Abstract
Bacterial cellulose has unique structural, functional, physical and chemical properties. The mass production of bacterial cellulose for industrial application has recently become more popular and attractive. The aim of this study was to develop an efficient scale-up process of bacterial cellulose production. Bacterial cellulose productivity of a new isolate Acetobacter pasteurianus RSV-4 (MTCC 25117) was investigated in whey medium without addition of any additives. Produced bacterial cellulose was characterized for its structure, purity, thermostability and strength by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, thermogravimetric analysis and Differential scanning calorimetry. β-galactosidase (1.5 IU/ml) was used to split whey lactose into its corresponding monomers glucose and galactose. Glucose was specifically consumed by Acetobacter pasteurianus for the production of BC. The highest concentration of 5.6 g cellulose/L of whey was obtained at 30 °C after 8 days of bacterial growth. Process was optimized for the production of bacterial cellulose on 120 L of whey medium under static conditions. Whey, a waste byproduct of milk processing industry was used in the process and therefore the developed process might be economical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is one of the most abundant inexhaustible polysaccharides with unique properties. These properties make it desirable for various applications (Chawla et al. 2009; Kim et al. 2006). Several bacterial species like Gluconacetobacter, Agrobacterium, Aerobacter, Azotobacter, Rhizobium, Sarcina, Salmonella, Enterobacter, Escherichia and other cyanobacterial species have been reported to produce extracellular cellulose called as bacterial cellulose (Jahan et al. 2012; Shoda and Sugano 2005). Unlike plant cellulose, bacterial cellulose (BC) has desirable properties like high purity as free from lignin and hemicelluloses, crystallinity, degree of polymerization, nano-structured network, wet tensile strength, good water holding capacity and biocompatibility (Blanco Parte et al. 2020; Brown 2004). Properties like low toxicity and high chemical stability make the BC a preferred material for manufacturing artificial skin, paint and a thickener for ink. A dextran/bacterial cellulose (BC) hydrogel has been reported useful in wound healing applications (Lin et al. 2017). Also, superior physical properties of BC attracted the manufacturers for its use in the preparation of speaker diaphragms, tourniquet and as dietary fibers (Klemm et al. 2001; Park et al. 2003).
The main factor for the high cost of the culture medium is the use of yeast extract and peptone. The agro-forestry industrial residues can be used as cheap carbon and nutrient source to overcome such limitations and keep alive the demand of this unique material. Some industrial waste material or byproducts like beet molasses, sugar-cane molasses and corn steep liquor and coconut water have already been used as carbon sources for BC production using different bacterial strains (Keshk et al. 2006; El-Saied et al. 2008; Kongruang 2008).
Cellulose production by various bacterial strains using carbon source such as glucose, fructose, arabitol, mannitol, ethanol, glycerol, citric acid and sucrose has been reported (Chawla et al. 2009; Brown 2004; Yoshino et al. 1996; Cherian et al. 2013). The commercial bacterial cellulose production from Acetobacter xylinus has been reported from a cheap and readily available substrate such as lactose (Kongruang 2008). No other member of Acetobacteraceae family has been reported for bacterial cellulose production by utilizing lactose.
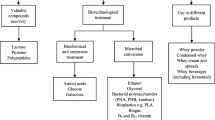
Lactose is the main constituent of whey, a byproduct of the cheese manufacturing industry. The global production of whey is about 110 million tons per annum and only 50% of whey produced worldwide is utilized and converted into useful products (Mohite et al. 2013). Remaining whey is disposed of and causes environmental issues. Whey has a high biological oxygen demand (BOD) of > 30 kg m− 3 and chemical oxygen demand (COD) of > 60 kg m− 3 and thus it must be treated before disposal into waterways and sewerage systems (Mohite et al. 2013). The disposal of whey is still an environmental problem. An alternative to chemical treatment and disposal could be the use of whey as a substrate for the growth of microorganisms.
Bacterial cellulose production by Acetobacter pasteurianus AP-1SK has been reported in static culture. For this a system has been developed where cellulose was formed on an oxygen-permeable synthetic membrane and on a synthetic medium with a yield of 1.45 g/2.6 L (Yoshino et al. 1996). Acetobacter pasteurianus strain PW1 has been reported for comparative efficacy of bacterial cellulose production on pineapple waste medium (PIWAM) and pawpaw waste medium (PAWAM). In PIWAM, yield of bacterial cellulose was in the range of 0.1 to 3.9 g/L and in PAWAM was in the range of 0.2 to 1.0 g/L (Adebayo-Tayo et al. 2017). Chemical mutagenesis has been used to develop cellulose overproducing strain of Acetobacter pasteurianus. The mutagenized strain has been reported for the production of double amount of bacterial cellulose compared to wild type strain on synthetic medium (Bertocchi et al. 1997).
In view of this, present study was aimed to identify a potent bacterial strain and to develop a process for bacterial cellulose production by using the identified strain on liquid whey medium under static condition. Produced bacterial cellulose (BC) was characterized by using Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), X-ray diffraction (XRD), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
Materials and methods
Microorganism
Acetobacter species used in this study was isolated from kinnow rich fruit residue. Potential bacterial isolate was identified as Acetobacter pasteurianus RSV-4 on the basis of cultural, morphological and 16S rDNA analysis. This bacterium was deposited to Microbial Type Culture Collection and Gene Bank (MTCC) and assigned number MTCC 25117. The organism was maintained on Hestrin-Schramm (HS) medium plates (Schramm and Hestrin 1954) at 4 °C and sub-cultured at regular intervals of 2 weeks.
Inoculum preparation
The inoculum was prepared by growing the bacterium in Sugar medium (Saxena and Jahan 2012), with slight modifications containing (g/L) table sugar, 20.0; cane molasses, 5.0; ammonium sulphate, 1.5; K2HPO4 1.15; MgSO4·7H2O, 0.7, pH 6.0 for 2 days under static condition. The bacterial cellulose (BC) mat produced was harvested from the medium and cut into pieces of uniform size having dimensions 10 mm × 10 mm. These mat pieces were used as inoculum for bacterial cellulose production at an inoculum size of 4 mat pieces per litre of production medium. The number of bacterial cells present in one mat piece (10 mm × 10 mm) of BC was calculated to be 1.6 × 106 cfu/ml.
Bacterial cellulose production
Whey was used as production medium without the addition of any other medium components. Here, whey medium comprising of lactose (4–5%), glucose (0.3–0.4%), galactose (0.1–0.2%) and protein (0.6%) was used as a basal BC production medium. Firstly β-galactosidase was added into the whey and further it was inoculated with mat pieces of bacterial cellulose which contains Acetobacter pasteurianus RSV-4 cells having 1.6 × 106 cfu/ml. Whey hydrolysate comprising of glucose (2.0–2.5%), galactose (2.0–2.5%) and protein (0.6%). To optimize temperature for maximum cellulose production, Acetobacter pasteurianus RSV-4 was grown for 8 days in 1 L of the production medium under static conditions at different temperatures from 20 to 35 °C. Produced cellulose was processed and maximum yield was found during 30 °C. All optimization experiments of bacterial cellulose production were carried out in 1 L medium in trays. Therefore, BC production was carried out at 30 °C for 8 days under static culture conditions.
Optimization of surface to volume ratio for bacterial cellulose production
To optimize surface to volume ratio (S/V ratio) for maximum cellulose production, two types of plastic trays of size 60 × 36 × 15 cm and 100 × 55 × 16 cm were filled with different volumes of the optimized medium. Produced cellulose by Acetobacter pasteurianus RSV-4 was processed and maximum yield was found after 8 days at 30 °C under static culture conditions. Beyond 8 days of Acetobacter pasteurianus RSV-4 in production medium, there was no increase in the yield of BC.
Prototype for scale-up production of bacterial cellulose
The BC production was carried out in 120 L of the production medium in two different size of trays specially designed tray type bioreactor. It is a horizontal prototype equipped with trays of holding capacity of 10 and 25 L of production medium constituting a total volume of 120 L. These trays are connected with half inch silicon tube for medium circulation and maintenance of medium level during the production of bacterial cellulose by the isolate. This tray type bioreactor has an in situ sterilization facility using steam. Bulk production (handling capacity) of BC was carried out in trays each containing 10 and 25 L of the optimized production conditions. The incubation was carried out in a room maintained at optimum temperature for cellulose production.
Estimation of lactose, glucose and galactose in whey medium
The qualitative and quantitative analysis of lactose, glucose and galactose during the production of BC in whey medium was performed by using HPLC (Agilent Model-1260) equipped with Hi-plex Ca column and refractive index detector (RID). For sugar estimation, the sample injection volume was 20 µL. The column was eluted with deionized water at a flow rate of 1 mL min− 1 at 85 °C.
Bacterial cellulose purification and quantification
The cellulose pellicle produced was rinsed 2–3 times with water. It was then treated with 0.5 N NaOH at 80 °C for 20 min. To neutralize NaOH, the pellicle was treated with 0.5% acetic acid solution. It was again washed with water for 2–3 times. The purified pellicle obtained was freeze dried and weighed for determining its yield (g/l).
Fourier transform infrared spectroscopy (FTIR) characterization
FT-IR spectra of the dried bacterial cellulose was recorded using a Perkin Elmer FTIR spectrophotometer [Spectrum GX & Autoimage, USA; spectral range: 4000–400 cm− 1; beam splitter: Ge-coated on KBr; detector: DTGS; resolution: 0.25 cm− 1 (step selectable)]. For analysis, the samples were mixed with KBr (IR grade, Merck, Germany) pellets and processed further to obtain IR data, which were transferred to a PC to acquire the spectra.
Scanning electron microscope (SEM) characterization
The SEM measurement was carried out using a field-emission scanning electron microscope JEOL, model JSM-7000F, instrument operated at 2 kV. The investigated BC membrane was covered with carbon.
X-ray diffraction (XRD) analysis
XRD pattern of BC produced by Acetobacter pasteurianus RSV-4 on HS medium and whey medium was performed with Xpert-Pro (PANalytica) for 2θ from 5 to 70 and with Cu anode.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis was performed using a thermogravimetric analyzer (SDT Q600 V20.9 Build 20), in an alumina crucible with approximately 5 mg of each sample. During analysis, temperature was varied between 25 and 600 °C with a heating rate of 10 °C/min in an oxidizing atmosphere (air) ASTM E-1868.
Differential scanning calorimetry (DSC)
The performance of BC such as thermal phase change, glass transition temperature (Tg), crystalline melt temperature (Tm), and thermal stability was measured by differential scanning calorimetry (DSC). Samples about 3–5 mg were sealed in an aluminum pan and used for analysis under a nitrogen atmosphere. To evaluate the curing behavior, nonisothermal DSC analysis of samples was performed according to ASTM D-3418 on DSC Q20 from TA Instruments. The samples were heated from − 100 to 250 °C at a rate of 10 °C/ min.
Elongation at break of bacterial cellulose
The maximum break strain of BC film was performed according to ASTM D-882 on Lloyd 2000R (Southampton, UK) universal testing machine. The break strain was the average values determined from five specimens.
Results and discussion
Isolation and identification of potential microbe for cellulose production
Potential isolate for BC production was isolated from kinnow rich fruit residues (Fig. 1a). The isolate was inoculated in HS medium for cellulose production under static condition. A compact mat was formed on the air-liquid interface of the medium (Fig. 1b). The mat was taken from the medium and examined for the presence of cellulose fibrils by SEM observation (Fig. 1c). The mat was found to be composed of cellulose fibrils. The isolate was identified through 16S rDNA sequence analysis as Acetobacter pasteurianus and submitted to MTCC 25117 with name Acetobacter pasteurianus RSV-4. The evolutionary tree was drawn to the scale with branch lengths in the same units as those of evolutionary distances were computed. The isolate showed close relation with the earlier identified Acetobacter pasteurianus from different sources (Fig. 1d). Earlier, a strain has been isolated from fermented fruit juice for bacterial cellulose (BC) production and identified by 16S rDNA sequencing analysis and biochemical characteristics test as Komagataeibacter intermedius FST213-1 (Lin et al. 2016a, b).
Isolation of potential bacterial cellulose producing microbe from kinnow rich fruit residue (a), cellulose like gel formation in kinnow rich fruit residue (b) and SEM micrograph of bacterial isolate showing fibrous material production (c). A neighbor-joining phylogenetic tree based on the 16S rRNA gene sequence showing the similarity between Acetobacter pasteurianus RSV4 and other strains (d)
Bacterial cellulose production by Acetobacter pasteurianus on whey medium
BC production by Acetobacter pasteurianus was carried out in 1L of production whey medium contained in a tray. The whey contained 2% glucose and its carbon-to-nitrogen ratio (C/N) ratio was 66.6. The C/N ratio of whey was mainly determined by the presence of lactose and proteins. The pH of the whey was found to be same (pH 4.0) before and after bacterial cellulose production by Acetobacter pasteurianus. This strain was found to produce 1.4 g/L of cellulose under static culture conditions in whey medium, without any other medium components (Table 1). While Komagataeibacter xylinus has been reported earlier for the production of only 0.78 g/L BC in whey medium. A mini-Tn10:lacZ:kan was used for transformation of a wild-type strain of Komagataeibacter xylinus through random transposon mutagenesis to generate a lactose-utilizing and cellulose-producing mutant strain designated as ITz3 (Battad-Bernardo et al. 2014). The mutant strain has been documented for production of BC with a yield of 1.78 g/L whey medium. Therefore, effect of β-galactosidase enzyme on the production of BC by Acetobacter pasteurianus was optimized. β-galactosidase plays a key role in the production of BC by splitting whey lactose into its corresponding monomers for easy use by the bacterium.
During optimization, 1.5 IU of β-galactosidase was found to split the lactose present in 1 ml of whey into glucose and galactose. With the use of 1.5 IU/ml β-galactosidase, Acetobacter pasteurianus was observed to produce maximum concentration 5.6 ± 0.27 g BC/L whey (Table 1). Generally, a nutritionally rich medium containing yeast extract and peptone supports the production of BC by Acetobacter strains. Previous studies have reported the requirement of complex medium (mix of 2% glucose, 0.5% yeast extract, 0.5% polypeptone, 0.675% Na2HPO4.12H2O and 0.115% citric acid monohydrate) for the efficient production of BC using Acetobacter sp. (Bertocchi et al. 1997; Fontana et al. 1990). Use of RSM in optimizing medium to increase BC production has been reported to enhance the yield by 3.82-fold compared to the standard HS medium in 6-days of culture (Santoso et al. 2020). The K. intermedius Similarly, a chemical mutagenized strain of Acetobacter pasteurianus has been reported for synthesizing double amounts of cellulose compared to its wild type strain on the synthetic GYE medium containing 20 g of D-glucose, 10 g of yeast extract, 2.7 g of Na2HPO4 and 1.2 g of citric acid in one liter of water at pH 6.0 (Hwang et al. 1999).
A comparative effect of sterilization (121 °C, 15 psi for 15 min) and heating (70 °C for 15 min) of whey on the yield of BC production by Acetobacter pasteurianus was conducted. Concentration of BC in sterilized whey was 5.8 g/l and heated whey was 5.6 g/l. Hence, there was insignificant difference in the concentration of BC production by Acetobacter pasteurianus on two types of whey media. Therefore, heated whey was recommended to be an economical substrate in the process for BC production by Acetobacter pasteurianus. In the whey medium, conversion of lactose into glucose and galactose as well as subsequent use of glucose by Acetobacter pasteurianus was observed. Concentration of various sugars was determined in whey during fermentation for bacterial cellulose production (Fig. 2a). HPLC profile of sugars present in the whey at 0 h of fermentation (Fig. 2b) and at 192 h or 8 days of fermentation (Fig. 2c). Furthermore, the BC production was observed to be dependent on consumption of glucose. Importantly, gluconic acid was not formed during the fermentative production of BC on whey medium (Fig. 2). This characteristic of Acetobacter pasteurianus has suggested its viable use for up-scale production of BC.
Some of the earlier studies have documented the influence of pH on bacterial cellulose production in synthetic medium. Glucose has been used for maximum production of BC by Acetobacter xylinum BRC5 at pH 5. While at pH 4, the concentration was decreased and gluconic acid was produced (Battad-Bernardo et al. 2014). In another study, the use of high glucose concentration (6%) has been reported for the production of BC to the level of 7 g/l. With time, pH of the medium was decreased and for sustained production of BC pH was required to be maintained otherwise some side products could have generated (Gullo et al. 2017). The importance of static culture condition has been reported by using Komagataeibacter xylinus for higher BC production and lower gluconic acid (Kuo et al. 2016).
Optimization of surface to volume ratio for bacterial cellulose production
In static conditions, the production of BC is dependent upon optimal aeration of the culture medium that can be calculated by the ratio surface to volume (S/V). In this study, 0.22 cm− 1 was found to be the best S/V for given culture conditions of relative humidity (RH) 65 ± 5% and 100 count for microbes (cfm) for the maximum production of BC (Tables 2 and 3). Similar response of static condition for BC production by using Acetobacter xylinum E25 has been reported (Krystynowicz et al. 2002a, b). In contrast, intensive agitation and aeration have been appeared to drastically reduce the cellulose synthesis. The effect of oxygen tension in the gaseous phase has also been monitored on production and physical properties of BC formed in static cultures. BC production has been reported much higher at the oxygen tensions of 10% and 15% than under atmospheric conditions (Watanabe and Yamanaka 1995).
The BC production occurred at the air–liquid interface because of the higher oxygen availability. This implied that an increase in the S/V ratio has enhanced the BC production. In present study, the 0.22 cm− 1 S/V ratio was chosen according to the highest BC productivity and the lowest by-product formation. Maximum thickness of bacterial cellulose membrane 2.6 cm was also observed between 0.13 and 0.22 cm− 1 S/V ratio. While at higher and lower S/V ratio, membrane thickness was decreased significantly. This result has documented the significance of S/V ratio on the yield and thickness of bacterial cellulose membrane (Tables 2 and 3). The concentration of BC produced (5.6 g/L) in these conditions was higher than that reported in earlier works using whey as medium (Dlamini and Peiris 1997; Jozala et al. 2015). Notably, increase in S/V ratio has increased the yield of BC but it has also increased the gluconic acid production (Béchard et al. 1995). Therefore, optimum level of S/V is crucial parameter in static condition for selective BC production.
Scale-up production of bacterial cellulose
The prototype was designed by considering surface to volume (S/V) ratio. Process was optimized for 120 L medium for BC production in whey medium. Medium, enzyme and inoculum were mixed in the reaction vessel and thereafter, it was connected to various trays as shown in prototype (Fig. 3). The developed prototype suggests that the optimized S/V can be performed at any scale irrespective of size of container. Production was carried out by Acetobacter pasteurianus in 120 L of whey production medium poured in two different size trays 25 L and 10 L (Fig. 3). The production medium in each tray was inoculated with slurry of mat pieces of size (4% w/v) under aseptic conditions and the trays were incubated at 30 ± 2 °C till 8 days. After the desired incubation period, cellulose mats formed were processed and dry weight of cellulose produced was estimated in terms of g/L. Cellulose production was successfully scaled up to 120 L of the production medium in different size of trays (Fig. 3). In 7 trays of 10L each and 2 trays of 25L each of whey in a single interconnected system accounting for a total 120 L as production medium, a cellulose mat of thickness 2.7 cm was produced in 8 days of incubation. Bacterial cellulose was synthesised on the surface of the whey medium. After 8 days, the volume of synthesised bacterial cellulose reached saturation and was observed to sink. After processing, the concentration of the produced cellulose was 620 g dry weight. The designing tray type bioreactor may be converted into incubation chambers/ room where bulk production of BC in kilogram scale can be efficiently carried out under static culture conditions.
Production of bacterial cellulose in different size interconnected trays of total volume 120 L. Dimensions of two different sizes of trays are shown at left side. Bacterial cellulose mat formed on whey medium in a tray (upper right) and wet bacterial cellulose mat obtained from whey medium (lower right)
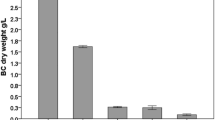
Recombinant Komagataeibacter xylinus containing lac Z gene from E. coli has been reported previously for the production of BC at a lower yield of 1.82 g/L whey (Battad-Bernardo et al. 2014). While use of carbon sources glycerol from biodiesel production and grape bagasse has been reported to produce 10 mg/mL and 8 mg/mL BC respectively after 14 days of cultivation (Vazquez et al. 2013). Use of residues from the wine and pulp industries have been reported to produce 0.6 and 0.3 g/L BC, respectively. While use of cheese whey has been shown to produce 0.1 g/L of BC after 96 h of incubation (Carreira et al. 2011). Production of BC with lactose as carbon source in the medium has been found to be very weak medium and produced only 0.070 g/L BC (Nguyen et al. 2008). On the other hand, HS broth supplemented with thin stillage (TS) has been reported to enhance the production of BC to 10.38 mg/mL after 7 days of cultivation (Wu and Liu 2012). Production of BC by Komagataeibacter xylinus on modified HS medium containing different carbon sources such mannitol, glucose, glycerol, fructose, sucrose or galactose has been studied (Mikkelsen et al. 2009). Sucrose and glycerol have been reported for the highest cellulose yields of 3.83 and 3.75 g/ L respectively after 96 h of incubation (Dai and Fan 2010). Hence, the isolated native bacterial strain Acetobacter pasteurianus has been found to produce BC at a quantity higher than reported previously on whey medium, suggesting economic feasibility of the process for BC production at pilot scale.
FT-IR analysis of bacterial cellulose
Conformational characteristics of BC obtained from HS medium and whey medium were analyzed by Perkin Elmer FTIR spectrophotometer (Fig. 4). The distinguished peaks at ~ 3300 cm− 1 indicates O–H stretching, ~ 2060 cm− 1 indicates C–H stretching, ~ 1600 cm− 1 indicates C–O–C stretching and ~ 1000 cm− 1 indicates C–O stretching. The bacterial cellulose produced by the Acetobacter pasteurianus on HS medium (Fig. 4a) and whey medium (Fig. 4b) showed similar FTIR spectra. Also, these FTIR spectra of BC shows similarity with earlier reported bacterial cellulose by microbes (El-Saied et al. 2008; Fan et al. 2012; Krystynowicz et al. 2002a, b). Results suggested the production of pure BC of high quality by Acetobacter pasteurianus on whey medium that was of equivalent quality to the one obtained on HS medium.
SEM micrograph of bacterial cellulose
Morphological characterization of the BC produced on HS medium and whey medium by Acetobacter pasteurianus was performed with scanning electron microscopy. BC mat was observed to be a dense network of interwoven ultrafine fibrils and the width of these cellulose fibrils was between 42 and 68 nm (Fig. 4). Morphology of BC produced by the isolate on either HS medium (Fig. 4c) or whey medium (Fig. 4d) was highly similar. Comparatively, thickness of BC fibrils produced on whey medium was more than the one produced on HS medium. The observed differences in the thickness of cellulose fibrils obtained from whey and HS medium could be responsible for their variable diffraction patterns (Sugiyama et al. 1991). The morphological study has suggested the production of more strong BC fibrils with better quality by Acetobacter pasteurianus in whey medium. Also, the morphology of BC produced in this study was similar to that of reported for BC produced by various other bacteria on different medium (Czaja et al. 2007; Jung et al. 2010; Tsouko et al. 2015).
X-ray diffraction of bacterial cellulose
X-ray diffraction analysis of BC produced by the Acetobacter pasteurianus on HS medium and whey medium has suggested their crystalline nature on both of the media (Fig. 5). The relative peak positions of the BC produced by the isolate on whey medium were in close agreement to the BC produced by the same strain on HS medium. Also, the XRD pattern of BC was highly similar to that of reported earlier (Premjet et al. 1996). However, there was a great difference in the orientation of the crysallites in the two samples, with the BC on whey medium providing a much more random orientation (Premjet et al. 1996). The diffraction pattern of BC on whey was nearly identical to the ideal pattern for Iα cellulose (French 2014). Also, the diffraction pattern of BC obtained from whey medium indicated a very random organization of the crystallites, as assumed during the calculation of the ideal pattern, whereas the BC from HS medium indicated a strong bias in the orientation that has put the (1 0 0) plane of the crystallites in the plane of the sample holder (French 2014). The obtained pattern and preferred orientation of the BC on whey medium documented it as Iα cellulose (Sugiyama et al. 1991). The d-spacing for three major hk0 peaks as well as the crystallite sizes, based on the Scherrer equation suggested the nature of BC produced on whey medium as Iα cellulose (Wada et al. 1997). The modification or improvement in material properties of BC produced in plastic composite support-rotating disk bioreactor PCS-RDB has been documented by incorporating different additives like microcrystalline cellulose (Avicel), carboxymethylcellulose (CMC), agar and sodium alginate (Lin et al. 2016a, b).
Thermal stability of produced bacterial cellulose
BC produced by the Acetobacter pasteurianus on HS medium and whey medium was subjected to thermal treatment in the temperature range of 25–500 °C. Thermogravimetric analysis (TGA) of cellulose particles has indicated multi-stage degradation behavior. At stage-1 between 25 and 100 °C, the initial weight loss could be due the removal of moisture from the BC. At stage-2 between 220 and 415 °C, significant weight loss could be due to depolymerisation and pyrolytic decomposition of BC (Fig. 6). Further, the TGA analysis revealed that BC produced by the isolate on HS medium (Fig. 6a) and whey medium (Fig. 6b) were stable upto 250 °C and beyond this temperature decomposition of BC was noticed. In the temperature range from 260 to 415 °C, about 43% BC obtained from whey medium was decomposed. While the decomposition of BC produced by the isolate on HS medium in the temperature range from 260 to 415 °C was about 98.2%. This data has suggested the better thermal stability of BC produced on whey medium compared to that obtained on HS medium by the Acetobacter pasteurianus isolate. Earlier studies have reported the similar thermal stability and temperature degradation behavior of bacterial cellulose (Cherian et al. 2013; George et al. 2005).
Differential scanning calorimetry (DSC) measures the heat absorbed or released by a material as a function of temperature or time. Glass transition temperature (Tg) of BC films produced by Acetobacter pasteurianus was found to be 53.29 °C (Fig. 6c). The observed Tg of BC was higher than reported for native bacterial cellulose (13.94 °C) and NaOH treated BC (41.41°C) (Dai and Fan 2010). Higher the Tg of BC, more is the stability and longer is the life (Hwang et al. 1999). Transformation due to thermal decomposition of crystalline phase of cellulose occurs at a temperature of 80–140 °C (Iguchi et al. 2000). However, higher Tg of BC produced by Acetobacter pasteurianus has supported their stability. Also, the observed Tm of BC was 72.38 °C. Furthermore, no degradation peaks were detected upto the temperature of 250 °C. These data suggested strong thermostability of the BC produced by Acetobacter pasteurianus on whey medium.
Elongation at break (%) of bacterial cellulose
BC produced by Acetobacter pasteurians on whey was observed to exhibit elongation of 13.82% at break. Elongation at break of BC produced by Acetobacter pasteurians RSV4 was higher as compared to that reported for other Gluconacetobacter/Acetobacter strains (Suwannapinunt et al. 2007; Nainggolan et al. 2013). The great physical strength of BC produced by Acetobacter pasteurians was also evinced through holding its different size in various ways without causing any damage to the mat.
Conclusions
In conclusion, process engineering has finally resulted in a very simple and economic whey medium for BC production by a novel and potent bacterium, Acetobacter pasteurians RSV-4 (MTCC 25117). At selected conditions and use of β-galactosidase, 5.6 g bacterial cellulose/L of whey production was achieved. Furthermore, process has also been optimized for scale up production of bacterial cellulose at 120 L by this natural isolate. Produced bacterial cellulose was highly pure, thermostable and had high breaking strength. These characteristic features of bacterial cellulose and its simple process of production at pilot scale using a very cheap whey medium make it a sustainable process to meet industrial demand.
References
Adebayo-Tayo BC, Akintunde MO, Alao SO (2017) Comparative effect of agrowastes on bacterial cellulose production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. Turkish J Agri Nat Sci 4:145–154
Battad-Bernardo E, McCrindle SL, Couperwhite I, Neilan BA (2014) Insertion of an E. coli lacZ gene in Acetobacter xylinus for the production of cellulose in whey. FEMS Microbiol Lett 231:253–260
Béchard SR, Levy L, Dorothée Clas S (1995) Thermal, mechanical and functional properties of cellulose acetate phthalate (CAP) coatings obtained from neutralized aqueous solutions. Int J Pharm 114:205–213
Bertocchi C, Delneri D, Signore S et al (1997) Characterization of microbial cellulose from a high-producing mutagenized Acetobacter pasteurianus strain. Biochim Biophys Acta 1336:211–217
Blanco Parte FG, Santoso SP, Chou CC et al (2020) Current progress on the production, modification, and applications of bacterial cellulose. Crit Rev Biotechnol 40:397–414
Brown RM (2004) Cellulose structure and biosynthesis: What is in store for the 21st century? J Polym Sci A Polym Chem 42:487–494
Carreira P, Mendes JAS, Trovatti E et al (2011) Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour Technol 102:7354–7360
Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47:107–124
Cherian BM, Leão AL, de Souza SF et al (2013) Bacterial nanocellulose for medical implants, Chap. 10. In: Sabu Thomas S, Visakh PM, Mathew AP (eds) Advances in Natural Polymers, Composites and Nanocomposites, 18. Springer-Verlag, Berlin Heidelberg, pp 337–359
Czaja WK, Young DJ, Kawecki M, Brown RM (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromol 8:1–12
Dai D, Fan M (2010) Characteristic and performance of elementary hemp fibre. Mater Sci Appl 1:336–342
Dlamini AM, Peiris PS (1997) Production of exopolysaccharide by Pseudomonas sp. ATCC 31461 (Pseudomonas elodea) using whey as fermentation substrate. Appl Microbiol Biotechnol 47:52–57
El-Saied H, El-Diwany AI, Basta AH et al (2008) Production and characterization of economical bacterial cellulose. Bioresource 3:1196–1217
Fan M, Dai D, Huang B (2012) Fourier transform infrared spectroscopy for natural fibers. In: Fourier transform—materials analysis, Croatia and Shanghai, China
Farah LFX, Podlech PAS, Archanjo CDR, Coral LA (2005) Continuous fermentation process to produce bacterial cellulosic sheets. WO2006066377A1
Fontana JD, Souza AM, Fontana CK et al (1990) Acetobacter cellulose pellicle as a temporary skin substitute. Appl Biochem Biotechnol 24–25:253–264
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
George J, Ramana K, Sabapathy S et al (2005) Characterization of chemically treated bacterial (Acetobacter xylinum) biopolymer: some thermo mechanical properties. Int J Biol Macromol 37:189–194
Gullo M, Sola A, Zanichelli G et al (2017) Increased production of bacterial cellulose as starting point for scaled-up applications. Appl Microbiol Biotechnol 101:8115–8127
Hwang JW, Yang YK, Hwang JK et al (1999) Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J Biosci Bioeng 88:83–188
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose- a masterpiece of nature’s arts. J Mat Sci 35:261–270
Jahan F, Kumar V, Rawat G, Saxena RK (2012) Production of microbial cellulose by a bacterium isolated from fruit. Appl Biochem Biotechnol 167:1157–1171
Jozala AF, Pértile RAN, dos Santos CA et al (2015) Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl Microbiol Biotechnol 99:181–190
Jung HI, Jeong JH, Lee OM et al (2010) Influence of glycerol on production and structural–physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour Technol 101:3602–3608
Keshk S, Razek T, Sameshima K (2006) Bacterial cellulose production from beet molasses. Afr J Biotechnol 5:1519–1523
Kim SY, Kim JN, Wee YJ et al (2006) Production of bacterial cellulose by Gluconacetobacter sp. RKY5 isolated from persimmon vinegar. Appl Biochem Biotechnol 131:705–715
Klemm D, Shumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose-artificial blood vessels for microsurgery. Prog Polym Sci 26:1561–1603
Kongruang S (2008) Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. Appl Biochem Biotechnol 148:245–256
Krystynowicz A, Czaja W, Wiktorowska-Jezierska A et al (2002a) Factors affecting the yield and properties of bacterial cellulose. J Microbiol Biotechnol 29:189–195
Krystynowicz A, Czaja W, Wiktorowska-Jezierska A et al (2002b) Factors affecting the yield and properties of bacterial cellulose. J Ind Micro Biotechnol 29:189–195
Kuo CH, Chen JH, Liou BK, Lee CK (2016) Utilization of acetate buffer to improve bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll 53:98–103
Lin SP, Huang YH, Hsu KD et al (2016a) Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit juice. Carbohydr Polym 151:827–833
Lin S, Liu C, Hsu K et al (2016b) Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose 23:367–377
Lin SP, Kung HN, Tsai YS et al (2017) Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 24:4927–4937
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 2:576–583
Mohite BV, Salunke BK, Patil SV (2013) Enhanced production of bacterial cellulose by using Gluconacetobacter hansenii NCIM 2529 strain under shaking conditions. Appl Biochem Biotechnol 169:1497–1511
Nainggolan H, Gea S, Bilotti E et al (2013) Mechanical and thermal properties of bacterial-cellulose-fibre-reinforced Mater-Bi(®) bio nanocomposite. Beilstein J Nanotechnol 4:325–329
Nguyen VT, Flanagan B, Gidley MJ, Dykes GA (2008) Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Curr Microbiol 57:449–453
Park JK, Park YH, Jung JY (2003) Production of bacterial cellulose by Gluconacetobacter hansenii PJK isolated from rotten apple. Biotechnol Bioprocess Eng 8:83–88
Premjet S, Ohtani Y, Sameshima K (1996) X-ray diffraction diagram of the bacterial cellulose membrane produced by Acetobacter xylinum in the medium with lignosulfonate. FIBER 52:169–174
Santoso SP, Chou C, Lin S et al (2020) Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 27:2497–2509
Saxena RK, Jahan F (2012) A novel isolated bacterial strain of gluconacetobacter oboediens and an optimized economic process for microbial cellulose production therefrom. WO2012110960A3
Schramm M, Hestrin S (1954) Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol 11:123–129
Shoda M, Sugano Y (2005) Recent advances in bacterial cellulose production. Biotechnol Bioprocess Eng 10:1–8
Sugiyama J, Vuong R, Chanzy H (1991) Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 1991 24(14):4168–4175
Suwannapinunt N, Burakorn J, Thaenthanee S (2007) Effect of culture conditions on bacterial cellulose (BC) production from Acetobacter xylinum TISTR976 and physical properties of BC parchment paper. Suranaree J Sci Technol 14:357–365
Tsouko E, Kourmentza CC, Ladakis D, Koutinas AA (2015) Bacterial cellulose production from industrial waste and by-product streams. Int J Mol Sci 16:14832–14849
Vazquez A, Foresti ML, Cerrutti P, Galvagno M (2013) Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J Polym Environ 21:545–554
Wada M, Okano T, Sugiyama J (1997) Synchrotron-radiated X-ray and neutron diffraction study of native cellulose. Cellulose 4:221–232
Watanabe KS, Yamanaka S (1995) Effects of oxygen tension in the gaseous phase on production and physical properties of bacterial cellulose formed under static culture conditions. Biosci Biotechnol Biochem 59:65–68
Wu JM, Liu RH (2012) Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr Polym 90:116–121
Yoshino T, Asakura T, Toda K (1996) Cellulose production by Acetobacter pasteurianus on silicone membrane. J Biosci Bioeng 81:32–36
Acknowledgments
The authors express their sincere thanks to the Department of Biotechnology (DBT), Government of India, New Delhi; BIRAC, GOI New Delhi and LSRB, DRDO, GOI for the financial support to successfully carry out this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, V., Sharma, D.K., Sandhu, P.P. et al. Sustainable process for the production of cellulose by an Acetobacter pasteurianus RSV-4 (MTCC 25117) on whey medium. Cellulose 28, 103–116 (2021). https://doi.org/10.1007/s10570-020-03519-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03519-6