Abstract
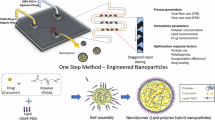
Taxane chemotherapeutics have played a key role in the treatment of various types of cancer throughout the past years. However, the drawbacks inherent to the pharmaceutical formulation of taxanes are still a reality and mainly due to the low aqueous solubility of these medicines, as well as to the nontargeted therapy and consequent side effects. Nanoparticles (NPs) of poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) have sparked broad interest in this field and demonstrated capacity of improving taxanes’ formulation. If, in one hand, the PLGA core of these NPs is able to solubilize drugs, on the other hand, the PEG shell promotes immune escape and presents chemical end groups for the attachment of targeting ligands. Advances in the design of these nanosystems resulted in the development of multitargeted PLGA/PEG NPs achieved by dual-ligand functionalization. The multitargeting offers a promising alternative to the delivery of taxanes across successive cell types or compartments and to the synergetic exploitation of more than one transporter on the cell surface. Besides the upgrade in the design of multitargeted PLGA/PEG NPs, their manufacturing has also evolved from bulk assembly to continuous-flow, high-throughput technologies such as microfluidics. This technology relies on microchannel platforms described to enable the production of large-scale batches of NPs in a better time-saving manner, with higher drug loading, reproducibility, and lower polydispersity. Herein, a detailed microfluidic method for the preparation of multitargeted, taxane-loaded PLGA/PEG NPs is described. Focus is given to the setting up of the microfluidic system and conditions required to manufacture these NPs by using polymers of PLGA and PEG previously elsewhere functionalized with two generic targeting ligands.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Perez EA (2009) Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 8(8):2086–2095. https://doi.org/10.1158/1535-7163.Mct-09-0366
Gligorov J, Lotz JP (2004) Preclinical pharmacology of the taxanes: implications of the differences. Oncologist 9(Suppl 2):3–8
Elhissi A, Mahmood R, Parveen I, Vali A, Ahmed W, Jackson MJ (2018) Taxane formulations: from plant to clinic. In: Jackson MJ, Ahmed W (eds) Micro and nanomanufacturing volume II. Springer, Cham, pp 23–33. https://doi.org/10.1007/978-3-319-67132-1_2
Yared JA, Tkaczuk KHR (2012) Update on taxane development: new analogs and new formulations. Drug Des Devel Ther 6:371–384. https://doi.org/10.2147/DDDT.S28997
Harrison MR, Holen KD, Liu G (2009) Beyond taxanes: a review of novel agents that target mitotic tubulin and microtubules, kinases, and kinesins. Clin Adv Hematol Oncol 7(1):54–64
Gaucher G, Marchessault RH, Leroux J-C (2010) Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Control Release 143(1):2–12. https://doi.org/10.1016/j.jconrel.2009.11.012
Feng L, Mumper RJ (2013) A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett 334(2):157–175. https://doi.org/10.1016/j.canlet.2012.07.006
Reul R, Renette T, Bege N, Kissel T (2011) Nanoparticles for paclitaxel delivery: a comparative study of different types of dendritic polyesters and their degradation behavior. Int J Pharm 407(1):190–196. https://doi.org/10.1016/j.ijpharm.2011.01.028
Enlow EM, Luft JC, Napier ME, DeSimone JM (2011) Potent engineered PLGA nanoparticles by virtue of exceptionally high chemotherapeutic loadings. Nano Lett 11(2):808–813. https://doi.org/10.1021/nl104117p
Rezvantalab S, Drude NI, Moraveji MK, Güvener N, Koons EK, Shi Y, Lammers T, Kiessling F (2018) PLGA-based nanoparticles in cancer treatment. Front Pharmacol 9:1260–1260. https://doi.org/10.3389/fphar.2018.01260
Rafiei P, Haddadi A (2017) Pharmacokinetic consequences of PLGA nanoparticles in docetaxel drug delivery. Pharm Nanotechnol 5(1):3–23. https://doi.org/10.2174/2211738505666161230110108
Khalil NM, TCFd N, Casa DM, Dalmolin LF, ACd M, Hoss I, Romano MA, Mainardes RM (2013) Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces 101:353–360. https://doi.org/10.1016/j.colsurfb.2012.06.024
Kroon J, Kooijman S, Cho N-J, Storm G, van der Pluijm G (2016) Improving taxane-based chemotherapy in castration-resistant prostate cancer. Trends Pharmacol Sci 37(6):451–462. https://doi.org/10.1016/j.tips.2016.03.003
Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G (2011) Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci 354(1):116–123. https://doi.org/10.1016/j.jcis.2010.10.024
Garg S, Heuck G, Ip S, Ramsay E (2016) Microfluidics: a transformational tool for nanomedicine development and production. J Drug Target 24(9):821–835. https://doi.org/10.1080/1061186x.2016.1198354
Martins C, Araújo F, Gomes MJ, Fernandes C, Nunes R, Li W, Santos HA, Borges F, Sarmento B (2018) Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur J Pharm Biopharm. https://doi.org/10.1016/j.ejpb.2018.01.014
Martins JP, Torrieri G, Santos HA (2018) The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin Drug Deliv 15(5):469–479. https://doi.org/10.1080/17425247.2018.1446936
Gdowski A, Johnson K, Shah S, Gryczynski I, Vishwanatha J, Ranjan A (2018) Optimization and scale up of microfluidic nanolipomer production method for preclinical and potential clinical trials. J Nanobiotechnol 16(1):12–12. https://doi.org/10.1186/s12951-018-0339-0
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41(7):2545–2561. https://doi.org/10.1039/c2cs15327k
Martins C, Sousa F, Araújo F, Sarmento B (2017) Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv Healthc Mater 7:1701035. https://doi.org/10.1002/adhm.201701035
Mout R, Moyano DF, Rana S, Rotello VM (2012) Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev 41(7):2539–2544. https://doi.org/10.1039/c2cs15294k
Feng L, Zhu C, Yuan H, Liu L, Lv F, Wang S (2013) Conjugated polymer nanoparticles: preparation, properties, functionalization and biological applications. Chem Soc Rev 42(16):6620–6633. https://doi.org/10.1039/C3CS60036J
Bastakoti BP, Liu Z (2017) Multifunctional polymeric micelles as therapeutic nanostructures: targeting, imaging, and triggered release. In: Ficai A, Grumezescu AM (eds) Nanostructures for cancer therapy. Elsevier, Amsterdam, pp 261–283. https://doi.org/10.1016/B978-0-323-46144-3.00010-6
Shi J, Xiao Z, Kamaly N, Farokhzad OC (2011) Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res 44(10):1123–1134. https://doi.org/10.1021/ar200054n
Pfister D, Morbidelli M (2014) Process for protein PEGylation. J Control Release 180:134–149. https://doi.org/10.1016/j.jconrel.2014.02.002
Chen Y, Xianyu Y, Wu J, Yin B, Jiang X (2016) Click chemistry-mediated nanosensors for biochemical assays. Theranostics 6(7):969–985. https://doi.org/10.7150/thno.14856
Q-s X, H-m D, Ma Y-q (2017) Can dual-ligand targeting enhance cellular uptake of nanoparticles? Nanoscale 9(26):8982–8989. https://doi.org/10.1039/C7NR01020F
Zhu Y, Feijen J, Zhong Z (2018) Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today 18:65–85. https://doi.org/10.1016/j.nantod.2017.12.007
Gao H (2017) Perspectives on dual targeting delivery systems for brain tumors. J Neuroimmune Pharmacol 12(1):6–16. https://doi.org/10.1007/s11481-016-9687-4
Xiong L, Du X, Kleitz F, Qiao SZ (2015) Cancer-cell-specific nuclear-targeted drug delivery by dual-ligand-modified mesoporous silica nanoparticles. Small 11(44):5919–5926. https://doi.org/10.1002/smll.201501056
Liu Y, Hui Y, Ran R, Yang G-Z, Wibowo D, Wang H-F, Middelberg APJ, Zhao C-X (2018) Synergetic combinations of dual-targeting ligands for enhanced in vitro and in vivo tumor targeting. Adv Healthc Mater 7(15):1800106. https://doi.org/10.1002/adhm.201800106
Elias DR, Poloukhtine A, Popik V, Tsourkas A (2013) Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 9(2):194–201. https://doi.org/10.1016/j.nano.2012.05.015
Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P (2013) Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev 42(3):1147–1235. https://doi.org/10.1039/c2cs35265f
Khmelnitsky YL, Belova AB, Levashov AV, Mozhaev VV (1991) Relationship between surface hydrophilicity of a protein and its stability against denaturation by organic solvents. FEBS Lett 284(2):267–269
Sousa F, Fonte P, Cruz A, Kennedy PJ, Pinto IM, Sarmento B (2018) Polyester-based nanoparticles for the encapsulation of monoclonal antibodies. In: Picanço-Castro V, Swiech K (eds) Recombinant glycoprotein production, vol 1674. Springer, New York, NY, pp 239–253. https://doi.org/10.1007/978-1-4939-7312-5_20
Clark AJ, Davis ME (2015) Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A 112(40):12486–12491. https://doi.org/10.1073/pnas.1517048112
Matsumoto A, Matsukawa Y, Suzuki T, Yoshino H (2005) Drug release characteristics of multi-reservoir type microspheres with poly(dl-lactide-co-glycolide) and poly(dl-lactide). J Control Release 106(1):172–180. https://doi.org/10.1016/j.jconrel.2005.03.026
Dragan U, Magdalena S (2009) Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci 5(1):1–14. https://doi.org/10.2174/157341309787314566
Kennedy PJ, Perreira I, Ferreira D, Nestor M, Oliveira C, Granja PL, Sarmento B (2018) Impact of surfactants on the target recognition of Fab-conjugated PLGA nanoparticles. Eur J Pharm Biopharm 127:366–370. https://doi.org/10.1016/j.ejpb.2018.03.005
Chiesa E, Dorati R, Pisani S, Conti B, Bergamini G, Modena T, Genta I (2018) The microfluidic technique and the manufacturing of polysaccharide nanoparticles. Pharmaceutics 10(4). https://doi.org/10.3390/pharmaceutics10040267
Lin J, Pan X, Yin Z, Ku X (2016) Solution of general dynamic equation for nanoparticles in turbulent flow considering fluctuating coagulation. Appl Math Mech 37(10):1275–1288. https://doi.org/10.1007/s10483-016-2131-9
Liu D, Zhang H, Fontana F, Hirvonen J, Santos HA (2017) Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 17(11):1856–1883. https://doi.org/10.1039/c7lc00242d
Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, Whitesides GM (2002) Chaotic mixer for microchannels. Science 295(5555):647–651. https://doi.org/10.1126/science.1066238
Sharp KV, Adrian RJ (2004) Transition from laminar to turbulent flow in liquid filled microtubes. Exp Fluids 36(5):741–747. https://doi.org/10.1007/s00348-003-0753-3
Acknowledgments
This work is a result of the project NORTE-01-0145-FEDER-000012, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). The work was also financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). C.M. gratefully acknowledges FCT—Fundação para a Ciência e a Tecnologia, Portugal, for financial support (grant SFRH/BD/137946/2018).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Martins, C., Sarmento, B. (2020). Microfluidic Manufacturing of Multitargeted PLGA/PEG Nanoparticles for Delivery of Taxane Chemotherapeutics. In: Jain, K. (eds) Drug Delivery Systems. Methods in Molecular Biology, vol 2059. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9798-5_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9798-5_11
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9797-8
Online ISBN: 978-1-4939-9798-5
eBook Packages: Springer Protocols