Abstract
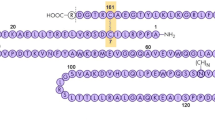
Erythropoietin (EPO) is a protein made by the kidneys in response to low red blood cell count that is secreted into the bloodstream and binds to a receptor on hematopoietic stem cells in the bone marrow inducing them to become new red blood cells. EPO made with recombinant DNA technology was brought to market in the 1980s to treat anemia caused by kidney disease and cancer chemotherapy. Because EPO infusion was able to replace blood transfusions in many cases, it rapidly became a multibillion dollar per year drug and as the first biologic created with recombinant technology it launched the biotech industry. For many years intense research was focused on creating a small molecule orally available EPO mimetic. The Robert Wood Johnson (RWJ) group seemed to definitively establish that only large peptides with a minimum of 60 residues could replace EPO, as anything less was not a full agonist. An intense study of the published work led me to hypothesize that the size of the mimetic is not the real issue, but the symmetry making and breaking of the EPO receptor induced by the ligand is the key to activating the stem cells. This analysis meant that residues in the binding site of the receptor deemed absolutely essential for ligand binding and activation from mutagenesis experiments, were probably not really that important. My fundamental hypotheses were: (a) the symmetric state of the homodimeric receptor is the most stable state and thus must be the off-state, (b) a highly localized binding site exists at a pivot point where the two halves of the receptor meet, (c) small molecules can be created that have high potency for this site that will be competitive with EPO and thus can displace the protein–protein interaction, (d) small symmetric molecules will stabilize the symmetric off-state of the receptor, and (e) a key asymmetry in the small molecule will stabilize a mirror image asymmetry in the receptor resulting in the stabilization of the on-state and proliferation of the stem cells into red blood cells. Researchers at Amgen published a co-crystal structure of EPO bound to the EPO receptor, which has a beautiful twofold symmetry—it was argued that this is the active state of the receptor. Activating the EPO receptor with EPO induces an almost instantaneous shutdown mechanism to sharply curtail any proliferative signal transduction, and thus, my hypotheses lead to the conclusion that the Amgen co-crystal is actually the state after receptor downregulation and thus an off-state. To put these hypotheses to the test, my computational method of Simulated Annealing of Chemical Potential was run using the co-crystal created at RWJ, which is the receptor trapped in a partial agonist state. The simulations predicted a previously unknown high affinity binding site at the pivot point where the two halves of the dimeric receptor meet, and detailed analysis of the fragment patterns led to the prediction of a molecule less than 300 MW that is basically twofold symmetric with a chiral center on one side and not the other. Thus, to the degree that computer simulations can be taken seriously, these results support my hypotheses on small molecule receptor activation. When this small molecule was synthesized and tested it indeed induced human hematopoietic stems cells to become red blood cells. When the predicted chiral center of this molecule was removed eliminating its one asymmetric feature, the resulting molecule was an antagonist—it could potently displace hot EPO but could no longer induce stem cell proliferation and differentiation. These results provided strong support for my theories on how to create potent small molecule EPO agonists and were used to launch the new company Locus Pharmaceuticals. These molecules, however, required significant chemical changes in order to make them stable in other in vitro assays and to be in vivo active, but these alterations had to be done in a way that maintained the symmetry–asymmetry considerations that led to the creation of an in vitro active molecule. The combination of changing functional groups to enable good pharmacokinetics, while not changing the key intrinsic symmetry properties were never seriously pursued at Locus and the program died. Investigations into how red blood cells are created have occupied many prominent researchers for the entire twentieth century. In the second half of the century EPO was discovered and by the end of the century it became a blockbuster commercial product that launched the biotech revolution.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Carnot P, Deflandre C (1906) The hemopoietic activity of serum during the regeneration of blood. C R Acad Sci 1434:384–387
Editorial (1972) Erythropoietin. Br Med J 1:263
Reissmann KR (1950) Studies on the mechanism of erythropoietic stimulation in parabiotic rats during hypoxia. Blood 5:372–380
Erslev A (1953) Humoral regulation of red cell production. Blood 8:349–357
Plzak LF, Fried W, Jacobson LO, Bethard WF (1955) Demonstration of stimulation of erythropoiesis by plasma from anemic rats using Fe59. J Lab Clin Med 46:671–678
Jacobson LO, Goldwasser E, Fried W, Plzak L (1957) Role of the kidney in erythropoiesis. Nature 179:633–634
Reissmann KR, Nomura T, Gunn RW, Brosius F (1960) Erythropoietic response to anemia or erythropoietin injection in uremic rats with or without functioning renal tissue. Blood 16:1411–1423
Hirashima K, Takaku F (1962) Experimental studies on erythropoietin. II. The relationship between juxtaglomerular cells and erythropoietin. Blood 20:1–8
Cotes PM, Bangham DR (1966) The international reference preparation of erythropoietin. Bull World Health Organ 35:751–760
Ward HP, Kurnick JE, Pisarczyk MJ (1971) Serum level of erythropoietin in anemias associated with chronic infection, malignancy, and primary hematopoietic disease. J Clin Invest 50:332–335
Radtke HW, Claussner A, Erbes PM, Scheuermann EH, Schoeppe W, Koch KM (1979) Serum erythropoietin concentration in chronic renal failure: relationship to degree of anemia and excretory renal function. Blood 54:877–884
Goldwasser E, Kung CK (1971) Purification of erythropoietin. Proc Natl Acad Sci U S A 68:697–698
Miyake T, Kung CK, Goldwasser E (1977) Purification of human erythropoietin. J Biol Chem 252:5558–5564
Lawn RM, Fritsch EF, Parker RC, Blake G, Maniatis T (1978) The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell 15:1157–1174
Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z et al (1985) Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A 82:7580–7584
Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF et al (1985) Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 313:806–810
Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW (1987) Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316:73–78
Pavlou AK, Reichert JM (2004) Recombinant protein therapeutics – success rates, market trends and values to 2010. Nat Biotechnol 22:1513–1519
Locatelli F, Vecchio LD (2001) Darbepoetin alfa. Amgen. Curr Opin Investig Drugs 2:1097–1104
Imai N, Kawamura A, Higuchi M, Oh-eda M, Orita T, Kawaguchi T, Ochi N (1990) Physicochemical and biological comparison of recombinant human erythropoietin with human urinary erythropoietin. J Biochem 107:352–359
Wasley LC, Timony G, Murtha P, Stoudemire J, Dorner AJ, Caro J, Krieger M, Kaufman RJ (1991) The importance of N- and O-linked oligosaccharides for the biosynthesis and in vitro and in vivo biologic activities of erythropoietin. Blood 77:2624–2632
Yamaguchi K, Akai K, Kawanishi G, Ueda M, Masuda S, Sasaki R (1991) Effects of site-directed removal of N-glycosylation sites in human erythropoietin on its production and biological properties. J Biol Chem 266:20434–20439
Delorme E, Lorenzini T, Giffin J, Martin F, Jacobsen F, Boone T, Elliott S (1993) Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31:9871–9876
Egrie JC, Browne JK (2001) Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 84(Suppl 1):3–10
Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, Johnson DL, Barrett RW, Jolliffe LK, Dower WJ (1996) Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273:458–464
Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, Dower WJ, Jolliffe LK, Wilson IA (1996) Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science 273:464–471
Johnson DL, Farrell FX, Barbone FP, McMahon FJ, Tullai J, Kroon D, Freedy J, Zivin RA, Mulcahy LS, Jolliffe LK (1997) Amino-terminal dimerization of an erythropoietin mimetic peptide results in increased erythropoietic activity. Chem Biol 4:939–950
Middleton SA, Johnson DL, Jin R, McMahon FJ, Collins A, Tullai J, Gruninger RH, Jolliffe LK, Mulcahy LS (1996) Identification of a critical ligand binding determinant of the human erythropoietin receptor. Evidence for common ligand binding motifs in the cytokine receptor family. J Biol Chem 271:14045–14054
Qureshi SA, Kim RM, Konteatis Z, Biazzo DE, Motamedi H, Rodrigues R, Boice JA, Calaycay JR, Bednarek MA, Griffin P, Gao YD, Chapman K, Mark DF (1999) Mimicry of erythropoietin by a nonpeptide molecule. Proc Natl Acad Sci U S A 96:12156–12161
Goldberg J, Jin Q, Ambroise Y, Satoh S, Desharnais J, Capps K, Boger DL (2002) Erythropoietin mimetics derived from solution phase combinatorial libraries. J Am Chem Soc 124:544–555
Middleton SA, Barbone FP, Johnson DL, Thurmond RL, You Y, McMahon FJ, Jin R, Livnah O, Tullai J, Farrell FX, Goldsmith MA, Wilson IA, Jolliffe LK (1999) Shared and unique determinants of the erythropoietin (EPO) receptor are important for binding EPO and EPO mimetic peptide. J Biol Chem 274:14163–14169
Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM (1998) Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 395:511–516
Bulut GB, Sulahian R, Ma Y, Chi NW, Huang LJ (2011) Ubiquitination regulates the internalization, endolysosomal sorting, and signaling of the erythropoietin receptor. J Biol Chem 286:6449–6457
Kamishimoto J, Tago K, Kasahara T, Funakoshi-Tago M (2011) Akt activation through the phosphorylation of erythropoietin receptor at tyrosine 479 is required for myeloproliferative disorder-associated JAK2 V617F mutant-induced cellular transformation. Cell Signal 23:849–856
Livnah O, Johnson DL, Stura EA, Farrell FX, Barbone FP, You Y, Liu KD, Goldsmith MA, He W, Krause CD, Pestka S, Jolliffe LK, Wilson IA (1998) An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat Struct Biol 5:993–1004
Adams DJ (1975) Grand canonical ensemble Monte Carlo for a Lennard-Jones fluid. Mol Phys 29:307–311
Guarnieri F (2004) Computational protein probing to identify binding sites. US Patent no. 6,735,530. Issued 11 May 2004
Guarnieri F, Mezei M (1996) Simulated annealing of chemical potential: a general procedure for locating bound waters. application to the study of the differential hydration propensities of the major and minor grooves of DNA. J Am Chem Soc 118:8493–8494
Kulp JL 3rd, Kulp JL Jr, Pompliano DL, Guarnieri F (2011) Diverse fragment clustering and water exclusion identify protein hot spots. J Am Chem Soc 133:10740–10743
Bradbury MW, Stump D, Guarnieri F, Berk PD (2011) Molecular modeling and functional confirmation of a predicted fatty acid binding site of mitochondrial aspartate aminotransferase. J Mol Biol 412:412–422
Mezei M (1987) Grand-canonical ensemble Monte Carlo study of dense liquid Lennard-Jones, soft spheres and water. Mol Phys 61:565–582
Kulp JL 3rd, Blumenthal SN, Wang Q, Bryan RL, Guarnieri F (2012) A fragment-based approach to the SAMPL3 Challenge. J Comput Aided Mol Des 26:583–594
Imai T, Ohura K (2010) The role of intestinal carboxylesterase in the oral absorption of prodrugs. Curr Drug Metab 11:793–805
Zawilska JB, Wojcieszak J, Olejniczak AB (2013) Prodrugs: a challenge for the drug development. Pharmacol Rep 65:1–14
Bursi R, Grootenhuis A, van der Louw J, Verhagen J, de Gooyer M, Jacobs P, Leysen D (2003) Structure-activity relationship study of human liver microsomes-catalyzed hydrolysis rate of ester prodrugs of MENT by comparative molecular field analysis (CoMFA). Steroids 68:213–220
Krosl J, Damen JE, Krystal G, Humphries RK (1996) Interleukin-3 (IL-3) inhibits erythropoietin-induced differentiation in Ba/F3 cells via the IL-3 receptor alpha subunit. J Biol Chem 271:27432–27437
Bugelski PJ, Makropoulos D, Sprinka-Doms T, Eirikis E, Volk A, Jiao Q, Huang C (2011) Differential effects of long-lived erythropoietin receptor agonists in rats. Pharm Anal Acta 2:2153–2435
Hara KY, Mori H (2006) An efficient method for quantitative determination of cellular ATP synthetic activity. J Biomol Screen 11:310–317
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Guarnieri, F. (2015). Designing a Small Molecule Erythropoietin Mimetic. In: Klon, A. (eds) Fragment-Based Methods in Drug Discovery. Methods in Molecular Biology, vol 1289. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-2486-8_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2486-8_14
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-2485-1
Online ISBN: 978-1-4939-2486-8
eBook Packages: Springer Protocols