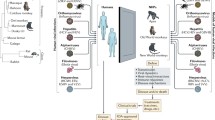
Abstract
Although human cytomegalovirus (HCMV) primary infection is generally asymptomatic, in immune-compromised patients HCMV increases morbidity and mortality. As a member of the betaherpesvirus family, in vivo studies of HCMV are limited due to its species specificity. CMVs from other species are often used as surrogates to express HCMV genes/proteins or used as models for inferring HCMV protein function in humans. Using innovative experiments, these animal models have answered important questions about CMV’s life cycle, dissemination, pathogenesis, immune evasion, and host immune response. This chapter provides CMV biologists with an overview of the insights gained using these animal models. Subsequent chapters will provide details of the specifics of the experimental methods developed for each of the animal models discussed here.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lafemina RL, Hayward GS (1988) Differences in cell-type-specific blocks to immediate early gene expression and DNA replication of human, simian and murine cytomegalovirus. J Gen Virol 69(Pt 2):355–374
Angulo A et al (1998) Enhancer requirement for murine cytomegalovirus growth and genetic complementation by the human cytomegalovirus enhancer. J Virol 72(11):8502–8509
Grzimek NK et al (1999) In vivo replication of recombinant murine cytomegalovirus driven by the paralogous major immediate-early promoter-enhancer of human cytomegalovirus. J Virol 73(6):5043–5055
Sandford GR et al (2001) Rat cytomegalovirus major immediate-early enhancer switching results in altered growth characteristics. J Virol 75(11):5076–5083
Tang Q, Maul GG (2006) Mouse cytomegalovirus crosses the species barrier with help from a few human cytomegalovirus proteins. J Virol 80(15):7510–7521
Lilja AE, Shenk T (2008) Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proc Natl Acad Sci U S A 105(50):19950–19955
Schleiss MR (2006) Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J 47(1):65–72
Schleiss MR (2002) Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J Clin Virol 25(Suppl 2):S37–S49
Powers C, Fruh K (2008) Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol 197(2):109–115
Loh HS et al (2006) Pathogenesis and vertical transmission of a transplacental rat cytomegalovirus. Virol J 3:42
Cheung KS, Lang DJ (1977) Transmission and activation of cytomegalovirus with blood transfusion: a mouse model. J Infect Dis 135:841–845
Stals FS et al (1990) An animal model for therapeutic intervention studies of CMV infection in the immunocompromised host. Arch Virol 114(1–2):91–107
Holtappels R et al (2008) CD8 T-cell-based immunotherapy of cytomegalovirus infection: “proof of concept” provided by the murine model. Med Microbiol Immunol 197(2):125–134
Craighead JE, Martin WB, Huber SA (1992) Role of CD4+ (helper) T cells in the pathogenesis of murine cytomegalovirus myocarditis. Lab Invest 66(6):755–761
Mutter W et al (1988) Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med 167(5):1645–1658
Osborn JE (1986) Cytomegalovirus and other herpesviruses of mice and rats. In: Bhatt PN et al (eds) Viral and mycoplasmal infections of laboratory rodents. Academic, London
Shellam GR et al (1985) The genetic background modulates the effect of the beige gene on susceptibility to cytomegalovirus infection in mice. Scand J Immunol 22(2):147–155
Rawlinson WD, Farrell HE, Barrell BG (1996) Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol 70(12):8833–8849
Cheeran MC, Lokensgard JR, Schleiss MR (2009) Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 22(1):99–126
Juanjuan C et al (2011) Murine model for congenital CMV infection and hearing impairment. Virol J 8:70
Medearis DN Jr (1964) Mouse cytomegalovirus infection. 3. Attempts to produce intrauterine infections. Am J Hyg 80:113–120
Woolf NK, Jaquish DV, Koehrn FJ (2007) Transplacental murine cytomegalovirus infection in the brain of SCID mice. Virol J 4:26
Vink C, Beuken E, Bruggeman CA (2000) Complete DNA sequence of the rat cytomegalovirus genome. J Virol 74(16):7656–7665
Schleiss MR (2008) Comparison of vaccine strategies against congenital CMV infection in the guinea pig model. J Clin Virol 41(3):224–230
Schleiss MR et al (2008) Analysis of the nucleotide sequence of the guinea pig cytomegalovirus (GPCMV) genome. Virol J 5:139
Lockridge KM et al (1999) Pathogenesis of experimental rhesus cytomegalovirus infection. J Virol 73(11):9576–9583
Tarantal AF et al (1998) Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta). J Infect Dis 177(2):446–450
Hansen SG et al (2003) Complete sequence and genomic analysis of rhesus cytomegalovirus. J Virol 77(12):6620–6636
Faber DW et al (1992) Role of HIV and CMV in the pathogenesis of retinitis and retinal vasculopathy in AIDS patients. Invest Ophthalmol Vis Sci 33(8):2345–2353
Britt WJ, Alford CA (2001) The human herpesviruses: cytomegalovirus. In: Knipe DM, Howley PM (eds) Fields virology, 4th edn. Lippincott-Raven, Philadelphia, PA, pp 2493–2523
Mocarski ES, Tan Courcelle C (2001) Cytomegaloviruses and their replication. In: Knipe DM, Howley PM (eds) Fields virology, 4th edn. Lippincott-Raven, Philadelphia, PA, pp 2629–2673
Istas AS et al (1995) Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin Infect Dis 20(3):665–670
Potena L et al (2006) Acute rejection and cardiac allograft vascular disease is reduced by suppression of subclinical cytomegalovirus infection. Transplantation 82(3):398–405
Demmler GJ (1994) Congenital cytomegalovirus infection. Semin Pediatr Neurol 1(1):36–42
Stagno S et al (1984) Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig Artic Ser 20(1):65–85
Fowler KB et al (1999) Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr 135(1):60–64
Pass RF (2005) Congenital cytomegalovirus infection and hearing loss. Herpes 12(2):50–55
Blum A et al (1998) High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol 81(7):866–868
Chiu B et al (1997) Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation 96(7):2144–2148
Espinola-Klein C et al (2002) Impact of infectious burden on progression of carotid atherosclerosis. Stroke 33(11):2581–2586
Grattan MT et al (1989) Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 261(24):3561–3566
Kishore J et al (2004) Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol 53(Pt 11):1155–1160
Papadakis KA et al (2001) Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol 96(7):2137–2142
Soderberg-Naucler C (2008) HCMV microinfections in inflammatory diseases and cancer. J Clin Virol 41(3):218–223
Cobbs CS et al (2002) Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62(12):3347–3350
Cinatl J et al (2004) Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol Med 10(1):19–23
Michaelis M, Doerr HW, Cinatl J (2009) The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia 11(1):1–9
Barami K (2010) Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J Clin Neurosci 17(7):819–823
Soroceanu L, Cobbs CS (2011) Is HCMV a tumor promoter? Virus Res 157(2):193–203
Helantera I et al (2003) The impact of cytomegalovirus infections and acute rejection episodes on the development of vascular changes in 6-month protocol biopsy specimens of cadaveric kidney allograft recipients. Transplantation 75(11):1858–1864
Koskinen PK et al (1993) Cytomegalovirus infection accelerates cardiac allograft vasculopathy: correlation between angiographic and endomyocardial biopsy findings in heart transplant patients. Transpl Int 6(6):341–347
Petrakopoulou P et al (2004) Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation 110(11 Suppl 1):II207–II212
Li RY, Tsutsui Y (2000) Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62(2):79–85
Tsutsui Y (1995) Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol Int 45(2):91–102
Tsutsui Y et al (1993) Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. Am J Pathol 143(3):804–813
Barry PA et al (2006) Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J 47(1):49–64
Vogel P et al (1994) Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab Anim Sci 44(1):25–30
Kumar ML, Nankervis GA (1978) Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis 138(5):650–654
London WT et al (1986) Experimental congenital disease with simian cytomegalovirus in rhesus monkeys. Teratology 33(3):323–331
Kashiwai A et al (1992) Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation stages. Arch Virol 127(1–4):37–48
Kosugi I et al (2000) Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab Invest 80(9):1373–1383
Malm G, Grondahl EH, Lewensohn-Fuchs I (2000) Congenital cytomegalovirus infection: a retrospective diagnosis in a child with pachygyria. Pediatr Neurol 22(5):407–408
Perlman JM, Argyle C (1992) Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann Neurol 31(1):64–68
van den Pol AN, Reuter JD, Santarelli JG (2002) Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system. J Virol 76(17):8842–8854
Matsukage S et al (2006) Mouse embryonic stem cells are not susceptible to cytomegalovirus but acquire susceptibility during differentiation. Birth Defects Res A Clin Mol Teratol 76(2):115–125
Kawasaki H et al (2002) The amount of immature glial cells in organotypic brain slices determines the susceptibility to murine cytomegalovirus infection. Lab Invest 82(10):1347–1358
Kosugi I et al (2002) Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am J Pathol 161(3):919–928
Mutnal MB et al (2011) Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One 6(1):e16211
Keithley EM, Woolf NK, Harris JP (1989) Development of morphological and physiological changes in the cochlea induced by cytomegalovirus. Laryngoscope 99(4):409–414
Tsutsui Y, Kosugi I, Kawasaki H (2005) Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol 15(5):327–345
Schachtele SJ et al (2011) Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol 17(3):201–211
Schraff SA et al (2007) The role of CMV inflammatory genes in hearing loss. Otol Neurotol 28(7):964–969
Cheeran MC et al (2004) Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J Neurovirol 10(3):152–162
Reuter JD et al (2004) Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J Virol 78(3):1473–1487
Harris JP et al (1984) Immunologic and electrophysiological response to cytomegaloviral inner ear infection in the guinea pig. J Infect Dis 150(4):523–530
Melnick JL et al (1983) Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet 2(8351):644–647
Speir E et al (1994) Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265(5170):391–394
Burnett MS et al (2001) Atherosclerosis in apoE knockout mice infected with multiple pathogens. J Infect Dis 183(2):226–231
Hsich E et al (2001) Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis 156(1):23–28
Vliegen I et al (2004) Cytomegalovirus infection aggravates atherogenesis in apoE knockout mice by both local and systemic immune activation. Microbes Infect 6(1):17–24
Vliegen I et al (2002) MCMV infection increases early T-lymphocyte influx in atherosclerotic lesions in apoE knockout mice. J Clin Virol 25(Suppl 2):S159–S171
Vliegen I et al (2004) Murine cytomegalovirus infection directs macrophage differentiation into a pro-inflammatory immune phenotype: implications for atherogenesis. Microbes Infect 6(12):1056–1062
Khoretonenko MV et al (2010) Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses. Am J Pathol 177(4):2134–2144
Melnychuk RM et al (2005) Mouse cytomegalovirus M33 is necessary and sufficient in virus-induced vascular smooth muscle cell migration. J Virol 79(16):10788–10795
Streblow DN et al (2005) Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant 5(3):436–442
Tikkanen J et al (2001) Cytomegalovirus infection-enhanced chronic rejection in the rat is prevented by antiviral prophylaxis. Transplant Proc 33(1–2):1801
Zeng H et al (2005) Mechanistic study of malononitrileamide FK778 in cardiac transplantation and CMV infection in rats. Transplantation 79(1):17–22
Orloff SL et al (2002) Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation 73(5):679–688
Orloff SL et al (2000) Tolerance induced by bone marrow chimerism prevents transplant vascular sclerosis in a rat model of small bowel transplant chronic rejection. Transplantation 69(7):1295–1303
Streblow DN et al (2003) Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J Virol 77(3):2182–2194
Soule JL et al (2006) Cytomegalovirus accelerates chronic allograft nephropathy in a rat renal transplant model with associated provocative chemokine profiles. Transplant Proc 38(10):3214–3220
Streblow DN, Orloff SL, Nelson JA (2007) Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol 19(5):577–582
Streblow DN et al (2007) Rat cytomegalovirus gene expression in cardiac allograft recipients is tissue specific and does not parallel the profiles detected in vitro. J Virol 81(8):3816–3826
Merigan TC et al (1992) A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. N Engl J Med 326(18):1182–1186
Valantine HA et al (1999) Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation 100(1):61–66
Orloff SL et al (2011) Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naive recipients. Am J Transplant 11(1):45–55
Duan Y, Ji Z, Atherton SS (1994) Dissemination and replication of MCMV after supraciliary inoculation in immunosuppressed BALB/c mice. Invest Ophthalmol Vis Sci 35(3):1124–1131
Zhang M et al (2005) Infection of retinal neurons during murine cytomegalovirus retinitis. Invest Ophthalmol Vis Sci 46(6):2047–2055
Zinkernagel MS et al (2010) In vivo imaging of ocular MCMV infection. Invest Ophthalmol Vis Sci 51(1):369–374
Zhang M, Xin H, Atherton SS (2005) Murine cytomegalovirus (MCMV) spreads to and replicates in the retina after endotoxin-induced disruption of the blood-retinal barrier of immunosuppressed BALB/c mice. J Neurovirol 11(4):365–375
Kercher L, Mitchell BM (2002) Persisting murine cytomegalovirus can reactivate and has unique transcriptional activity in ocular tissue. J Virol 76(18):9165–9175
Zinkernagel MS et al (2011) Kinetics of ocular and systemic antigen-specific T-cell responses elicited during murine cytomegalovirus retinitis. Immunol Cell Biol 90(3):330–336
Zhang M, Atherton SS (2002) Apoptosis in the retina during MCMV retinitis in immunosuppressed BALB/c mice. J Clin Virol 25(Suppl 2):S137–S147
Zhou J, Zhang M, Atherton SS (2007) Tumor necrosis factor-alpha-induced apoptosis in murine cytomegalovirus retinitis. Invest Ophthalmol Vis Sci 48(4):1691–1700
Bigger JE et al (1999) Protection against murine cytomegalovirus retinitis by adoptive transfer of virus-specific CD8+ T cells. Invest Ophthalmol Vis Sci 40(11):2608–2613
Igietseme JU et al (1991) Mechanisms of protection against herpes simplex virus type 1-induced retinal necrosis by in vitro-activated T lymphocytes. J Virol 65(2):763–768
Bigger JE, Thomas CA III, Atherton SS (1998) NK cell modulation of murine cytomegalovirus retinitis. J Immunol 160(12):5826–5831
Henrickson RV et al (1983) Epidemic of acquired immunodeficiency in rhesus monkeys. Lancet 1(8321):388–390
Baskin GB (1987) Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol 129(2):345–352
Kaup F et al (1998) Gastrointestinal pathology in rhesus monkeys with experimental SIV infection. Pathobiology 66(3–4):159–164
Kuhn EM et al (1999) Immunohistochemical studies of productive rhesus cytomegalovirus infection in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol 36(1):51–56
Kaur A et al (2002) Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J Virol 76(8):3646–3658
Kaur A et al (2003) Direct relationship between suppression of virus-specific immunity and emergence of cytomegalovirus disease in simian AIDS. J Virol 77(10):5749–5758
Sequar G et al (2002) Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J Virol 76(15):7661–7671
Dix RD, Podack ER, Cousins SW (2003) Loss of the perforin cytotoxic pathway predisposes mice to experimental cytomegalovirus retinitis. J Virol 77(6):3402–3408
Aquino-de Jesus MJ, Griffith BP (1989) Cytomegalovirus infection in immunocompromised guinea pigs: a model for testing antiviral agents in vivo. Antiviral Res 12(4):181–193
Polic B et al (1998) Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188(6):1047–1054
Kaur A et al (1996) Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J Virol 70(11):7725–7733
Schlub TE et al (2011) Comparing the kinetics of NK cells, CD4, and CD8 T cells in murine cytomegalovirus infection. J Immunol 187(3):1385–1392
Chan KS, Kaur A (2007) Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques. J Immunol Methods 325(1–2):20–34
Yue Y et al (2006) Characterization and immunological analysis of the rhesus cytomegalovirus homologue (Rh112) of the human cytomegalovirus UL83 lower matrix phosphoprotein (pp65). J Gen Virol 87(Pt 4):777–787
Cekinovic D et al (2008) Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J Virol 82(24):12172–12180
Yue Y, Zhou SS, Barry PA (2003) Antibody responses to rhesus cytomegalovirus glycoprotein B in naturally infected rhesus macaques. J Gen Virol 84(Pt 12):3371–3379
Pass RF et al (2009) Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360(12):1191–1199
Abel K et al (2011) Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques. J Virol 85(6):2878–2890
Jonjic S et al (1990) Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol 64(11):5457–5464
Jonjic S et al (1994) Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med 179(5):1713–1717
Arens R et al (2008) Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol 180(10):6472–6476
Snyder CM et al (2009) CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol 183(6):3932–3941
Bukowski JF, Woda BA, Welsh RM (1984) Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol 52(1):119–128
Scalzo AA et al (1992) The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J Immunol 149(2):581–589
Dokun AO et al (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2(10):951–956
Humphreys IR et al (2007) Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med 204(5):1217–1225
Jonjic S et al (1989) Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 169(4):1199–1212
Lucin P et al (1992) Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol 66(4):1977–1984
Powers C et al (2008) Cytomegalovirus immune evasion. Curr Top Microbiol Immunol 325:333–359
Miller-Kittrell M, Sparer TE (2009) Feeling manipulated: cytomegalovirus immune manipulation. Virol J 6:4
Fleming P et al (1999) The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J Virol 73(8):6800–6809
Saederup N et al (2001) Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J Virol 75(20):9966–9976
Noda S et al (2006) Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood 107(1):30–38
Sparer TE et al (2004) Expression of human CXCR2 in murine neutrophils as a model for assessing cytomegalovirus chemokine vCXCL-1 function in vivo. J Interferon Cytokine Res 24(10):611–620
Miller-Kittrell M et al (2007) Functional characterization of chimpanzee cytomegalovirus chemokine, vCXCL-1(CCMV). Virology 364(2):454–465
Vischer HF, Leurs R, Smit MJ (2006) HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol Sci 27(1):56–63
Cardin RD et al (2009) The M33 chemokine receptor homolog of murine cytomegalovirus exhibits a differential tissue-specific role during in vivo replication and latency. J Virol 83(15):7590–7601
Davis-Poynter NJ, Degli-Esposti M, Farrell HE (1999) Murine cytomegalovirus homologues of cellular immunomodulatory genes. Intervirology 42(5–6):331–341
Beisser PS et al (1998) The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J Virol 72(3):2352–2363
Farrell HE et al (2011) Partial functional complementation between human and mouse cytomegalovirus chemokine receptor homologues. J Virol 85(12):6091–6095
Rivailler P et al (2006) Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. J Virol 80(8):4179–4182
Penfold ME et al (2003) Characterization of the rhesus cytomegalovirus US28 locus. J Virol 77(19):10404–10413
Oxford KL et al (2008) Protein coding content of the U(L)b′ region of wild-type rhesus cytomegalovirus. Virology 373(1):181–188
Lesniewski M et al (2006) Primate cytomegalovirus US12 gene family: a distinct and diverse clade of seven-transmembrane proteins. Virology 354(2):286–298
Alcendor DJ et al (2009) Patterns of divergence in the vCXCL and vGPCR gene clusters in primate cytomegalovirus genomes. Virology 395(1):21–32
Chang WL et al (2009) Human cytomegalovirus suppresses type I interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology 390(2):330–337
Chang WL et al (2007) Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J Immunol 178(12):7794–7804
Chang WL et al (2004) Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol 78(16):8720–8731
Raftery MJ et al (2004) Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol 173(5):3383–3391
Spencer JV et al (2008) Stimulation of B lymphocytes by cmvIL-10 but not LAcmvIL-10. Virology 374(1):164–169
Spencer JV et al (2002) Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol 76(3):1285–1292
Chang WL, Barry PA (2010) Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci U S A 107(52):22647–22652
Lockridge KM et al (2000) Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268(2):272–280
Kloover JS et al (2002) A rat cytomegalovirus strain with a disruption of the r144 MHC class I-like gene is attenuated in the acute phase of infection in neonatal rats. Arch Virol 147(4):813–824
Farrell HE et al (1997) Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature 386(6624):510–514
Farrell H et al (2000) Cytomegalovirus MHC class I homologues and natural killer cells: an overview. Microbes Infect 2(5):521–532
Babic M et al (2010) Cytomegalovirus immunoevasin reveals the physiological role of “missing self” recognition in natural killer cell dependent virus control in vivo. J Exp Med 207(12):2663–2673
Beisser PS et al (2000) The r144 major histocompatibility complex class I-like gene of rat cytomegalovirus is dispensable for both acute and long-term infection in the immunocompromised host. J Virol 74(2):1045–1050
Pande NT et al (2005) Rhesus cytomegalovirus contains functional homologues of US2, US3, US6, and US11. J Virol 79(9):5786–5798
Hansen SG et al (2010) Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328(5974):102–106
Hengel H, Koszinowski UH (2010) Virology. A vaccine monkey wrench? Science 328(5974):51–52
Bohm V et al (2008) The immune evasion paradox: immunoevasins of murine cytomegalovirus enhance priming of CD8 T cells by preventing negative feedback regulation. J Virol 82(23):11637–11650
Zhu H et al (2002) Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc Natl Acad Sci U S A 99(6):3932–3937
Rue CA et al (2004) A cyclooxygenase-2 homologue encoded by rhesus cytomegalovirus is a determinant for endothelial cell tropism. J Virol 78(22):12529–12536
Mocarski ES, Upton JW, Kaiser WJ (2012) Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol 12(2):79–88
Upton JW, Kaiser WJ, Mocarski ES (2010) Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7(4):302–313
Manzur M et al (2009) Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ 16(2):312–320
McCormick AL et al (2003) Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316(2):221–233
McCormick AL (2008) Control of apoptosis by human cytomegalovirus. Curr Top Microbiol Immunol 325:281–295
Goldmacher VS et al (1999) A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A 96(22):12536–12541
Schmidt GM et al (1991) A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N Engl J Med 324(15):1005–1011
Schopfer K, Lauber E, Krech U (1978) Congenital cytomegalovirus infection in newborn infants of mothers infected before pregnancy. Arch Dis Child 53(7):536–539
Stagno S et al (1977) Congenital cytomegalovirus infection. N Engl J Med 296(22):1254–1258
Stagno S et al (1982) Maternal cytomegalovirus infection and perinatal transmission. Clin Obstet Gynecol 25(3):563–576
Diosi P et al (1967) Cytomegalovirus infection associated with pregnancy. Lancet 2(7525):1063–1066
Dworsky M et al (1983) Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 72(3):295–299
Hamprecht K et al (2001) Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 357(9255):513–518
Stagno S et al (1980) Breast milk and the risk of cytomegalovirus infection. N Engl J Med 302(19):1073–1076
Vochem M et al (1998) Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J 17(1):53–58
Adler SP (1991) Cytomegalovirus and child day care: risk factors for maternal infection. Pediatr Infect Dis J 10(8):590–594
Meyers J (1985) Cytomegalovirus infection after organ allografting. The Herpesviruses 4:201
Pass RF (2001) Cytomegalovirus. In: Knipe DM, Howley PM (eds) Fields virology, 4th edn. Lippincott-Raven, Philadelphia, PA
Farroway LN et al (2005) Transmission of two Australian strains of murine cytomegalovirus (MCMV) in enclosure populations of house mice (Mus domesticus). Epidemiol Infect 133(4):701–710
Cheung KS et al (1981) Murine cytomegalovirus infection: hematological, morphological, and functional study of lymphoid cells. Infect Immun 33(1):239–249
Ho M (1991) Cytomegalovirus: biology and infection, 2nd edn. Plenum Medical Books, New York
Hudson JB (1979) The murine cytomegalovirus as a model for the study of viral pathogenesis and persistent infections. Arch Virol 62(1):1–29
Osborn JE, Shahidi NT (1973) Thrombocytopenia in murine cytomegalovirus infection. J Lab Clin Med 81(1):53–63
Klotman ME et al (1990) Detection of mouse cytomegalovirus nucleic acid in latently infected mice by in vitro enzymatic amplification. J Infect Dis 161(2):220–225
Mercer JA, Wiley CA, Spector DH (1988) Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol 62(3):987–997
Reddehase MJ, Podlech J, Grzimek NK (2002) Mouse models of cytomegalovirus latency: overview. J Clin Virol 25(Suppl 2):S23–S36
Collins TM, Quirk MR, Jordan MC (1994) Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol 68(10):6305–6311
Hanson LK et al (2001) Products of US22 genes M140 and M141 confer efficient replication of murine cytomegalovirus in macrophages and spleen. J Virol 75(14):6292–6302
Sacher T et al (2008) Conditional gene expression systems to study herpesvirus biology in vivo. Med Microbiol Immunol 197(2):269–276
Sacher T et al (2011) The role of cell types in cytomegalovirus infection in vivo. Eur J Cell Biol 91(1):70–77
McGregor A, Liu F, Schleiss MR (2004) Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83). J Virol 78(18):9872–9889
Jarvis MA, Nelson JA (2007) Molecular basis of persistence and latency. In: Campadelli-Fiume G, Arvin A, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (eds) Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge
Reddehase MJ et al (2008) Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol 325:315–331
Koffron AJ et al (1998) Cellular localization of latent murine cytomegalovirus. J Virol 72(1):95–103
Podlech J et al (2000) Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 74(16):7496–7507
Podlech J et al (1998) Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol 79(Pt 9):2099–2104
Reddehase MJ et al (1985) Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 55(2):264–273
Griffith BP et al (1981) Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun 32(2):857–863
Tessmer MS, Reilly EC, Brossay L (2011) Salivary gland NK cells are phenotypically and functionally unique. PLoS Pathog 7(1):e1001254
Walton SM et al (2011) T-cell help permits memory CD8(+) T-cell inflation during cytomegalovirus latency. Eur J Immunol 41(8):2248–2259
Seckert CK et al (2011) Antigen-presenting cells of haematopoietic origin prime cytomegalovirus-specific CD8 T-cells but are not sufficient for driving memory inflation during viral latency. J Gen Virol 92(Pt 9):1994–2005
Li Z et al (2012) A mouse model of CMV transmission following kidney transplantation. Am J Transplant 12(4):1024–1028
Baskar JF, Stanat SC, Huang ES (1985) Congenital defects due to reactivation of latent murine cytomegaloviral infection during pregnancy. J Infect Dis 152(3):621–624
Mayo DR, Rapp F (1980) Leukaemia reactivates mouse cytomegalovirus. J Gen Virol 51(Pt 2):401–404
Zhang M et al (2005) Ocular reactivation of MCMV after immunosuppression of latently infected BALB/c mice. Invest Ophthalmol Vis Sci 46(1):252–258
Busche A et al (2009) The mouse cytomegalovirus immediate-early 1 gene is not required for establishment of latency or for reactivation in the lungs. J Virol 83(9):4030–4038
Hummel M, Abecassis MM (2002) A model for reactivation of CMV from latency. J Clin Virol 25(Suppl 2):S123–S136
Hummel M et al (2001) Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J Virol 75(10):4814–4822
Kloover JS et al (2002) Persistent rat cytomegalovirus (RCMV) infection of the salivary glands contributes to the anti-RCMV humoral immune response. Virus Res 85(2):163–172
Klotman ME, Starnes D, Hamilton JD (1985) The source of murine cytomegalovirus in mice receiving kidney allografts. J Infect Dis 152(6):1192–1196
Woolf NK et al (1985) Hearing loss in experimental cytomegalovirus infection of the guinea pig inner ear: prevention by systemic immunity. Ann Otol Rhinol Laryngol 94(4 Pt 1):350–356
Harrison CJ et al (1995) Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J Infect Dis 172(5):1212–1220
Schleiss MR et al (2004) Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis 189(8):1374–1381
Schleiss MR et al (2000) Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral Immunol 13(2):155–167
Schleiss MR, Bourne N, Bernstein DI (2003) Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis 188(12):1868–1874
Schleiss MR et al (2007) Preconceptual administration of an alphavirus replicon UL83 (pp 65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis 195(6):789–798
Yue Y et al (2007) Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65-2, and viral interleukin-10 in rhesus macaques. J Virol 81(3):1095–1109
Slavuljica I et al (2010) Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest 120(12):4532–4545
Mohr CA et al (2010) A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. J Virol 84(15):7730–7742
Morello CS et al (2005) Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge. J Virol 79(1):159–175
Snyder CM et al (2010) Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One 5(3):e9681
MacDonald MR et al (1998) Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by using an attenuated MCMV mutant. J Virol 72(1):442–451
Sandford GR, Burns WH (1988) Use of temperature-sensitive mutants of mouse cytomegalovirus as vaccines. J Infect Dis 158(3):596–601
Kern ER (2006) Pivotal role of animal models in the development of new therapies for cytomegalovirus infections. Antiviral Res 71(2–3):164–171
Shanley JD, Morningstar J, Jordan MC (1985) Inhibition of murine cytomegalovirus lung infection and interstitial pneumonitis by acyclovir and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother 28(2):172–175
Rybak RJ et al (1999) Effective treatment of murine cytomegalovirus infections with methylenecyclopropane analogues of nucleosides. Antiviral Res 43(3):175–188
Kern ER et al (2004) Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob Agents Chemother 48(12):4745–4753
Matthews T, Boehme R (1988) Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis 10(Suppl 3):S490–S494
Beadle JR et al (2002) Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Chemother 46(8):2381–2386
Bourne N, Bravo FJ, Bernstein DI (2000) Cyclic HPMPC is safe and effective against systemic guinea pig cytomegalovirus infection in immune compromised animals. Antiviral Res 47(2):103–109
White DR et al (2006) The effect of cidofovir on cytomegalovirus-induced hearing loss in a Guinea pig model. Arch Otolaryngol Head Neck Surg 132(6):608–615
Schleiss MR, Anderson JL, McGregor A (2006) Cyclic cidofovir (cHPMPC) prevents congenital cytomegalovirus infection in a guinea pig model. Virol J 3:9
Schleiss MR et al (2005) The non-nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus (CMV) disease and mortality in immunocompromised guinea pigs. Antiviral Res 65(1):35–43
North TW et al (2004) Rhesus cytomegalovirus is similar to human cytomegalovirus in susceptibility to benzimidazole nucleosides. Antimicrob Agents Chemother 48(7):2760–2765
Allen LB et al (1992) Novel method for evaluating antiviral drugs against human cytomegalovirus in mice. Antimicrob Agents Chemother 36(1):206–208
Gao L et al (2007) An animal model of human cytomegalovirus infection. Transplant Proc 39(10):3438–3443
Mocarski ES et al (1993) Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci U S A 90(1):104–108
DiLoreto D Jr et al (1994) Cytomegalovirus infection of human retinal tissue: an in vivo model. Lab Invest 71(1):141–148
Bidanset DJ et al (2001) Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J Infect Dis 184(2):192–195
Kern ER et al (2001) Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother 12(Suppl 1):149–156
Bidanset DJ et al (2004) Efficacy of ganciclovir and cidofovir against human cytomegalovirus replication in SCID mice implanted with human retinal tissue. Antiviral Res 63(1):61–64
Wang W et al (2005) Human cytomegalovirus genes in the 15-kilobase region are required for viral replication in implanted human tissues in SCID mice. J Virol 79(4):2115–2123
Laycock KA et al (1997) An in vivo model of human cytomegalovirus retinal infection. Am J Ophthalmol 124(2):181–189
Smith MS et al (2010) Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe 8(3):284–291
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Dogra, P., Sparer, T.E. (2014). What We Have Learned from Animal Models of HCMV. In: Yurochko, A., Miller, W. (eds) Human Cytomegaloviruses. Methods in Molecular Biology, vol 1119. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-788-4_15
Download citation
DOI: https://doi.org/10.1007/978-1-62703-788-4_15
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-787-7
Online ISBN: 978-1-62703-788-4
eBook Packages: Springer Protocols