar AA (2012) Enhancement of phenolics, flavonoids and glucosinolates of broccoli (Brassica oleracea, var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J Saudi Soc Agric Sci 11:135–142
Dauda SN, Ajayi FA, Ndor E (2008) Growth and yield of water melon (citrullus lanatus) as affected by poultry manure application. J Agric Soc Sci 4:121–124
Dhull S, Goyal S, Kapoor K, Mundra M (2004) Microbial biomass carbon and microbial activities of soils receiving chemical fertilizers and organic amendments. Arch Agron Soil Sci 50:641–647
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86:373–389
Matich AJ, Mckenzie MJ, Lill RE, Brummell DA, Mcghie TK, Chen RKY, Rowan DD (2012) Selenoglucosinolates and their metabolites produced in Brassica spp. fertilised with sodium selenate. Phytochemistry 75:140–152
Ávila FW, Yong Y, Faquin V, Ramos SJ, Guilherme LRG, Thannhauser TW, Li L (2014) Impact of selenium supply on Se -methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem 165:578–586
Tian M, Xu X, Liu Y, Xie L, Pan S (2016) Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem 190:374–380
Anna P, Dominik K, Tadeusz P et al (2014) The influence of selenium addition during germination of Brassica seeds on health-promoting potential of sprouts. Int J Food Sci Nutr 65:692–702
Sepúlveda I, Barrientos H, Mahn A, Moenne A (2013) Changes in SeMSC, glucosinolates and sulforaphane levels, and in proteome profile in broccoli (Brassica oleracea var. Italica) fertilized with sodium selenate. Molecules 18:5221–5234
Jones RB, Faragher JD, Winkler S (2006) A review of the influence of postharvest treatments on quality and glucosinolate content in broccoli (Brassica oleracea var. italica) heads. Postharvest Biol Technol 41:1–8
Xu C-J, Guo D-P, Yuan J, Yuan G-F, Wang Q-M (2006) Changes in glucoraphanin content and quinone reductase activity in broccoli (Brassica oleracea var. italica) florets during cooling and controlled atmosphere storage. Postharvest Biol Technol 42:176–184
Rangkadilok N, Tomkins B, Nicolas ME, Premier RR, Bennett RN, Eagling DR, Taylor PWJ (2002) The effect of post-harvest and packaging treatments on glucoraphanin concentration in broccoli (Brassica oleracea var. italica). J Agric Food Chem 50:7386–7391
Rodrigues AS, Rosa EAS (1999) Effect of post-harvest treatments on the level of glucosinolates in broccoli. J Sci Food Agric 79:1028–1032
Howard LA, Jeffery EH, Wallig MA, Klein BP (1997) Retention of phytochemicals in fresh and processed broccoli. J Food Sci 62:1098–1104
Hansen M, Moller P, Sorensen H, Detrejo MC (1995) Glucosinolates in broccoli stored under controlled atmosphere. J Am Soc Hortic Sci 120:1069–1074
Verkerk R, Dekker M, Jongen WMF (2001) Post-harvest increase of indolyl glucosinolates in response to chopping and storage of Brassica vegetables. J Sci Food Agric 81:953–958
Yuan G, Sun B, Yuan J, Wang Q (2010) Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem 118:774–781
Volden J, Bengtsson GB, Wicklund T (2009) Glucosinolates, l-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem 112:967–976
Song LJ, Thornalley PJ (2007) Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem Toxicol 45:216–224
Guo Y, Gao Z, Li L, Wang Y, Zhao H, Hu M, Li M, Zhang Z (2013) Effect of controlled atmospheres with varying O2/CO2 levels on the postharvest senescence and quality of broccoli (Brassica oleracea L. var. italica) florets. Eur Food Res Technol 237:943–950
Fernández-León MF, Fernández-León AM, Lozano M, Ayuso MC, González-Gómez D (2013) Different postharvest strategies to preserve broccoli quality during storage and shelf life: controlled atmosphere and 1-MCP. Food Chem 138:564–573
Dunford NT, Temelli F (1996) Effect of supercritical CO2 on myrosinase activity and glucosinolate degradation in canola. J Agric Food Chem 44:2372–2376
Hansen ME, Sorensen H, Cantwell M (2001) Changes in acetaldehyde, ethanol and amino acid concentrations in broccoli florets during air and controlled atmosphere storage. Postharvest Biol Technol 22:227–237
Izumi H, Watada AE, Douglas W (1996) Optimum O2 or CO2 atmosphere for storing broccoli florets at various temperatures. J Am Soc Hortic Sci 121:127–131
Fernández-León MF, Fernández-León AM, Lozano M, Ayuso MC, Amodio ML, Colelli G, González-Gómez D (2013) Retention of quality and functional values of broccoli ‘Parthenon’ stored in modified atmosphere packaging. Food Control 31:302–313
Vallejo F, Tomas-Barberan F, Garcia-Viguera C (2003) Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J Agric Food Chem 51:3029–3034
Schreiner MC, Peters PJ, Krumbein AB (2006) Glucosinolates in mixed-packaged mini broccoli and mini cauliflower under modified atmosphere. J Agric Food Chem 54:2218–2222
Ku VVV, Wills RBH (1999) Effect of 1-methylcyclopropene on the storage life of broccoli. Postharvest Biol Technol 17:127–132
Xu F, Chen X, Yang Z, Jin P, Wang K, Shang H, Wang X, Zheng Y (2013) Maintaining quality and bioactive compounds of broccoli by combined treatment with 1-methylcyclopropene and 6-benzylaminopurine. J Sci Food Agric 93:1156–1161
Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA (2003) Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol 131:298–308
Luo Z, Xu X, Cai Z, Yan M (2007) Effects of ethylene and 1-methylcyclopropene (1-MCP) on lignification of postharvest bamboo shoot. Food Chem 105:521–527
Jin P, Yao D, Xu F, Wang H, Zheng Y (2015) Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chem 172:705–709
Xu F, Yang Z, Chen X, Jin P, Wang X, Zheng Y (2012) 6-Benzylaminopurine delays senescence and enhances health-promoting compounds of harvested broccoli. J Agric Food Chem 60:234–240
Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B (2007) Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. P Nutr Soc 66:69–81
Andress EL, Harrison JA (2006) So easy to preserve. University of Georgia Cooperative Extension Service, Athens
Dosz EB, Jeffery EH (2013) Commercially produced frozen broccoli lacks the ability to form sulforaphane. J Funct Foods 5:987–990
Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B (2008) Influence of blanching and freezing broccoli (Brassica oleracea var. italica) prior to storage and cooking on glucosinolate concentrations and myrosinase activity. Eur Food Res Technol 227:37–44
Alanis-Garza PA, Becerra-Moreno A, Mora-Nieves JL, Mora-Mora JP, Jacobo-Velazquez DA (2015) Effect of industrial freezing on the stability of chemopreventive compounds in broccoli. Int J Food Sci Nutr 66:282–288
Perez C, Barrientos H, Roman J, Mahn A (2014) Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem 145:264–271
Hwang E-S, Kim G-H (2013) Effects of various heating methods on glucosinolate, carotenoid and tocopherol concentrations in broccoli. Int J Food Sci Nutr 64:103–111
Sones K, Heaney RK, Fenwick GR (1984) An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J Sci Food Agric 35:712–720
Bongoni R, Verkerk R, Steenbekkers B, Dekker M, Stieger M (2014) Evaluation of different cooking conditions on broccoli (Brassica oleracea var. italica) to improve the nutritional value and consumer acceptance. Plant Foods Hum Nutr 69:228–234
Lopez-Berenguer C, Carvajal M, Moreno DA, Garcia-Viguera C (2007) Effects of microwave cooking conditions on bioactive compounds present in broccoli inflorescences. J Agric Food Chem 55:10001–10007
Vallejo F, Tomas-Barberan FA, Garcia-Viguera C (2002) Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol 215:310–316
Yuan GF, Sun B, Yuan J, Wang QM (2009) Effects of different cooking methods on health-promoting compounds of broccoli. J Zhejiang Univ Sci B 10:580–588
Gliszczynska-Swiglo A, Ciska E, Pawlak-Lemanska K, Chmielewski J, Borkowski T, Tyrakowska B (2006) Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit Contam 23:1088–1098
Moreno DA, Lopez-Berenguer C, Garcia-Viguera C (2007) Effects of stir-fry cooking with different edible oils on the phytochemical composition of broccoli. J Food Sci 72:S64–S68
Francisco M, Velasco P, Moreno DA, Garcia-Viguera C, Elena Cartea M (2010) Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res Int 43:1455–1463
Jones RB, Frisina CL, Winkler S, Imsic M, Tomkins RB (2010) Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem 123:237–242
Wathelet JP, Mabon N, Foucart M, Marlier M (1996) Influence of blanching on the quality of Brussels sprouts (Brassica oleracea L cv gemmifera). Sci Aliment 16:393–402
Oerlemans K, Barrett DM, Suades CB, Verkerk R, Dekker M (2006) Thermal degradation of glucosinolates in red cabbage. Food Chem 95:19–29
Matusheski NV, Juvik JA, Jeffery EH (2004) Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 65:1273–1281
Ghawi SK, Methven L, Niranjan K (2013) The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem 138:1734–1741
Rosa EAS, Heaney RK, Fenwick GR, Portas CAM (1997) 7-Methylsulffinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 21:1983–1988
Aguilo-Aguayo I, Suarez M, Plaza L, Hossain MB, Brunton N, Lyng JG, Rai DK (2015) Optimization of pulsed electric field pre-treatments to enhance health-promoting glucosinolates in broccoli flowers and stalk. J Sci Food Agric 95:1868–1875
Zhang Y, Huai D, Yang Q, Cheng Y, Ma M, Kliebenstein DJ, Zhou Y (2015) Overexpression of three glucosinolate biosynthesis genes in Brassica napus identifies enhanced resistance to sclerotinia sclerotiorum and Botrytis cinerea. Plos One 10, e0140491. doi:10.1371/journal.pone.0140491
Augustine R, Mukhopadhyay A, Bisht NC (2013) Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnol J 11:855–866
Liu Z, Hirani AH, McVetty PBE, Daayf F, Quiros CF, Li G (2012) Reducing progoitrin and enriching glucoraphanin in Brassica napus seeds through silencing of the GSL-ALK gene family. Plant Mol Biol 79:179–189
Qian H, Sun B, Miao H, Cai C, Xu C, Wang Q (2015) Variation of glucosinolates and quinone reductase activity among different varieties of Chinese kale and improvement of glucoraphanin by metabolic engineering. Food Chem 168:321–326
Liu Z, Hammerlindl J, Keller W, McVetty PBE, Daayf F, Quiros CF, Li G (2011) MAM gene silencing leads to the induction of C3 and reduction of C4 and C5 side-chain aliphatic glucosinolates in Brassica napus. Mol Breed 27:467–478
Acknowledgments
This work was supported by grants from National Science Foundation of China (NO. 30370974, 31270343, 31470385).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Miao, H., Wang, J., Cai, C., Chang, J., Zhao, Y., Wang, Q. (2017). Accumulation of Glucosinolates in Broccoli. In: Mérillon, JM., Ramawat, K. (eds) Glucosinolates. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-25462-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-25462-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25461-6
Online ISBN: 978-3-319-25462-3
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics