Abstract
The quantification of circulating thrombin is a valuable tool to accurately assess the activity of the blood coagulation system. Here, we describe the combined application of the thrombin-specific reversible active-site inhibitor argatroban and the DNA-aptamer HD1-22 for conduction of an enzyme capture assay for reliable measurement of plasma thrombin levels.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Thrombin is a multifunctional enzyme that plays an important role within the process of blood coagulation. Besides the activation of blood platelets and plasmatic coagulation cofactors, one of the main functions of thrombin is the conversion of fibrinogen to fibrin, leading to the formation of a stable clot. To prevent the development of thrombosis, the activity of thrombin is tightly regulated by several endogenous inhibitory mechanisms [1].
The plasma level of active thrombin represents a useful biomarker for the assessment of the activity of a patient’s blood coagulation system. To date, the generation of thrombin is determined indirectly by the measurement of plasma thrombin-antithrombin complexes (TAT) or prothrombin activation peptides (F1.2) [2]. Since both markers accumulate in the circulation, however, corresponding concentrations do not reflect the coagulation status at the time point of sample taking.
In comparison to the quantification of plasma TAT or F1.2, the measurement of circulating levels of active thrombin is far more challenging. In addition to the need for a specific ligand that allows for the discrimination between the enzyme and its structural similar zymogen, a strategy to prevent the fast inhibition of free thrombin ex vivo is required [3].
In this chapter, the combined application of a thrombin-specific DNA-aptamer and a reversible active site inhibitor is described that allows for the quantification of plasma thrombin levels. Besides the basic methodological details, we also go into details about the potential pitfalls that come with this oligonucleotide-based enzyme capture assay.
2 Materials
Prepare all solutions using sterile ultrapure water and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise).
2.1 Blood Sampling
-
1.
Blood sampling set: Sterile (winged) blood collection/infusion set with tubing (up to 30 cm) and short (up to 20 mm) cannula with an inner diameter of at least 0.8 mm (21G) but preferable 1.2 mm (18G) (see Note 1 ).
-
2.
Basic syringe-like blood collection tubes: e.g., S-Monovette® (Sarstedt, Nürnbrecht, Germany) containing sodium-citrate (10.6 mM final concentration in e.g., 3 ml of taken whole blood) (see Note 2 ).
-
3.
190 mM Argatroban : e.g., Argatra® (Mitsubishi Pharma, Düsseldorf, Germany) (see Note 3 ). Store stock solution at 4 °C upon opening of original vial.
-
4.
154 mM NaCl solution.
2.2 Oligonucleotide-Based Enzyme Capture Assay (OECA)
-
1.
Fluorometer for 96-well plates with appropriate filter set for measurement of 7-amino-4-methyl coumarins (AMC) (Ex/Em = 340–360/440–460 nm) (see Note 4 ).
-
2.
Framed Maxisorp Fluoronunc 8 × 12 microtiter modules (white) (Nunc, Thermo Fisher Scientific) (see Note 5 ).
-
3.
Adhesive polyester film (Platemax®, Axygen, Union City, CA, USA).
-
4.
Biotin -labeled BSA: Store lyophilized powder at 4 °C until dissolved. Aliquot and store resolved stock solution at ≤−40 °C. Prevent freeze–thaw cycles.
-
5.
Streptavidin (ultrapure) (AppliChem, Darmstadt, Germany): Store lyophilized powder at 4 °C until dissolved. Aliquot and store resolved stock solution at ≤ −40 °C. Prevent freeze–thaw cycles.
-
6.
Protease-free bovine serum albumin (BSA): Store at 4 °C.
-
7.
3′-Biotinylated thrombin-binding DNA-aptamer HD1-22 (5′-GGTTGGTGTGGTTGGAAAAAAA-AAAAAAAAGTCCGTGGTAGGGCAGGTTGGGGTGACT-3′; Page purified) (Microsynth, Balgach, Switzerland) (see Note 6 ). Store lyophilized powder at 4 °C until dissolved. Aliquot and store resolved stock solutions (e.g., 100 μM) at ≤ −20 °C until used.
-
8.
Human α-thrombin with defined specific activity (Haematologic Technologies, Essex Junction, VT, USA) (see Note 7 ): Store original vial at −20 °C. For long-term storage, dilute material in PBS, pH 7.4, containing 10 mg/ml BSA. Prepare aliquots and store at < −70 °C until use. Prevent freeze–thaw cycles.
-
9.
Normal citrate-anticoagulated human plasma (see Note 8 ): Store at <−40 °C until used. Prevent freeze–thaw cycles.
-
10.
Highly pure sterile-filtered dimethyl sulfoxide (DMSO).
-
11.
Sensitive fluorogenic peptide substrate for thrombin detection (Boc-Asp(OBzl)-Pro-Arg-AMC [I-1560]) (Bachem, Bubendorf, Switzerland) (see Note 9 ): Store lyophilized powder at <−15 °C. Dissolve in 10 % DMSO in sterile water: remove powder from the original plastic tube and add into a glass tube. Dissolve powder to reach a concentration of 10 mM. Store aliquots at −20 °C until used. Prevent freeze–thaw cycles.
-
12.
Coating buffer: 30 mmol/L Na2CO2, 200 mmol/L NaHCO3, pH 9.0.
-
13.
PBS buffer (general composition): 137 mmol/L NaCl, 2.7 mmol/L KCl, 9.6 mmol/L Na2HPO4, and 1.5 mmol/L KH2PO4, pH 7.4.
-
14.
Aptamer buffer: PBS, pH 7.4, 3 mmol/L MgCl2, 1 mg/ml BSA.
-
15.
Washing buffer: PBS, pH 7.4, 3 mmol/L MgCl2, 0.05 % Tween 20.
-
16.
Dilution buffer: PBS, pH 7.4, 1 mg/ml BSA; 200 μM Argatroban .
-
17.
Blocking buffer: PBS, pH 7.4, 20 mg/ml BSA, 0.05 % Tween 20.
-
18.
Substrate buffer: 10 mM Tris–HCl, 154 mM NaCl, pH 8.5.
3 Methods
Once formed and released into the circulation, thrombin is promptly inactivated, mainly by complex formation with the serine protease inhibitor (serpin) antithrombin [1]. Accordingly, the half-life of thrombin in plasma has been found to be about 1 min [4]. Since this process of inactivation also takes place ex vivo, a strategy to stabilize the amount of free thrombin during and after the sampling of whole blood is needed [3].
The interaction between thrombin and a serpin involves the enzyme’s active site [5]. Thus, blocking of the latter efficiently prevents complex formation. During the assay described here, the thrombin-specific, reversible active site inhibitor argatroban is added to the citrate blood collection tubes. While citrate binds Ca2+-ions and thereby prevents the clotting of taken blood ex vivo, argatroban binds to the active site of thrombin molecules, thereby preventing their inactivation by complex formation [3]. After centrifugation of whole blood, processed plasma is added to the wells of microtiter-modules that have been coated with the thrombin-specific, bivalent DNA-aptamer HD1-22 [6, 7]. Since HD1-22 recognizes two distinct exosites of the thrombin molecule, the presence of argatroban does not interfere with binding. During the subsequent washing step, argatroban is completely removed together with plasma remains, making the active sites accessible for a fluorogenic peptide substrate that is finally used for the quantification of the immobilized thrombin molecules [3].
In this paragraph, all steps needed for the measurement of plasma thrombin levels by the aptamer-based enzyme capture assay are described. Carry out all procedures at room temperature unless otherwise specified. During incubation steps, wells should be generally sealed with adhesive polyester film and stored in the dark. Care should be taken to avoid temperature differences within the microtiter modules. For washing, if not otherwise stated, discard incubated solution and rinse wells three times with 300 μL of washing buffer. An automated plate washer might be used for this purpose (e.g., Biotek ELx50, Biotek, Bad Friedrichshall, Germany). After, washing, tap inverted wells gently on clean adsorbent paper to remove any excess solution.
3.1 Preparation of Thrombin Blood Collection Tubes
-
1.
Add Argatroban to citrate blood collection tubes in order to yield a final concentration of 100 μM in the blood sample: For example, pre-dilute Argatra® 1:50 in 0.9 % NaCl solution (e.g., 490 μl NaCl + 10 μl Argatra®) to reach a concentration of 3.8 mM (see Note 10 ). Subsequently, add 79 μl of this solution to a 3 ml citrate blood collection tube. Store tubes at 2–8 °C for up to 2 months. Bring to RT before use.
3.2 Blood Collection and Handling of Samples
-
1.
Collect blood into prepared thrombin blood collection tubes (see Note 11 ).
-
2.
Store blood samples at RT and centrifuge within 8 h (see Note 12 ).
-
3.
Centrifuge the blood sample at 2500 × g for 15 min.
-
4.
Store processed plasma at ≤ −40 °C for up to 6 months until analyzed (see Note 12 ).
3.3 Preparation of Aptamer -Loaded Microtiter Modules
-
1.
Add 100 μl of coating buffer containing 10 μg/ml of biotin-labeled BSA into designated wells. Incubate overnight at 4 °C.
-
2.
Wash wells and add 100 μL of washing buffer containing 1 mg/ml BSA and 10 μg/ml streptavidin into the wells and incubate for 1 h.
-
3.
Wash wells and block by adding 200 μL/well of blocking buffer. Incubate for 2 h.
-
4.
Aspirate/remove the blocking buffer and tap inverted wells gently on clean adsorbent paper to remove excess solution.
-
5.
Load 3′-biotinylated aptamers into the primed wells: dilute 3′-biotinylated HD1-22 to a concentration of 10 nmol/L using aptamer buffer and add 100 μl of this solution to each well. Incubate for 1 h.
-
6.
Wash wells and store sealed framed aptamer-loaded modules at 4 °C for up to 2 month until use.
3.4 Preparation of Plasma-Based Thrombin Calibrators
-
1.
Prepare Argatroban -primed (200 μM final concentration) normal citrate-anticoagulated human plasma: For the preparation of 2000 μl, pre-dilute Argatra® 1:50 in 0.9 % NaCl solution (e.g., 490 μl NaCl + 10 μl Argatra®) to reach a concentration of 3.8 mM (see Note 10 ). Subsequently, add 105 μl of this solution to 1895 μl of citrate-anticoagulated human plasma and mix well.
-
2.
For preparation of plasma thrombin calibrators, perform ½-log dilution series of human α-thrombin in Argatroban -primed plasma, starting from 40 mU/ml (see Note 7 ). Prepare 250 μl of each dilution, covering a concentration range down to 0.13 U/ml. Apply remaining Argatroban-primed plasma as the blank calibrator.
3.5 Oligonucleotide-Based Enzyme Capture Assay (OECA)
-
1.
Frame needed number of aptamer-loaded microtiter modules and bring to RT.
-
2.
Thaw frozen calibrators and samples in a water bath at 37 °C. Subsequently, bring to RT and assay as described below. Samples and calibrators are tested undiluted and should be run in duplicate.
-
3.
Pipette 100 μl of calibrators and samples into designated wells. Incubate for 1 h.
-
4.
Prepare 1 ml of substrate solution for each 8 wells right in time before the end of the incubation. Dilute the stock solution of the fluorogenic substrate (30 mM) 1 in 100 using substrate buffer to yield a final substrate concentration of 300 μM. Store at RT in the dark until use.
-
5.
Empty the wells using an eight-channel multi-pipette. Take care that fresh tips for each module (strip) are used to avoid cross-contamination. Subsequently, manually add 250 μl of washing buffer to each well and wash additional three times with washing buffer (300 μl/well) (see Note 13 ).
-
6.
Pipette 100 μl of the freshly prepared substrate solution to each well. Measure fluorescence at 360[ex]/460[em] nm within 1–2 min.
-
7.
Seal plate and incubate for 2 h.
-
8.
Measure fluorescence at 360[ex]/460[em]. Calculate the mean net change in fluorescence (dFU) for each calibrator.
-
9.
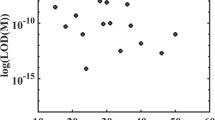
Construct a standard curve by plotting the dFU observed for each calibrator versus the corresponding concentration of thrombin in mU/ml. Use an appropriate curve fit algorithm (e.g., 4-parameter logistic function) to calculate the amount of thrombin present in the plasma samples (Fig. 1a). Calculate mean values from duplicate samples after conversion of dFU to corresponding plasma thrombin concentrations (Fig. 1b). Ensure that quantitative values are above the lower limit of quantification (LLOQ) of the assay. Thrombin plasma levels in healthy individuals are typically below the LOD (see Note 14 ). Consider any medications or conditions that may directly or indirectly (artificially) influence assay results (see Note 15 ).
Fig. 1 Typical standard curve and test results. (a) Half-logarithmic dilution series of human alpha-thrombin in argatroban-primed plasma. Shown interpolation was done by 4-parameter-logistic curve fit. Inlet shows the lower concentration range. (b) Typical results as measured during hip replacement surgery. While levels before surgery were found to be below the LOD of the assay, plasma thrombin concentrations continuously increased during surgery and rapidly decreased afterwards
-
10.
Samples which yield values above the highest standard must be pre-diluted with dilution buffer and retested. Multiply the results by the dilution factor in order to obtain the concentration of thrombin in the original sample (see Note 16 ).
4 Notes
-
1.
We found that the use of cannula with an inner diameter of less than 0.8 mm increases the risk of artificial generation of thrombin during the course of blood collection (see Note 11 ).
-
2.
Use syringe-based (Monovette) rather than vacuum-based (Vacutainer) blood collection tubes to allow for convenient addition of Argatroban solution (see Note 10 ). Furthermore, higher shear rates which result from the use of vacuum tubes may also facilitate artificial thrombin generation during blood sampling.
-
3.
We use medical-grade Argatroban (Argatra®) for all experiments. Consider that Argatra® is a prescription drug. In case of legal difficulties, Argatroban might also be purchased as a research reagent. However, we do not have any experience with the use of such preparations.
-
4.
Use appropriate filter sets for the fluorophore AMC (see Note 9 ) and assess optimal gain settings. For the thrombin assay described here, white microtiter modules should be used (see Note 5 ). Accordingly, gain settings of the fluorometer must be adjusted accordingly in order to prevent saturation of signals.
-
5.
The AMC-based fluorogenic substrate (see Note 9 ) leads to significant background fluorescence of the solution. Thus, the general benefit of very-low-background fluorescence of black microtiter modules becomes secondary. In fact, the white modules, which are applicable for both fluorescence and luminescence measurements, yielded better performance with respect to assay sensitivity and reproducibility.
-
6.
The aptamer HD1-22 must be ordered with a 3′-biotin tag. It has been previously shown that the modification of the 5′-end of the HD1 aptamer sequence leads to reduced binding affinity [8]. Page purification should be chosen to ensure best possible purity of full-length molecules.
-
7.
The application of well-defined preparations of highly purified human alpha-thrombin for preparation of thrombin standards is critical to ensure reproducible results between batches and runs. Only order purified human alpha thrombin. Each batch must come with a certificate that states the specific activity of the preparation in terms of (NIH) units/mg [9]. Do not use batches that show an activity of < 3500 units/mg. Thrombin may be supplied in 50 % (vol/vol) glycerol/H2O (to be stored at −20 °C). However, we observed highest long-term stability when storing appropriately diluted material at ≤ −70 °C.
-
8.
We use in-house preparations of citrate-anticoagulated plasma: blood from healthy donors is taken into donation bags containing sodium-citrate solution (10 mM final concentration in taken whole blood). After separation of cells by centrifugation, processed plasma of individual donations is mixed to achieve pooled normal plasma. Aliquots are stored at −40 °C until use. Alternatively, (pooled) citrate-anticoagulated plasma may be purchased from commercial sources.
-
9.
We found that 7-amino-4-methylcoumarin (AMC)-based peptide substrates with high conversion rates show the best compromise between assay sensitivity and reproducibility. As an alternative to the substrate listed in Subheading 2.2, also the fluorogenic substrate H-D-CHA-Ala-Arg-AMC, as offered by Pentapharm (Basel, Switzerland) can be used. Due to inadequate sensitivity, the use of chromogenic substrates is not recommended. In comparison to AMC, the use of a rhodamine-110 (Rh110)-based peptide substrate may improve overall assay sensitivity. However, we observed an unsatisfactory reproducibility of results at low concentrations when using an Rh-110-based substrate for quantification of plasma thrombin levels.
-
10.
Medical-grade Argatroban (Argatra®) is supplied by Mitsubishi Pharma at a concentration of 100 mg/ml (190 mM) in ethanol-based solution. Due to its limited solubility in water, Argatroban will precipitate during the described first dilution step. In order to ensure complete dissolving, the following measures should be taken: Use a 100 μl pipette tip to take up 10 μl of the original Argatra® solution. Wipe off any droplets remaining on the outside of the tip and pipette the 10 μl quickly into 490 μl of 0.9 % NaCl solution without coming into contact with the reaction tube. Precipitates will be clearly visible. Directly close and vortex the reaction tube to completely redissolve the aragtroban. Inspect the used pipette tip and reaction tube: discard and repeat described procedure in case of any remaining precipitates. If problems remain, warm both Argatra® and the NaCl solution to 37 °C before mixing them together as described above.
-
11.
Please consider that prepared thrombin blood collection tubes might not be sterile after addition of the argatroban-solution and should therefore not be used for blood collection via direct venipuncture/phlebotomy. Blood should be collected using a sterile collection/infusion set with tubing. Also keep in mind that the process of venipuncture represents a vascular lesion itself and therefore bears the risk of artificial thrombin generation that may interfere with the measurement of circulating thrombin levels. Thus, in order to minimize such effects, the following measures should be taken: the first blood sample (up to 3 ml) should not be used for thrombin determination, therefore use other blood collection tube, e.g. EDTA-tube, for other analysis than thrombin determination first and then immediately draw blood into the thrombin blood collection tube(s). Discard material in case of initially unsuccessful puncture attempt or obviously impaired blood flow during sampling. Do not puncture the same vein again but preferably change to the contralateral limb for second attempt.
-
12.
Ensure storage of filled thrombin blood collection tube at RT. We observed a drop in thrombin activity to 90 % in case tubes were cooled before centrifugation [3]. However, processed plasma should be stored on ice or frozen at ≤ −40 °C until analyzed.
-
13.
Due to the high sensitivity of the assay, there is a risk of cross-contamination between wells when using an automated plate washer to aspirate the samples from the wells. Thus, original samples should be manually removed from the wells using disposable tips. After subsequent manual addition of washing buffer, the automated washing procedure might be applied.
-
14.
Lab specific validation of the assay’s key characteristics should be performed. Besides reproducibility, also the LLOQ and the limit of detection (LOD) of the assay should be determined [3, 10]. Argatroban -primed plasma should be used for the preparation of corresponding thrombin dilutions [3]. In our lab, the following values were determined: LLOQ: 0.039 ng/ml (1.08 pM). LOD: 0.017 ng/ml (0.47 pM). According to the specific activity of the used thrombin preparation, these values correspond to 0.15 mU/ml (LLOQ) and 0.06 mU/ml (LOD). When plasma samples obtained from 20 healthy blood donors were studied, levels of free thrombin were found to below the LLOQ (n = 5) or the LOD (n = 15) of the assay. In contrast, highly increased plasma thrombin levels were found during the course of hip replacement surgery [3].
-
15.
The DNA aptamer HD1-22 simultaneously binds to exosites I and II of thrombin [6, 7]. Since exosite II is the heparin-binding site, the presence of heparins might lead to artificially decreased levels of plasma thrombin due to impaired binding of the heparin-thrombin complex. The same is true for direct thrombin inhibitors that simultaneously target exosite I and the active site (e.g., bivalirudin). In contrast, the presence of active site inhibitors like argatroban or dabigatran [11] may lead to artificially increased levels due to impaired complex-formation between thrombin and the serpins [5]. However, since all directly or indirectly acting anticoagulants lead to down-regulation of thrombin generation in vivo, such effects may be secondary due to resulting non-detectable plasma thrombin levels.
-
16.
Since the activity of free thrombin is highly regulated in vivo, observed plasma thrombin levels are normally found within the 40 mU/ml range as covered by the standard curve. Especially within the lower concentration range, plasma (matrix) calibrators are needed to correct for enzymatic plasma background activity, which trigger unspecific fluorescence generation [3, 10]. In case the pre-dilution of samples due to high plasma thrombin levels is needed; however, this aspect becomes secondary, allowing the use of buffer instead of plasma for sample dilution.
References
Mann KG (2003) Thrombin formation. Chest 124(3 Suppl):4S–10S
Bailey MA, Griffin KJ, Sohrabi S, Whalley DJ, Johnson AB, Baxter PD, Ariëns RA, Scott DJ (2013) Plasma thrombin-antithrombin complex, prothrombin fragments 1 and 2, and D-dimer levels are elevated after endovascular but not open repair of infrarenal abdominal aortic aneurysm. J Vasc Surg 57:1512–1518
Müller J, Becher T, Braunstein J, Berdel P, Gravius S, Rohrbach F, Oldenburg J, Mayer G, Pötzsch B (2012) Profiling of active thrombin in human blood by supramolecular complexes. Angew Chem Int Ed 50:6075–6078
Rühl H, Müller J, Harbrecht U, Fimmers R, Oldenburg J, Mayer G, Pötzsch B (2012) Thrombin inhibition profiles in healthy individuals and thrombophilic patients. Thromb Haemost 107:848–853
Huntington JA (2013) Thrombin inhibition by the serpins. J Thromb Haemost 11(Suppl 1):254–264
Müller J, Wulffen B, Pötzsch B, Mayer G (2007) Multidomain targeting generates a high-affinity thrombin-inhibiting bivalent aptamer. Chembiochem 8:2223–2226
Müller J, Freitag D, Mayer G, Pötzsch B (2008) Anticoagulant characteristics of HD1-22, a bivalent aptamer that specifically inhibits thrombin and prothrombinase. J Thromb Haemost 6:2105–2112
Buff MC, Schäfer F, Wulffen B, Müller J, Pötzsch B, Heckel A, Mayer G (2010) Dependence of aptamer activity on opposed terminal extensions: improvement of light-regulation efficiency. Nucleic Acids Res 38:2111–2118
Whitton C, Sands D, Lee T, Chang A, Longstaff C (2005) A reunification of the US (“NIH”) and International Unit into a single standard for Thrombin. Thromb Haemost 93:261–266
Müller J, Friedrich M, Becher T, Braunstein J, Kupper T, Berdel P, Gravius S, Rohrbach F, Oldenburg J, Mayer G, Pötzsch B (2012) Monitoring of plasma levels of activated protein C using a clinically applicable oligonucleotide-based enzyme capture assay. J Thromb Haemost 10:390–398
Harbrecht U (2011) Old and new anticoagulants. Hamostaseologie 31:21–27
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Müller, J., Becher, T., Mayer, G., Pötzsch, B. (2016). Aptamer-Based Enzyme Capture Assay for Measurement of Plasma Thrombin Levels. In: Mayer, G. (eds) Nucleic Acid Aptamers. Methods in Molecular Biology, vol 1380. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3197-2_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3197-2_15
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3196-5
Online ISBN: 978-1-4939-3197-2
eBook Packages: Springer Protocols