Abstract
Bacterial cellulose (BC) is a high-strong cellulose with high-purity produced by bacteria. The aim of this study was to explore the feasibility of using tofu soy whey as a novel and cheap culture medium to produce bacterial cellulose (BC) through the fermentation of kombucha. In this study, the statistical optimization of the culture medium for producing BC from kombucha was carried out by selecting different parameters. A three-level, three-factor Box-Behnken design (BBD) was used to determine the optimal levels for three significant variables (sucrose addition, kombucha inoculation amount and fermentation temperature). According to the results, the optimal fermentation conditions were found as follows: sucrose addition 8.5%, kombucha inoculation amount 10%, fermentation temperature 32℃, the BC yield can be up to 4.20 g/100 mL (D.W) under 11d fermentation. Besides, the BC was determined with strong tensile strength and water absorption capacity. By scanning electron microscopy (SEM) observation, Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and differential scanning calorimetry (DSC) determination, BC produced by soy whey and Hestrin-Schramm (HS) medium were compared. The results showed that BC produced from soy whey has a typical cellulose structure, characteristic peaks of typical functional groups of cellulose, and crystal diffraction peaks of type I natural cellulose. In conclusion, this study utilized the nutrients in the soy whey to obtain a high yield of bacterial cellulose, make full use of industrial waste water, which was more environmentally friendly and cheaper.
Graphical Abstract

Highlights
• Open a novel way for the recycling and upgrading of soy whey.
• Provide a new environment-friendly,high-yielding culture medium for the production of BC.
• SW-BC presents good tensile strength and water absorption capacity.
• SW-BC shows high thermal stability and high crystallinity
Similar content being viewed by others
Introduction
Tofu is made from soymilk, which is the liquid filtered from soybean slurry after the beans are soaked in water and blended. After heating the soy milk to boil, the precipitating agent is added to form curd and pressed into tofu cake. During the progress of pressing into tofu cake, a lot of water was squeezed out, and the pressed water is soy whey (Chua & Liu 2019; Han et al. 2004). But the BOD (biochemical oxygen demand) and COD (chemical oxygen demand) of this industrial wastewater are 100 times higher than the discharge standard. Under the traditional process, 1 kg of soybeans were produced to 2.5 kg of tofu and released 8.95 kg of soy whey (Tu et al. 2019). So the treatment of a large amount of this wastewater has long puzzled the soybean products industry (Chua et al. 2017). However, the soy whey from tofu still contains a significant amount of the nutrients found in soy milk, including carbohydrates, protein, and isoflavones (Dai et al. 2021; Undhad Trupti et al. 2021). According to our previous analysis, the soy whey purchased at the market is valuable in nutrients: 11 g/L carbohydrate, including 8.8 g/L reducing sugar; 5.45 g/L protein; 0.13 g/L isoflavone. These compounds can provide nutrients to the microorganisms. And some of them can confer certain health benefits, such as reduced cholesterol Mia, reduced risk of atherosclerosis, cancer, hypertension, and osteoporosis, and relief of postmenopausal symptoms (Chua & Liu 2019; Makwana et al. 2023).
Kombucha is a probiotic beverage, which is produced through the fermentation of sugared tea with an inoculum of SCOBY (symbiotic community of bacteria and yeast) (Abuduaibifu & Tamer 2019). In the past decade, significant progress has been made in research on kombucha, and it has been reported that drinking it can prevent various cancers and cardiovascular diseases, promote liver function, and stimulate the immune system (Jayabalan et al. 2014). Bacterial cellulose (BC) is an extremely pure and robust cellulose produced by some bacterial strains, such as Acetobacter (Yang et al. 2020). It is well known that kombucha bacterial cellulose is the bacterial cellulose on the surface of the medium by the acetic acid bacteria after the yeast in the kombucha consortium metabolizes the carbon source in the medium (Haghighi et al. 2021; Nguyen et al. 2015). Although bacterial cellulose and plant cellulose share the same structural type, their distinct nanofibrous structures give them different physical, mechanical, and chemical properties than plant cellulose (Abdelraof et al. 2019a, 2019b; Abdelraof et al. 2019a, 2019b; Carreira et al. 2011). It can be applied in medicine, such as skin dressing (Lemnaru et al. 2020), and can also be applied to food, such as Nata de coco, jelly (Ul-Islam et al. 2012). Stirred culture and static culture are two basic methods to produce BC. In general, the technical characteristics required for special food and biomedical applications are obtained under static culture, which can obtain smoother and thicker BC, but the yield is lower than that of stirred culture (Gao et al. 2020; Kojima et al. 1997; Watanabe et al. 1998). Therefore, it is necessary to increase the production of BC under static culture to meet the industrial requirements.
Presently, some research works on BC production with unconventional medium mainly focuses on production performance related aspects. The main goal of these researches is to optimize culture conditions, and this way, reduce the costs by improving the efficiency of process. Kumbhar et al. (2015) used pineapple and watermelon rinds to produce BC, they compared the resulting BC to those produced in Hestrin-Schramm (HS) media and showed that varying the medium component resulted in different BC structures and properties. Qiu et al. (2022) used the enzymatic solution of kitchen waste as a low-cost alternative source to produce BC, and the prepared BC film had a typical microfiber network, realizing the efficient conversion of kitchen waste into high-performance BC. To the best of our knowledge, there is no report on using soy whey as the culture medium to produce BC with kombucha consortium. Taken together, this study provides a new environment-friendly culture medium to produce BC, and achieved a considerable yield. This study also opens a novel way for the recycling and upgrading of soy whey.
Materials and methods
Materials
Black tea was purchased from Chengdu Mingxiangtang Tea Co., Ltd. Soy whey was purchased from Gaochun Tofu Shop at Zijincheng Vegetable Market in Nanjing, Jiangsu, China. PI propidium iodide and green cyanine SYT0 dye solutions were obtained from Biosharp Co., Ltd. All other reagents were in analytical grade, and purchased from Shanghai Macley Biochemical Technology Co., Ltd. and Beijing Aoboxing Biotechnology Co., Ltd.
Microorganism and inoculum preparation
The native culture of Kombucha was obtained from a household at Zhanjiang, Guangdong, and was used as the starter culture. The stock culture based on black tea medium was prepared and maintained at static conditions at 30℃ and subcultured periodically (14–20 days) in the same liquid medium. The kombucha to be inoculated can be stored in a 4℃ refrigerator (no more than 7d).
Black tea medium (Jayabalan et al. 2007): The 0.5% (w/v) black tea and 10% (w/v) sucrose was weighted and added to water, stirred while heating until sucrose was completely dissolved, and kept in a boiling state for 1 min, filtered with 400 mesh gauze.
Hestrin-Schramm (HS) medium (g/L) (Schramm & Hestrin 1954): The 1L of the culture medium contains glucose 30 g, peptone 5 g, yeast extract 5 g, citric acid 1 g,and Na2HPO4·12H2O 7.5 g. The pH of the culture medium was adjusted to 6 using 1.0 M HCl.
The 10% (v/v) inoculation concentration, 30℃ culture temperature and 6-8d of culture time was required for bacterial cellulose production (Ben Taheur et al. 2020).
Pretreatment of soy whey
The soy whey from the market was centrifuged at 6500xg, 4℃ for 10 min in centrifuge (CT15RT, Hitachi Corporation, Japan), then the precipitation was discarded, and the supernatant was taken and placed in 4℃ refrigerators for use (no more than 1 day).
Optimization test design of fermentation conditions
There were five independent variables that may affect BC yield was selected: a) carbon source added to soy whey; b) concentration of added carbon source; c) kombucha inoculation amount; d) fermentation temperature; e) days of fermentation.
Firstly, the ability of kombucha to convert different carbon sources into BC was explored. The pretreated soy whey was split in bottles and 10% (w/v) sucrose, glucose, glycerol, starch, and stachyose were added separately. Then 10% kombucha liquid was inoculated, and cultured at 30℃ for 8 d, comparing with the soy whey without additional carbon source. After identifying the carbon source, the effect of its added concentration on the BC yield was explored. The identified carbon sources of 4, 6, 8, 10, 12, and 14% (w/v) were added, 10% kombucha liquid were inoculated, then cultured at 30℃ for 14d, to make sure the fermentation was over. The final BC yield was recorded. Then, the proper kombucha inoculation amount was explored. 10% (w/v) of sucrose was added to the soy whey, inoculated with 4, 6, 8, 10, 12 and 14% of kombucha liquid, and cultured at 30℃ for 14d to record the yield of the final BC. After determining the amount of kombucha inoculation, the effect of temperature on fermentation was explored. 10% (w/v) of sucrose was added in soy whey, 10% kombucha liquid was inoculated, cultured at 24, 26, 28, 30, 32 and 34℃ for 14d and the final BC yield was incubated. In order to explore the appropriate fermentation days, 10% (w/v) of sucrose was added and 10% kombucha liquid was inoculated, the fermentation temperature was 30℃, cultured for 6, 7, 8, 9, 10, 11, 12 and 13d, the daily synthesis amount of BC was recorded.
Using the method of Design-Expert V8.0.6 response surface optimization design software design of a three-factor three-level experiment, the Box Behnken Design (BBD) is used to determine the optimal level of each parameter and its interaction in the production of BC (Zhuang et al. 2021). A total of 17 all-factorial BBD trials were used to study the separate and synergistic effects of the three selected significant parameters. The selected parameters were sucrose addition, kombucha inoculation amount, and fermentation temperature. And the fermentation time was 11d. Each variable was studied at three different levels (− 1, 0, + 1), where 0 represented the central value for each variable, while + 1 and − 1 represented the high and low values for each variable, respectively. Test factors and parameter levels were shown in Table 1. BC yield (dry weight) was used as a response (Y) and culture conditions as an independent variable (X). The experimental results were fitted by regression (non-linear optimization algorithm) to a predictive quadratic polynomial. The 3D plots were plotted to show the interactions between significant variables and their best values.
The above cellulose production was recorded as the dry weight after freeze-drying. The drying method was to wipe the surface of the obtained wet cellulose film clean, put it at -20℃ for pre-freezing, and then vacuumed freeze-drying for 48 h (Biosafer-10D, Biosafer Biotechnology Co., Ltd, Nanjing, Jiangsu, China).
Purification of BC
The BC film was heated in 0.5% NaOH solution at 90 °C for 30 min to remove residual bacteria, medium, and other impurities in BC, and then washed with distilled water until the pH of the wash solution was neutral. It is worth noting that the solution is stirred slowly during the heating. The reason of stirring slowly is to prevent the BC structure being destroyed.
Water content and rehydration degree of BC
The purified sample was placed in a natural environment for 1 min to let the surface water drip naturally, and then the weight of initial sample was recorded as W1. The sample was freeze-dried for 48 h and recorded weight of dry sample as W2. Then the dry sample was mixed with water and incubated for 48 h at room temperature, weighed again, and recorded as W0.
Water content and rehydration degree are calculated by the following formula:
Scanning electron microscope (SEM) observation
The BC were characterized using scanning electron microscope (EVO-LS10, Zeiss Technology Co. Ltd, Germany). Dry BC was fixed and coated with a thin layer of gold nanoparticles during the preparation of the SEM imaging, and the SEM experiments were imaged at a 10,000 × magnification at an accelerating voltage of 10 kV.
Fourier Transform infrared spectrum (FT-IR)
A dry thin film of BC was taken and analyzed by FT-IR spectroscopy in the transmission mode by using a Fourier transform infrared spectrometer (Nicolet iS50, Thermo Technology Co., Ltd., USA), Each sample was scanned for 46 times, and the scan range was from 4000 to 500 cm−1.
X-ray diffraction (XRD)
Dry BC was ground into powder for X-ray diffraction analysis, and the sample was analyzed with ni-filtered Cu-Kα (α = 0.15418 nm). Diffraction maps were recorded at room temperature using an X-ray diffrtometer (D2 PHASER, Bruker Technology Co. Ltd, Germany). Operating voltage and current were 40 kV and 40 mA, respectively. The 2θ range was 5–60° and was scanned at 5°/min with a step length of 0.02° (2θ). The crystallinity was calculated according to the Segal formula (Segal et al. 1959), as follows:
Among I200 is the maximum diffraction intensity of the crystallization peak around 2θ = 22°, and Iam is the minimum diffraction intensity of the crystallization peak around 2θ = 18°.
Thermo gravimetric analysis (TGA)
Dry BC was ground with a mortar and pestle, and then dumped through a 100-mesh sieve (0.149 mm). Thermo weight analysis was performed with a thermal weight analyzer (TG/DTA7200, Hitachi Technology Co. Ltd, Japan). The temperature range, ranging between 30 and 800℃, has a warming rate of 10℃/min.
Confocal laser scanning microscope (CLSM)
BC before and after purification were stained with PI propidium iodide and green cyanine SYT0 dye solutions (Zou et al. 2020), and the samples were soaked in both dyes and placed in a 37℃ incubator for 15 min. The treated samples were cut into thin sheets on glass slides, and the distribution of cells in BC was observed using a rotary-table laser confocal microscope (Ultra View VOX, PerkinElmer Co., Ltd, USA) before and after purification.
Tensile properties
Referring to the research method of Wang et al. (2021), the mechanical characterization of BC was carried out by using a texture meter (TA.XT Plus, Stable Micro Systems Technology Co., Ltd, UK) with a mechanical probe of 150 N. The tensile properties of BC were tested by TA-TGA stretching fixture, and the thickness of BC was controlled at 1.5 ± 0.05 mm, the width was 10 mm, and the length was 20 mm. The sample was clamped with an initial force of 0.15N and a speed of 60 mm/min. A force sensing unit was used to record the variation of tension with stretching distance. The hardness and elasticity of BC were determined by the Texture Profile Analysis (TPA) program of the texture analyzer. A 25 mm cylindrical probe was used, the deformation depth of BC was 60%, the probe was lowered at a speed of 60 mm/min, and the time interval of two presses was 30 s.
Statistical analysis
All single-factor tests were repeated three times and the mean values were taken. Statistical analysis of data was performed using the SAS system software. Duncan test confirmed significant difference (P < 0.05). Origin 2022b was used to graph. The response surface test was analyzed by Design-Expert V8.0.6. The analysis of variance (ANOVA) was applied to establish the linear, quadratic, and interaction effects of the independent variables on the response (BC yield). Various statistical parameters such as coefficient of determination (R2), adjusted coefficient of determination (adjusted R2), and adequate precision were used to evaluate the adequacy and precision of the developed models.
Results and discussion
Univariate experimental results
Only a small amount of sugar in industrial soy whey so as to require additional carbon sources to serve as a medium for fermenting bacterial cellulose. As shown in Table 2, similar to the results of Ramana et al. (2000), sucrose was the most efficient cellulose carbon source in the selected carbon sources for this experiment, and BC produced by sucrose had a smooth surface and better thickness. The complete BC film with smooth surface cannot be formed with glycerol as carbon (c) source or no added carbon source (f). Although the BC obtained by using starch as carbon (d) source is whiter, the surface is not smooth. Therefore, sucrose was selected as the carbon source for subsequent research. The reason for this result is kombucha consortium includes acetic acid bacteria, yeast and lactic acid bacteria, there isa complex relationship between these microorganisms. The yeast in kombucha bacteria convert sucrose into glucose and fructose for use, and the product of which can stimulate the metabolism of yeast, this relationship makes sucrose more suitable as the carbon source of kombucha producing bacterial cellulose (Chakravorty et al. 2016).
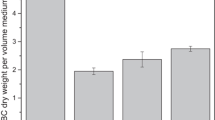
The results of the univariate experiment were shown in Fig. 1. In summary, in the experimental group, when the amount of sucrose added was 8%, the inoculum amount was 10%, and the fermentation temperature was 32℃, the maximum BC was produced. After fermentation for 11d, the cellulose yield did not increase with the increase of fermentation days, and it could be basically judged that the fermentation was complete at 11d.
Optimization of fermentation conditions based on Box-Behnken design
Before the design of Box-Behnken, this study analyzed the correlation between five variables and BC yield through statistical literature data. The results showed that carbon source with soy and fermentation days had low correlation with BC output. Therefore, in the subsequent design of Box-Behnken, only three other variables were retained in this study.
To determine the optimal level for each fermentation condition, three-factor three-levels were performed according to the BBD to achieve the maximum yield of BC production, from the initial 2.56 g/100 mL (D.W) to 4.20 g/100 mL (D.W), an improvement of 64%.In the univariate experiment, the three factors on the bacterial cellulose yield were: sucrose addition (X1), kombucha inoculation amount (X2), and the fermentation temperature (X3). As shown in Table 3, these variables were all studied at three different levels (− 1, 0, + 1), with design matrices for 17 different test combinations and experimental results for BC production.
The effect of the interaction on the BC production was shown visually, by drawing the 3D response surface curve for any two independent variables, while keeping the others at the intermediate level (Fig. 2). Polynomial regression analysis was performed using BC yield as the response value and fitted to obtain a multiple regression model as:
where Y value represented BC yield (g/100 mL), X1, X2, and X3 represented sucrose addition, kombucha inoculation amount, and fermentation temperature, respectively. The statistical analysis of the ANOVA for the whole quadratic model of the response surface was shown in Table 4. The regression model of BC yield was significant (P < 0.0001), the lack of fit (P = 0.2572) was insignificant and high R2 (0.9808), and the adjusted R2 was 0.9562. It showed that the regression model fits the data very well. The fermentation time (X3) and its quadratic term (X32) have a significant impact on the yield of BC (P < 0.001). (Kumari et al. 2022; Saleh et al. 2020).
In addition, using the Design-Expert V8.0.6 to estimate the optimal level of the three variables obtained from the maximum point of the polynomial model, and showed that the relative values of sucrose addition, kombucha inoculation amount, and fermentation temperature were 8.36% (w/v), 10.18% (v/v), and 31.5℃, respectively. For production convenience, the optimized fermentation conditions were: 8.5% of sucrose addition, 10% of kombucha inoculation amount, fermentation temperature of 32℃, and incubation time of 11d.
To determine the accuracy of the quadratic polynomial, three experiments were repeated under this fermentation condition yielded a bacterial cellulose production of 4.20 g/100 mL (D.W), being close to the predicted value of 4.30 g/100 mL (D.W). The BC yield of 4.20 g/100 mL (D.W) equal to 42 g/L (D.W), was higher than that of single strain k2G30 cultivated in black tea (23 g/L) (La China et al. 2021) and Acetobacter xylinum cultivated in coconut milk (35 g/L) (Liu et al. 2019). The result indicated that the capacity of kombucha to produce BC may be higher than that of single strains, and soy whey contains nutrients that allow microbes to thrive, which can be considered as a potential low-cost, high-yield medium for BC preparation..
Water content and rehydration degree of BC
The moisture content of BC from different batches was calculated, and it was found that the moisture content of BC from the fermentation of soy whey was between 83 and 92%. It indicated the membrane structure of BC has strong water retention ability, which is suitable for water bonded food additives and biological materials. Moreover, the larger the volume of BC, the higher the water content, which can be explained by the loose network structure of BC, the larger the volume of bacterial cellulose membrane, the looser the structure, the larger the void.
The rehydration degree of the BC was close to 100%, it is consistent with the results of Fan et al. (2011). The network structure of BC obtained by vacuum freeze-drying is the most complete, and the dehydrated BC could almost re-hydrate to its initial state.
SEM analysis
BC produced by HS (HS-BC) was milky white, and BC produced by soy whey (SW-BC) was yellow. This was related to the medium containing part of the pigment. These pigments bind to BC in situ during fermentation, giving BC different colors, but can be removed by the purification process (Supplementary Fig. 1).
The morphological structure of purified bacterial cellulose was studied by SEM imaging (Fig. 3). BC obtained from different media all had uniform three-dimensional networks of cellulose nanofibers and microfibers, which were similar to that from modified Hestrin Schramm medium (Mikkelsen et al. 2009) and oat hulls (Skiba et al. 2020). The purification method of BC treated with NaOH and then washed with water can remove all impurities and bacterial cells (Iguchi et al. 2000). The SEM image of purified bacterial cellulose sample (Fig. 3B2) showed that the culture medium and bacteria in BC were almost completely removed by purification (Lee et al. 2015).
As shown in the Fig. 3A2, B2, SW-BC has a more compact structure than that of HS-BC. To further distinguish the morphological differences of BC synthesized by different media, 100 random fiber widths were manually calculated from SEM images according to the scale to determine the diameter distribution and average diameter of BC (Fig. 3A3, B3). The diameter of the fiber band of HS-BC ranged from 20 to 100 nm, with an average diameter of 53 nm. While SW-BC ranged from 20 to 160 nm, resulting in a higher average fiber diameter (70 nm) than that from HS medium and beverage industrial waste of citrus peel and pomace (50 nm) (Fan et al. 2016).
XRD analysis
BC is a homogeneous polycrystalline macromolecular compound composed of ordered crystal region and disordered amorphous region (French, 2014). In the ordered crystal region, different BCS have strong broad peaks at 2θ = 14.5° and 22.7°, which is almost the same as the results of Tyagi and Suresh et al. (2016). The XRD patterns (Fig. 4) show three reflections at 14.76,16.79 and 22.75, respectively. These are typical diffraction peaks of type I cellulose, which reflected the crystal and amorphous structure of BC components, and also proved that kombucha can produce natural cellulose. According to the Segal formula, the crystallinity of BC produced by soy whey was 82.7%, which was higher than that of BC produced by HS medium (79.8%). In contrast to the literature (Fan et al. 2016), BC from SW medium was higher than that produced by citrus peel and pomace enzymolysis medium(63%).
FT-IR analysis
The FT-IR spectra can be used to determine the chemical structures, such as the functional groups of the substances, and to use the bond vibration frequencies to characterize the bonding properties of the molecules (Lin et al. 2016). The FT-IR spectra of the SW-BC were recorded at the wave number range of 500 to 4000 cm−1 as shown in Fig. 5. The strong absorption band at 3345.6 cm−1 in the BC spectrum was due to the presence of the hydroxyl group (-OH) in BC, which was consistent with the literature. The strong absorption band at 2896.9 cm−1was attributed to the presence of CH2 telescopic vibration. The absorption band at 1650.5 cm−1was attributed to the presence of carboxyl(C = O) functional group. Bands were also observed at 1422.6 cm−1 (bending vibration of C-H), 1316.5 cm−1 (bending vibration of C-H bonds), 1155.9 cm−1 (asymmetric expansion vibration of C–O–C glycosidic bonds), 1105.9 cm−1 and 1033.1 cm−1 (expansion vibration of C–OH and C–C-OH bonds in secondary alcohol and primary alcohol). These FT-IR band data analysis is largely consistent with the literature (Chang & Chen 2016). The presence of the crystal region and the purity of the BC were illustrated (Fan et al. 2016; Halib et al. 2012).
Notably, bacterial cellulose (BC) produced by soy whey (SW) medium and Hestrin Schramm (HS) medium showed a significant leftwards shift near 1650 cm−1, indicating that -C = O produced by SW medium was slightly different from that produced by HS medium.
Thermal-weight analysis
The TGA degradation curves of dry BC membranes indicated three phases of mass loss during the thermal analysis of the test samples (Fig. 6). The first stage, from room temperature to 150℃, was attributed to evaporation of residual water in BC resulting in a small weight loss. The second stage, which occurred in the 250,400 °C range, was associated with a series of reactions associated with cellulose degradation, including dehydration, depolymerization of glycoside units, and breakdown of glucose units, followed by the formation of carbon residues. The second mass loss phase was associated with a high mass loss of BC, characterized by the starting temperature (TOnset). The third stage occurs between 450 and 650 °C, and was associated with the oxidation and decomposition of the carbon-containing residues (El-Saied et al. 2008; Vasconcelos et al. 2017).
The TOnset of SW-BC was 343.37℃. These results were consistent with those reported in the literature, and the TOnset of SW-BC was slightly higher than that reported in the literature (335℃) (Vasconcelos et al. 2017), indicating that SW-BC had high thermal stability.
Mechanical properties
Mechanical properties are an important factor when evaluating the applicability of BC as wound dressings, tissue engineering, and other biomedical fields(Chen et al. 2019). As shown in Fig. 7, SW-BC had better tensile resistance. It has been proved that the arrangement of fibrils is positively correlated with the tensile strength (Khattak et al. 2015). This is the same as the result of SEM. The hardness of SW-BC film was 4.32 ± 0.27N, which was significantly higher than that of HS-BC film (3.43 ± 0.14N). The elasticity of HS-BC (0.68 ± 0.08) was higher than that of SW-BC (0.62 ± 0.12).
The CLSM analysis performed
SYTO green cyanine live cell nucleic acid dye is a nucleic acid dye with cell membrane permeability, and the fluorescence intensity is significantly enhanced after nucleic acid binding, and can be used to dye RNA and DNA from dead or alive eukaryotic cells. The PI dye solution cannot penetrate the cell membrane and is excluded from the living cells, but it can cross the damaged cell membrane and stain the nuclei, and so it can be used to dye the dead cells. As shown in Fig. 8, a large number of bacteria were present in the bacterial cellulose membrane before purification (Fig. 8A) and were more evenly distributed within the membrane. After purification (Fig. 8B), the bacterial cellulose membrane was not basically detected, indicating that the purification process can remove impurities and bacteria in the membrane.
Conclusion
In this study, only carbon sources were added to the soy whey, and no other medium components were added. Through the optimization of fermentation conditions, sucrose addition 8.5%, kombucha inoculation amount 10%, fermentation temperature 32℃ are the optimal levels, and the fermentation time is 11d, the yield of SW-BC was increased from 2.57 g/100 mL (D.W) to 4.20 g/100 mL (D.W). The SEM observation showed that BC from soy whey medium had the same structure as BC from HS medium and other fermentation medium. The average diameters of BC produced from soy whey and HS media were 72 nm and 53 nm. Furthermore, SW-BC had characteristic peaks of typical cellulose functional groups using FTIR analysis. Additionally, the XRD patterns indicated SW-BC had crystal diffraction peak of type I natural cellulose. Overall, this study provided a new, high-yielding and environmentally friendly medium for BC production.
Availability of data and materials
All data supporting this study are included in this manuscript. Further details are available from the corresponding author upon request.
Abbreviations
- BC:
-
Bacterial cellulose
- BBD:
-
Box-BehnkenDesign
- SEM:
-
Scanning electron microscopy
- FT-IR:
-
Fourier transform infrared
- XRD:
-
X-ray diffraction
- DSC:
-
Differential scanning calorimetry
- HS:
-
Hestrin-Schramm
- SW:
-
Soy whey
- HS-BC:
-
Bacterial cellulose from kombucha-fermented Hestrin-Schramm medium
- SW-BC:
-
Bacterial cellulose from kombucha-fermented soy whey medium
- BOD:
-
Biochemical oxygen demand
- COD:
-
Chemical oxygen demand
- TGA:
-
Thermogravimetric analysis
- CLSM:
-
Confocal laser scanning microscope
- TPA:
-
Texture Profile Analysis
- ANOVA:
-
Analysis of Variance
References
Abdelraof, M., Hasanin, M. S., & El-Saied, H. (2019a). Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydrate Polymers, 211, 75–83. https://doi.org/10.1016/j.carbpol.2019.01.095
Abdelraof, M., Hasanin, M. S., Farag, M. M., & Ahmed, H. Y. (2019b). Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. International Journal of Biological Macromolecules, 138, 975–985. https://doi.org/10.1016/j.ijbiomac.2019.07.144
Abuduaibifu, A., & Tamer, C. E. (2019). Evaluation of physicochemical and bioaccessibility properties of goji berry kombucha. Journal of Food Processing and Preservation, 43(9). https://doi.org/10.1111/jfpp.14077
Ben Taheur, F., Mansour, C., Ben Jeddou, K., Machreki, Y., Kouidhi, B., Abdulhakim, J. A., & Chaieb, K. (2020). Aflatoxin B-1 degradation by microorganisms isolated from Kombucha culture. Toxicon, 179, 76–83. https://doi.org/10.1016/j.toxicon.2020.03.004
Carreira, P., Mendes, J. A. S., Trovatti, E., Serafim, L. S., Freire, C. S. R., Silvestre, A. J. D., & Neto, C. P. (2011). Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresource Technology, 102(15), 7354–7360. https://doi.org/10.1016/j.biortech.2011.04.081
Chakravorty, S., Bhattacharya, S., Chatzinotas, A., Chakraborty, W., Bhattacharya, D., & Gachhui, R. (2016). Kombucha tea fermentation: Microbial and biochemical dynamics. International Journal of Food Microbiology, 220, 63–72. https://doi.org/10.1016/j.ijfoodmicro.2015.12.015
Chang, W. S., & Chen, H. H. (2016). Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocolloids, 53, 75–83. https://doi.org/10.1016/j.foodhyd.2014.12.009
Chen, J. B., Chen, C. T., Liang, G. Y., Xu, X. R., Hao, Q. L., & Sun, D. P. (2019). In situ preparation of bacterial cellulose with antimicrobial properties from bioconversion of mulberry leaves. Carbohydrate Polymers, 220, 170–175. https://doi.org/10.1016/j.carbpol.2019.05.062
Chua, J. Y., & Liu, S. Q. (2019). Soy whey: more than just wastewater from tofu and soy protein isolate industry. Trends in Food Science & Technology, 91, 24–32. https://doi.org/10.1016/j.tifs.2019.06.016
Chua, J. Y., Lu, Y. Y., & Liu, S. Q. (2017). Biotransformation of soy whey into soy alcoholic beverage by four commercial strains of & ITSaccharomyces cerevisiae&IT. International Journal of Food Microbiology, 262, 14–22. https://doi.org/10.1016/j.ijfoodmicro.2017.09.007
Dai, Y., Zhou, J., Wang, L., Mingsheng, & Xia, X. (2021). Biotransformation of soy whey into a novel functional beverage by Cordyceps militaris SN-18. Food Production Processing and Nutrition, 3, 1. https://doi.org/10.1186/s43014-021-00054-0
El-Saied, H., El-Diwany, A. I., Basta, A. H., Atwa, N. A., & El-Ghwas, D. E. (2008). Production and characterization of economical bacterial cellulose. BioResources, 3(4), 1196–1217.
Fan, H., Wu, Y., Hu, X., Wu, J., & Liao, X. (2011). Characteristics of thin-layer drying and rehydration of nata de coco. International Journal of Food Science and Technology, 46(7), 1438–1444. https://doi.org/10.1111/j.1365-2621.2011.02642.x
Fan, X., Gao, Y., He, W. Y., Hu, H., Tian, M., Wang, K. X., & Pan, S. Y. (2016). Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydrate Polymers, 151, 1068–1072. https://doi.org/10.1016/j.carbpol.2016.06.062
French, A. D. (2014). Idealized powder diffraction patterns for cellulose polymorphs. Cellulose, 21(2), 885–896. https://doi.org/10.1007/s10570-013-0030-4
Gao, H. L., Sun, Q., Han, Z. B., Li, J. H., Liao, B. W., Hu, L. L., . . . Jin, M. F. (2020). Comparison of bacterial nanocellulose produced by different strains under static and agitated culture conditions. Carbohydrate Polymers, 227. https://doi.org/10.1016/j.carbpol.2019.115323
Haghighi, H., Gullo, M., La China, S., Pfeifer, F., Siesler, H. W., Licciardello, F., & Pulvirenti, A. (2021). Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocolloids, 113. https://doi.org/10.1016/j.foodhyd.2020.106454
Halib, N., Amin, M., & Ahmad, I. (2012). Physicochemical properties and characterization of nata de coco from local food industries as a source of cellulose. Sains Malaysiana, 41(2), 205–211.
Han, B. Z., Rombouts, F. M., & Nout, M. J. R. (2004). Amino acid profiles of sufu, a Chinese fermented soybean food. Journal of Food Composition and Analysis, 17(6), 689–698. https://doi.org/10.1016/j.jfca.2003.09.012
Iguchi, M., Yamanaka, S., & Budhiono, A. (2000). Bacterial cellulose - a masterpiece of nature’s arts. Journal of Materials Science, 35(2), 261–270. https://doi.org/10.1023/a:1004775229149
Jayabalan, R., Malbasa, R. V., Loncar, E. S., Vitas, J. S., & Sathishkumar, M. (2014). A Review on Kombucha TeaMicrobiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Comprehensive Reviews in Food Science and Food Safety, 13(4), 538–550. https://doi.org/10.1111/1541-4337.12073
Jayabalan, R., Marimuthu, S., & Swaminathan, K. (2007). Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chemistry, 102(1), 392–398. https://doi.org/10.1016/j.foodchem.2006.05.032
Khattak, W. A., Khan, T., Ul-Islam, M., Wahid, F., & Park, J. K. (2015). Production, characterization and physico-mechanical properties of bacterial cellulose from industrial wastes. Journal of Polymers and the Environment, 23(1), 45–53. https://doi.org/10.1007/s10924-014-0663-x
Kojima, Y., Seto, A., Tonouchi, N., Tsuchida, T., & Yoshinaga, F. (1997). High rate production in static culture of bacterial cellulose from sucrose by a newly isolated Acetobacter strain. Bioscience Biotechnology and Biochemistry, 61(9), 1585–1586. https://doi.org/10.1271/bbb.61.1585
Kumari, A., Gupta, A., & Chauhan, A. K. (2022). Optimization of the iron-enriched extruded snack containing jackfruit seed flour, mung bean flour and ferrous ammonium phosphate by using response surface methodology. Food Production Processing and Nutrition, 4(1). https://doi.org/10.1186/s43014-022-00108-x
Kumbhar, J. V., Rajwade, J. M., & Paknikar, K. M. (2015). Fruit peels support higher yield and superior quality bacterial cellulose production. Applied Microbiology and Biotechnology, 99(16), 6677–6691. https://doi.org/10.1007/s00253-015-6644-8
La China, S., De Vero, L., Anguluri, K., Brugnoli, M., Mamlouk, D., & Gullo, M. (2021). Kombucha Tea as a reservoir of cellulose producing bacteria: assessing diversity among komagataeibacter isolates. Applied Sciences-Basel, 11(4). https://doi.org/10.3390/app11041595
Lee, C. M., Gu, J., Kafle, K., Catchmark, J., & Kim, S. H. (2015). Cellulose produced by Gluconacetobacter xylinus strains ATCC 53524 and ATCC 23768: Pellicle formation, post-synthesis aggregation and fiber density. Carbohydrate Polymers, 133, 270–276. https://doi.org/10.1016/j.carbpol.2015.06.091
Lemnaru, G. M., Trusca, R. D., Ilie, C. I., Tiplea, R. E., Ficai, D., Oprea, O., . . . Ditu, L. M. (2020). Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules, 25(18). https://doi.org/10.3390/molecules25184069
Lin, S. P., Huang, Y. H., Hsu, K. D., Lai, Y. J., Chen, Y. K., & Cheng, K. C. (2016). Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit juice. Carbohydrate Polymers, 151, 827–833. https://doi.org/10.1016/j.carbpol.2016.06.032
Liu, D. M., Yao, K., Li, J. H., Huang, Y. Y., Brennan, C. S., Chen, S. M., Li, L. (2019). The effect of ultraviolet modification of Acetobacter xylinum (CGMCC No. 7431) and the use of coconut milk on the yield and quality of bacterial cellulose. International Journal of Food Science and Technology, 54(11), 3099–3108. https://doi.org/10.1111/ijfs.14225
Makwana, S., Prajapati, J. B., Pipaliya, R., & Hati, S. (2023). Effects of probiotic fermented milk on management of obesity studied in high-fat-diet induced obese rat model. Food Production Processing and Nutrition, 5(1). https://doi.org/10.1186/s43014-022-00112-1
Mikkelsen, D., Flanagan, B. M., Dykes, G. A., & Gidley, M. J. (2009). Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. Journal of Applied Microbiology, 107(2), 576–583. https://doi.org/10.1111/j.1365-2672.2009.04226.x
Nguyen, N. K., Nguyen, P. B., Nguyen, H. T., & Le, P. H. (2015). Screening the optimal ratio of symbiosis between isolated yeast and acetic acid bacteria strain from traditional kombucha for high-level production of glucuronic acid. Lwt-Food Science and Technology, 64(2), 1149–1155. https://doi.org/10.1016/j.lwt.2015.07.018
Qiu, W., Ren, H., & Wang, Q. (2022). Production of bacterial cellulose from enzymatic hydrolysate of kitchen waste by fermentation with kombucha. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02903-5
Ramana, K. V., Tomar, A., & Singh, L. (2000). Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World Journal of Microbiology & Biotechnology, 16(3), 245–248. https://doi.org/10.1023/a:1008958014270
Saleh, A. K., Soliman, N. A., Farrag, A. A., Ibrahim, M. M., El-Shinnawy, N. A., & Abdel-Fattah, Y. R. (2020). Statistical optimization and characterization of a biocellulose produced by local Egyptian isolate Komagataeibacter hansenii AS.5. International Journal of Biological Macromolecules, 144(C), 198-207
Schramm, M., & Hestrin, S. (1954). Synthesis of cellulose by Acetobacter xylinum. I. Micromethod for the determination of celluloses. The Biochemical Journal, 56(1), 163–166. https://doi.org/10.1042/bj0560163
Segal, L., Creely, J. J., Martin, A., Jr., & Conrad, C. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal, 29(10), 786–794.
Skiba, E. A., Budaeva, V. V., Ovchinnikova, E. V., Gladysheva, E. K., Kashcheyeva, E. I., Pavlov, I. N., & Sakovich, G. V. (2020). A technology for pilot production of bacterial cellulose from oat hulls. Chemical Engineering Journal, 383. https://doi.org/10.1016/j.cej.2019.123128
Tu, C., Tang, S., Azi, F., Hu, W., & Dong, M. (2019). Use of kombucha consortium to transform soy whey into a novel functional beverage. Journal of Functional Foods, 52, 81–89. https://doi.org/10.1016/j.jff.2018.10.024
Tyagi, N., & Suresh, S. (2016). Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: Optimization & characterization. Journal of Cleaner Production, 112, 71–80. https://doi.org/10.1016/j.jclepro.2015.07.054
Ul-Islam, M., Khan, T., & Park, J. K. (2012). Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydrate Polymers, 88(2), 596–603. https://doi.org/10.1016/j.carbpol.2012.01.006
Undhad Trupti, J., Das, S., Solanki, D., Kinariwala, D., & Hati, S. (2021). Bioactivities and ACE-inhibitory peptides releasing potential of lactic acid bacteria in fermented soy milk. Food Production Processing and Nutrition, 3, 1. https://doi.org/10.1186/s43014-021-00056-y
Vasconcelos, N. F., Feitosa, J. P. A., da Gama, F. M. P., Morais, J. P. S., Andrade, F. K., de Souza, M. D. M., & Rosa, M. D. (2017). Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydrate Polymers, 155, 425–431. https://doi.org/10.1016/j.carbpol.2016.08.090
Wang, Q., Nnanna, P. C., Shen, F., Huang, M., Tian, D., Hu, J. G., . . . Deng, S. H. (2021). Full utilization of sweet sorghum for bacterial cellulose production: A concept of material crop. Industrial Crops and Products, 162. https://doi.org/10.1016/j.indcrop.2021.113256
Watanabe, K., Tabuchi, M., Morinaga, Y., & Yoshinaga, F. (1998). Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose, 5(3), 187–200. https://doi.org/10.1023/a:1009272904582
Yang, Y. N., Lu, K. Y., Wang, P., Ho, Y. C., Tsai, M. L., & Mi, F. L. (2020). Development of bacterial cellulose/chitin multi-nanofibers based smart films containing natural active microspheres and nanoparticles formed in situ. Carbohydrate Polymers, 228. https://doi.org/10.1016/j.carbpol.2019.115370
Zhuang, C.C., Liu, C. R., Shan, C. B., Liu, Z., Liu, L., & Ma, C. M. (2021). High-yield production of secoisolariciresinol diglucoside from flaxseed hull by extraction with alcoholic ammonium hydroxide and chromatography on microporous resin. Food Production Processing and Nutrition, 3(1). https://doi.org/10.1186/s43014-021-00079-5
Zou, X. Z., Zhang, S., Chen, L., Hu, J. Q., & Hong, F. F. (2020). Determination of live and dead Komagataeibacter xylinus cells and first attempt at precise control of inoculation in nanocellulose production. Microbial Biotechnology, 13(2), 458–469. https://doi.org/10.1111/1751-7915.13494
Acknowledgements
Not applicable.
Funding
This work was supported by the Jiangsu Agriculture Science and Technology Innovation Fund (Grant No.CX(21)2003).
Author information
Authors and Affiliations
Contributions
X. Feng: Formal analysis, Visualization, Datacuration, Software,Writing-Original draft preparation. Zhiwen Ge: Validation, Writing-Review and Editing. Y.Wang: Investigation, Validation. Baomin Zhao: Conceptualization, Methodology. X. Xia: Project administration, Supervision, Writing- Review and Editing. M. Dong: Conceptualization, Supervision, Fund acquisition, Writing-Review and Editing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
SEM imaging of purified SW-BC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, X., Ge, Z., Wang, Y. et al. Production and characterization of bacterial cellulose from kombucha-fermented soy whey. Food Prod Process and Nutr 6, 20 (2024). https://doi.org/10.1186/s43014-023-00188-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43014-023-00188-3