Abstract
Immunological protection of the individual from infection requires the coordinated involvement of numerous tissues, cell types, and secreted factors collectively referred to as the immune system. This is equally true for the immune response to environmental antigens that is the underlying cause of diseases such as allergic asthma and food allergies. This sequence of events is set in motion by a T cell response defined by the cytokines that it produces and their impact on neighboring B cells to produce antibody that uniquely sensitizes the host to the offending allergen. Granule-laden cells of the innate immune system are the ultimate sources of the powerful inflammatory mediators of the allergic response. To fully understand the factors which contribute to the development of allergies and that are discussed in this book, this chapter provides the reader with an introduction to the cellular and chemical mediators of the immune response, beginning with those of the innate immune system. The chapter then works its way to the mediators of the adaptive immune system, along the way covering important topics such as major histocompatibility complex (MHC), antigen processing and presentation, and lymphocyte development. The chapter concludes with an introduction to hypersensitivity mechanisms, focusing on the allergic response.
The author wishes to acknowledge Suzanne Jones for reviewing and editing this submission.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
A key component of the maintenance of health and physiological homeostasis is the inherent ability of the host to protect itself from the many potential pathogens with which we share our environment. These measures of protection are diverse and range from keratinized-stratified skin offering a physical barrier to infection to cytotoxic T lymphocytes (CTL) capable of discerning the presence of a foreign cytosolic virus and triggering apoptosis of the infected cell. Collectively, the tissues, cells (known as leukocytes), and secreted factors which provide protection against infection are referred to as the immune system. The mobilization of this system of secreted factors and bone marrow-derived cells, some of which are resident to tissues and others of which circulate through the blood and lymph, takes place in two waves of recruitment and activation. The first wave consists of fairly nonspecific cellular and chemical mediators whose job it is to rapidly eliminate microbes that have entered host tissues. Because this protection is provided by components of the immune system that are always present at maximum capacity to rapidly respond to an infectious threat, this is termed innate or natural immunity. While the benefit of the innate immune response is its rapid mobilization, its limitation is that its mechanisms of protection are fairly nonspecific in that it deals with many different microbes using a fairly restricted set of mechanisms. Additionally, protection by the innate immune system is not enhanced by repeated exposure to a pathogen (i.e., it has no immunological memory). The second wave of the immune response is more finely adapted to deal with the specific pathogen encountered, and as such takes more time to develop. Because this form of immunity adapts to specific infectious agents and is shaped by the host’s history of pathogen encounter, it is referred to as adaptive or acquired immunity. It is the adaptive immune response that provides enhanced protection from a pathogen that the host has already encountered, and as such is the functional basis of the practice of vaccination. It is also the adaptive immune system that is responsible for targeting the harmless antigens of the environment known to trigger the allergic response. This chapter begins with an overview of the mediators of the innate and adaptive immune system, followed by an in-depth discussion of their recruitment, activation, and effector function during the antimicrobial response. The chapter also includes a section on the development of the antigen receptors of the adaptive system so that the reader better appreciates the capacity of the immune system to recognize and respond to a virtually unlimited number of antigens. The chapter concludes with an overview of the ways in which the immune response can cause tissue injury with an emphasis on the allergic response.
2 Features of the Innate Immune System
The “ever-present” protection provided by the innate immune system utilizes different anatomical and chemical barriers of the host, including the skin and the epithelial lining of the mucosal surfaces of the airway, gastrointestinal, and reproductive tracts. This barrier is enhanced in the airways by the mucociliary escalator, whereby would-be pathogens are trapped in secreted mucus and forced up by the continuous beating of the cilia which extend from the respiratory epithelium. Low physiological pH, secretion of antimicrobial peptides (defensins and cathelicidins), and the production of opsonizing surfactants are additional chemical measures in the digestive tract and mucous membranes that provide continuous protection from infection. In the event of an epithelial injury providing the opportunity for infection, or the penetration of this first line of defense by a pathogen, additional components of the innate immune system are rapidly mobilized to actively respond to the infectious threat. This includes neutrophils, which are the first cells of the immune system recruited to the site, followed by monocytes-macrophages. Both cell types phagocytose (engulf) and then use proteolytic enzymes and reactive oxygen species to break down pathogens and cellular debris. Natural killer (NK) cells are also rapidly recruited to the site of an infection and are important in response to invading viruses (Brown et al. 2001). Dendritic cells (DCs) in their immature state are resident to the epithelial tissues of the host. These cells also take up pathogens and debris but serve a different ultimate purpose, which is to transport bits of the infectious invader to the draining lymph nodes for recognition by lymphocytes of the adaptive immune system. For this reason, DCs are seen as the bridge between innate and adaptive immunity (Mellman and Steinman 2001). Other cells of the innate immune system, such as mast cells, eosinophils, and basophils, play an important role in the immune response to parasites and helminths but in the absence of such types of pathogens are best known for their roles in acute and chronic allergic responses (Eckman et al. 2010; Galli et al. 2005; Blanchard and Rothenberg 2009). Finally, the innate immune response also includes secreted factors found in blood and tissue fluids. Principal among these are proteins of the complement system, which are produced by the liver and circulate in an inactive form. Activation of the complement system can be triggered in different ways, but ultimately it contributes to the recruitment of neutrophils and destruction of bacterial targets. Additional secreted factors produced early during the immune response include the inflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin-1 (IL-1), which together have numerous effects on the developing immune response, such as promoting the recruitment and activation of neutrophils and macrophages and the induction of fever.
3 Features of the Adaptive Immune System
If the innate immune response does not remove the infectious threat, an adaptive immune response is triggered. The cellular mediators of the adaptive immune response are called lymphocytes. Lymphocytes are found in the bloodstream, lymph, peripheral lymphoid organs (such as the spleen and lymph nodes), mucosal epithelial surfaces, and sites of infection. There are two principal types of lymphocytes: B cells and T cells. Both B and T cells express a diverse repertoire of receptors of the immunoglobulin superfamily that differentiate between normal tissue constituents (i.e., self) and foreign material derived from microbes, referred to as antigen. It is estimated that the cells of the B and T cell compartments have the potential to express as many as 1011–1016 different antigen receptors, thereby providing the host with the capacity to recognize and respond to a virtually limitless array of foreign antigen and guaranteeing that no infectious threat will go unchallenged (Abbas et al. 2012). If there is a downside to this design, it is that maintaining such a diversity of lymphocytes limits the number of cells specific for any one particular microbe. To compensate for this apparent limitation, antigen recognition triggers multiple rounds of clonal expansion of that cell, thereby creating many daughter cells bearing an identical antigen receptor with specificity for the offending microbe. Once the microbe has been cleared and the need for so many cells has passed, a process called “activation-induced cell death” (AICD) will result in the contraction of lymphocyte numbers and the re-establishment of homeostasis. With such an enormous diversity of antigen receptors and aggressive amplification of lymphocyte numbers upon stimulation comes the additional concern of unintended injury to the host. The likelihood of this is dramatically reduced, however, by an elaborate process of “negative selection” that purges the repertoire of developing lymphocytes that express antigen receptors which bind to self-antigen with high affinity, thereby helping to establish tolerance to the components of normal tissue.
Morphologically, B and T cells are indistinguishable but are phenotypically quite unique. To begin with, B and T cells can be distinguished by the expression of certain molecules on their cell surface: B cells uniquely express CD19, CD20, and CD21, whereas T cells express CD2 and CD3. Furthermore, all CD3 T cells can be further subdivided into those which express CD4 and CD8. Before encounter with their foreign antigen, lymphocytes circulate between the blood, peripheral lymphoid organs, and lymph in an inactivated or “naïve” state. Such cells do not exhibit an effector function and instead continuously follow this pattern of recirculation in search of their cognate foreign antigen. Antigen recognition in the peripheral lymphoid organs will interrupt this pattern, and instead of passing from these sites to the lymph and later back to the blood, the lymphocytes will be sequestered as they become activated, undergo clonal expansion, and differentiate into the powerful effector cells of the adaptive immune system (Springer 1994; Zhu et al. 2010). This process takes time, and in an individual who has not been previously exposed to a particular pathogen, a “primary immune response” can take 7–10 days to develop. Thus, it becomes clear why the rapid response of the innate immune system is so important to limiting the early growth of a pathogen while the adaptive immune response develops. The T and B cell response to subsequent exposure to the same pathogen will occur with hastened kinetics (3–5 days), such that the infection may be cleared before the individual feels ill. This enhanced “secondary immune response” is the result of the creation of memory T and B cells during the primary response that respond rapidly and in greater numbers to repeated exposure to the same pathogen (Murphy and Weaver 2017).
Humoral immunity is the term used to describe B cell-mediated immunological protection, which is most effective against extracellular microbes and their toxins. B cells have the capacity to recognize a diverse array of foreign antigenic compounds that may be constituent parts of an invading microbe, including polysaccharides, proteins, lipids, and nucleic acids. Once fully activated, B cells differentiate into plasma cells which produce large amounts of antibody (also called immunoglobulin) that bind with high affinity to the original cognate antigen, thereby neutralizing the pathogen or its toxin or marking it for destruction by the complement system or a phagocyte. Alternatively, T cells are most capable of dealing with intracellular pathogens through mechanisms collectively referred to as cellular immunity. T cell antigen receptors recognize microbial proteins that have been broken down into small peptides and are displayed on the surface of infected cells or professional antigen-presenting cells (APCs). Central to this process of antigen presentation are the major histocompatibility complex (MHC) proteins that serve as the scaffolding used to display foreign peptides to T cells. Once activated, CD4 T cells are most helpful in dealing with intracellular bacteria and fungal pathogens that have the capacity to survive within macrophages after being phagocytosed, whereas CD8 T cells are adept at killing host cells harboring intracellular microbes in their cytoplasm. In addition, CD4 T cells help to orchestrate a fully functional humoral immune response by providing signals to B cells that induce a process of antibody affinity maturation and functional class switching. A third and functionally distinct subset of T cells is best known for its capacity to suppress the immune response rather than to promote it. These regulatory T cells express the CD4 and CD25 cell surface proteins and have been the focus of intense research over the past 20 years that has clarified some of the ways in which the immune system balances pro- and anti-inflammatory signals (Sakaguchi et al. 2008; Roncarolo et al. 2001).
This chapter will introduce the reader to the principle cellular and chemical mediators of the innate and adaptive waves of the immune response. Because it is the mediators of the adaptive immune system that determine one’s likelihood of developing allergies, the focus of this book, more emphasis will be placed on the development, recruitment, and function of these mediators in health and disease.
4 The Innate Immune Response
4.1 Recruitment and Function of the Cellular Mediators of the Innate Immune Response
The function of the cellular and chemical mediators of the innate immune system is to recognize the presence of tissue injury or microbes that have defeated the epithelial barriers of the body and to quickly control, or even eradicate, the infectious threat. The cellular mediators derive from common myeloid precursor cells in the bone marrow under the influence of growth factors, such as granulocyte colony-stimulating factor (G-CSF) and monocyte colony-stimulating factor (M-CSF), that drives the production of neutrophils and monocytes/macrophages, respectively (Abbas et al. 2012; Wynn et al. 2013).
Activating an innate immune response begins with the tissue macrophages, DCs, and mast cells that are optimally located in the epithelium and other tissues of the body to serve as sentinel cells responsible for sensing the presence of invading microbes and cellular damage (Wynn et al. 2013; Shortman and Liu 2002). Activation of these cells is triggered by the recognition of common structural motifs shared among broad groups of pathogens, referred to as pathogen-associated molecular patterns, or PAMPs. PAMPs are not expressed by host tissues and are often structures that are essential for the survival and infectivity of the microbes (Beutler and Rietschel 2003). Examples are lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, and flagellin, a central component of bacterial flagella. Recognition of these structures is imparted by a collection of germline DNA-encoded receptors referred to as pathogen recognition receptors (PRRs) (Takeuchi and Akira 2010). PRRs are less diverse than lymphocyte antigen receptors and are found widely expressed among different cells of the innate immune system. A major family of PRRs are called Toll-like receptors (TLR), so named because of homology with the Toll gene, first identified by its role during drosophila embryogenesis and later found to be critical for adult fly antimicrobial defense (Beutler and Rietschel 2003; Lemaitre et al. 1996, 1997). TLRs are numbered one through nine, with each recognizing distinct bacterial or viral PAMPs such as LPS, bacterial peptidoglycan, or viral single-stranded or double-stranded RNA (Takeuchi and Akira 2010). TLRs specific for microbial components encountered as part of the cell wall of extracellular bacteria are expressed at the cell surface, while those specific for motifs associated with microbial nucleic acids are found within endosomes, where ingested microbes are digested and their nucleic acids released.
PRR binding to its cognate microbial ligand triggers a cascade of intracellular signaling events resulting in the activation of transcription factors, most notably NF-κB and interferon regulatory factor (IRF). Following activation and translocation into the nucleus, NF-κB will promote the expression of various cytokines and adhesion molecules important for the early recruitment of neutrophils and monocytes to the site of infection, while IRFs drive the production of the antiviral type I interferons (Hiscott et al. 2006; Honda and Taniguchi 2006). Two of the most important cytokines produced as a result of PRR engagement and NF-κB activation are IL-1 and TNFα (Svanborg et al. 1999). These cytokines play an early role in the recruitment of neutrophils to the site of infection by activating the endothelial cells of local venules. In response, endothelial cells express the adhesion molecules E-selectin and ICAM-1 and VCAM-1 (Alon and Feigelson 2002; Bunting et al. 2002). Carbohydrates expressed by circulating neutrophils bind with relatively low affinity to E-selectin. Stable adhesion to the endothelium requires the local secretion of the chemokine CXCL-8 (IL-8) which triggers a conformational change in the leukocyte integrins LFA-1, MAC-1, and VLA-4, allowing for high-affinity binding to the endothelial ligands, ICAM-1 and VCAM-1 (Yoshie 2000; Luster 2002). Once firmly attached to the endothelium, neutrophils, and later monocytes, will extravasate into the tissue and follow the chemokine gradient to the site of infection. Once in the infected tissue, neutrophils and macrophages engage the pathogen via their PRRs which triggers phagocytosis (uptake) of the microbe into phagosomes. These loaded phagosomes will fuse with lysosomes that contain the reactive oxygen species, nitric oxide, and proteolytic enzymes that destroy the bacteria. In addition to phagocytosis and killing of the pathogen, activated macrophages are significant sources of IL-1 and TNFα, which further enhance the inflammatory reaction and recruitment of inflammatory mediators. IL-6 and IL-12 are also produced by macrophages and are important for triggering the production of acute phase proteins such as C-reactive protein (CRP) by the liver and promoting a cell-mediated adaptive immune response, respectively. IL-12 produced by macrophages is also significant for its activation of NK cells, which in turn enhance macrophage function through their production of the critical cytokine IFNγ (Godshall et al. 2003).
In addition to enhancing the macrophage response, NK cells are important for the innate killing of infected or neoplastic host cells. NK cells are unique in that they derive from the same lymphoid progenitor as T and B cells; however, they do not express a similar repertoire of diverse antigen receptors. Rather, NK cells utilize a complement of killer inhibitory and activating receptors to differentiate between healthy and injured or infected cells, and it is the balance of signals delivered through these receptors that determine the activation of NK cell-mediated killing. One well-described activating receptor, NKG2D, recognizes the MHC class I-like proteins MIC-A and MIC-B that are found on virally infected and tumor cells but not on healthy cells (Bauer et al. 1999; Gonzalez et al. 2006). NKG2D recognition of these molecules delivers signals that activate NK cytolytic killing of infected cell. In contrast, inhibitory receptors recognize the cell surface molecule MHC class I, which is expressed by all healthy nucleated cells and is critical for presentation of cytosolic antigen to CD8 T cells (Borrego et al. 2002; Long 2008). Engagement of inhibitory receptors suppresses NK cell-mediated killing of the MHC class I-bearing cell; however, viruses that replicate in the cytosol and whose antigen is displayed to CD8 T cells via the MHC class I molecule have mechanisms to suppress MHC class I expression. In this situation, inhibition of NK cell activation is lost and signals delivered through the activating receptors will dominate, resulting in NK cell killing of the infected target cell (Vivier et al. 2008).
4.2 Complement System
Vasodilation and increased vascular permeability at the site of infection or injury not only will result in the recruitment of cellular mediators such as neutrophils and monocytes but also will draw in components of the complement system which are constitutively found in the blood. The complement system is composed of multiple plasma proteins, many of which are proteolytic enzymes that circulate in an inactive form. Activation of the complement system results in the sequential activation of these enzymes that serve to cleave other complement proteins into “a” and “b” fragments that, as a result, acquire specific functions in the immune response. The culmination of these actions is the creation of a transmembrane channel, called the membrane attack complex (MAC), in bacterial cell walls that disrupts osmotic balance and cause lysis of the microbes.
The cascade of complement protein activation can be initiated by three different pathways. The alternative pathway of activation is initiated as part of the innate immune response and depends upon the low-level spontaneous hydrolysis of the C3 complement protein and formation of C3b. C3b is readily hydrolyzed in the blood and tissue fluid and is only stabilized upon attachment to the surface of a microbe. The microbe-bound C3b then becomes the nucleus for the formation of convertase enzymes that cleave large amounts of C3 and later C5 into biologically active “a” and “b” cleavage products (Gros et al. 2008). The lectin pathway of complement activation is also considered part of the innate immune response; however, it takes longer to become activated because it requires the production of mannose-binding lectin (MBL), an acute phase protein, by the liver. When MBL binds to terminal mannose residues on the surface of microbes, it becomes enzymatically active and cleaves circulating C2 and C4 complement proteins, the products of which contribute to the pathway’s C3 and C5 convertase enzymes (Fujita 2002). The classical complement pathway is unique in that it requires the presence of antibody bound to the surface of a microbe to become activated and is therefore considered to be an effector function of the adaptive immune response (McGrath et al. 2006). The bound antibody becomes a target for the C1 complement protein, which is very similar in function to MBL in that once bound to antibody, it becomes enzymatically active to cleave C2 and C4 complement proteins which lead to the pathway’s C3 and C5 convertase enzymes. The three complement pathways converge following the formation of the C5 convertases and the creation of large amounts of C5b, which initiates the formation of the (MAC) . In addition to microbial lysis by the formation of the MAC, activated complement proteins such as C3a and C5a promote recruitment of neutrophils, while C3b and C4b are highly effective opsinins that enhance microbe phagocytosis (Schraufstatter et al. 2002; Helmy et al. 2006). Complement activation not only plays an important role in immunity to pathogens but also is an important player in tissue damage caused by dysregulated immune responses, such as those seen during autoimmune disease (Hadders et al. 2007).
5 The Adaptive Immune Response
5.1 Organs and Tissues of the Immune System
While innate immunity is optimal for quickly recognizing the presence of an infectious agent and controlling the infection, it is often insufficient by itself to completely eradicate an infection. In this regard, the importance of the adaptive immune system is illustrated in those individuals with a congenital immunodeficiency that prevents normal T and B cell development and who often succumb to infectious disease within the first year of life unless corrective action is taken, such as a bone marrow transplant.
T and B cells are derived from common lymphoid precursors found in the bone marrow, committing to either lineage under the influence of signals delivered through several cell surface receptors. Signals downstream of these receptors induce lineage-specific transcription factors that promote the creation of either T or B cell antigen receptors. Although both cell types derive from precursors in the bone marrow, they undergo the majority of their differentiation in two different locations: T cells in the thymus and B cells in the bone marrow. The thymus and bone marrow are collectively known as the generative, or primary, lymphoid organs. Lymphocyte development in both sites follows a similar sequence of progenitor proliferation under the influence of cytokines such as IL-7, gene segment rearrangement for the purpose of creating a diverse repertoire of antigen receptor-bearing cells, and selection of cells with useful antigen receptors (described in more detail below).
Following export to the blood from the primary lymphoid organs, mature naïve B and T cells on the lookout for their cognate microbial antigens home to peripheral or secondary lymphoid organs of the body, including the lymph nodes and spleen. Lymph, derived from plasma that has leaked from blood vessels and contains debris from dying cells and antigens of any infectious organisms, drains from tissues via a network of lymphatic vessels. These vessels direct the lymph into regional lymph nodes where antigen is captured in T and B cell zones. In addition to antigen carried passively from infected tissues by lymph, it is also actively picked up and transported to the draining lymph nodes by mature DCs. It is therefore in the regional lymph nodes, as well as in the spleen, in which blood-borne antigen is filtered, where antigen is concentrated from larger regions of the body and first displayed to the lymphocytes that continuously home there. Mucosal epithelial surfaces, such as the small intestine, which are the portals of entry for the vast majority of infectious agents that invade the human body, have developed equally elaborate processes to surveil for the presence of pathogenic microbes. Specialized intestinal epithelial cells, called M cells, actively take up and transport antigens from the intestinal lumen to the underlying Peyer’s patches, which consist of aggregates of macrophages, DCs, and circulating lymphocytes.
5.2 Lymphocyte Trafficking
Similar to the trafficking of neutrophils to sites of infection and inflammation, lymphocytes migrate to specific sites based upon their particular expression pattern of select homing and chemokine receptors. L-selectin and CCR7 are homing receptors found to be highly expressed by naïve T cells. Interaction between L-selectin and its ligand (PNAd) expressed by lymph node high endothelial venules (HEVs) initiates a low-affinity, transient interaction along the local endothelium (Rosen 2004). CCL19 and CCL21 chemokines, which are produced in the paracortical T cell areas of the lymph node, bind to the CCR7 receptor, which triggers a conformational change in the LFA-1 integrin allowing it to firmly bind to its endothelial ligand, ICAM-1, thereby arresting the lymphocyte. Collectively, these events allow the naïve T lymphocyte to enter the lymph node which will now follow the CCL19 and CCL21 gradient to the structure’s T cell area (Cyster 1999, 2000). In a beautifully choreographed interaction, DCs carrying antigen captured from regional tissue sites and also bearing the CCR7 chemokine receptor will be equally drawn in by the CCL19 and CCL21 gradients to the T cell zone, thereby allowing for the DC presentation of captured antigen to the circulating T cells. Alternatively, circulating naïve B cells, which localize to the follicles of the lymph node cortex, express the chemokine receptor CXCR5, which specifically recognize and follow the CXCL13 chemokine produced only in the follicles (Cyster et al. 2000). In this way, naïve T and B cells localize to specific areas of the lymph node to test their antigen receptors against antigen originating from surrounding tissues. As we will see, a fully functional B cell response to an antigen requires signals provided by CD4 T cells. To facilitate this interaction, a cohort of the activated B and CD4 T cells will “flip” their chemokine receptor expression causing the CD4 T cells to move toward the follicles and B cells to move toward the paracortical T cell areas.
It is estimated that each of the body’s 1 × 1012 lymphocytes passes through each lymph node about once a day in search of their cognate antigen (Abbas et al. 2012). In the absence of an antigen-recognition event, naïve lymphocytes will exit the lymph node via the efferent lymphatic and enter the next lymph node in the chain, surveilling for foreign antigen before eventually returning to the blood by way of the thoracic duct. Recognition of foreign antigen triggers the activation of transcription factors and genes that drive the clonal expansion and differentiation of the particular lymphocyte into effector cells. As part of this program of expansion and differentiation, the activated cells will decrease expression of the cell surface molecules responsible for recirculation through the peripheral lymphoid organs (CCR7 and L-selectin) and instead increase those ligands (E- and P-selectin ligand) and chemokine receptors (e.g., CXCR3) that will allow them to traffic to the site of infection. At the same time, TNFα and IL-1 produced at the site of infection will activate the local endothelium to express the complementary selectins and cell adhesion molecules which, along with local chemokine production, will efficiently draw in the powerful effector cells required to fight the infection.
A more recent advancement in our understanding of the mechanisms that control lymphocyte circulation is the elucidation of the role that the molecule termed phospholipid sphingosine 1-phosphate (S1P) and its receptor plays in the egress of T cells from the lymph node (Cyster and Schwab 2012). S1P’s influence on T cell’s trafficking behavior is due to its high level of expression in the blood and lymph and low level inside the lymph node. In response to the high level of S1P in the blood, circulating naïve T cells express low levels of the S1P receptor. Upon entering the lymph node, where the S1P level is lower, naïve T cells begin to upregulate the receptor, thereby becoming more sensitive to its gradient, causing the T cell to leave the node through the efferent lymphatic. T cell recognition of antigen in the lymph node will delay the increased expression of the S1P receptor, thereby causing the cell and its progeny to be sequestered in the lymph node during the clonal expansion and differentiation phase of the T cell response. Upon completion of this process, the level of S1P receptor on the effector cells is allowed to increase, thereby drawing the cells back into the lymph and blood after which they will selectively traffic to the site of infection (Cyster and Schwab 2012).
5.3 Antigen and Antigen Receptors
Pathogen recognition by cells of the innate immune system is accomplished through PRRs that detect common motifs shared among broad groups of pathogens such as lipopolysaccharide and double-stranded viral RNA. It is therefore outside the scope of the function of the innate immune system to differentiate between closely related organisms or to mount an immune response tailored to the characteristics of a unique pathogen. The adaptive immune system, however, is designed for such tasks. As discussed, naïve T and B cells first encounter their cognate antigen as they circulate through the peripheral lymphoid organs of the body. Recognition of antigen occurs through antigen receptors which triggers the activation of the lymphocyte on which the receptor is expressed. As we will see, this activation leads to the transcription and translation of genes that encode cytokines and other effector molecules that allow the lymphocyte to divide, differentiate, and mediate its effector function. The activation signal is delivered through invariant accessory molecules associated with the antigen receptors, including the CD3 complex and CD4 and CD8 molecules for T cells, and the immunoglobulin alpha and beta (Igα and Igβ) proteins for B cells.
B and T cells recognize different types of antigen. B cell antigen receptors can recognize the three-dimensional structure of a variety of microbe-derived macromolecules, including polysaccharides, nucleic acids, lipids, and proteins. These may be present on the microbe’s cell surface or be in soluble form (e.g., a secreted microbial toxin). The antigen receptors of most T cells, however, recognize peptide fragments from microbial protein antigens that have been broken down and displayed to the cells in the context of MHC proteins. Because a fully functional B cell response requires activation signals that can only be provided by antigen-stimulated CD4 T cells, foreign protein antigens are most effective at eliciting all functions of cellular and humoral immunity and are referred to as T-dependent antigens. Large macromolecular antigens may contain multiple different epitopes, which are the precise parts of the macromolecule recognized by different antigen receptors. A macromolecule with multiple identical epitopes is referred to as poly- or multivalent, an example of which are the long-chain polysaccharides of some bacteria capsules. The repeating sugar subunits of these polysaccharide chains engage multiple B cell antigen receptors of a cell at once, thereby generating a strong activation signal. Such antigens can activate a limited B cell response independent of T cell help and are therefore called T-independent antigens. Clinically, this is very significant because capsular polysaccharide vaccines induce protective immunity against encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae by eliciting opsonizing antibodies that promote phagocytosis and killing of the microbe. Newborns mount poor T-independent responses, and, therefore, pure capsular polysaccharide vaccines fail to induce protective immunity in this population. The development of conjugated vaccines, whereby bacterial polysaccharides are conjugated to foreign proteins thereby creating a T cell-dependent antigen, has dramatically decreased morbidity and mortality in newborns and infants caused by infection from encapsulated bacteria (Klein Klouwenberg and Bont 2008).
An additional significant difference between T and B cell antigen receptors is that T cell antigen receptors are only found in a membrane-bound form and do not serve any additional role beyond recognition of peptide antigen displayed by MHC. In contrast, the B cell antigen receptors are a membrane-bound form of antibody which is secreted prodigiously by fully differentiated plasma cells as the principle effector molecule of the humoral immune response. This antibody may be produced as one of five different “isotypes.” While all secreted antibody must bind to its target antigen to contribute to the immune response, the mechanism by which it contributes will vary depending upon the isotype. Antibody effector functions include activation of the classical complement pathway, opsonization of pathogens for enhanced phagocytosis, mast cell sensitization, mucosal immunity, and pathogen/toxin neutralization.
5.4 Properties of MHC
The MHC gene was first identified for its role in the immunological rejection of tissue transplanted between genetically disparate individuals, whereby, in experimental mouse models, skin graft transplants were only accepted if the recipient and donor strains exhibited the same MHC genes (Rosenberg and Singer 1992). Today, we understand that MHC molecules are membrane proteins whose physiological function is to display peptides derived from protein antigens to T lymphocytes and that their significance in the setting of tissue transplantation is because of the polymorphic nature of the MHC genes across the population. The presented peptides may be derived from either microbial or self-proteins; however, normally only microbial peptides will be recognized by T cell antigen receptors. In humans, the MHC proteins are called human leukocyte antigens (HLA) and are encoded on chromosome 6, where two sets of genes are found, called the class I and class II MHC genes.
The MHC class I locus includes three HLA genes: HLA-A, HLA-B, and HLA-C. Each of the genes displays extensive polymorphism across the population, as evidenced by over 2600 known HLA-B alleles (Murphy and Weaver 2017). Each MHC class I molecule consists of an alpha chain covalently bound to a β2-microglobulin molecule. The class II locus includes the HLA-DP, HLA-DQ, and HLA-DR genes, each of which encodes an alpha chain and a beta chain that together form an MHC class II molecule. HLA-DR is the most polymorphic of the class II genes, represented by over 1200 different alleles (Murphy and Weaver 2017). MHC class I molecules are expressed by all nucleated cells. This is in contrast to MHC class II molecules, which are expressed mainly by antigen-presenting cells, which are dendritic cells, B cells, and macrophages.
Both MHC class I and class II gene products demonstrate a tertiary protein structure that includes a peptide-binding cleft accommodating peptides of 8–10 and 10–30 amino acids in length, respectively. Many of the polymorphic differences between unique MHC class I and class II alleles translate into structural differences in the molecules’ peptide-binding clefts determining the complementary array of antigenic peptides that are presented by each MHC. The existence of many different MHC alleles is therefore beneficial at the level of the population as it provides the capability to display a vast array of peptide antigens within the group, thereby ensuring that members will be able to display and mount effective responses to the diversity of microbes in the environment. This is further ensured at the level of each individual by the fact that MHC genes are codominantly expressed, meaning that alleles inherited from both parents are transcribed and translated. This maximizes the number of different MHC molecules in an individual, thereby expanding the breadth of antigenic peptides presented.
5.5 Antigen Processing and Presentation
Different microbes may establish infections in different locations inside the host. For example, bacteria such as Haemophilus influenza and Staphylococcus pneumoniae replicate outside of host cells, while viruses, including rabies, hepatitis B, and HIV, establish infections inside of host cells where they utilize different components of the cell’s own machinery to create new viral particles. Microbial antigen may, therefore, originate in either extracellular or intracellular locations within the host. This becomes significant as the extra- or intracellular habitat of the microbe not only has implications for how antigen is processed by host cells and presented to T cells but also for what mechanisms the immune system must use to clear the infection. Accordingly, antigen derived from organisms such as bacteria, fungi, and parasites that originate outside of host cells will be taken up by antigen-presenting cells (macrophages and dendritic cells) and enzymatically digested in the cell’s phagolysosomes. The resulting microbial peptide fragments will be loaded onto MHC class II molecules as they are transported to the cell surface from the endoplasmic reticulum, where they are synthesized (Guermonprez et al. 2002). Once at the cell surface, the MHC class II-peptide complexes are surveyed for recognition by circulating CD4 T cells. On the other hand, antigen produced in the cytoplasm of virally infected cells is digested by the proteasome. The resulting peptide fragments are then shuttled into the endoplasmic reticulum by way of the transporter associated with antigen presentation (TAP), where they are loaded onto MHC class I molecules as they are being synthesized (Williams et al. 2002). The stable MHC class I-peptide complex then makes it way to the cell surface via the exocytic pathway. Once at the surface, the peptide-MHC complexes are interrogated for recognition by CD8 T cells.
The physical interaction of the T cell antigen receptor with the MHC-peptide complex spans both the peptide and the MHC molecule. As a result, T cell antigen receptors can only recognize peptide antigens when presented in the context of one’s MHC class I or II molecules, a concept called MHC restriction. Antigen recognition above a threshold affinity triggers a cascade of intracellular signaling events that leads to the activation of the lymphocyte on which the receptor is expressed and the development of effector functions that are specific to the type of pathogen encountered. Therefore, CD4 T cells activated by microbial antigen taken up in phagosomes and presented by MHC class II may respond by producing cytokines (e.g., IL-17 and IFNγ) that enhance the recruitment and killing ability of phagocytes, whereas CD8 T cells activated by viral antigen produced in the cytosol and presented by MHC class I will respond by producing perforin and granzyme and the expression of Fas ligand (FasL) which trigger the induction of apoptosis of the virally infected cell (Murphy and Reiner 2002; Russel and Ley 2002).
5.6 Molecular Structure of Antigen Receptors
The molecular structure of B and T cell antigen receptors is similar in that they both include variable and constant regions. The variable region is so named because of the extent to which antigen receptors from different B and T cell clones demonstrate amino acid sequence variability in this area. Within each variable region, variability of the amino acid sequences is concentrated in what are termed hypervariable regions, also known as complementarity determining regions (CDRs), as these are the regions of the receptor that determine antigen specificity based upon their complementary interaction with antigen. On the other hand, the constant region of the B and T cell antigen receptors demonstrates minimal variability between different clones. This region is required for structural integrity and, in the case of secreted antibody, their specific protective function.
The B cell receptor is built from four polypeptide chains, two identical larger (heavy) chains and two identical smaller (light) chains. Each chain has a variable region and a constant region. The assembled antibody molecule has a “Y” shape with two antigen-binding sites at the top, each of which is formed by the association of one heavy chain and one light chain variable region. The lower portion of the “Y” is built from the constant region of the heavy chains, which consists of three to four constant domains. As a membrane-bound antigen receptor, it is this C-terminal end of the heavy chain that is anchored in the plasma membrane; however, activated B cells will produce antibodies that lack the membrane anchor and are therefore produced as a secreted protein. Early investigation into antibody structure and function identified the portion of the antibody responsible for antigen binding as the Fab (fragment, antigen binding) fragment and that portion responsible for its biologic activity as the Fc (fragment, crystalline) fragment (Porter 1991). Therefore, each antibody has two identical Fab fragments and a single Fc fragment. As we will see, the phagocytosis of antibody-coated microbes is facilitated through neutrophil and macrophage recognition of antibody via a number of different Fc receptors.
Antibody heavy chains contain one of five different constant regions, termed μ, δ, γ, ε, and α. Antibodies produced with the different heavy chain constant regions are grouped together into classes or isotypes named according to their heavy chain: IgM, IgD, IgG, IgA, and IgE. Each of the different isotypes is characterized by its specialized role(s) in providing immunological protection to the host. IgM and IgD are remarkable because these are the isotypes specifically used by naïve B cells as membrane-bound antigen receptors (Abney et al. 1978). Once activated, B cells will produce secreted IgM which is characterized by its pentameric form and low affinity for antigen. Cooperation between CD4 T cells and B cells responding to the same microbe will result in CD4 T cell-derived activation signals that lead to full B cell activation and switching to the production of either IgG, IgA, or IgE.
The most common form of the T cell receptor is built from two polypeptide chains, termed the α and β chains. Each chain contains a single variable and constant region, with the antigen-binding site formed by the association of the alpha and beta chain variable regions. As mentioned already, the T cell antigen receptor recognizes antigenic peptide displayed by one’s MHC molecules, a feature known as “MHC restriction” of the TCR. Thus, portions of the TCR interact with the MHC, while others interact with the antigenic peptide. Remarkably, a T cell response can occur as a result of the TCR recognizing as few as 1–3 amino acid residues of the bound antigenic peptide (Sant’Angelo et al. 1996). Additionally, not all of the potential epitopes of a complex antigen will be recognized to stimulate a T cell response. Those that do trigger an immune response are referred to as “immunodominant epitopes” (Kjer-Nielsen et al. 2003).
5.7 Development of T Cells and B Cells
As mentioned earlier, development of B cells in the bone marrow and T cells in the thymus follows a sequence of progenitor proliferation, recombination of antigen-receptor gene segments, and selection of cells with useful antigen receptors. Developing lymphocytes are characterized according to their stepwise progression through this process. The early proliferation of lymphocyte progenitors driven by cytokines such as IL-7 gives rise to a large number of progenitors called pro-B and pro-T cells. Generation of such a large number of cells is critical because only a fraction will fully mature to competent lymphocytes. It is during the pro-B and pro-T cell stage when gene segment recombination begins in order to create the genetic code for each cell’s unique antigen receptor, beginning with the immunoglobulin (Ig) heavy chain and TCR β chain, respectively. Prior to this recombination, the “germline” configuration of these loci includes multiple adjacent gene segments belonging to the variable (V), diversity (D), and joining (J) families. For example, on the Ig heavy chain locus, there are about 45 different V gene segments, 23 D segments, and 6 J segments, among which is the potential for a million of different V-D-J combinations, each of which yields a unique Ig heavy chain variable domain (Abbas et al. 2016). In addition, the heavy chain locus also includes a number of constant (C) region genes which encode for the heavy chain constant domains that specify the different antibody isotypes. The germline TCR β chain locus is similarly constructed, however, with a different number of V, D, and J segments and fewer constant region genes.
Genetic recombination during the pro-B and pro-T cell stage includes the random selection of one each of the V, D, and J DNA segments which are spliced together to create an in-frame V-D-J coding exon of the Ig heavy chain or TCR β chain DNA loci, respectively, which then undergo gene transcription (Early et al. 1980; Shinkai et al. 1992). It is at the level of the RNA transcript that the recombined antigen-receptor variable region is connected to the heavy chain or β chain constant (C) region gene, thereby creating a complete set of RNA instructions for the first half of the B cell and T cell antigen receptor. For pro-B cells, this RNA splicing always occurs between the recombined heavy chain variable region and the mu (μ) constant region RNA. As a result, Ig recombination during the pro-B cell stage results in the production of a heavy chain of the IgM isotype (i.e., the μ heavy chain), a central feature of B cell development (Goding et al. 1977). Translation of the recombined μ heavy chain and TCR β chain proteins marks the progression of the developing lymphocytes from the pro- to the pre-B and pre-T cell stage, at which point an almost identical process of random genetic recombination and subsequent expression occurs at the Ig light chain and TCR α chain loci. Successful expression of the fully recombined IgM B cell receptor and TCR marks advancement to the immature lymphocyte stage.
For B cells, the final step to full maturity may take place in the bone marrow or the spleen and involves the co-expression of both IgM and IgD isotype antigen receptors (Abney et al. 1978). Immature T cells express both the CD4 and CD8 co-receptor (referred to as “double-positive” thymocytes) and have the potential to terminally differentiate into either subset, a fate determined by which self-MHC molecule the randomly generated TCR recognizes (von Boehmer et al. 1989). As a result of this process, called positive selection, double-positive thymocytes that recognize peptide antigen presented by one’s own MHC class II molecules become CD4 T cells, while those that recognize peptide antigen presented by MHC class I molecules become CD8 T cells. Importantly, only cells that recognize peptide/MHC complexes with low to moderate affinity will become positively selected to either the CD4 or CD8 subset. Cells that do not recognize either MHC would not be helpful during an immune response in that individual and therefore die by apoptosis. On the other hand, double-positive lymphocytes that bind with high affinity to self-peptides in the thymus presented by either MHC class I or class II pose a significant threat to the individual because of the likelihood of becoming activated by self-antigens and initiating an autoimmune response. Therefore, these cells are also removed from the maturation process, either by apoptosis or redirection of their development into regulatory T cells, a population of CD4 T cells identified by their constitutive expression of CD25 (Shortman et al. 1990; Jordan et al. 2001). Regulatory T cells enter the peripheral tissues and function to control T cell reactivity to self-antigens through the production of inhibitory cytokines IL-10 and TGF-β, and expression of CTLA-4 (Sakaguchi et al. 2008; Saraiva and O’Garra 2010). This process of purging potentially autoreactive immature lymphocytes from the repertoire is called negative selection. Immature B cells are also screened against self-antigen found in the bone marrow; however, developing B cells that bind to self-antigen at this site have the opportunity to create a different light chain (an event called receptor editing) and thus change the specificity of the antigen receptor.
This elaborate process of building antigen receptors from randomly selected gene segments spliced together from the germline DNA creates a tremendously diverse repertoire of millions of different antigen receptors, a concept termed “combinatorial diversity.” The diversity of antigen receptors is further enhanced by a process whereby nucleotides are randomly deleted and added from the V-D-J sites of recombination, thereby expanding many times the number of unique antigen receptors generated during the process of lymphocyte development. This so-called “junctional diversity” increases the number of unique antigen receptors by a million fold or more. The incredible number of different antigen receptors generated as a result of this complex process ensures that the immune system has the capacity to recognize and respond to any infectious threat it may encounter.
The genetic recombination process requires a lymphoid-specific enzyme called VDJ recombinase that is composed of the recombinase-activating gene 1 and 2 proteins, termed RAG 1 and RAG 2 (Jung et al. 2006). The critical role of these proteins in the development of B and T cells is highlighted by the fact that mutation in the RAG genes is responsible for an autosomal recessive form of severe combined immune deficiency (SCID), characterized by a deficiency in T and B cell numbers.
5.8 T Cell Activation
After reaching full maturity and export from the central lymphoid organs, naïve B and T cells will begin a pattern of circulation, discussed earlier under Lymphocyte Trafficking, which facilitates the antigen receptor–mediated screening of the antigens concentrated in the peripheral lymphoid organs. Our attention now turns to the molecular interactions involved in T and B cell activation and the effector mechanisms brought to bear by these cell types during the immune response, beginning with T cells.
As already established, activation of naïve T cells during an immune response depends upon their recognition of foreign antigen by way of their T cell antigen receptor (TCR). Antigen is displayed to T cells within the context of MHC class I and class II molecules, which are presented on the surface of antigen-presenting cells. DCs are the most critical APCs for the activation of naïve T cells as they are either transporting antigen to the regional lymph nodes from the peripheral tissues or capturing antigen in the lymph nodes that is carried there by the flowing lymph (Banchereau and Steinman 1998). A complementary interaction between the TCR and the MHC-peptide complex that is of high enough affinity and long enough duration will lead to the biochemical signals needed to activate a T cell response. The strength of the interaction is assisted by integrins, such as LFA-1, on the T cell binding to integrin ligands, such as ICAM-1, expressed by the DCs (Friedl and Brocker 2002). As seen earlier, under resting conditions, integrins such as LFA-1 are in a low-affinity conformation and only bind to their ligands with high affinity under the influence of chemokines, as well as, in this case, antigen recognition.
If antigen recognition occurs, then T cell activation is achieved as a result of a biochemical signaling cascade involving the CD3 complex and its associated ζ chains, as well as the CD4 and CD8 co-receptors (described below). The activation of naïve T cells also requires a “costimulatory” signal delivered to the T cell when its cell surface CD28 molecule engages either of its ligands, B7-1 and B7-2 (Bour-Jordan and Bluestone 2002). Because B7-1 and B7-2 are only expressed by DCs which have become activated through innate recognition of pathogens via their pathogen recognition receptors, this co-stimulation requirement for naïve T cells to become activated serves as a checkpoint to help ensure that T cells are responding to foreign, and not self, antigens. T cells whose receptors engage self-antigen in the absence of costimulation may become unresponsive to antigen stimulation going forward, a condition referred to as anergy. Cells can be maintained in the anergic state by the T cell expression of CD28-like molecules that deliver an inhibitory (rather than costimulatory) signal, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) or programmed death protein1 (PD-1) (Schneider et al. 2008). While CTLA-4 and PD-1 prevent the response to self-antigens and the development of autoimmunity, they are also seen to suppress potentially helpful immune responses against tumor cells, an area of study that led to the development of drugs termed “checkpoint inhibitors” that block the immunosuppressive function of CTLA-4 and PD-1, thereby unleashing the immune system to more aggressively attack developing and established tumors. So significant was this body of work that it earned immunotherapy pioneers James Allison and Tasuku Honjo the 2018 Nobel Prize in Medicine (Peggs et al. 2009).
Successful T cell antigen recognition and costimulation will result in a cell-signaling cascade initiated by the Lck tyrosine kinase, which is associated with the cytoplasmic domain of the CD4 and CD8 co-receptors. The Lck kinase phosphorylates tyrosine residues of the CD3 ζ chains, which become docking sights for another tyrosine kinase, Zap-70, which also becomes activated when phosphorylated by Lck (Au-Yeung et al. 2009). The activation of ZAP-70 leads to the activation of the transcription factors NFAT, NFκB, and AP-1, which move into the nucleus and activate genes required for T cell proliferation, cytokine production, and effector function (Brownlie and Zamoyska 2013). Key among these genes are those that encode the cytokine IL-2 and the IL-2 receptor. IL-2 is important early during the T cell response as it stimulates the clonal proliferation and survival of activated T cells, thereby expanding many fold the number of antigen-specific cells capable of responding to current infectious threat.
5.9 T Cell Effector Functions
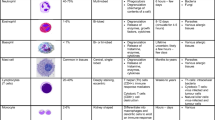
It is critical at this point to recognize the diversity of the different kinds of pathogens that the immune system must be able to protect against, including bacteria that survive in phagosomes, viruses that replicate in the cytoplasm, and parasites that infect the lumen of the gut. The immune response to these different types of infections must be tailored to their unique features and vulnerabilities. To provide this capability, activated T cells differentiate under the influence of signals, some of which were generated during the innate immune response, into specific effector cell populations capable of mounting an effective immune response to the particular offending pathogen. We will begin this discussion with the different subsets of activated CD4 T cells and the pathogens that they defend against (Table 1).
Once activated by antigen, CD4 T cells differentiate into different subsets of effector cells that are primarily defined by the cytokines that they produce. These cytokines are the critical factors responsible for recruiting and/or activating unique effector functions of the immune system that are optimal for responding to different pathogens. The first CD4 T cell subsets to be defined were referred to as T helper subsets 1 and 2 (Th1 and Th2). Subsequently, a third, Th17, subset was identified. Today, we understand that Th1 cells are critical for the immune response to intracellular microbes, Th2 cells are most protective against parasitic helminth infections, and Th17 cells are best at combating extracellular bacteria and fungal infections (Annunziato and Romagnani 2009).
CD4 T cells differentiate into Th1 cells under the influence of the cytokines produced during the innate immune response to intracellular bacteria and viruses. These cytokines include IL-12 produced by DCs and interferon γ (IFNγ produced by NK cells. At the molecular level, this Th1 differentiation is driven by the activation of the transcription factors STAT1, STAT4, and T-bet that occurs in response to the early secreted IL-12 and IFNγ (Murphy and Reiner 2002). By this mechanism, naïve CD4 T cells which recognize antigens of these microbes will be triggered by IL-12 and IFNγ to differentiate into Th1 cells. IFNγ is not only important for the differentiation of the Th1 cells, but it is also the principal effector cytokine produced by this CD4 T cell subset and is critical in their role of enhancing macrophage killing of microbes that have been phagocytosed (Annunziato and Romagnani 2009). Th1 cell-derived IFNγ works in concert with a molecule found on the surface of Th1 cells, called CD40L. The CD40L receptor, CD40, is expressed by macrophages and B cells, and this ligand-receptor interaction is necessary for Th1-mediated help during the immune response, as evidenced by the cellular and humoral immunodeficiency observed when CD40L is not sufficiently expressed (Kamanaka et al. 1996). The enhanced ability of Th1-activated macrophages to kill phagocytosed microbes is due to their increased production of reactive oxygen species, nitric oxide, and damaging lysosomal enzymes. Th1 cells also enhance the antigen-presenting cell function of macrophages by inducing their expression of MHC class II and the B7-1/B7-2 costimulatory molecules (Annunziato and Romagnani 2009).
Whereas pathogens such as intracellular bacteria and viruses induce strong innate immune responses that drive the production of inflammatory cytokines, parasitic helminths are much less of a trigger for the innate immune response. In the absence of these strong inflammatory signals, it appears that antigen-activated CD4 T cells may default to producing low levels of the cytokine IL-4, which activates the transcription factors GATA-3 and STAT6 that promote the differentiation to the Th2 subset (Paul and Zhu 2010). As noted above, Th2 cells are especially protective against helminth infections, which is achieved via a number of different mechanisms. To begin with, committed Th2 cells are strong producers of IL-4 and IL-5, which trigger B cells to class switch to the IgE antibody isotype and promote eosinophils responses, respectively. Eosinophils and mast cells express high levels of the Fc receptor for IgE (FcεR1) and, therefore, will be activated through these receptors by IgE-coated helminths. Once activated, eosinophils release their granule contents which destroy the helminths. IL-4, along with another Th2 cytokine, IL-13, induce intestinal mucus secretion and peristalsis, which also contribute to helminth expulsion (Anthony et al. 2007). Another effect of the IL-4/IL-13 cytokine pair is that they promote the development of so-called alternatively activated macrophages that have anti-inflammatory and tissue repair functions (Van Dyken and Locksley 2013).
The most recent Th cell subset to be described are the Th17 cells. Th17 cells are characterized by secretion of the cytokines IL-17 and IL-22. IL-17 is most well-known for promoting the recruitment of phagocytes, mainly neutrophils, to the site of an infection and, therefore, are important contributors to defense against extracellular bacteria and fungal infections (Littman and Rudensky 2010). Differentiation of Th17 cells requires a number of different cytokines, including TGF-β and the inflammatory cytokines IL-1 and IL-6 (McGeachy and Cua 2008).
A fundamental feature of CD4 T cell responses is the extent to which the balance between the production of Th1, Th2, and Th17 cytokines impacts the outcome of the immune response. This is because many of the cytokines produced by one subset are inhibitory to the others. For example, IFNγ produced by Th1 cells inhibits the development of Th2 and Th17 responses, while IL-4, IL-10, and IL-13 produced by Th2 cells inhibit the killing ability of macrophages, thereby suppressing Th1-mediated cellular immunity (Murphy and Reiner 2002; Annunziato and Romagnani 2009). This can be demonstrated experimentally using different strains of mice with either a Th1 or Th2 predisposition, but it is most profoundly demonstrated in different populations of people infected with Mycobacterium leprae. This pathogen has the capacity to survive and replicate in phagosomes once taken up by macrophages. Control of the infection requires macrophage activation by a dominant Th1 response. If this occurs, the result is the tuberculoid leprosy form of disease, characterized by localized infection and low infectivity. On the other hand, Mycobacterium leprae infection of an individual who mounts a more dominant Th2 response that impedes macrophage activation and strong cellular immunity results in the more severe lepromatous leprosy, characterized by unchecked growth of mycobacterium, disseminated infection, and high infectivity (Modlin 1995).
As opposed to bacteria and fungi that originate outside of host cells and, therefore, can be phagocytosed and killed, opsonized, neutralized, or lysed, viruses that replicate in the cell’s cytoplasm are comparatively more difficult to reach. CD8 T cells have evolved to meet this challenge. As described earlier, viral antigen synthesized in the cytoplasm during the viral replication is presented to CD8 T cells via MHC class I molecules. Antigen recognition by CD8 T cells will result in their activation and expression of cell membrane and secreted proteins which will be used to induce apoptosis in the infected cell, thereby preventing the production of new virons. The CD8 T cell membrane protein is called Fas ligand (FasL), which binds to its receptor, called Fas, on the infected cell. This receptor-ligand interaction will trigger the activation of caspases in the infected cell, resulting in its apoptosis. Target cell apoptosis can also be induced through the CD8 T cell release of the granule proteins: granzyme B and perforin. Granzyme B is the protein responsible for the activation of caspases and induction of apoptosis, while perforin is required to facilitate entry of granzyme B into the infected cell (Russell and Ley 2002).
The final phase to the T cell response to infection will be the contraction of effector T cell numbers and the establishment of a much smaller number of long-lived memory T cells, which will surveil for the re-occurrence of infection. Memory cells, which can be found in lymphoid organs or the peripheral tissues, do not continue to exhibit their effector functions during this period of surveillance but are poised to rapidly expand and re-establish effector function upon re-encounter with their target antigens. The survival of memory CD4 and CD8 T cells does not require antigen stimulation; however, their maintenance is dependent on stimulation by the cytokines IL-7 and IL-15 (Murali-Krishna et al. 1999; Seddon et al. 2003).
5.10 B Cell Activation
While different types of B cells have been described, such as marginal zone B cells and B-1 B cells that uniquely reside in certain areas of the spleen and mucosal tissues and respond to polysaccharides and lipids, the discussion on B cell activation and effector function in this chapter will focus on follicular B cells that are the source of high-affinity class-switched antibodies, the principal mediators of humoral immunity. Follicular B cells reside in and circulate through the lymphoid follicles and become activated by protein or protein-associated antigen that has been transported to and concentrated here. We will see that antigen recognition is just the first step in the activation of follicular B cells that also involves interaction with helper CD4 T cells responding to the same microbial antigens.
Similar to the CD3 complex on T cells, the Igβ and Igα chains associated with the B cell antigen receptor serve important roles in the cascade of signaling events induced upon B cell recognition of antigen. The phosphorylation of Igβ and Igα tyrosine residues, recruitment of kinases, and activation of adaptor proteins lead to the activation of transcription factors that control genes involved in B cell proliferation and differentiation. As seen with T cells, B cells also benefit from innate costimulatory signals during the activation process, provided by complement receptors such as CR2 and TLRs which engage components of the microbe (Pasare and Medzhitov 2005). Collectively, these events induce the early phase of the B cell response, characterized by increased survival and proliferation and functional changes that will facilitate the B cell-T cell interaction that will occur next. These changes include the B cell’s transition into an antigen-presenting cell, accomplished by the internalization of the receptor-bound antigen and the increased expression of MHC class II and B7-1/B7-2 costimulatory ligands. The activated B cell will also increase its expression of the CCR7 chemokine receptor at the same time that activated helper T cells in the paracortex are increasing expression of CXCR5 (Okada and Cyster 2006). Recall that the CCR7 and CXCR5 chemokine receptors direct leukocyte trafficking to the T cell and B cell areas of the lymph node, respectively. Therefore, the outcome of this flip in chemokine receptor expression will be that B and helper CD4 T cells responding to antigen will migrate toward each other. At this point, the B cell is functioning as a professional antigen-presenting cell, expressing high levels of MHC Class II/peptide complexes and costimulatory ligands. If CD4 T cell recognition of antigen presented by the B cell occurs, the CD4 T cell will provide activating signals through its secretion of cytokines and expression of CD40L, which will bind to CD40 expressed by the activated B cell (Meng et al. 2018). As a result, the fully activated B cell will undergo clonal expansion and antibody synthesis and secretion. Following this T-B cell interaction, a smaller number of activated CD4 T and B cells will be drawn into the B cell follicle. These CD4 cells, referred to as follicular helper T cells, provide signals to the B cells that induce their rapid division, creating clusters of dividing B cells referred to as germinal centers (Crotty 2014). A sequence of somatic mutation of the B cell Ig genes followed by selection of those clones producing the antibody with highest affinity or antigen now occurs, a process referred to as affinity maturation. The selected high-affinity clones will differentiate into long-lived antibody-producing plasma cells and memory B cells.
As noted earlier, antibodies are produced in a number of different forms called isotypes. The isotype of an antibody is significant as different isotype antibodies have different immunological functions. While the first antibody produced during a primary B cell response is always IgM, isotype class switching during the immune response, and subsequent exposures to the antigen (secondary response), will lead to the production of larger amounts of other isotypes, including IgG, IgA, and IgE. This isotype class switching is under the control of the cytokines produced by follicular helper T cells providing help to germinal center B cells (Crotty 2014). For example, IFNγ, the signature Th1 cytokine, causes isotype switching to the IgG1 and IgG3 isotypes. During the immune response to extracellular bacteria, these isotypes are notable for their role as effective opsonins, which work in concert with IFNγ−activated macrophages that have enhanced phagocytic and killing ability. In contrast, the IL-4 produced by Th2 CD4 T cells stimulates class switching to IgE, which works together with eosinophils to eliminate helminths (Anthony et al. 2007).
The ability of B cells to class switch from IgM to other Ig isotypes, as directed by the CD4 T cells, allows the humoral response to be optimized to fight a particular infection (Davies and Metzger 1983). At the molecular level, the isotype is determined by the unique constant region (μ, δ, γ, ε, or α) incorporated into the antibody’s heavy chain. Isotype class switching, therefore, requires recombination of the heavy chain DNA such that the variable region is combined with the appropriate constant region. The importance of isotype class switching is underscored by the occurrence of X-linked hyper-IgM syndrome, an immune deficiency caused by a mutation in the gene encoding the T cell CD40L molecule (Meng et al. 2018). In this syndrome, activated B cells receive early activation signals through their antigen receptor but do not get help from CD4 T cells because of the CD40L mutation, therefore preventing class switching from occurring. Patients of this disease produce mainly low-affinity IgM that has limited protective function and therefore suffer from recurrent infections with pyogenic bacteria due to reduced opsonizing IgG. Importantly, these people also experience reduced cell-mediated immunity because of the important role of CD40L in providing CD4 T cell help to macrophages, as described in Sect. 5.9 of this chapter.
5.11 Antibody Effector Function
The importance of antibody to the immunological protection of the host is illustrated by the increased frequency of infectious disease in those individuals with compromised B cell development. These individuals commonly suffer from recurrent respiratory infections by pyogenic bacteria, such as Streptococcus pneumonia and Haemophilus influenzae. The main virulence factor of these encapsulated bacteria is their polysaccharide capsule that protects them from phagocytosis. The B cell response to antigens of the polysaccharide capsule results in the production of antibody that when bound to the capsule facilitates the effective phagocytosis of the bacteria by neutrophils and macrophages (Klein Klouwenberg and Bont 2008). Antibodies that facilitate phagocytosis of coated microbes are referred to as opsonins. When bound to the microbe, the Fc portion of the antibody extends away from the microbe’s surface. Antibodies of the IgG (IgG1 and IgG3) isotype are the most effective opsonins because their Fc region readily binds to a high-affinity Fc receptor, called FcγR1, expressed by phagocytes. This interaction between the Fc receptor and its ligand triggers the phagocytosis of the coated microbe.
Antibodies of the IgM and IgG isotypes that have coated a microbe can also indirectly facilitate its phagocytosis by the activation of the complement system, discussed in detail during the section on innate immunity (Diebolder et al. 2014). It is the classical complement pathway that is activated by antibody bound to a microbe, resulting in the deposition of the C3b complement protein on the microbial cell membrane, a potent opsonin recognized by the CR1 complement receptor, expressed on phagocytes. In addition to the deposition of the C3b opsonins, activation of complement also results in the production of factors chemotactic for neutrophils (C3a and C5a) and the formation of the bactericidal MAC.
IgG antibodies may also coat host cells during the course of an infection with enveloped viruses, such as influenza. In this situation, antibodies are binding to viral glycoproteins that are embedded in the host cell membrane as part of the viral life cycle. These IgG isotype antibodies can be recognized by FcγRIII, an Fc receptor expressed uniquely by NK cells. When engaged these receptors generate signals that activate the cytolytic function of NK cells resulting in the induction of apoptosis of virally infected cell, a process called antibody-dependent cell-mediated cytotoxicity (ADCC) (Chung et al. 2009).
Individuals with humoral immune deficiencies are also susceptible to infections by viruses which are normally neutralized by antibody, such as the enteroviruses (e.g., poliovirus and coxsackievirus). Neutralization refers to an antibody’s capacity to block the infectivity of a microbe by binding to and neutralizing microbial surface molecules required to establish infection. Antibodies can also attach to microbial toxins, thereby preventing them from mediating their dangerous effects. This is exemplified by the use of the tetanus vaccine, where recipients are vaccinated with an inactivated version of the tetanus toxin (toxoid) in order to induce production of antibodies capable of binding to and neutralizing the toxin. Although any isotype antibody can neutralize, most neutralizing antibodies in the blood and tissue are IgG (Ward and Ghetie 1995). In the mucocal organs, this job is performed by IgA, the principal class of antibody produced in mucosal tissues (Suzuki et al. 2004). The vast majority of infectious agents invade the human body via the mucosal organs, underscoring the importance of strong immunological protection at these sites. The plasma cells responsible for the production of mucosal IgA are found in the lamina propria, beneath the mucosal epithelium. Once secreted by the plasma cell, the dimeric IgA is ferried across the mucosal epithelium into the organ lumen by a special Fc receptor called the poly-Ig receptor (Lamm 1998). Once released into the lumen, the IgA will neutralize would-be pathogens, preventing them from crossing the epithelial barrier and establishing an infection. In the lactating mother, dimeric IgA binds to the same poly-Ig receptor to get transcytosed across the glandular epithelium and released into the breast milk, thereby providing an important measure of immunological protection against intestinal and respiratory infection in the newborn.
Whereas antibody coating a bacterial cell may facilitate its phagocytosis, most helminths are too large to be taken up by a macrophage or neutrophil. The immune response to such parasites depends upon the activation of eosinophils. The recruitment and activation of eosinophils to the infection require the production of IgE isotype antibody, the principal isotype produced in response to a helminth infection. As described earlier, B cells class switch to IgE under the direction of IL-4 produced during a dominant Th2 helper CD4 T cells response to the helminth. IgE bound to the helminth will activate the eosinophils through the high-affinity Fc receptor for IgE, called FcεR1, expressed on the eosinophil surface. In response the eosinophils release granules containing major basic protein and eosinophilic cationic protein, which are toxic to parasites. Mast cells also express FcεR1 and therefore will also become activated and participate during the anti-helminth response (Anthony 2007).
5.12 Immunological Tolerance
As we have discussed, the B and T cells of the adaptive immune system are created with a tremendous capacity to discern the presence of any of the many microbes with which we share our environment. Of course, creating the diverse repertoire of antigen receptor–bearing B and T cells that makes this possible comes with the risk that some of those cells will bear receptors with an affinity for normal molecules expressed by the host, otherwise referred to as self-antigens. The concept of Immunological Tolerance refers to the fact that although the immune system would appear to walk a fine line between highly sensitive surveillance for foreign microbes and mistaking a harmless self-antigen as a threat, it does so successfully because of multiple built-in mechanisms and checkpoints. These mechanisms include the active process of removing developing lymphocytes that express receptors found to strongly bind self-antigen in the bone marrow and thymus, a mechanism referred to as central tolerance. On the other hand, peripheral tolerance refers to mechanisms that prevent activation by self-antigens in the periphery.
The underlying concepts of central tolerance were discussed earlier in Sect. 5.7. Essentially, lymphocytes at the immature stage of development found to interact strongly with self-antigen are directed to undergo apoptosis, a process referred to as negative selection. Negative selection is an active process in that self-antigens must be displayed to developing lymphocytes in order to identify and delete those with potentially autoreactive antigen receptors. One potential challenge, therefore, is establishing central tolerance to antigens only expressed in certain specialized peripheral tissues, for example, those of endocrine organs. The protein called AIRE (autoimmune regulator) assists in this regard by activating the expression of these peripheral tissue genes in the thymus. In doing so, AIRE assures that peptide derived from these self-proteins will be presented to developing T cells and that cells with antigen receptors that bind with high affinity to these peptides when presented by host MHC will be removed. The importance of AIRE is underscored by the development of autoimmune polyendocrine syndrome (APS) type I, a disorder caused by a defective AIRE gene and characterized by a constellation of autoimmune assaults on tissues of the endocrine system (Anderson et al. 2005).
Importantly, not every immature B and T cell that encounters self-antigen during development will undergo apoptosis. For example, CD4 T cells with high affinity for self-antigens may be triggered to differentiate into regulatory T cells, a unique and specialized population of CD4 T cells that will help maintain peripheral tolerance, as described below. Additionally, immature B cells that recognize self-antigens in the bone marrow may go through a process called receptor editing, whereby the cell re-expresses the RAG genes for the purpose of recombining a second light chain. Replacement of the original with the newly recombined light chain will alter the antigen receptor such that a new antigen-binding site will be created. If the edited B cell receptor still recognizes self-antigen with high affinity, the cell will die by apoptosis.
Despite the intricate mechanism of negative selection and function of proteins such as AIRE, the thymus and bone marrow are sources of autoreactive B and T cells that enter the periphery. The evidence for this are the multiple mechanisms designed to prevent the activation of circulating mature lymphocytes by self-antigens (peripheral tolerance), and the unfortunate autoimmune diseases that develop when those mechanisms are deficient in some way. These mechanisms include the functional inactivation and deletion of autoreactive cells, as well as their suppression by regulatory T cells or other inhibitory elements.
As discussed earlier, the activation of naïve T cells requires not only antigen recognition through the TCR but also engagement of the CD28 costimulatory receptor with its ligand B7-1 and B7-2 expressed by the dendritic cell. Because B7 is expressed when the dendritic cell is activated by a microbe through a pathogen recognition receptor, or by the local production of inflammatory cytokines (also an indication of an infectious threat in the region), this costimulation requirement assures naïve T cell activation occurs as a result of recognizing microbial antigen. It is therefore likely that T cells engaging antigen presented in the absence of costimulation are recognizing peptide derived from self-proteins. Since these T cells represent a potential autoimmune threat, they are functionally inactivated, a state that is referred to as anergy. Anergic T cells survive but are unresponsive to antigen going forward. This unresponsiveness is believed to be due to expression of molecules such as CTLA-4 and PD-1, which deliver inhibitory signals to the T cell. CTLA-4 may also suppress the T cell responses by binding to and removing the B7 costimulatory molecules from the surface of APCs. The importance of CTLA-4 to peripheral tolerance is underscored by the fact that the development of autoimmune diseases such as Grave’s disease and Hashimoto’s thyroiditis is associated with polymorphisms in the CTLA-4 gene, which potentially impact its function (Ueda et al. 2003). In addition to becoming anergic, T cells that recognize self-antigen presented without costimulation may be triggered to undergo apoptosis. This is because costimulation normally induces the production of anti-apoptotic proteins, protecting fully activated T cells from induced cell death. Recognition of self-antigens in the absence of costimulation would therefore fail to stimulate this increase in anti-apoptotic proteins and be more likely to lead to apoptosis of the autoreactive T cell. Anergy and apoptosis are also potential outcomes for self-reactive B cells. The main determining factor in this case is whether or not an antigen-engaged B cells receives adequate help from CD4 T cells. A B cell activated by recognition of self-antigen may fail to elicit CD4 T cell help, in which case the tolerogenic mechanisms of anergy and apoptosis are more likely to follow.
Another important mediator of peripheral tolerance are regulatory CD4 T cells. Although regulatory T cells may develop as a result of negative selection mechanisms in the thymus, as already discussed, they are also thought to arise in the periphery following CD4 T cell recognition of self-antigen. Regulatory CD4 T cells are phenotypically identified by their constitutive expression of the IL-2 receptor α chain, CD25, a hint to the important role that IL-2 plays in the survival and function of these cells, along with the cytokine TGF-β. The transcription factor FoxP3 is required for the development and function of regulatory T cells, a finding that has contributed to our appreciation of their importance to maintaining peripheral tolerance, as mutations in the FoxP3 gene is known to result in the development of an aggressive autoimmune syndrome called IPEX, for immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome (Wildin et al. 2001). Regulatory T cell control over the immune response is accomplished via several mechanisms, including expression of CTLA-4 and the secretion of IL-10 and TGF-β, cytokines known for their inhibitory effect on T cells, B cells, macrophages, and dendritic cells (Sakaguchi et al. 2008).
6 Introduction to Allergy and the Immunological Mechanisms of Hypersensitivity
Although all of the mechanisms of innate and adaptive immunity and tolerance discussed above are constantly functioning in the background, we don’t often think about the day-to-day activity of the immune system until it seems to not be working quite right. While congenital or acquired immune deficiencies are indicated clinically by an abnormally high frequency of infections, hypersensitivity reactions in many ways represent the opposite problem, injury caused by excessive or dysregulated immune responses. This may occur as a result of aberrant responses to environmental or self (auto)-antigens when the mechanisms of tolerance discussed above are not fully functional. Utilizing the basics of immunology described in this chapter, this final section will provide an overview of the mechanisms of hypersensitivity reactions, with a focus toward the type I hypersensitivity reaction, otherwise known as the allergic response (Table 2).
Hypersensitivity reactions, whereby the immune system causes injury to host tissues, are classified based upon the immunopathogenic mechanism responsible for the disease. Four principle hypersensitivity mechanisms are usually described. The type I hypersensitivity reaction underlies the response to harmless environmental antigens which trigger diseases such as allergic asthma, hay fever and food allergies. As we will discuss in more detail below, diseases caused by hypersensitivity mechanisms II–IV often involve the aberrant immune responses to self-antigens and, therefore, are the mechanistic basis for autoimmunity.
6.1 Introduction to the Allergic Response
The immediate type I hypersensitivity (allergic) response is triggered by an apparent excessive response to harmless environmental antigens, referred to as allergens. These may include pollen, dust mites, insect venom or animal dander. Before a clinically significant allergic response can occur, an individual must be sensitized to the allergen. During sensitization, individuals who develop allergies default to a strong Th2 CD4 T cell response when exposed to the allergen (Fahy 2015). As discussed in the T and B Cell Effector Function sections, Th2 cells are principally characterized by the production of the cytokines IL-4, IL-5, and IL-13. At this point, IL-4 and IL-13 are the critical players as they instruct the allergen-specific B cells to class switch to IgE (Gould and Sutton 2008). The IgE antibodies with specificity for the offending allergen will coat the surface of mast cells located in connective and subepithelial tissues throughout the body. Mast cells bind the IgE via their cell surface FcεRI, resulting in the antigen-binding regions of the antibody oriented away from the cell surface (Gould and Sutton 2008). The mast cells are now said to be sensitized against the offending allergen. Re-exposure to the sensitizing allergen will cause cross-linking of the IgE on the mast cell surface by the allergen, which will stimulate the mast cell’s activation and release of the chemical mediators of the allergic response (Gould and Sutton 2008).
The chemical mediators released from the mast cell include histamine, which is preformed and stored in cytoplasmic granules (Gandhi and Wasserman 2009; Smuda and Bryce 2011). Histamine’s biological effects include dilation of blood vessels, increased vascular permeability, and transient contraction of smooth muscles (Gandhi and Wasserman 2009; Smuda and Bryce 2011). Mast cell activation also stimulates the rapid synthesis and secretion of eicosanoids, including prostaglandins and leukotrienes, both of which are derived from arachidonic acid. Leukotrienes have effects similar to those of histamine; however, molecule-for-molecule leukotrienes are much more powerful stimulants of smooth muscle contraction (Gandhi and Wasserman 2009). The final set of mediators released by the mast cells are cytokines that mobilize and recruit inflammatory cells, including eosinophils and neutrophils, to the site of allergen exposure. These cytokines include TNFα, IL-5, IL-4, and IL-13 (Eifan and Durham 2016).
The earliest clinical effects of the allergic response can be experienced minutes after allergen exposure (hence the term immediate hypersensitivity reaction is used), reflecting the rapid release of histamine and synthesis of eicosanoids by activated mast cells (Eifan and Durham 2016). These effects include edema, mucus secretion, and smooth muscle spasm. For example, the classical wheal and flare response observed minutes after the subcutaneous injection of allergen to a sensitized individual is caused by histamine-mediated vasodilation and vascular leakage. Similarly, airway obstruction experienced during the immediate phase of the asthmatic response is caused by smooth muscle bronchoconstriction, mucus secretion, vasodilation, and increased blood vessel permeability.
As compared to the immediate hypersensitivity reaction, which is caused by mediators released from resident tissue mast cells, the later phase of the allergic reaction is caused by cells recruited to the site by the mast cell-derived cytokines. The infiltrate is heavy with eosinophils, as one might predict by the IL-5 produced by mast cells and Th2 cells. When activated, eosinophils produce two unique proteins called major basic protein and eosinophil cationic protein, which cause additional epithelial damage and more airway constriction (Costa et al. 1997).
The clinical manifestations of allergic responses vary with the anatomical site of the allergic reaction. For example, allergic rhinitis develops in response to inhaled allergens such as pollen that stimulate mast cells in the nasal mucosa, resulting in increased mucus secretion. On the other hand, allergic asthma is caused by the activation of bronchial mast cells and is characterized by airway obstruction caused by mucus secretion, inflammation, and bronchial smooth muscle contraction. The most severe form of allergy is anaphylaxis, caused by the systemic activation of sensitized mast cells. This may occur in response to bee stings or ingested nuts or shell fish, the allergens from which get absorbed into the circulation. The systemic reaction causes edema in many tissues, accompanied by a fall in blood pressure and bronchoconstriction, creating a potentially life-threatening situation.
It is unknown why some individuals originally mount the strong Th2 responses to environmental antigens that are the determining factor for the development of allergies. For certain, there is a genetic basis for the development of allergic disease, as evidenced by the large number of genetic polymorphisms that appear to associate with the development of disease. As one might expect, a number of the genes implicated are associated with CD4 T cell differentiation, cytokine signaling pathways, and the high-affinity IgE receptor (Holloway et al. 2010).
6.2 Antibody and T Cell-Mediated Hypersensitivity Reactions
Type II and type III hypersensitivity reactions ar e both mediated by antibodies directed against either tissue-bound antigens or antigens circulating in the blood or tissue fluid, respectively. Type II hypersensitivity diseases include autoimmune diseases such as Graves’ disease, myasthenia gravis, and Goodpasture syndrome, whereby the autoantibody’s attachment to a cell surface or extracellular matrix component (e.g., collagen) either disrupts normal physiological function of the target cell or elicits an inflammatory response via the activation of complement and recruitment of neutrophils. Once on the scene, neutrophils become activated by the bound antibody and complement (Binks et al. 2016). Type III hypersensitivity reactions are initiated by the binding of antibody to antigen circulating in the blood and tissue fluids. Such immune complexes are normally removed by the phagocytic cells of the spleen and liver. However, when phagocytic cells are not functional or are overwhelmed, or the immune complexes are of a certain size or electrical charge, the circulating immune complexes may be deposited in the walls of blood vessels. This especially happens in the kidney because of the high pressure at which blood flows though this organ. An inflammatory response ensues when the deposited immune complexes trigger the activation of complement, leading to the generation of C5a which effectively recruits neutrophils that become activated by the deposited immune complexes and complement. Systemic lupus erythematous (SLE) is a striking example of a type III hypersensitivity disease, characterized by the widespread deposition of immune complexes composed of autoantibody bound to a number of different nuclear autoantigens, most notably double-stranded DNA (Fairhurst et al. 2006).
Finally, type IV hypersensitivity reactions are mediated by CD4 or CD8 T cells activated by either self-antigens or persistent environmental or microbial antigens. Type I (autoimmune) diabetes is a classic example of a type IV hypersensitivity disease caused by the response of autoreactive CD4 and CD8 T cells to autoantigens expressed by β cells of the pancreas. As a result, the β cells are killed directly either by the infiltrating CD8 T cells or by the inflammatory cytokines produced by macrophages activated by the autoreactive helper CD4 T cells (Roep 2003).
7 Conclusion
This chapter endeavored to provide the reader with an overview of the principle mediators of the immune response as it has evolved to protect the host from multiple kinds of infectious threats. This included a discussion of the early acting innate immune response, which effectively functions to control the spread of an infection while the T and B cells of the adaptive immune system expand in number and differentiate into effectors specialized at clearing the current infection. We then discussed the different mechanisms by which the immune system can itself cause tissue injury, focusing on the allergic response. With this foundation of basic immunology knowledge, the reader is better prepared to understand the applied clinical immunology that follows in the proceeding chapters of this comprehensive text.
References
Abas A, Lichtman A, Pillai S. Basic immunology, function and disorders of the immune system. 5th ed. St Louis: Elsevier; 2016.
Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 7th ed. Philadelphia: Elsevier Saunders; 2012.
Abney ER, Cooper MD, Kearney JF, Lawton AR, Parkhouse RM. Sequential expression of immunoglobulin on developing mouse B lymphocytes: a systematic survey that suggests a model for the generation of immunoglobulin isotype diversity. J Immunol. 1978;120:2041–9.
Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104.
Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of aire control of T cell tolerance. Immunity. 2005;23:227–39.
Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther. 2009;11:267–4.
Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87.
Au-Yeung BB, Deindl S, Hsu L-Y, Palacios EH, Levin SE, Kuriyan J, Weiss A. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress inducible MICA. Science. 1999;285:727–9.
Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–76.
Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol. 2016;263:826–34.
Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121.
Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–60.
Bour-Jordan H, Bluestone JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7.
Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–7.
Brownlie RJ, Zamoyska R. T cell receptor signaling networks: branched, diversified, and bonded. Nat Rev Immunol. 2013;13:257–69.
Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving β2 integrins and selectin ligands. Curr Opin Hematol. 2002;9:30–5.
Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–10.
Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278:1815–22.
Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–42.
Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102.
Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol. 2000;10:R30–3.
Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94.
Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–93.
Davies DR, Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117.
Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–3.
Early P, Huang H, Davis M, Calame K, Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980;19:981–92.
Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, MacGlashan DW, Saini SS. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–95.
Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46:1139–51.
Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69.
Friedl P, Brocker EB. TCR triggering on the move: diversity of T-cell interactions with antigen-presenting cells. Immunol Rev. 2002;186:83–9.
Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–53.
Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42.
Gandhi C, Wasserman SI. Biochemical mediators of allergic reactions. In: Grammer LC, Greenberger PA, editors. Patterson’s allergic diseases. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
Goding JW, Scott DW, Layton JE. Genetics, cellular expression and function of IgD and IgM receptors. Immunol Rev. 1977;37:152–86.
Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–9.
Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol. 2006;298:121–38.
Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17.
Gros P, Milder FJ, Janssen BJ. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58.
Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67.
Hadders MA, Beringer DX, Gros P. Structure of C8α-MACPF reveals mechanism of membrane attack in complement immune defense. Science. 2007;317:1552–4.
Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–27.
Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. Manipulation of the nuclear factor-κB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–67.
Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125:S81–94.
Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–58.
Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6.
Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–70.
Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at Two Distinct Phases of Cell-Mediated Immunity. Immunity. 1996;4:275–81.
Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity. 2003;18:53–64.
Klein Klouwenberg PM, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol. 2008;2008:628963.
Lamm ME. Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am J Phys. 1998;274:G614–7.
Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83.
Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–9.
Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58.
Long EO. Negative signalling by inhibitory receptors: the NK cell para‑ digm. Immunol Rev. 2008;224:70–84.
Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–35.
McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–53.
McGrath FD, Brouwer MC, Arlaud GJ, Daha MR, Hack CE, Roos A. Evidence that complement protein C1q interacts with C-reactive protein through its globular head region. J Immunol. 2006;176:2950–7.
Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen presenting machines. Cell. 2001;106:225–58.
Meng X, Yang B, Suen WC. Prospects for modulating the CD40/CD40L pathway in the therapy of the hyper-IgM syndrome. Innate Immun. 2018;24:4–10.
Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1995;102:828–32.
Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–81.
Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44.
Murphy K, Weaver C. Janeway’s immunobiology. 9th ed. New York: Garland Science; 2017.
Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–85.
Pasare C, Medzhitov R. Control of B-cell responses by toll-like receptors. Nature. 2005;438:364–8.
Paul WE, Zhu J. How are Th2 responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35.
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25.
Porter RR. Structural studies of immunoglobulins. Scand J Immunol. 1991;34:382–9.
Roep BO. The role of T cells in the pathogenesis of type-1 diabetes: from cause to cure. Diabetologia. 2003;46:305–21.
Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79.
Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–56.
Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–58.
Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87.
Sant’Angelo DB, Waterbury G, Preston-Hurlburt P, Yoon ST, Medzhitov R, Hong SC, Janeway CA Jr. The specificity and orientation of a TCR to its peptide-MHC class II ligands. Immunity. 1996;4:367–76.
Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81.
Schneider H, Valk E, Leung R, Rudd CE. CTLA-4 activation of phosphatidylinositol 3-kinase (PI 3-K) and protein kinanse B (PKB/AKT) sustains T cell anergy without cell death. PLoS One. 2008;3:e3842.
Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement C3a and C5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102–10.
Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6.
Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67.
Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61.
Shortman K, Egerton M, Spangrude GJ, Scollay R. The generation and fate of thymocytes. Semin Immunol. 1990;2:3–12.
Smuda C, Bryce PJ. New development in the use of histamine and histamine receptors. Curr Allergy Asthma Rep. 2011;11:94–100.
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14.
Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–6.
Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999;2:99–105.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, DiGenova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11.
Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–43.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10.
von Boehmer H, Kisielow P, Lishi H, Scott B, Borgulya P, Teh HS. The expression of CD4 and CD8 accessory molecules on mature T cells is not random but correlates with the specificity of the α:β receptor for antigen. Immunol Rev. 1989;109:143–51.
Ward ES, Ghetie V. The effector functions of immunoglobulins: implications for therapy. Ther Immunol. 1995;2:77–94.
Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, LevyLahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20.
Williams A, Peh CA, Elliott T. The cell biology of MHC class I antigen presentation. Tissue Antigens. 2002;59:3–17.
Wynn TA, Chawla A, Pollard JW. Origins and hallmarks of macrophages: development, homeostasis, and disease. Nature. 2013;496:445–55.
Yoshie O. Role of chemokines in trafficking of lymphocytes and dendritic cells. Int J Hematol. 2000;72:399–407.
Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89.
Author information
Authors and Affiliations
Corresponding author
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Jones, S.C. (2019). Overview of Immunology and Allergy. In: Allergy and Asthma. Springer, Cham. https://doi.org/10.1007/978-3-030-05147-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-05147-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05146-4
Online ISBN: 978-3-030-05147-1
eBook Packages: MedicineReference Module Medicine