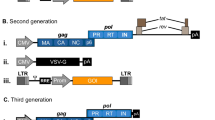
Abstract
In clinical gene transfer applications, lentiviral vectors (LV) have rapidly become the primary means to achieve permanent and stable expression of a gene of interest or alteration of gene expression in target cells. This status can be attributed primarily to the ability of the LV to (1) transduce dividing as well as quiescent cells, (2) restrict or expand tropism through envelope pseudo-typing, and (3) regulate gene expression within different cell lineages through internal promoter selection. Recent progress in viral vector design such as the elimination of unnecessary viral elements, split packaging, and self-inactivating vectors has established a significant safety profile for these vectors. The level of GMP compliance required for the manufacture of LV is dependent upon their intended use, stage of drug product development, and country where the vector will be used as the different regulatory authorities who oversee the clinical usage of such products may have different requirements. As such, successful GMP manufacture of LV requires a combination of diverse factors including: regulatory expertise, compliant facilities, validated and calibrated equipments, starting materials of the highest quality, trained production personnel, scientifically robust production processes, and a quality by design approach. More importantly, oversight throughout manufacturing by an independent Quality Assurance Unit who has the authority to reject or approve the materials is required. We describe here the GMP manufacture of LV at our facility using a four plasmid system where 293T cells from an approved Master Cell Bank (MCB) are transiently transfected using polyethylenimine (PEI). Following transfection, the media is changed and Benzonase added to digest residual plasmid DNA. Two harvests of crude supernatant are collected and then clarified by filtration. The clarified supernatant is purified and concentrated by anion exchange chromatography and tangential flow filtration. The final product is then diafiltered directly into the sponsor defined final formulation buffer and aseptically filled.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Milone MC, O'Doherty U (2018) Clinical use of lentiviral vectors. Leukemia 32(7):1529–1541
Coffin J (1997) In: Hughes S, Varmus H (eds) Retroviruses. Cold Spring Harbor Laboratory Press, New York
Rosenberg S, Aebersold P, Cornetta K et al (1990) Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med 323:570–578
Blaese R, Culver K, Miller A et al (1995) T lymphocyte-directed gene therapy for ADA–SCID: initial trial results after 4 years. Science 270:475–480
Kohn D, Weinberg K, Nolta J et al (1995) Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med 1:1017–1023
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G et al (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288:669–672
Aiuti A, Slavin S, Aker M et al (2002) Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296:2410–2413
Hacein-Bey-Abina S, Von Kalle C, Schmidt M et al (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302:415–419
Bushman F (2007) Retroviral integration and human gene therapy. J Clin Invest 117(8):2083–2086
Cattoglio C, Pellin D, Rizzi E et al (2010) High definition mapping of retroviral integration sites identifies active regulatory elements in human multipotent hematopoietic progenitors. Blood 116:5507–5517
Raper SE, Chirmule N, Lee FS et al (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80:148–158
Schröder A, Shinn P, Chen H et al (2002) HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110(4):521–529
Mitchell R, Beitzel B, Schroder A et al (2004) Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2(8):E234
https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/C120309. Accessed 1 Nov 2018
Schambach A, Swaney W, van der Loo J (2009) Design and production of retro- and lentiviral vectors for gene expression in hematopoietic cells. Methods Mol Biol 506:191–205
Dull T, Zufferey R, Kelly M et al (1998) A third-generation lentivirus vector with a conditional packaging system. J Virol 72(11):8463–8471
Modlich U, Bohne J, Schmidt M et al (2006) Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 108:2545–2553
Gándara C, Affleck V, Stoll EA (2018) Manufacture of third-generation Lentivirus for preclinical use, with process development considerations for translation to good manufacturing practice. Hum Gene Ther Methods 29(1):1–15
Gama-Norton L, Botezatu L, Herrmann S et al (2011) Lentivirus production is influenced by SV40 large T-antigen and chromosomal integration of the vector in HEK293 cells. Hum Gene Ther 22:1269–1279
Valkama AJ, Leinonen HM, Lipponen EM et al (2018) Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther 25(1):39–46
Merten OW, Hebben M, Bovolenta C (2016) Production of lentiviral vectors. Mol Ther Methods Clin Dev 3:16017. https://doi.org/10.1038/mtm.2016.17
Tomás HA, Rodrigues AF, Carrondo MJT, Coroadinha AS (2018) LentiPro26: novel stable cell lines for constitutive lentiviral vector production. Sci Rep 8(1):5271. https://doi.org/10.1038/s41598-018-23593-y
Slepushkin V, Chang N, Cohen R et al (2003) Large-scale purification of a lentiviral vector by size exclusion chromatography or mustang Q ion exchange chromatography. Bioprocess J 2(5):89–95
Leath A, Cornetta K (2012) Developing novel lentiviral vectors into clinical products. Methods Enzymol 507:89–108
Acknowledgments
We are grateful to Prof. H. Trent Spencer, Department of Pediatrics, School of Medicine, Emory University, Atlanta GA, for his critical review of this book chapter. Our facility is generously supported by the Cincinnati Children’s Hospital Research Foundation and we would like to acknowledge the hard work and dedication of the entire staff of the Translational Core Laboratories at Cincinnati Children’s Hospital; without it, our contributions to the gene therapy field would not be possible.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Dasgupta, A. et al. (2020). Phase I/II Manufacture of Lentiviral Vectors Under GMP in an Academic Setting. In: Swiech, K., Malmegrim, K., Picanço-Castro, V. (eds) Chimeric Antigen Receptor T Cells. Methods in Molecular Biology, vol 2086. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0146-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0146-4_3
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0145-7
Online ISBN: 978-1-0716-0146-4
eBook Packages: Springer Protocols