Abstract
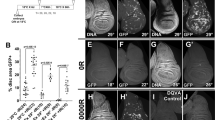
Under genotoxic stress, activation of cell cycle checkpoint responses leads to cell cycle arrest, which allows cells to repair DNA damage before continuing to cycle. Drosophila larval epithelial sacs, called imaginal discs, are an excellent in vivo model system for studying radiation-induced cell cycle arrest. Larval imaginal discs go into cell cycle arrest after being subjected to low-dose irradiation, are subject to easy genetic manipulation, are not crucial for survival of the organism, and can be dissected easily for further molecular or cellular analysis. In this chapter, we describe methods for assessing low-dose irradiation-induced cell cycle arrest. Mitotic cells are identified by immunofluorescence staining for the mitotic marker phosphorylated histone H3 (phospho-histone H3 or pH3). When wandering third-instar control larvae, without transgene expression, are exposed to 500 rads of X-ray or γ-ray irradiation, the number of pH3-positive cells in wing imaginal discs is reduced from hundreds before irradiation to approximately 30 after irradiation, with an equal distribution between the anterior and posterior compartments (Yan et al., 2011, FASEB J). Using the GAL4/UAS system, RNAi, cDNA, or microRNA sponge transgenes can be expressed in the posterior compartment of the wing disc using drivers such as engrailed (en)-Gal4, while the anterior compartment serves as an internal control. This approach makes it possible to do genome-wide genetic screening for molecules involved in radiation-induced cell cycle arrest.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Yan, S. J., Lim, S. J., Shi, S., Dutta, P., and Li, W. X. (2011) Unphosphorylated STAT and heterochromatin protect genome stability, FASEB J. 1, 232–241.
Morgan, T. H. (1910) Sex limited inheritance in Drosophila, Science 32, 120–122.
Bellen, H. J., Tong, C., and Tsuda, H. (2010) 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future, Nat Rev Neurosci 11, 514–522.
Cohen, B. (1995) Nobel committee rewards pioneers of development studies in fruitflies, Nature 377, 465.
Raju, T. N. (1999) The Nobel chronicles. 1933: Thomas Hunt Morgan (1866–1945), Lancet 353, 157.
Elledge, S. J. (1996) Cell cycle checkpoints: preventing an identity crisis, Science 274, 1664–1672.
Song, Y. H. (2005) Drosophila melanogaster: a model for the study of DNA damage checkpoint response, Mol Cells 19, 167–179.
Weatherbee, S. D., and Carroll, S. B. (1999) Selector genes and limb identity in arthropods and vertebrates, Cell 97, 283–286.
Yan, S. J., Gu, Y., Li, W. X., and Fleming, R. J. (2004) Multiple signaling pathways and a selector protein sequentially regulate Drosophila wing development, Development 131, 285–298.
Garcia-Bellido, A., and Merriam, J. R. (1971) Parameters of the wing imaginal disc development of Drosophila melanogaster, Dev Biol 24, 61–87.
Brand, A. H., and Perrimon, N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes, Development 118, 401–415.
Dietzl, G., Chen, D., Schnorrer, F., Su, K. C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S., Couto, A., Marra, V., Keleman, K., and Dickson, B. J. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila, Nature 448, 151–156.
Loya, C. M., Lu, C. S., Van Vactor, D., and Fulga, T. A. (2009) Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms, Nat Methods 6, 897–903.
Pospisilik, J. A., Schramek, D., Schnidar, H., Cronin, S. J., Nehme, N. T., Zhang, X., Knauf, C., Cani, P. D., Aumayr, K., Todoric, J., Bayer, M., Haschemi, A., Puviindran, V., Tar, K., Orthofer, M., Neely, G. G., Dietzl, G., Manoukian, A., Funovics, M., Prager, G., Wagner, O., Ferrandon, D., Aberger, F., Hui, C. C., Esterbauer, H., and Penninger, J. M. (2010) Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate, Cell 140, 148–160.
Neely, G. G., Kuba, K., Cammarato, A., Isobe, K., Amann, S., Zhang, L., Murata, M., Elmen, L., Gupta, V., Arora, S., Sarangi, R., Dan, D., Fujisawa, S., Usami, T., Xia, C. P., Keene, A. C., Alayari, N. N., Yamakawa, H., Elling, U., Berger, C., Novatchkova, M., Koglgruber, R., Fukuda, K., Nishina, H., Isobe, M., Pospisilik, J. A., Imai, Y., Pfeufer, A., Hicks, A. A., Pramstaller, P. P., Subramaniam, S., Kimura, A., Ocorr, K., Bodmer, R., and Penninger, J. M. (2010) A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function, Cell 141, 142–153.
Bodenstein, D. (1994) The postembryonic development of Drosophila. In Biology of Drosophila (ed. M. Demerec), Facsimile edn., pp. 275–367, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Acknowledgments
We are grateful to Christine Summers in the Bohmann lab for providing recipes and protocols for making fly food and apple juice agar plates. We thank Dr L. Silver-Morse for critical reading of the manuscript. S.J.Y. is supported by a postdoctoral training grant (T32CA009363) from the National Institutes of Health. The Li lab is supported by grants from the National Institutes of Health, an American Cancer Society Research Scholar Grant, and a Leukemia & Lymphoma Society Research Scholar Grant to W.X.L.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Yan, SJ., Li, W.X. (2011). Using Drosophila Larval Imaginal Discs to Study Low-Dose Radiation-Induced Cell Cycle Arrest. In: Li, W. (eds) Cell Cycle Checkpoints. Methods in Molecular Biology, vol 782. Humana Press. https://doi.org/10.1007/978-1-61779-273-1_8
Download citation
DOI: https://doi.org/10.1007/978-1-61779-273-1_8
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-61779-272-4
Online ISBN: 978-1-61779-273-1
eBook Packages: Springer Protocols