Abstract
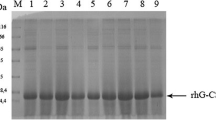
The human granulocytic colony-stimulating factor (hG-CSF) acts mainly by promoting the maturation of granulocytes and stimulating their phagocytic and chemotactic activity. It has been used in the treatment of many diseases, in particular in neutropenic conditions. Here, we describe the purification process of the recombinant protein hG-CSF expressed in Pichia pastoris. The protein purification proved to be efficient using the nickel affinity chromatography method described in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Clark SC, Kamen R (1987) The human hematopoietic colony-stimulating factors. Science 236:1229–1237
Callard RE (1994) The cytokine facts book, vol 24. Academic Press, San Diego, p 265
Iwasaki H, Shimoda K, Okamura S, Otsuka T, Nagafuji K, Harada N et al (1999) Production of soluble granulocyte colony-stimulating factor receptors from myelomonocytic cells. J Immunol 163:6907–6912
Hubel K, Engert A (2003) Clinical applications of granulocyte colony-stimulating factor: an update and summary. Ann Hematol 87:207–213
Hammerling U, Kroon R, Sjodin L (1995) In vitro bioassay with bioassay with enhanced sensitivity for human granulocyte colony-stimulating factor. J Pharm Biomed Anal 13:9–20
Babaeipour V, Khanchezar S, Mofid MR, Abbas MPH (2015) Efficient process development of recombinant human granulocyte colony-stimulating factor (rh-GCSF) production in Escherichia coli. Iran Biomed J 2:102–110
Karimi Z, Nezafat N, Negahdaripour M, Aydin B, Hemmati S, Ghasemi Y (2015) The effect of rare codons following the ATG start codon on expression of human granulocyte-colony stimulating factor in Escherichia coli. Protein Expr Purif 114:108–114
Maity N, Thawani A, Sharma A, Gautam A, Mishira S, Sahai V (2015) Expression and control of codon-optimized granulocyte colony-stimulating factor in Pichia pastoris. Appl Biochem Biotechnol 178:159–172
Apte-Deshpande A, Somani S, Mandal G, Soorapaneni S, Padmanabhan S (2009) Over expression and analysis of O-glycosylated recombinant human granulocyte colony stimulating factor in Pichia Pastoris using Agilent 2100 Bioanalyzer. J Biotechnol 143:44–50
Bashir S, Sadaf S, Ahmad S, Akhtar MW (2015) Enhanced and secretory expression of human granulocyte colony stimulating factor by Bacillus subtilis SCK6. BioMed Res Int. ID 636249:9p
Talebkhan Y, Samadi T, Samie A, Barkhordari F, Azizi M, Khalaj V, Mirabzadeh E (2016) Expression of granulocyte colony stimulating factor (GCSF) in Hansenula polymorpha. Iran J Microbiol 8:21–28
Codevilla CF et al (2004) Biological potency evaluation and characterization of rhG-CSF in pharmaceutical products. Braz J Hematol Hemother 26:104–108
Gomes FR, Maluenda AC, Tapias JO, Oliveira FL et al (2012) Expression of recombinant human mutant granulocyte colony stimulating factors (Nartograstim) in Escherichia coli. World J Microbiol Biotechnol 28:2593–2600
Vans ALS, Renard G, Palma MS et al (2008) Human granulocyte colony stimulating factor (hG-CSF): cloning, overexpression, purification and characterization. Microb Cell Factories 7:13
Vemula S, Thunuguntla A, Dedaniya A, Kokkiligadda S, Palle C, Ronda SR (2015) Improved production and characterization of recombinant human granulocyte colony stimulating factor from E. coli under optimized downstream processes. Protein Expr Purif 108:62–72
Acknowledgments
This work was supported by University of São Paulo (USP) and by CAPES.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Archangelo, B.S., de Sousa Russo, E.M. (2018). Purification Method for Recombinant hG-CSF by Affinity Chromatography. In: Picanço-Castro, V., Swiech, K. (eds) Recombinant Glycoprotein Production. Methods in Molecular Biology, vol 1674. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7312-5_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7312-5_16
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7311-8
Online ISBN: 978-1-4939-7312-5
eBook Packages: Springer Protocols