Abstract
Estrogen receptors play critical roles in regulating genes responsible for development and maintenance of reproductive tissues and other physiological function. The interaction of ERs with DNA sequences, known as estrogen response elements (EREs) (a palindromic repeat separated by three-base spacer, 5′GGTCAnnnTGACC-3′), is required for estrogen regulation of target gene expression. Here, we describe a simple “mix-and-measure”-based method for detecting ER:ERE interactions using ERE-immobilized metal nanoparticles and water-soluble conjugated polyelectrolytes (CPEs) as cooperative sensing elements. This method can differentiate the distinct DNA-binding affinity between ERα and ERβ, and determine ER:ERE-binding stoichiometry. This method can also accurately detect all 15 singly mutated EREs (i.e., three possible base substitutions at each of one to five positions from left to right of the 5′ end half site, GGTCA) for their binding energy to ER. This method is compatible with 96-well plate format for high-throughput analysis.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Förster resonance energy transfer
- Gold nanoparticle s
- Water-soluble conjugated polyelectrolytes
- Transcription factor
- Protein:DNA interactions
- Estrogen receptor
1 Introduction
Estrogen receptor s play critical roles in regulating genes responsible for development and maintenance of reproductive tissues and other physiological function. Two major ER subtypes (ERα and ERβ) are arranged into similar domains and the degree of homology varies widely among the regions. Despite the high degree of similarity in the DNA binding domain (96 % amino acid identity) and biochemical properties, ERα and ERβ differ substantially in tissue distribution [1]. The interaction of ERα and ERβ with DNA sequences, known as estrogen response elements (EREs) (a palindromic repeat separated by three-base spacer, 5′GGTCAnnnTGACC-3′), is required for estrogen regulation of target gene expression [2].
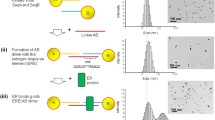
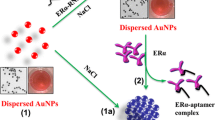
Here we introduce a rapid and quantitative method for measuring ER:ERE interactions in a “mix-and-measure” manner [3]. In this method, double-stranded DNA (ERE)-conjugated gold nanoparticle (denoted as dsDNA-AuNPs or ERE-AuNPs) and water-soluble conjugated polyelectrolytes (CPEs) are used as collaborative sensing elements. The unique optical and charge properties of CPEs [4, 5] and the prominent property of 13 nm AuNPs as superquencher [6, 7] are exploited. The detection of ER binding to ERE is based on the Förster resonance energy transfer (FRET) and/or nanoparticle surface energy transfer (NSET) between the CPEs and ERE-AuNPs. The signal readout is the differential CPE emission determined by ER binding to ERE that changes the interaction between CPEs and ERE-AuNPs (Fig. 1).
Three water-soluble CPEs of different charge properties (cationic and anionic) and emission wavelengths (i.e., 410, 430, and 560 nm) have been chosen to demonstrate the concept. Through a proper selection of CPEs in terms of charge properties and emission wavelengths, ER binding can be detected by fluorescence recovery (e.g., light-on, Fig. 1a), fluorescence quenching (e.g., light-off, Fig. 1b), or both ways (e.g., two-way, Fig. 1c). With this method, we can differentiate the distinct DNA-binding affinity between ERα and ERβ, and determine ER:ERE-binding stoichiometry. We can also accurately detect singly mutated EREs for their binding strength to ER. The binding energy matrix measured for the total of 15 singly mutated EREs (each of them carrying one base variation in the 5′ end half-site with all possible base substitutions at each of the five possible sites) correlates to an in vivo energy model determined by thermodynamic modeling of ChIP-seq (TherMos) with a rank correlation coefficient of 0.98 [2]. This method has been validated for other transcription factors, for example, FoxA1 and Ap2γ and DNA-proteins of known and/ or unknown charge properties [8], which is not always feasible with FRET assays using other donor-acceptor pair [9].
2 Materials and Apparatus
-
1.
Purified recombinant human estrogen receptors ERα (3770 nM) in 50 mM Tris–HCl, pH 8.0, 500 mM KCl, 1 mM EDTA, 1 mM Na3VO4, 2 mM DTT, and 10 % glycerol and ERβ (4500 nM) in 50 mM Bis-Tris-propane, pH 9.0, 400 mM KCl, 2 mM DTT, 1 mM EDTA, and 10 % glycerol (seeNote 1 ).
-
2.
Custom-synthesized ERE sequences (35 bp oligonucleotides: one wild-type ERE “wtERE,” one scrambled ERE “scrERE,” 15 singly mutated EREs—“mut1” to “mut15”) are listed in Table 1. The wtERE contains perfect core sequence, 5′-GGTCAnnnTGACC-3′, and scrERE has both the 5′ and 3′ half-sites completely scrambled. The thiolated version of these sequences (used for conjugation on AuNPs) are denoted as thiol-wtERE and thiol-scrERE, respectively.
Table 1 ERE sequences -
3.
Water-soluble conjugated polyelectrolytes (CPEs) were utilized, including two anionic CPEs (ACPE) and one cationic CPE (CCPE). CPEs are designated by their charge and peak emission wavelengths as ACPE-430 (synthesized in our lab [9]), ACPE-560, and CCPE-410 (Sigma Aldrich) (Fig. 2a). The degree of quenching of CPEs by dsDNA(ERE)-conjugated AuNPs (Fig. 2b) is 15 %, 50 %, and 85 %, respectively, depending on the spectral integral, charge property, and quantum yield [3].
-
4.
40 mM sodium citrate, 1 mM gold (III) chloride trihydrate (HAuCl4), round-bottomed flask, reflux condenser, large stir bar, aqua regia.
-
5.
Tris (2-carboxyethyl) phosphine, 3 kDa molecular weight cut-off (3 kDaMWCO) centrifugal filtration device (e.g., Microcon YM-3).
-
6.
0.1 M HCl, 1.71 M NaCl, 0.1 M NaOH, 5 mM HEPES buffer.
-
7.
100 μM thiazole orange, 1 mM potassium cyanide (KCN), 10 mM PBS buffer: 2.7 mM KCl, 137 mM NaCl, pH 7.4.
-
8.
Nanodrop 3300 fluorescent spectrophotometer (Thermo Scientific).
-
9.
Infinite M200 monochromator microplate reader (TECAN).
3 Methods
3.1 Preparation of Gold Nanoparticles
Gold nanoparticle s (AuNPs) of 13 nm in diameter are prepared by the citrate reduction of HAuCl4.
-
1.
Prepare 1 mM (50 mL) gold chloride HAuCl4 solution and 40 mM (5 mL) sodium citrate solution.
-
2.
Heat and stir HAuCl4 solution at 100 °C under reflux until it boils and starts condensation.
-
3.
Add 5 mL of sodium citrate solution rapidly. Within several minutes, the color of the solution will change from pale yellow to red.
-
4.
Continue heating for 30 min to ensure complete reduction.
-
5.
Continue stirring for an additional 15 min after heating is stopped and allow to cool to room temperature (seeNote 2 ).
3.2 Preparation of dsDNA-Conjugated AuNPs
-
1.
Prepare 50 μM thiolated single-stranded DNA (ssDNA) in 5 mM HEPES buffer.
-
2.
Activate thiolated ssDNA with tris (2-carboxyethyl) phosphine (TCEP, 10 fold molar excess to the ssDNA) and stir for 10 min (seeNote 3 ).
-
3.
Centrifuge the final solution through a 3 kDa MWCO centrifugal filter device at 19,064 × g for 30–45 min to remove TCEP before conjugation to AuNPs. The final volume should be around 5–10 μL. Afterwards, top up the solution with 5 mM HEPES buffer and repeat the washes for another 2–3 times (seeNote 4 ).
-
4.
Conjugate activated thiolated DNA to AuNP [10]. To prepare 500 μL of DNA conjugated AuNP solution, first mix activated DNA with AuNPs at desired ratio (100:1, for example, 500 nM:5 nM) and incubate for 5 min (seeNote 5 ). Then add 5 μL of 0.1 M HCl to lower the pH of the solution to pH 3 and wait for 5 min before adding 9 μL of 1.71 M NaCl to make the final salt concentration of 30 mM. Incubate the sample for another 5 min and bring the pH back to 7 by adding 10 μL of 0.1 M NaOH. Top up the final solution to 500 μL with distilled water.
-
5.
Anneal the ssDNA-AuNPs conjugates (500 nM) with their complementary DNAs (500 nM) at 90 °C for 5 min before cooling down to room temperature (seeNote 6 ).
-
6.
Wash the obtained conjugation mixture through three rounds of centrifugation and rinsing with 5 mM HEPES buffer (pH 7.4) to remove the unbound DNA strands and stored at 4 °C (seeNote 7 ).
3.3 Quantification of dsDNA on AuNPs
Quantification of dsDNA coverage on dsDNA-AuNP conjugates was done using thiazole orange (TO) intercalation method [10].
-
1.
Dilute 25 μL of DNA-AuNP conjugate solution to 100 μL with HEPES buffer.
-
2.
Add 5 μL of 1 mM KCN to dissolve the AuNPs completely and release attached dsDNA (seeNote 8 ).
-
3.
Mix 0.5 mL of 100 μM thiazole orange with the released dsDNA in the supernatant for 15 min, and record the emission of thiazole orange at 530 nm using a Nanodrop 3300 fluorescent spectrophotometer. The fluorescence intensity from thiazole orange will be proportional to the amount of dsDNA since thiazole orange dye only gives appreciable emission as it intercalates into the dsDNA. Use a series of known concentrations of dsDNA to generate a calibration curve, then measure, and quantify the samples accordingly.
3.4 ACPE-430-Based Detection of ERα and ERβ Binding to wtERE-AuNPs
Conduct the ER:ERE-binding assay in four main steps.
-
1.
Incubate 10 μL of 500 nM ERE-AuNPs with 25 μL of ER ranging from 0 to 1 μM in a microplate (384-well flat clear-bottom black polystyrene TC-treated microplate, Corning) containing 55 μL PBS buffer, for 30 min at room temperature. (For determination of the binding constant ( K d ) and the stoichiometry (n), titrate the protein with increasing concentration from 0 to 250 nM.)
-
2.
Add 10 μL of 1 μM ACPE-430 solution into above solution in step 1 and incubate for another 10 min. In the solution the final concentrations of ERE-AuNPs, ER, and CP are 50 nM, 0–250 nM, and 100 nM, respectively.
-
3.
Measure the fluorescence spectra of the final solution, using a microplate reader (Infinite M200, Tecan) from 300 to 700 nm at an excitation wavelength of 370 nm, and compare the spectra in the presence and absence of protein. (In control experiments of testing CPE emissions in the presence of DNA or protein without AuNPs, 50 nM dsDNA or 250 nM protein were added into 100 nM ACPE-430 in PBS buffer.) (SeeNote 9 .)
-
4.
With the [ER] dependent fluorescence intensity scales, calculate the protein-DNA binding affinity constant K d quantitatively through (F0 − F)/(F − Fsat) = ([protein]/Kd)n (Fig. 3c). Obtain the Kd by plotting log [(F0 − F)/(F − Fsat)] versus log [protein], where F0 and Fsat are the relative fluorescence intensities in the absence of protein and in protein saturation, respectively. The value of log [protein] at log [(F0 − F)/(F − Fsat)] = 0 equals to the logarithm of the Kd. The slope, n, is the binding stoichiometry of protein to DNA. The Kd values and stoichiometric (n) values (Table 2) confirm the following characteristics: ERα binds to wtERE as a dimer (n ~2) and more strongly than ERβ (Kd, ERα < Kd, ERβ), and ERβ binds with a ratio of approximately 4 to 1 molar ratio with wtERE as determined previously using SPR spectroscopy [1]. The Kd obtained for ERα correlates well to the value of 46 nM obtained using Sepharose chromatography [11].
Fig. 3 Fluorescence emission spectra of ACPE-430 mixed with wtERE-AuNPs without ER (black dashed line) and with increasing molar concentration of (a) ERα and (b) ERβ relative to DNA (0, 0.5, 1.0, 2.0, 3.0, 4.0, and 5.0fold). (c) Logarithmic plot of log [(F0 − F)/(F − Fsat)] vs. log [ERα (diamond) or ERβ (square)] for deducing binding constant ( K d ) and binding stoichiometry (see text for more detail)
Table 2 Binding constant ( K d ) of ERα and ERβ with wtERE measured by three CPE/DNA-AuNP hybrid sensorsa,b
3.5 CCPE-410-Based Detection of ERα and ERβ Binding to wtERE-AuNPs
Relative to the “light-off” principle, a “light-on” assay can offer an additional benefit, that is, the solution glows upon protein binding. The human eye can quickly adapt to the background illumination and rapidly catch the change in intensity under UV light to distinguish objects from background. CCPE-410-based detection of ERα and ERβ binding to wtERE-AuNPs was conducted by the same steps as in ACPE-430-based detection, except that the polyelectrolyte used was CCPE-410.
CCPE-410, which exhibits opposite charge from DNA, initially is quenched by 85 % (Fig. 2). Since ERα and ERβ are positively charged at pH 7.4, ERs binding to ERE lower the charge of DNA-AuNPs conjugates and thus reduce the electrostatic attraction or the affinity of CCPE-410 to AuNP. Thereby fluorescence recovery is observed as the measure of ER-ERE interactions (Fig. 4a, b). This CCPE could be the polymer of choice if visual detection is desirable through the noticeable fluorescence recovery (Fig. 4c). Quantitative measurement of ER affinity with this CCPE-410/AuNPs hybrid sensor gives the same result as the previous ACPE-430 sensor; that is, ERα has a stronger affinity to wtERE than ERβ, and ERα binds to ERE as a dimer, whereas ERβ binds to ERE as a tetramer (Table 2).
Fluorescence emission spectra of CCPE-410 mixed with ERE-AuNPs without ER (black dashed line) and with increasing molar concentration of (a) ERα and (b) ERβ relative to DNA (0, 0.5, 1.0, 2.0, 3.0, 4.0, and 5.0 fold). The insets summarize change in fluorescence intensity at 410 nm as a function of [ER]. (c) Change in fluorescence emission of CCPE-410 as the ratio of ERα to ERE is varied from 0, 1, 2 to 5, respectively, under UV light
3.6 ACPE-560-Based Detection of ERα and ERβ Binding to wtERE-AuNPs
ACPE-560-based detection of ERα and ERβ binding to wtERE-AuNPs was conducted by the same steps as in ACPE-430-based detection, except that the polyelectrolyte used was ACPE-560.
The ACPE-560 has a weak hydrophobic interaction that is not favorable for distance dependent energy transfer. However, the higher overlap integral of its emission spectrum with the absorption spectrum of AuNP renders an enhanced quenching to ~50 % of the original fluorescence intensity (Fig. 2). The intermediate fluorescence quenching basically gives ACPE-560 its unique dual applicability, in which it can be used to detect protein binding either by fluorescence quenching or by recovery. This “two-way” fashion allows for detecting of proteins of positive or negative charge or proteins of unknown charge properties.
At pH 7.4, ERα is positively charged; this renders the fluorescence of the ACPE-560 to be further quenched with its binding to DNA (Fig. 5a). At pH 9.5, ERα is negatively charged. The binding to ERE-AuNPs at this pH manifests as the recovery of the anionic ACPE-560 fluorescence intensity (Fig. 5b) due to the repulsion between the CPE and the bound protein. Figure 5c shows a summary of ER concentration dependent ACPE-560 intensity change at pH 7.4 and 9.5 for ERα and ERβ. The K d values measured by ACPE-560 (Table 2) are the average values from both methods. The results from ACPE-560 closely agree to those measured using the other two CPEs.
(a, b) Fluorescence spectra of ACPE-560 mixed with ERE-AuNPs without ER (black dashed line) and with increasing molar concentration of ERα at (a) pH 7.4 and (b) pH 9.5. (c) The relative change in fluorescent intensity at 560 nm of ACPE-560 as the function of ER concentration for ERα at pH 7.4 (triangle), pH 9.5 (diamond) and ERβ at pH 7.4 (circle), pH 9.5 (square)
3.7 Binding Energy of ERα to Singly Mutated ERE: Using a Competition Approach
A perfect ERE contains a palindromic consensus sequence separated by a three-base pair spacer, 5′-GGTCAnnnTGACC-3′. Despite the consensus ERE delineated from conserved cis-regulatory elements, the majority of in vivo EREs deviate from the consensus, with one half-site identical to that in the consensus and the second half-site having nucleotide variant(s). To detect the binding energy of ERα to singly mutant EREs (one nucleotide variant in one of the half-sites), a competition approach is used with the CCPE-410 “light-on” probe.
-
1.
Incubate 10 μL of 1 μM ERα solution with 5 μL of 1 μM DNA solution (competitor DNA, i.e., the mutEREs) in a microplate (384-well flat clear-bottom black polystyrene TC-treated microplate, Corning) containing 65 μL PBS buffer for 10 min.
-
2.
Add 10 μL of 500 nM wtERE-AuNPs conjugate to above mixture and incubate for another 30 min at RT.
-
3.
Add 10 μL of 1 μM CCPE-410 solution (100 nM CCPE-410) (e.g., 2:1 molar ratio to dsDNA-AuNPs) to the mixture of step 2 and incubate for an additional 10 min to reach equilibrium.
-
4.
Measure and compare the fluorescence spectra of the final solution (same as in step 3, ACPE-430-based detection) in the presence and absence of protein-mutERE mixture (Fig. 6). When the competitor has a weaker affinity to ERα (e.g., the scrERE), more ERα (positively charged at pH 7.4) will be available to bind to wtERE-AuNPs, which leads to a stronger repulsion of the CCPE-410 and thus high emission intensity (“light-on”), and vice versa (Fig. 6a for scrERE, mut12, mut2, or mut7). The intensity trend for these competitors correlates well with the trend of their free energy value (Table inset in Fig. 6a, the larger the value, the lower the affinity), giving the following affinity order wtERE > mut7 > mut2/mut12 > scrERE. For all 15 singly mutant ERE variants, the position- and type-specific affinity trend measured by the DNA-AuNPs/CCPE-410 hybrid sensor correlates with the energy matrix derived from the in vivo model using TherMos (Fig. 6b) with a rank correlation efficient of 0.98.
Fig. 6 (a) Fluorescence intensity plot of CCPE-410 responding to ERα binding to wtERE-AuNPs in the presence of various competitors DNA, i.e., wtERE, scrERE, smut2, smut7, and smut12. The red dashed line refers to emission intensity with ERα binding without any free competitor DNA in solution. (b) Relative fluorescence intensity bar plot for all single mutated EREs as competitor (red bars (bars with error bars)), with that without competitor DNA as reference (leftmost bar). The blue bars (the bars without error bars) are calculation free energy using TherMos thermodynamic prediction [2]
4 Notes
-
1.
At first use, dispense the proteins into 10 μL aliquots, as is, without additional storage buffer and store at −80 °C to avoid multiple freeze-thaw cycles. The proteins should be mixed gently and not vortexed. Before use, thaw the aliquots in a room temperature water bath and return to 4 °C to maintain activity.
-
2.
Before setting up for AuNPs synthesis, a round-bottom flask, a reflux condenser, and a large stir bar should be washed with first aqua regia and then thoroughly with water. Aqua regia is noxious and extremely caustic. Handle with extreme care in a well-ventilated fume hood. Make sure that HAuCl4 solution is boiling before adding the sodium citrate solution. Condensation droplets should be seen inside the flask. Sodium citrate solution should be added to the mixture as rapidly as possible. If the addition is done correctly, the solution should turn from yellow to purple and then to deep red.
-
3.
Thiol-modified oligonucleotides are supplied in the protected form with the disulfide linkage intact to minimize the potential for oxidation, which results in oligo dimer formation. To use the free thiol (-SH) in the application the disulfide linkage must be reduced with supplier’s recommended reducing agent.
-
4.
Time and rpm of the centrifugation needs to be optimized accordingly but do not exceed 21,885 × g.
-
5.
The incubation time can be lengthened if necessary.
-
6.
The caps of the centrifuge tubes should be sealed with parafilm to prevent sample loss due to evaporation during heating. After heating to 90 °C, turn the heating block off and leave the tubes in the block until it cools down to achieve a gradual cooling step. For sequences with significant hairpin potential, a gradual cooling/annealing step is beneficial.
-
7.
The conjugates should be stable and no appearance of any precipitation over several weeks. The stability of AuNPs after DNA conjugation can be tested using high-salt solution [3, 8].
-
8.
Care must be taken when using potassium cyanide since it is highly toxic. Work involving sodium or potassium cyanide should only be conducted in an operational fume hood. Adequate personal protective equipment must be worn and great care should be taken that cyanide salts do not come into contact with acid, which liberates highly toxic hydrogen cyanide gas. Cyanide waste solutions must be collected in a dedicated waste bottle and labeled as hazardous waste.
-
9.
ERα and ERβ (isoelectric point, pI ~8.3 and 8.8, respectively) [12] are positively charged at pH 7.4. The anionic ACPE-430 was initially quenched merely by 15 % by wtERE-AuNPs (Fig. 2b), due to the limited interaction with the ERE. Binding of ERα and ERβ to wtERE (Fig. 3a, b) lowers the overall negative charge of DNA-AuNPs (more positive), leading to the increased magnitude of quenching with “light-off” principle.
References
Su X, Lin CY, O’Shea SJ et al (2006) Combinational study of estrogen receptors-response elements interactions using SPR and QCM. Anal Chem 78:5552–5558
Song HY, Sun W, Prabhakar S et al (2013) Study sequence rules of ERα–DNA interactions using dual polarisation interferometry and computational modeling. Anal Biochem 433:121–128
Lukman S, Aung KMM, Liu J et al (2014) Hybrid sensor using gold nanoparticles and conjugated polyelectrolytes for studying sequence rule in protein–DNA interactions. ACS Appl Mater Interfaces 5:12725–12734
Liu B, Bazan GC (2004) Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem Mater 16:4467–4475
Zhu C, Liu LB, Yang Q, Lv F, Wang S (2012) Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem Rev 112:4687–4735
Su S, Zuo XL, Pan D et al (2013) Design and applications of gold nanoparticle conjugates by exploiting biomolecule–gold nanoparticle interactions. Nanoscale 5:2589–2599
Wang J, Qu XG (2013) Recent progress in nanosensors for sensitive detection of biomolecules. Nanoscale 5:3589–3600
Lukman S, Aung KMM, Lim M et al (2014) Hybrid assembly of DNA-coated gold nanoparticles with water soluble conjugated polymers for studying protein-DNA interaction and ligand inhibition. RSC Adv 4:8883
Wang J, Liu B (2009) Fluorescence resonance energy transfer between an anionic conjugated polymer and a dye-labeled lysozyme aptamer for specific lysozyme detection. Chem Commun 17:2284–2286
Zhang X, Servos MR, Liu J (2012) Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route. J Am Chem Soc 134:7266–7269
Bond JP, Notides AC (1987) Estrogen receptor purification by affinity chromatography using an orange triazine dye. Anal Biochem 163:385–390
Hornbeck PV, Kornhauser JM, Tkachev S et al (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res 40:D261–D270
Acknowledgements
The authors would like to acknowledge the Agency for Science, Technology and Research (A*STAR), Singapore, for the financial support (JCO 1131CFG001).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Su, X., Aung, K.M.M., Lukman, S., Liu, B. (2016). Gold Nanoparticle-Based Förster Resonance Energy Transfer (FRET) Analysis of Estrogen Receptor: DNA Interaction. In: Eyster, K.M. (eds) Estrogen Receptors. Methods in Molecular Biology, vol 1366. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3127-9_17
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3127-9_17
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3126-2
Online ISBN: 978-1-4939-3127-9
eBook Packages: Springer Protocols