Abstract
Lipids from microalgae present a promising feedstock for biodiesel production, but deployment for economical production requires cost reductions for microalgae cultivation, dewatering, and lipid extraction. Lipid recovery from wet concentrated algal slurry, rather than from dried microalgae, is therefore desirable to avoid the need for secondary dewatering and/or drying. This chapter provides two protocols to enable extraction of cellular lipids from wet algal biomass. Depending on the cell wall composition, pretreatment of biomass is recommended prior to lipid extraction to facilitate better solvent penetration. While the chloroform/methanol-based Bligh and Dyer method may give higher lipid recovery yields, the use of safer solvents and in particular solvent combinations, such as a mixture of hexane and ethanol, may be more suitable for large-scale extraction.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords
1 Introduction
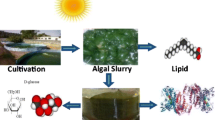
Microalgae have been considered a promising feedstock of biodiesel based on their high areal productivity and their ability to grow in most types of water (fresh, brackish, seawater, and even wastewater) while not requiring arable land [1–3]. However, their cultivation, harvesting, and downstream processing require cost reductions to enable economical production of microalgal fuels and by-products [4]. One of the main contributors of costly downstream processes of microalgal biodiesel production is dewatering and drying of the biomass [5–7]. In many of the current lipid extraction/recovery techniques, moisture can hinder the process efficiency significantly. The main reason is that moisture avoids efficient contact between microalgal cells and recovery solvent [8, 9]. On the other hand, most microalgal cells are composed of rigid cell walls which prohibit the access of recovery solvent to the intracellular lipid contents. To overcome all these obstacles, a process should be developed in which microalgal cells are ruptured in their wet condition and their lipids recovered by a suitable solvent mixture (Fig. 1).
First, to avoid dealing with large volumes of water, primary dewatering or concentrating of microalgae is required (e.g., by settling [10]). Among others, thermal and microwave-assisted pretreatments have been shown to be effective in rupturing or weakening microalgal cell walls [8, 11]. In some microalgal species with weak cell walls, osmotic shock can be applied to open up the cells [12]. After applying pretreatments, the lipid bodies of the microalgal cells can be recovered through applying a suitable solvent. Target compound polarity is the main factor determining the suitable solvent. We and others have previously shown that mixing polar and nonpolar solvents can enhance the lipid recovery efficiency [9, 11]. Increasing the polarity of the solvent not only facilitates the solvent penetration into the microalgal cells but also results in better access to microalgal lipids when moisture is present. In this protocol, we will describe and compare two solvent recovery techniques: (1) Bligh and Dyer’s technique [13] that is widely used in laboratories but not considered practical for large-scale use as it involves chloroform and (2) a solvent mixture of hexane and ethanol (3:1 ratio).
2 Materials
-
Hexane, methanol, chloroform, and ethanol; all were HPLC grade sourced from Merck KGaA
-
40 mL glass vials with polytetrafluoroethylene (PTFE)-lined caps
-
Corex glass centrifuge tubes
-
2 mL soda glass tubes
-
Avanti centrifuge (HP-20 XPI) (Beckman Coulter) for centrifugation of volumes higher than 1 L
-
Techno Spin R centrifuge (Sorvall Instruments) for less than 100 mL volumes
-
Vacuum desiccator
-
Digital Mettler AM50 scale with 0.1 mg accuracy
-
Agilent 6890 GC coupled to a 5975 MSD
-
LG Microwave Oven Model no. MS3447GR
-
Hot plate/magnetic stirrer
3 Methods
3.1 Lipid Extraction and Gravimetric Measurement
Microalgal slurry (e.g., from Scenedesmus sp.) is prepared after primary dewatering of microalgal culture that has been stimulated for cellular triacylglycerol accumulation in lipid bodies to result in a 16 g dry weight/L mixture. Alternatively, microalgal slurry can be reconstituted for experimental purposes by mixing biomass harvested by centrifugation with water (paste/water ratio of 1:5). After applying suitable pretreatments (e.g., microwave-assisted or thermal; see notes), 8 mL of the algal slurry is placed in a 40 mL glass vial with PTFE (polytetrafluoroethylene)-lined caps.
3.1.1 Extraction via Bligh and Dyer Method
For wet lipid extraction according to an adapted Bligh and Dyer method, 5 mL chloroform and 10 mL methanol are added to 8 mL algal slurry, and the mixture is vortexed for 4 min. Then additional 5 ml chloroform is added, followed by vortexing for another 4 min (ratio of chloroform/methanol/water, 2:2:1.8). Then the content of the vial is transferred to Corex glass centrifuge tubes followed by centrifugation at 1,000 × g for 7 min to form phase separation. At least five times, 2 mL of the organic layer (chloroform with extracted lipids on bottom) is pipetted and transferred to five pre-weighted soda glass tubes, and the chloroform is evaporated using a vacuum desiccator.
3.1.2 Extraction via a Solvent Mixture
Volumes of 15 mL hexane and 5 mL ethanol (3:1 ratio) are added to 8 mL algal slurry, followed by 4 min of vortexing. Then the vial content is transferred to Corex glass centrifuge tubes and centrifuged at 1,000 × g for 7 min for phase separation. At least five times, 2 mL of the organic layer (hexane with extracted lipids on top) is pipetted and transferred to five pre-weighted soda glass tubes, and the hexane is evaporated using a vacuum desiccator.
3.1.3 Calculating Total Amount of Extracted Lipids
Weighing the samples and gravimetric measuring of the lipid extractions are carried out using a digital Mettler AM50 scale with 0.1 mg accuracy, subtracting the weight of each soda glass tube.
3.2 Fatty Acid Analysis
Around 2 mg of extracted lipids are redissolved 1:1000 in chloroform, and a 100 μL aliquot is taken and dried down. Then, the lipids are hydrolyzed and methyl esterified with 300 μL of 2% H2SO4 in methanol solution at 80°C by shaking (480 rpm) for 2 h on a thermal mixer. Prior to esterification, 50 μg of heneicosanoic acid (C21) is added to the pellet in each sample as an internal standard. After esterification, 300 μL of 0.9% (w/v) NaCl and 300 μL of HPLC grade hexane are added and vortexed for 20 s. Phase separation is performed by centrifugation at 16,000 × g for 3 min, and the hexane layer is used for fatty acid methyl ester profile analysis by gas chromatography/mass spectroscopy (GC/MS). GC/MS analyses are carried out on an Agilent 6890 GC coupled to a 5975 MSD using 1 μL injection. A DB-Wax column (Agilent, 122-7032) is used with running conditions as described in Agilent’s RTL DB-Wax method (Application note: 5988-5871EN). Identification of fatty acid methyl esters is based on mass spectral profiles and retention times in the Agilent’s RTL DB-Wax method.
4 Notes
4.1 Pretreatments
4.1.1 Thermal Pretreatment
Microalgal slurry is transferred to an Erlenmeyer flask and subsequently placed on a hot plate with a magnetic stirrer. The culture has to be heated up to 80°C temperature and kept at this temperature for 10 min while it is continuously stirred.
4.1.2 Microwave-Assisted Pretreatment
A beaker is filled with microalgal slurry and placed in a microwave (LG Microwave Oven Model no. MS3447GR) at a setting of 1.1 kW for 3 min. The slurry is then stirred to a homogenous mixture and immediately placed in the microwave under the same settings for another 2 min. The temperature of the slurry after the microwave treatment ranges typically between 80°C and 90°C.
4.2 Precautions To Be Taken During Transferring of the Organic Layer
In lipid recovery by hexane and ethanol, the centrifugation results in the hexane layer on top, microalgal debris in the middle, and a mixture of water and ethanol in the bottom of the Corex tube. In this case, pipetting the hexane layer is straightforward. However, for the Bligh and Dyer method, the phase separation after centrifugation is different with a mixture of water and methanol on top, algal debris in the middle, and the chloroform organic phase in the bottom layer of the Corex tube. In this case, extra care should be taken when pipetting the chloroform layer to avoid water and methanol and more importantly algal debris. To make this happen, as soon as the pipet reaches the top layer of water and methanol, positive pressure should be applied to the pipet which causes bubbling in the tube, and this pressure should be released upon the contact of the pipet with the bottom chloroform layer.
References
Hu Q et al (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Schenk PM et al (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1(1):20–43
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14(1):217–232
Lardon L et al (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43(17):6475–6481
Uduman N et al (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J Renewable Sustainable Energy 2(1):012701
Sharma KK et al (2013) Critical analysis of current microalgae dewatering techniques. Biofuels 4(4):397–407
Passell H et al (2013) Algae biodiesel life cycle assessment using current commercial data. J Environ Manage 129:103–111
Lee AK, Lewis DM, Ashman PJ (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenergy 46:89–101
Ranjith Kumar R, Hanumantha Rao P, Arumugam M (2015) Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res 2:61
Sharma K, Li Y, Schenk PM (2014) UV-C-mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem 16(7):3539–3548
Ghasemi Naghdi F et al (2014) Comparative effects of biomass pre-treatments for direct and indirect transesterification to enhance microalgal lipid recovery. Front Energy Res 2:57
Yoo G et al (2012) Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour Technol 123:717–722
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Acknowledgments
We wish to thank Meat & Livestock Australia and the Australian Research Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this protocol
Cite this protocol
Naghdi, F.G., Schenk, P.M. (2015). Protocols on Lipid Extraction from Wet Algal Biomass. In: McGenity, T., Timmis, K., Nogales , B. (eds) Hydrocarbon and Lipid Microbiology Protocols . Springer Protocols Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8623_2015_131
Download citation
DOI: https://doi.org/10.1007/8623_2015_131
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49135-5
Online ISBN: 978-3-662-49137-9
eBook Packages: Springer Protocols