Abstract
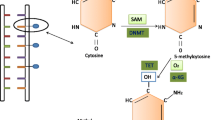
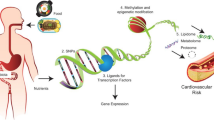
Precision medicine is a revolutionary approach to disease prevention and treatment that takes into account individual differences in lifestyle, environment, and biology. The US National Institutes of Health has recently launched The All of Us Research Program (2016) to extend precision medicine to all diseases by building a national research cohort of one million or more US participants. This review is limited to how the human microbiome factors into precision medicine from the applied aspect of preventing and managing cancer. The Precision Medicine Initiative was established in an effort to address particular characteristics of each person with the aim to increase the effectiveness of medical interventions in terms of prevention and treatment of multiple diseases including cancer. Many factors contribute to the response to an intervention. The microbiome and microbially produced metabolites are capable of epigenetic modulation of gene activity, and can influence the response through these mechanisms. The fact that diet has an impact on microbiome implies that it will also affect the epigenetic mechanisms involving microbiota. In this chapter, we review some major epigenetic mechanisms, notably DNA methylation, chromatin remodeling and histone modification, and noncoding RNA, implicated in cancer prevention and treatment. Several examples of how microbially produced metabolites from food influence cancer risk and treatment response through epigenetic mechanisms will be discussed. Some challenges include the limited understanding of how diet shapes the microbiome and how to best evaluate those changes since both, diet and the microbiota, exhibit daily and seasonal variations. Ongoing research seeks to understand the relationship between the human microbiome and multiple diseases including cancer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
The White House (2015) President Obama’s Precision Medicine Initiative. https://obamawhitehouse.archives.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative
Hullar MA, Fu BC (2014) Diet, the gut microbiome, and epigenetics. Cancer J 20(3):170–175
David LA, Maurice CF, Carmody RN, Gootenberg DB, JE B, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–563
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489(7415):220–230
Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C, Tollefsbol TO (2015) Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics 7:112. https://doi.org/10.1186/s13148-015-0144-7
Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, Nelson KE (2017) The human microbiome and cancer. Cancer Prev Res 10(4):226–234
Linus Pauling Institute (2017) Micronutrient information: folate. http://lpi.oregonstate.edu/mic/vitamins/folate
Bueno O, Molloy AM, Fernandez-Ballart JD, García-Minguillán CJ, Ceruelo S, Ríos L, Ueland PM, Meyer K, Murphy MM (2016) Common polymorphisms that affect folate transport or metabolism modify the effect of the MTHFR 677C> T polymorphism on folate status. J Nutr 146(1):1–8
Rossi M, Amaretti A, Raimondi S (2011) Folate production by probiotic bacteria. Nutrients 3(1):118–134
Pompei A, Cordisco L, Amaretti A, Zanoni S, Raimondi S, Matteuzzi D, Rossi M (2007) Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J Nutr 137(12):2742–2746
Strozzi GP, Mogna L (2008) Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J Clin Gastroenterol 42(S3):S179–S184
Thomas CM, Saulnier DMA, Spinler JK, Hemarajata P, Gao C, Jones SE, Grimm S, Balderas MA, Burstein MD, Morra C, Roeth D, Kalkum M, Versalovic J (2016) FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiology 5(5):802–818
Matherly LH, Hou Z, Deng Y (2007) Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev 26:111–128
Zhao R, Matherly LH, Goldman ID (2009) Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11:e4. https://doi.org/10.1017/S1462399409000969
Hurst NR, Kendig DM, Murthy KS, Grider JR (2014) The short chain fatty acids, butyrate and propionate have differential effects on the motility of the Guinea pig colon. Neurogastroenterol Motil 26(11):1586–1596
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227
Canani RB, Costanzo M, Leone L (2012) The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics 4(1):4. https://doi.org/10.1186/1868-7083-4-4
Liu D, Andrade SP, Castro PR, Treacy J, Ashworth J, Slevin M (2016) Low concentration of sodium butyrate from ultrabraid + NaBu suture, promotes angiogenesis and tissue remodelling in tendon-bones injury. Sci Rep 6:346–349
Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M (2017) Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J 11:841–852
Miquel S, Martín R, Bridonneau C, Robert V, Sokol H, Bermúdez-Humarán LG, Thomas M, Langella P (2014) Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 5(2):146–151
Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7:979. https://doi.org/10.3389/fmicb.2016.00979
Wu N, Yang X, Zhang R, , Li J, Xiao X, Hu Y, Chen Y, Yang F, Lu N, Wang Z, Luan C, Liu Y, Wang B, Xiang C, Wang Y, Zhao F, Gao GF, Wang S, Li L, Zhang H, Zhu B (2013) Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol 66(2):462–470
Roth SY, Denu JM, Allis CD (2001) Histone acetyltransferases. Annu Rev Biochem 70(1):81–120
Licciardi PV, Ververis K, Karagiannis TC (2011) Histone deacetylase inhibition and dietary short-chain fatty acids. ISRN Allergy 2011:869647. https://doi.org/10.5402/2011/869647
Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D et al (2008) Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem 19(9):587–593
Tang Y, Wang J, Lian Y, Fan C, Zhang P, Wu Y, Li X, Xiong F, Li X, Li G, Xiong W, Zeng Z (2017) Linking long non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancer. Mol Cancer 16(1):42. https://doi.org/10.1186/s12943-017-0612-0
Reisman D, Glaros S, Thompson EA (2009) The SWI/SNF complex and cancer. Oncogene 28:1653–1668
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10(3):155–159
Myzak MC, Dashwood WM, Orner GA et al (2006) Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J 20(3):506–508
American Cancer Society (2017) Key statistics for colorectal cancer. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
American Institute for Cancer Research (2017) Learn about colorectal cancer. http://www.aicr.org/learn-more-about-cancer/colorectal-cancer/
Ohigashi S, Sudo K, Kobayashi D, Takahashi O, Takahashi T, Asahara T, Nomoto K, Onodera H (2013) Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci 58(6):1717–1726
Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T (2011) Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343:d6617. https://doi.org/10.1136/bmj.d6617
Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP (2013) Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8(8):e70803. https://doi.org/10.1371/journal.pone.0070803
Tjalsma H, Boleij A, Marchesi JR, Dutilh BE (2012) A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol 10:575–582
Brabban AD, Edwards C (1994) Isolation of glucosinolate degrading microorganisms and their potential for reducing the glucosinolate content of rapemeal. FEMS Microbiol Lett 119:83–88
Elfoul L, Rabot S, Khelifa N, Quinsac A, Duguay A, Rimbault A (2001) Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol Lett 197:99–103
Holst B, Williamson G (2003) A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep 21(3):425–447
Kundu S, Kumar S, Bajaj A (2015) Cross-talk between bile acids and gastrointestinal tract for progression and development of cancer and its therapeutic implications. IUMBM Life 67(7):514–523
Khare T, Khare S (2014) Controversies in chemoprevention of colorectal cancer with ursodeoxycholic. Acid JSM Gastroenterol Hepatol 2(1):1009
Dziedzic K, Szwengie A, Górecka D, Gujska E, Kaczkowska J, Drożdżyńska A, Walkowiak J (2016) Effect of wheat dietary fiber particle size during digestion in vitro on bile acid, faecal bacteria and short-chain fatty acid content. Plant Foods Hum Nutr 71:151–157
Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI (2010) Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res 70(20):7960–7969
Hodge AM, Williamson EJ, Bassett JK, MacInnis RJ, Giles GG, English DR (2015) Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int J Cancer 137(5):1224–1223
Young TK, Kelly JJ, Friborg J, Soininen L, Wong KO (2016) Cancer among circumpolar populations: an emerging public health concern. Int J Circumpolar Health 75(1):29787
O’Brien DK, Upton L (2008) Cancer incidence and mortality in Alaska, 1996–2004. Alaska Department of Health and Social Services, Section of Chronic Disease and Health Promotion, Alaska Cancer Registry. Depdhss, Anchorage (AK alaska.gov/dph/Chronic/Documents/Cancer/assets/cancerRegistry1996-2004.pdf
Hofmanová J, Vaculová A, Lojek A, Kozubík A (2005) Interaction of polyunsaturated fatty acids and sodium butyrate during apoptosis in HT-29 human colon adenocarcinoma cells. Eur J Nutr 44:40–51
Kolar SSN, Barhoumi R, Callaway ES, Barhoumi R, Callaway ES, Fan Y-Y, Wang N, Lupton JR, Chapkin RS (2007) Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol 293:G935–G943
O'Keefe SJ (2016) Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 13(12):691–706
Turner ND, Lloyd SK (2017) Association between red meat consumption and colon cancer: a systematic review of experimental results. Exp Biol Med 242(8):813–839
Sun J, Kato I (2016) Gut microbiota, inflammation and colorectal cancer. Genes Dis 3(2):130–113
Corredoira-Sánchez J, García-Garrote F, Rabuñal R, López-Roses L, García-País MJ, Castro E, González-Soler R, Coira A, Pita J, López-Álvarez MJ, Alonso MP, Varela J (2012) Association between bacteremia due to streptococcus gallolyticus subsp. Gallolyticus (streptococcus bovis I) and colorectal neoplasia: a case control study. Clin Infect Dis 55(4):491–494
Sharara AI, Hamdan AT, Malli A, El-Halabi MM, Hashash JG, Ghaith OA, Kanj SS (2013) Association of Streptococcus bovis endocarditis and advanced colorectal neoplasia: a case-control study. J Dig Dis 14(7):382–387
zur Hausen H (2006) Streptococcus bovis: causal or incidental involvement in cancer of the colon? Int J Cancer 119(9):xi–xii. https://doi.org/10.1002/ijc.22314
National Cancer Institute (2017) Cancer stats facts: female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html
National Cancer Institute (2017) Cancer stats facts: prostate cancer. https://seer.cancer.gov/statfacts/html/prost.html
Shimizu K, Muranaka Y, Fujimura R, Ishida H, Tazume S, Shimamura T (1998) Normalization of reproductive function in germfree mice following bacterial contamination. Exp Anim 47(3):151–158
Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goeder JJ (2014) Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab 99(12):4632–4640
Li F, Hullar MA, Beresford SA, Lampe JW (2011) Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr 106(3):408–416
de Figueiredo SM, Binda NS, Nogueira-Machado JA, Vieira-Filho SA, Caligiorne RB (2015) The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat Endocr Metab Immune Drug Discov 9(1):24–39
Meeran SM, Patel SN, Tollefsbol TO (2010) Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One 5(7):e11457
Vázquez L, Flórez AB, Guadamuro L, Mayo B (2017) Effect of soy isoflavones on growth of representative bacterial species from the human gut. Nutrients 9(7):E727. https://doi.org/10.3390/nu9070727
Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA (2000) Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer 36(1):27–32
Itsumi M, Shiota M, Takeuchi A, Kashiwagi E, Inokuchi J, Tatsugami K, Kajioka S, Uchiumi T, Naito S, Eto M, Yokomizo A (2016) Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci 107(7):1022–1028
Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I (2001) Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21(4):504–516
Zhang W, Hong L, Graham DY (2014) An update on helicobacter pylori as the cause of gastric cancer. Gastrointest Tumors 1(3):155–165
Moss SF (2016) The clinical evidence linking helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol 3(2):183–191
Khalsa J, Duffy LC, Riscuta G, Starke-Reed P, Hubbard VS (2017) Omics for understanding the gut-liver-microbiome axis and precision medicine. Clin Pharmacol Drug Dev 6(2):176–185
Kuntz TM, Gilbert JA (2017) Introducing the microbiome into precision medicine. Trends Pharmacol Sci 38(1):81–91
Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB (2015) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64(5):731–742
Riscuta G, Dumitrescu RG (2012) Nutrigenomics: implications for breast and colon cancer prevention. Methods Mol Biol 863:343–358
Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O'Hanlon DM, Burton JP, Francis KP, Tangney M, Reid G (2014) Microbiota of human breast tissue. Appl Environ Microbiol 80(10):3007–3014
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Riscuta, G., Xi, D., Pierre-Victor, D., Starke-Reed, P., Khalsa, J., Duffy, L. (2018). Diet, Microbiome, and Epigenetics in the Era of Precision Medicine. In: Dumitrescu, R., Verma, M. (eds) Cancer Epigenetics for Precision Medicine . Methods in Molecular Biology, vol 1856. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-8751-1_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8751-1_8
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-8750-4
Online ISBN: 978-1-4939-8751-1
eBook Packages: Springer Protocols