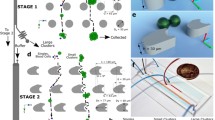
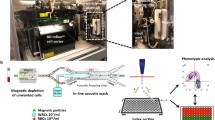
Abstract
Isolation of extremely rare circulating tumor cell (CTC) clusters from the bloodstream of patients enables minimally invasive diagnosis and prognosis while providing information on their role in metastasis. A few technologies specifically developed for the enrichment of CTC clusters fail to achieve a high enough processing throughput to be practical in clinical settings or risk damaging large clusters owing to their structural design producing high shear forces. Here, we outline a methodology developed for rapid and effective enrichment of CTC clusters from cancer patients, independent of the cluster size and cell surface markers. Minimally invasive access to tumor cells in hematogenous circulation will be an integral part of cancer screening and personalized medicine.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Fidler IJ (1993) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A et al (2011) Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29(12):1556–1563
Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S et al (2009) Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 15(22):6980–6986
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695
Stott SL, Richard L, Nagrath S, Min Y, Miyamoto DT, Ulkus L et al (2010) Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2(25)
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN et al (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci 113:E854–E863
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell:1110–1122
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK et al (2012) Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 30(5):525–532
Vona G, Sabile A, Louha M, Sitruk V, Romana S, Franco D et al (2000) Isolation by Size of Epithelial Tumor Cells 156(1):57–63
Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI et al (2010) Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res 16:5011–5018
Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA et al (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci 107(43):18392–18397
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L et al (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450:1235–1239
Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T et al (2012) Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 12:178
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T (2012) Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 14(1):1–9
Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B et al (2015) A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods 12(7):685–691
Au SH, Edd J, Stoddard AE, Wong KHK, Fachin F, Maheswaran S et al (2017) Microfluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci Rep 7:2433
Edd JF, Mishra A, Dubash TD, Herrera S, Mohammad R, Williams EK et al (2020) Microfluidic concentration and separation of circulating tumor cell clusters from large blood volumes. Lab Chip 20(3):558–567
Boya M, Ozkaya-Ahmadov T, Swain BE, Chu CH, Asmare N, Civelekoglu O et al (2022) High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells. Nat Commun 13(1):3385
Acknowledgments
This work was supported by the start-up funds provided to A.F.S. by Georgia Institute of Technology. The research was also supported in part by the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award No. W81XWH-20-1-0649, the Dunwoody Golf Club Prostate Cancer Research Award, and Winship Invest$ Pilot Grant from the Winship Cancer Institute of Emory University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Boya, M., Sarioglu, A.F. (2023). Cluster-Wells: A Technology for Routine and Rapid Isolation of Extremely Rare Circulating Tumor Cell Clusters from Unprocessed Whole Blood. In: Garcia-Cordero, J.L., Revzin, A. (eds) Microfluidic Systems for Cancer Diagnosis . Methods in Molecular Biology, vol 2679. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3271-0_18
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3271-0_18
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3270-3
Online ISBN: 978-1-0716-3271-0
eBook Packages: Springer Protocols