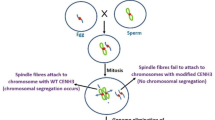
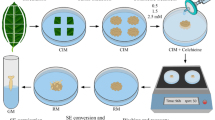
Abstract
The completely homozygous genetic background of doubled haploids (DHs) has many applications in breeding programs and research studies. Haploid induction and chromosome doubling of induced haploids are the two main steps of doubled haploid creation. Both steps have their own complexities. Chromosome doubling of induced haploids may happen spontaneously, although usually at a low rate. Therefore, artificial/induced chromosome doubling of haploid cells/plantlets is necessary to produce DHs at an acceptable level. The most common method is using some mitotic spindle poisons that target the organization of the microtubule system. Colchicine is a well-known and widely used antimitotic. However, there are substances alternative to colchicine in terms of efficiency, toxicity, safety, and genetic stability, which can be applied in in vitro and in vivo pathways. Both pathways have their own advantages and disadvantages. However, in vitro-induced chromosome doubling has been much preferred in recent years, maybe because of the dual effect of antimitotic agents (haploid induction and chromosome doubling) in just one step, and the reduced generation of chimeras. Plant genotype, the developmental stage of initial haploids, and type–concentration–duration of application of antimitotic agents, are top influential parameters on chromosome doubling efficiency. In this review, we highlight different aspects related to antimitotic agents and to plant parameters for successful chromosome doubling and high DH yield.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Broughton S, Castello M, Liu L, Killen J, Hepworth A, O’Leary R (2020) The effect of caffeine and trifluralin on chromosome doubling in wheat anther culture. Plan Theory 9(105):1–14
Germanà MA (2011) Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Rep 30(5):839–857
Seguí-Simarro JM (2016) Androgenesis in Solanaceae. In: Germana M, Lambardi M (eds) In Vitro embryogenesis in higher plants. Methods in molecular biology, vol 1359. Humana, New York, NY
Segui-Simarro JM, Nuez F (2008) Pathways to doubled haploidy: chromosome doubling during androgenesis. Cytogenet Genom Res 120:358–369
Ren J, Wu P, Trampe B, Tian X, Lübberstedt T, Chen S (2017) Novel technologies in doubled haploid line development. Plant Biotechnol J 15(11):1361–1370
Shariatpanahi ME, Ahmadi B (2016) Isolated microspore culture and its applications in plant breeding and genetics. In: Anis M, Ahmad N (eds) Plant tissue culture: propagation, conservation and crop improvement. Springer, Singapore
Kumlehn J, Serazetdinova L, Hensel G, Becker D, Loerz H (2006) Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with agrobacterium tumefaciens. Plant Biotechnol J 4(2):251–261
Eudes F, Shim YS, Jiang F (2014) Engineering the haploid genome of microspores. Biocatal Agric Biotechnol 3(1):20–23
Chugh A, Amundsen E, Eudes F (2009) Translocation of cell-penetrating peptides and delivery of their cargoes in triticale microspores. Plant Cell Rep 28(5):801–810
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142(1–2):169–196
Collard BC, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 363(1491):557–572
Sanchez DL, Liu S, Ibrahim R, Blanco M, Lübberstedt T (2018) Genome-wide association studies of doubled haploid exotic introgression lines for root system architecture traits in maize (Zea mays L.). Plant Sci 268:30–38
Seyis F, Aydin E, Çatal Mİ (2014) Haploids in the improvement of crucifers. Türk Tarım Doğa Bilimleri 7(7):1419–1424
Dong YQ, Zhao WX, Li XH, Liu XC, Gao NN, Huang JH, Wang WY, Xu XL, Tang ZH (2016) Androgenesis, gynogenesis, and parthenogenesis haploids in cucurbit species. Plant Cell Rep 35(10):1991–2019
Dirks R, Van Dun K, De Snoo CB, Van Den Berg M, Lelivelt CL, Voermans W, Woudenberg L, De Wit JP, Reinink K, Schut JW, Van Der Zeeuw E (2009) Reverse breeding: a novel breeding approach based on engineered meiosis. Plant Biotechnol J 7(9):837–845
Kasha KJ (2005) Chromosome doubling and recovery of doubled haploid plants. In: Palmer CE, Keller WA, Kasha KJ (eds) Haploids in crop improvement II, vol 56. Springer-Verlag, Berlin, pp 123–152
Kasha KJ, Maluszynski M (2003) Production of doubled haploids in crop plants. An introduction. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Springer, Dordrecht
Santeramo D, Howell J, Ji Y, Yu W, Liu W, Kelliher T (2020) DNA content equivalence in haploid and diploid maize leaves. Planta 251(1):30
Ahmadi B, Ebrahimzadeh H (2020) In vitro androgenesis: spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep 39(3):299–316
Castillo AM, Cistué L, Vallés MP, Soriano M (2009) Chromosome doubling in monocots. In: Touraev A, Forster BP, Jain SM (eds) Advances in haploid production in higher plants. Springer, Dordrecht
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104(3):359–373
Yuan S, Su Y, Liu Y, Li Z, Fang Z, Yang L, Zhuang M, Zhang Y, Lv H, Sun P (2015) Chromosome doubling of microspore-derived plants from cabbage (Brassica oleracea var. capitata L.) and broccoli (Brassica oleracea var. italica L.). Front Plant Sci 6:1118
Palmer CE, Keller WA, Arnison PG (1996) Utilization of Brassica haploids. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants. Kluwer Academic Publishers, Dordrecht, pp 173–192
Hofinger BJ, Huynh OA, Jankowicz-Cieslak J, Müller A, Otto I, Kumlehn J, Till BJ (2013) Validation of doubled haploid plants by enzymatic mismatch cleavage. Plant Methods 9(43):1–10
Daghma DES, Hensel G, Rutten T, Melzer M, Kumlehn J (2014) Cellular dynamics during early barley pollen embryogenesis revealed by time-lapse imaging. Front Plant Sci 5(675):1–14
Kahrizi D, Mohammadi R (2009) Study of androgenesis and spontaneous chromosome doubling in barley (Hordeum vulgare L.) genotypes using isolated microspore culture. Acta Agron Hung 57(2):155–164
Li JR, Zhuang FY, Ou CG, Hu H, Zhao ZW, Mao JH (2013) Microspore embryogenesis and production of haploid and doubled haploid plants in carrot (Daucus carota L.). Plant Cell Tissue Organ Cult 112:275–287
Kleiber D, Prigge V, Melchinger AE, Burkard F, San Vicente F, Palomino G, Gordillo GA (2012) Haploid fertility in temperate and tropical maize germplasm. Crop Sci 52:623–630
Kasha KJ, Hu TC, Oro R, Simion E, Shim YS (2001) Nuclear fusion leads to chromosome doubling during mannitol pretreatment of barley (Hordeum vulgare L.) microspores. J Exp Bot 52(359):1227–1238
Maluszynska J (2003) Cytogenetic tests for ploidy level analyseschromosome counting. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants: a manual. Kluwer Academic Publishers, Dordrecht, pp 391–395
Cistue L, Castan MS, Castillo A, Valles MP, Sanz JM, Echavari B (2006) Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep 25(4):275–264
Weber S, Nker F, Friedt W (2005) Improved doubled haploid production protocol for Brassica napus using microspore colchicine treatment in vitro and ploidy determination by flow cytometry. Plant Breed 124:511–513
da Silva Dias JC (2003) Protocol for broccoli microspore culture. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants: a manual. Kluwer Academic, Dordrecht, pp 195–204
Martin B, Widholm JM (1996) Ploidy of small individual embryo-like structures from maize anther cultures treated with chromosome doubling agents and calli derived from them. Plant Cell Rep 15:781–785
Gervais C, Newcomb W, Simmonds DH (2000) Rearrangement of the actin filament and microtubule cytoskeleton during induction of microspore embryogenesis in Brassica napus L. cv. Topas. Protoplasma 213(3–4):194–202
Dubas E, Custers J, Kieft H, Wędzony M, van Lammeren AAM (2011) Microtubule configurations and nuclear DNA synthesis during initiation of suspensor-bearing embryos from Brassica napus cv Topas microspores. Plant Cell Rep 30(11):2105–2116
Li H, Devaux P (2003) High frequency regeneration of barley doubled haploid plants from isolated microspore culture. Plant Sci 164(3):379–386
Soriano M, Cistue L, Valles MP, Castillo AM (2007) Effects of colchicine on anther and microspore culture of bread wheat (Triticum aestivum L.). Plant Cell Tissue Organ Cult 91(3):225–234
Sato S, Katoh N, Iwai S, Hagimori M (2005) Frequency of spontaneous polyploidization of embryos regenerated from cultured anthers or microspores of Brassica rap a var. pekinensis L. and B. oleracea var. capitata L. Breed Sci 55:99–102
Hu T, Kasha KJ (1996) Performance of isolated microspore-derived doubled haploids of wheat (Triticum aestivum L.). Can J Plant Sci 77(4):549–555
Jauhar PP, Dogramacı-Altuntepe M, Peterson TS, Almouslem AB (2000) Seedset on synthetic haploids of durum wheat: cytological and molecular investigations. Crop Sci 40:1742–1749
Jauhar PP (2007) Meiotic restitution in wheat polyhaploids and amphihaploids: a potent force in evolution of the Triticeae. J Hered 98:188–193
Takahira J, Cousin A, Nelson MN, Cowling WA (2011) Improvement in efficiency of microspore culture to produce doubled haploid canola (Brassica napus L.) by flow cytometry. Plant Cell Tissue Organ Cult 104(1):51–59
Joubés J, Chevalier C (2000) Endoreduplication in higher plants. Plant Mol Biol 43:735–745
Weber J, Georgiev V, Pavlov A, Bley T (2008) Flow cytometric investigations of diploid and tetraploid plants and in vitro cultures of Datura stramonium and Hyoscyamus niger. Cytometry A 73:931–939
Perera PIP, Ordoñez CA, Lopez-Lavalle LAB, Dedicova B (2014) A milestone in the doubled haploid pathway of cassava. Protoplasma 251:233–246
Testillano PS, Georgiev S, Mogensen HL, Coronado MJ, Dumas C, Risueno MC, Matthys-Rochon E (2004) Spontaneous chromosome doubling results from nuclear fusion during in vitro maize induced microspore embryogenesis. Chromosoma 112(7):342–349
Shim YS, Kasha KJ, Simion E, Letarte J (2006) The relationship between induction of embryogenesis and chromosome doubling in microspore cultures. Protoplasma 228(1–3):79–86
Acharya BC, Ramji MV (1977) Experimental androgenesis in plants – a review. Proc Indian Acad Sci 86(6):337–360
Sarto GE, Stubblefield PA, Therman E (1982) Endomitosis in human trophoblast. Human Genet 62(3):228–232
Lee HO, Davidson JM, Duronio RJ (2009) Endoreplication: polyploidy with purpose. Gen Dev 23:2461–2477
Malallah GA, Afzal M, Attia TA, Abraham D (1996) Tapetal cell nuclear characteristics of some Kuwaiti plants. Cytologia 61:259–267
Sharma SK, Kumaria S, Tandon P, Rao SR (2012) Endomitosis in tapetal cells of some Cymbidiums (Orchidaceae). Nucleus 55:21–25
Hüsemann LC, Reese A, Radine C, Piekorz RP, Budach W, Sohn D, Jänicke RU (2020) The microtubule targeting agents eribulin and paclitaxel activate similar signaling pathways and induce cell death predominantly in a caspase-independent manner. Cell Cycle 19(4):464–478
Silva P, Barbosa J, Nascimento AV, Faria J, Reis R, Bousbaa H (2011) Monitoring the fidelity of mitotic chromosome segregation by the spindle assembly checkpoint. Cell Prolif 44(5):391–400
Dube D, Tiwari P, Kaur P (2016) The hunt for antimitotic agents: an overview of structure-based design strategies. Expert Opin Drug Discov 11(6):579–597
Schmidt M, Bastians H (2007) Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Up 10(4–5):162–181
Downing KH (2000) Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev Cell Dev Biol 16:89–111
Chaikam V, Molenaar W, Melchinger AE, Boddupalli PM (2019) Doubled haploid technology for line development in maize: technical advances and prospects. Theor Appl Genet 132:3227–3243
Ebrahimzadeh H, Soltanloo H, Shariatpanahi ME, Eskandari A, Ramezanpour SS (2018) Improved chromosome doubling of parthenogenetic haploid plants of cucumber (Cucumis sativus L.) using colchicine, trifluralin, and oryzalin. Plant Cell Tissue Organ Cult 135(3):407–417
Grosso V, Farina A, Giorgi D, Nardi L, Diretto G, Lucretti S (2018) A high-throughput flow cytometry system for early screening of in vitro made polyploids in Dendrobium hybrids. Plant Cell Tissue Organ Cult 132(1):57–70
Sood S, Dwivedi S (2015) Doubled haploid platform: an accelerated breeding approach for crop improvement. In: Bahadur B, Venkat RM, Sahijram L, Krishnamurthy K (eds) Plant biology and biotechnology, volume II: plant genomicsand biotechnology. Springer, New Delhi, pp 89–111
Noori SAS, Norouzi M, Karimzadeh G, Shirkool K, Niazian M (2017) Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult 130(3):543–551
Sivakumar G, Alba K, Phillips GC (2017) Biorhizome: a biosynthetic platform for colchicine biomanufacturing. Front Plant Sci 8:1137
Eng WH, Ho WS (2019) Polyploidization using colchicine in horticultural plants: a review. Sci Hortic 246:604–617
Melchinger AE, Molenaar WS, Mirdita V, Schipprack W (2016) Colchicine alternatives for chromosome doubling in maize haploids for doubled-haploid production. Crop Sci 56(2):559–569
Pintos B, Manzanera JA, Bueno MA (2007) Antimitotic agents increase the production of doubled-haploid embryos from cork oak anther culture. J Plant Physiol 164(12):1595–1604
Pazuki A, Aflaki F, Gürel S, Ergül A, Gürel E (2018) Production of doubled haploids in sugar beet (Beta vulgaris): an efficient method by a multivariate experiment. Plant Cell Tissue Org Cult 132(1):85–97
Aqafarini A, Lotfi M, Norouzi M, Karimzadeh G (2019) Induction of tetraploidy in garden cress: morphological and cytological changes. Plant Cell Tissue Organ Cult 137(3):627–635
Fu L, Zhu Y, Li M, Wang C, Sun H (2019) Autopolyploid induction via somatic embryogenesis in Lilium distichum Nakai and Lilium cernuum Komar. Plant Cell Tissue Organ Cult 139:237–248
Khalili S, Niazian M, Arab M, Norouzi M (2019) In vitro chromosome doubling of African daisy, Gerbera jamesonii bolus cv. Mini red. Nucleus 63:59–65
Sabzehzari M, Hoveidamanesh S, Modarresi M, Mohammadi V (2019) Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of Plantago psyllium. Plant Cell Tissue Organ Cult 139(1):131–137
Ye YM, Tong J, Shi XP, Yuan W, Li GR (2010) Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci Hortic 124(1):95–101
He M, Gao W, Gao Y, Liu Y, Yang X, Jiao H, Zhou Y (2016) Polyploidy induced by colchicine in Dendranthema indicum var. Aromaticum, a scented chrysanthemum. Eur J Hortic Sci 81(4):219–226
Xu C, Zhang Y, Huang Z, Yao P, Li Y, Kang X (2018) Impact of the leaf cut callus development stages of Populus on the tetraploid production rate by colchicine treatment. J Plant Growth Regul 37(2):635–644
Rao PS, Suprasanna P (1996) Methods to double haploid chromosome numbers. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants. Current plant science and biotechnology in agriculture, vol 23. Springer, Dordrecht
Qi-Qing LI, Zhang J, Ji-Hua LIU, Bo-Yang YU (2018) Morphological and chemical studies of artificial Andrographis paniculata polyploids. Chin J Nat Med 16(2):81–89
Sun FY, Liu L, Yu Y, Ruan XM, Wang CY, Hu QW, Wu DX, Sun G (2020) MicroRNA-mediated responses to colchicine treatment in barley. Planta 251(2):1–14
Jensen CJ (1975) Barley monoploids and doubled monoploids: technique and experience. In: H. Gaul Proceedings of the 3rd international barley genetics symposium., 316–345. Verlag Karl Thiemeg, Munich
Subrahmanyam NC, Kasha KJ (1975) Chromosome doubling of barley haploids by nitrous oxide and colchicine treatments. Can J Genet Cytol 17(4):573–583
Premvaranon P, Vearasilp S, Thanapornpoonpong S, Karladee D, Gorinstein S (2011) In vitro studies to produce double haploid in Indica hybrid rice. Biologia 66(6):1074–1081
Mohammadi PP, Moini A, Ebrahimi A, Javidfar F (2012) Doubled haploid plants following colchicine treatment of microspore-derived embryos of oilseed rape (Brassica napus L.). Plant Cell Tissue Organ Cult 108:251–256
Yemets AI, Blume YB (2008) Progress in plant polyploidization based on antimicrotubular drugs. Open Hortic J 1(1):15–20
Sood S, Dhawan R, Singh K, Bains NS (2003) Development of novel chromosome doubling strategies for wheat × maize system of wheat haploid production. Plant Breed 122(6):493–496
Pan-pan H, Wei-xu L, Hui-hui L (2018) In vitro induction and identification of autotetraploid of Bletilla striata (Thunb.) Reichb. f. by colchicine treatment. Plant Cell Tissue Organ Cult 132(3):425–432
Ochatt SJ, Patat-Ochatt EM, Moessner A (2011) Ploidy level determination within the context of in vitro breeding. Plant Cell Tissue Organ Cult 104(3):329–341
Dwivedi SL, Britt AB, Tripathi L, Sharma S, Upadhyaya HD, Ortiz R (2015) Haploids: constraints and opportunities in plant breeding. Biotechnol Adv 33(6):812–829
Niazian M, Sadat-Noori SA, Abdipour M, Tohidfar M, Mortazavian SMM (2018) Image processing and artificial neural network-based models to measure and predict physical properties of embryogenic callus and number of somatic embryos in ajowan (Trachyspermum ammi (L.) Sprague). In Vitro Cell Dev Biol Plant 54(1):54–68
Niazian M, Shariatpanahi ME, Abdipour M, Oroojloo M (2019) Modeling callus induction and regeneration in an anther culture of tomato (Lycopersicon esculentum L.) using image processing and artificial neural network method. Protoplasma 256(5):1317–1332
Wang H, Dong B, Jiang J, Fang W, Guan Z, Liao Y, Chen S, Chen F (2014) Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern. Front Plant Sci 5:738
Vanous K, Vanous A, Frei UK, Lübberstedt T (2017) Generation of maize (Zea mays) doubled haploids via traditional methods. Curr Protoc Plant Biol 2(2):147–157
Warchoł M, Skrzypek E, Nowakowska A, Marcińska I, Czyczyło-Mysza I, Dziurka K, Juzoń K, Cyganek K (2016) The effect of auxin and genotype on the production of Avena sativa L. doubled haploid lines. Plant Growth Regul 78(2):155–165
Marcińska I, Nowakowska A, Skrzypek E, Czyczyło-Mysza I (2013) Production of double haploids in oat (Avena sativa L.) by pollination with maize (Zea mays L.). Open Life Sci 8(3):306–313
Warchoł M, Czyczyło-Mysza I, Marcińska I, Dziurka K, Noga A, Skrzypek E (2018) The effect of genotype, media composition, pH and sugar concentrations on oat (Avena sativa L.) doubled haploid production through oat× maize crosses. Acta Physiol Plant 40(5):93
Dunwell JM, Wilkinson MJ, Nelson S, Wening S, Sitorus AC, Mienanti D, Alfiko Y, Croxford AE, Ford CS, Forster BP, Caligari PD (2010) Production of haploids and doubled haploids in oil palm. BMC Plant Biol 10(1):218
Arzani A, Darvey NL (2001) The effect of colchicine on triticale anther-derived plants: microspore pre-treatment and haploid-plant treatment using a hydroponic recovery system. Euphytica 122(2):235–241
Ślusarkiewicz-Jarzina A, Pudelska H, Woźna J, Pniewski T (2017) Improved production of doubled haploids of winter and spring triticale hybrids via combination of colchicine treatments on anthers and regenerated plants. J Appl Genet 58(3):287–295
Tayeng T, Chaudhary HK, Kishore N (2012) Enhancing doubled haploid production efficiency in wheat (Triticum aestivum L. em. Thell) by in vivo colchicine manipulations in Imperata cylindrica-mediated chromosome elimination approach. Plant Breed 131(5):574–578
Srivastava P, Gill RS, Sharma A, Kumar S, Raghupati N, Mahal GS, Bains NS (2012) Colchicine administered as post pollination tiller injection is deleterious for doubled haploid production in durum wheat x maize crosses. Crop Improv 39(1):60–64
Srivastava P, Bains NS (2018) Accelerated wheat breeding: doubled haploids and rapid generation advance. In: Gosal S, Wani S (eds) Biotechnologies of crop improvement, volume 1. Springer, Cham
Foschi ML, Martinez LE, Ponce MT, Galmarini CR, Bohanec B (2013) Effect of colchicine and amiprophos-methyl on the production of in vitro doubled haploid onion plants and correlation assessment between ploidy level and stomatal size. Rev FCA UNCUYO 45(2):155–164
Alan AR, Lim W, Mutschler MA, Earle ED (2007) Complementary strategies for ploidy manipulations in gynogenic onion (Allium cepa L.). Plant Sci 173:25–31
Ferrie AMR, Irmen KI, Beattie AD, Rossnagel BG (2014) Isolated microspore culture of oat (Avena sativa L.) for the production of doubled haploids: effect of pre-culture and post-culture conditions. Plant Cell Tissue Organ Cult 116(1):89–96
Bossoutrot D, Hosemans D (1985) Gynogenesis in Beta vulgaris L.: from in vitro culture of unpollinated ovules to the production of doubled haploid plants in soil. Plant Cell Rep 4:300–303
Lionneton E, Beuret W, Delaitre V, Ochatt S, Rancillac M (2001) Improved microspore culture and doubled-haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rep 20:126–130
Klutschewski S (2012) Methodical improvements in microspore culture of Brassica napus L. Dissertation. University of Gottingen, Gottingen
Bhatia R, Dey SS, Sood S, Sharma K, Parkash C, Kumar R (2017) Efficient microspore embryogenesis in cauliflower (Brassica oleracea var. botrytis L.) for development of plants with different ploidy level and their use in breeding programme. Sci Hortic 216:83–92
Herrera JC, Moreno LG, Acuna JR, De Pena M, Osorio D (2002) Colchicine-induced microspore embryogenesis in coffee. Plant Cell Tissue Organ Cult 71(1):89–92
Lotfi M, Alan AR, Henning MJ, Jahn MM, Earle ED (2003) Production of haploid and doubled haploid plants of melon (Cucumis melo L.) for use in breeding for multiple virus resistance. Plant Cell Rep 21:1121–1128
Burun B, Emiroglu U (2008) A comparative study on colchicine application methods in obtaining doubled haploids of tobacco (Nicotiana tabacum L.). Turk J Biol 32(2):105–111
Islam SMS (2010) The effect of colchicine pretreatment on isolated microspore culture of wheat (Triticum aestivum L.). Austr J Crop Sci 4(9):660–665
Würschum T, Tucker MR, Reif JC, Maurer HP (2012) Improved efficiency of doubled haploid generation in hexaploid triticale by in vitro chromosome doubling. BMC Plant Biol 12(109):1–7
Obert B, Barnabas B (2004) Colchicine induced embryogenesis in maize. Plant Cell Tissue Organ Cult 77(3):283–285
Ren X, Ci J, Cui X, Yang W (2018) Doubling effect of anti-microtubule herbicides on the maize haploid. Emirates J Food Agric 30(10):903–908
Grzebelus E, Adamus A (2004) Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos. Plant Sci 167:569–574
Fayos O, Valles MP, Graces-Claver A, Moller C, Castillo AM (2015) Doubled haploid production from Spanish onion (Allium cepa L.) germplasm: embryogenesis induction, plant regeneration and chromosome doubling. Front Plant Sci 6:384
Hansen AL, Gertz A, Joersbo M, Andersen SB (2000) Chromosome doubling in vitro with amiprophos-methyl in Beta vulgaris ovule culture. Acta Agric Scand Sect B Soil Plant Sci 50:89–95
Hansen NJP, Andersen SB (1998) Efficient production of doubled haploid wheat plants by in vitro treatment of microspores with trifluralin or APM. Plant Breed 117:401–405
Wan Y, Duncan DR, Rayburn AL, Petolino JF, Widholm JM (1991) The use of anti-microtubule herbicides for the production of doubled haploid plants from anther-derived maize callus. Theor Appl Genet 81(2):205–211
Soriano M, Cistué L, Castillo AM (2008) Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant Cell Rep 27(5):805–811
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Shariatpanahi, M.E., Niazian, M., Ahmadi, B. (2021). Methods for Chromosome Doubling. In: Segui-Simarro, J.M. (eds) Doubled Haploid Technology. Methods in Molecular Biology, vol 2287. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1315-3_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1315-3_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1314-6
Online ISBN: 978-1-0716-1315-3
eBook Packages: Springer Protocols