Abstract
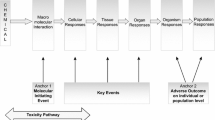
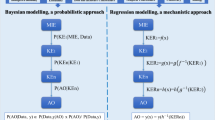
Adverse outcome pathways (AOPs) are tools to capture and visualize mechanisms driving toxicological effects. They share a common structure consisting of a molecular initiating event, a series of key events connected by key event relationships and an adverse outcome. Development and evaluation of AOPs ideally comply with guidelines issued by the Organization for Economic Cooperation and Development. AOPs have been introduced for major types of hepatotoxicity, which is not a surprise, as the liver is a frequent target for systemic adversity. Various applications for AOPs have been proposed in the areas of toxicology and chemical risk assessment, in particular in relation to the establishment of quantitative structure–activity relationships, the elaboration of prioritization strategies, and the development of novel in vitro toxicity screening tests and testing strategies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
OECD (2013) OECD series on testing and assessment number 184: guidance document on developing and assessing adverse outcome pathways ENV/JM/MONO(2013)6. OECD Publishing, Paris, pp 1–45
US EPA (2005) Guidelines for carcinogen risk assessment. US EPA, Washington DC
Bogdanffy MS, Daston G, Faustman EM, Kimmel CA, Kimmel GL, Seed J et al (2001) Harmonization of cancer and noncancer risk assessment: proceedings of a consensus-building workshop. Toxicol Sci 61:18–31
Julien E, Boobis AR, Olin SS (2009) The key events dose-response framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit Rev Food Sci Nutr 49:682–689
Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD et al (2003) A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol 33:591–653
Seed J, Carney EW, Corley RA et al (2005) Overview: using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol 35:664–672
NRC (2007) Toxicity testing in the 21st century: a vision and a strategy. The National Academies Press, Washington DC
Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741
Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA et al (2014) Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci 142:312–320
Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B et al (2015) Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol 72:514–537
Burden N, Sewell F, Andersen ME, Boobis A, Chipman JK, Cronin MTD et al (2015) Adverse outcome pathways can drive non-animal approaches for safety assessment. J Appl Toxicol 35:971–975
Vinken M, Knapen D, Vergauwen L, Hengstler JG, Angrish M, Whelan M (2017) Adverse outcome pathways: a concise introduction for toxicologists. Arch Toxicol 91:3697–3707
Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA (2016) Adverse outcome pathways: organizing toxicological information to improve decision making. J Pharmacol Exp Ther 356:170–181
Perkins EJ, Antczak P, Burgoon L, Falciani F, Garcia-Reyero N, Gutsell S et al (2015) Adverse outcome pathways for regulatory applications: examination of four case studies with different degrees of completeness and scientific confidence. Toxicol Sci 148:14–25
OECD (2017) OECD series on testing and assessment number 184: revised guidance document on developing and assessing adverse outcome pathways ENV/JM/MONO(2013)6. OECD Publishing, Paris, pp 1–32
http://aopkb.org/ (consulted July 2020)
OECD (2016) OECD series on adverse outcome pathways number 1: users’ handbook supplement to the guidance document for developing and assessing adverse outcome pathways. OECD Publishing, Paris, pp 1–53
Delrue N, Sachana M, Sakuratani Y, Gourmelon A, Leinala E, Diderich R (2016) The adverse outcome pathway concept: a basis for developing regulatory decision-making tools. Altern Lab Anim 44:417–429
https://aopwiki.org/ (consulted July 2020)
Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA et al (2014) Adverse outcome pathway development II: best practices. Toxicol Sci 142:321–330
Ankley GT, Edwards SW (2018) The adverse outcome pathway: a multifaceted framework supporting 21st century toxicology. Curr Opin Toxicol 1:1–7
Leist M, Ghallab A, Graepel R, Marchan R, Hassan R, Bennekou SH et al (2017) Adverse outcome pathways: opportunities, limitations and open questions. Arch Toxicol 91:3477–3505
Carusi A, Davies MR, De Grandis G, Escher BI, Hodges G, Leung KMY et al (2018) Harvesting the promise of AOPs: an assessment and recommendations. Sci Total Environ 628-629:1542–1556
Gijbels E, Vinken M (2017) An update on adverse outcome pathways leading to liver injury. Appl In Vitro Toxicol 3:283–285
Bell SM, Angrish MM, Wood CE, Edwards SW (2016) Integrating publicly available data to generate computationally predicted adverse outcome pathways for fatty liver. Toxicol Sci 150:510–520
Oki NO, Edwards SW (2016) An integrative data mining approach to identifying adverse outcome pathway signatures. Toxicology 350-352:49–61
Oki NO, Nelms MD, Bell SM, Mortensen HM, Edwards SW (2016) Accelerating adverse outcome pathway development using publicly available data sources. Curr Environ Health Rep 3:53–63
Perkins EJ, Ashauer R, Burgoon L, Conolly R, Landesmann B, Mackay C et al (2019) Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environ Toxicol Chem 38:1850–1865
Spinu N, Cronin MTD, Enoch SJ, Madden JC, Worth AP (2020) Quantitative adverse outcome pathway (qAOP) models for toxicity prediction. Arch Toxicol 94:1497–1510
Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L et al (2018) Adverse outcome pathway networks I: development and applications. Environ Toxicol Chem 37:1723–1733
Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L et al (2018) Adverse outcome pathway networks II: network analytics. Environ Toxicol Chem 37:1734–1748
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med 58:295–300
Vinken M, Maes M, Vanhaecke T, Rogiers V (2013) Drug-induced liver injury: mechanisms, types and biomarkers. Curr Med Chem 20:3011–3021
AbdulHameed MDM, Pannala VR, Wallqvist A (2019) Mining public toxicogenomic data reveals insights and challenges in delineating liver steatosis adverse outcome pathways. Front Genet 10:1007
Mellor CL, Steinmetz FP, Cronin MT (2016) The identification of nuclear receptors associated with hepatic steatosis to develop and extend adverse outcome pathways. Crit Rev Toxicol 46:138–152
Vinken M, Landesmann B, Goumenou M, Vinken S, Shah I, Jaeschke H et al (2013) Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol Sci 136:97–106
Gijbels E, Vilas-Boas V, Annaert P, Vanhaecke T, Devisscher L, Vinken M (2020) Robustness testing and optimization of an adverse outcome pathway on cholestatic liver injury. Arch Toxicol 94:1151–1172
Horvat T, Landesmann B, Lostia A, Vinken M, Munn S, Whelan M (2017) Adverse outcome pathway development from protein alkylation to liver fibrosis. Arch Toxicol 91:1523–1543
Gadaleta D, Manganelli S, Roncaglioni A, Toma C, Benfenati E, Mombelli E (2018) QSAR modelling of ToxCast assays relevant to the molecular initiating events of AOPs leading to hepatic steatosis. J Chem Inf Model 58:1501–1517
Hirano H, Kurata A, Onishi Y, Sakurai A, Siato H, Nakagawa H et al (2006) High-speed screening and QSAR analysis of human ATP-binding cassette transporter ABCB11 (bile salt export pump) to predict drug-induced intrahepatic cholestasis. Mol Pharm 2:252–265
Saito H, Osumi M, Hirano H, Shin W, Nakamura R, Ishikawa T (2009) Technical pitfalls and improvements for high-speed screening and QSAR analysis to predict inhibitors of the human bile salt export pump (ABCB11/BSEP). AAPS J 11:581–589
Warner DJ, Chen H, Cantin LD, Kenna JG, Stahl S, Walker CL et al (2012) Mitigating the inhibition of human bile salt export pump by drugs: opportunities provided by physicochemical property modulation, in silico modeling, and structural modification. Drug Metab Dispos 40:2332–2341
Honório KM, Salum LB, Garratt RC, Polikarpov I, Andricopulo AD (2008) Two- and three-dimensional quantitative structure–activity relationships studies on a series of liver X receptor ligands. Open Med Chem J 2:87–96
Judson R, Kavlock R, Martin M, Reif D, Houck K, Knudsen T et al (2013) Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX 30:51–56
Yozzo KL, McGee SP, Volz DC (2013) Adverse outcome pathways during zebrafish embryogenesis: a case study with paraoxon. Aquat Toxicol 126:346–354
Oki NO, Farcal L, Abdelaziz A, Florean O, Doktorova TY, Exner T et al (2019) Integrated analysis of in vitro data and the adverse outcome pathway framework for prioritization and regulatory applications: an exploratory case study using publicly available data on piperonyl butoxide and liver models. Toxicol In Vitro 54:23–32
Rooney J, Hill T 3rd, Qin C, Sistare FD, Corton JC (2018) Adverse outcome pathway-driven identification of rat liver tumorigens in short-term assays. Toxicol Appl Pharmacol 356:99–113
Lichtenstein D, Luckert C, Alarcan J, de Sousa G, Gioutlakis M, Katsanou E et al (2020) An adverse outcome pathway-based approach to assess steatotic mixture effects of hepatotoxic pesticides in vitro. Food Chem Toxicol 139:111283
Khadka KK, Chen M, Liu Z, Tong W, Wang D (2020) Integrating adverse outcome pathways (AOPs) and high throughput in vitro assays for better risk evaluations, a study with drug-induced liver injury (DILI). ALTEX 37:187–196
Acknowledgments
This work was financially supported by Cosmetics Europe, the European Chemical Industry Council, the European Marie-Curie Sklodowska Action programme (Individual Fellowship 833095), the Center for Alternatives to Animal Testing at Johns Hopkins University Baltimore-USA, the Research Foundation Flanders-Belgium (FWO-Vlaanderen), and the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-Brussel).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Arnesdotter, E., Gijbels, E., dos Santos Rodrigues, B., Vilas-Boas, V., Vinken, M. (2022). Adverse Outcome Pathways as Versatile Tools in Liver Toxicity Testing. In: Benfenati, E. (eds) In Silico Methods for Predicting Drug Toxicity. Methods in Molecular Biology, vol 2425. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1960-5_20
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1960-5_20
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1959-9
Online ISBN: 978-1-0716-1960-5
eBook Packages: Springer Protocols