Abstract
Phytoestrogens are naturally occurring constituents of plants present in the significant proportion of our diet. They have been extensively studied due to their potential as pharmacological targets and nutraceutical benefits. The potential pharmacological applications of these molecules include cardioprotection, antimicrobial, anticancer, anti-obesity, antiosteoporosis, antidiabetic, and neuroprotection. Phytoestrogens are polyphenolic nonsteroidal compounds of plant origin with estrogen-like biological activity. They mimic estradiol-like effects in several tissue/tissues of the mammalian body. The health benefits accredited to them are due to their ability to mimic estrogenic actions. In this chapter, we aim to provide comprehensive coverage of the pharmacological aspects of the most pronounced phytoestrogens of our daily life. We will discuss different classes of phytoestrogens under the subcategory of flavonoids and non-flavonoids. Numerous plant-derived compounds like genistein, daidzein, 8-prenylnaringenin, equol, quercetin, coumestrol, isoliquiritigenin, and resveratrol belonging to different classes of phytoestrogens will be discussed with their therapeutic values and clinical applications in a broad range of health-related problems and disorders.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phytoestrogens are naturally occurring constituents of plants, having structural similarity to mammalian female sex hormones estrogens (xenoestrogens). They exhibit estradiol-like effects in one or more target tissues in mammalian body [1]. Under the classical definition of phytoestrogens fall those compounds, which can induce estrus, exert estrogenic effects on the central nervous system (CNS), and stimulate growth of the genital tract of female animals. Broadly, the phytoestrogen can be defined as the chemicals which can display effects suggestive of estrogenicity which include binding to the estrogen receptors, thereby inducing specific estrogen-responsive gene products leading to stimulation of estrogen receptor(s)-positive breast cancer cell growth [2]. They cause both estrogenic and antiestrogenic effects [3] by binding to estrogen receptors alpha and beta with a preference estrogen receptor [1, 4]. Since estrogen receptors are present in different tissues all over the mammalian body, central nervous system (CNS), reproductive system, mammary gland, bones, lungs, and ovary, phytoestrogens have specific hormonal effects on different tissues [5]. Phytoestrogens act as natural defense against herbivorous animals by controlling their female fertility, attributed to their mimicry and action as antagonists of estrogen [3]. Phytoestrogens exert their effects mediated via multiple modes of actions including both genomic and non-genomic mechanisms [6]. Estrogenic and antiestrogenic effects on estrogen receptors (ERs) are some genomic mechanisms of action, while other effects’ direct interaction with ERs may not be involved [7]. Phytoestrogens have stable structure and low molecular weight which gains them access through cell membranes [8]. They interact with enzymes and receptors, bind to ERs and provoke specific estrogen-responsive gene products, and stimulate ER-mediated effects in the body. Non-genomic effects exerted by phytoestrogens do not involve direct interaction with ERs. Some of the non-genomic actions include inhibition of tyrosine kinase, antioxidant effects and suppression of angiogenesis, induction of cancer cell differentiation, and DNA topoisomerase activities [9]. Among these, the most well-characterized mode of phytoestrogen action appears to be through estrogen receptor (ER) binding.
A plethora of information related to health benefits of phytoestrogens is available in the literature. The first major attributed health benefits of phytoestrogen are an alternative to hormone replacement therapy (HRT) to alleviate premenopausal symptoms and osteoporosis. Phytoestrogens have also been suggested to have health benefits in cardiovascular diseases, metabolic disorders [10], and breast cancer, but their beneficial role in breast cancer is contradicted by some adverse effects. They have been reported to possess antiviral [11] and antimicrobial properties [12]. In this chapter, we have presented the classification and a detailed description of the role of phytoestrogens in human health along with their positive and negative effects.
2 Clinical Importance of Different Classes of Phytoestrogens
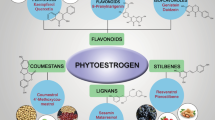
Phytoestrogens classification is based on their chemical structures, which resemble estradiol (E2). There are several classes of phytoestrogens found in plants. Broadly, phytoestrogens are classified into two major classes such as flavonoids and non-flavonoids. Flavonoids are further divided into isoflavones, flavones, isoflavans, flavonols, and coumestans. Non-flavonoid consists mainly of lignans, stilbenes, and chalcones. The detailed classification of phytoestrogens along with their clinical significance is presented below.
2.1 Flavonoids
2.1.1 Isoflavones
The isoflavonoids are 3-phenyl chromones having C6-C3-C6 skeleton in which each C6 moiety is a benzene ring linked via a heterocyclic pyrone ring (Fig. 15.1). They can exist as glucosides or as aglycones. The glucosides are further hydrolyzed in the gut to their respective aglycones.
Isoflavones are substituted derivatives of isoflavonoids where two or three hydrogen atoms are replaced by hydroxyl groups. Their structures encompass a planar ring system with a substituted p-hydroxy aromatic ring and second in-plane hydroxyl group which are approximately 12Å away from each other. This distance is almost identical to that between the C-3 and C-17 hydroxyls of estradiol [13] (Fig. 15.2). This is why they can bind to receptors. Two ring structures separated with two carbon atoms as well as spacing between two hydroxyls play an important role in binding affinity of isoflavones to ERs [14].
Hydrogen bond interactions with amino acids of the ligand-binding site of the ER are determined by these distances [15]. Isoflavones have gained considerable importance in the areas of clinical nutrition and disease prevention during last few decades. They show beneficial effects on cancer, lipid metabolism, cardiovascular diseases, osteoporosis, and blood coagulation and act as antioxidants [16]. They proved their efficacy as insecticidal, piscicidal, antifungal, and antimicrobial agents. Two of the most extensively studied isoflavones are genistein and daidzein.
2.1.1.1 Genistein
Genistein (5,7,4′-trihydroxyisoflavone) (Fig. 15.3) is an isoflavone first isolated from the dyer’s broom, Genista tinctoria , and is naturally found in a number of plants including soybeans, lupin, fava beans, kudzu, and other legumes and commonly exists as inactive glucoside genistein [17]. It is derived from a precursor molecule, biochanin A, that is converted to genistein after breakdown by intestinal glucosidases.
Genistein is one of the phytoestrogens to show potent receptor binding affinity with the estrogen receptors. It can prop up tissue specific as agonist or antagonist depending on the estrogen receptor and concentration of circulating endogenous estrogens [18]. Despite the similarity, genistein can bind to ERα with relative binding affinity of only 0.05–1% to the binding affinity of 17β-estradiol, while it’s greater in case of ERβ [19]. This differential binding affinity of genistein (estrogen receptor-β seven times more than that of estrogen receptor-α) makes it a selective estrogen receptor modulator [20] and thus promotes multiple effects on the target tissue.
Synthetic derivatives have been prepared to test the potency of genistein. Among numerous biological effects displayed by genistein, the most pronounced one is anticancer property. It has been reported to exert antiproliferative properties on different types of cancer. Several mechanisms for the anticancer effects have been proposed including inhibition of angiogenesis, affecting cellular transduction signaling pathways inhibiting carcinogenesis, inactivation of nuclear factor kappa B (NF-κB), inhibition of metastasis of cancer cells by controlling proteolytic enzymes matrix metalloproteinases (MMPs), topoisomerase, tyrosine kinase activity, and antioxidant properties. Genistein has also been known to cause cell cycle arrest in various human cancer cell lines as described below.
2.1.1.1.1 Cancer
The proliferative (estrogenic) and antiproliferative (antiestrogenic) effects in human breast cancer cell lines due to genistein made it the phytoestrogen of greatest interest. It imparts biphasic effect in estrogen receptor (ER)-positive MCF-7 breast cancer cell line which is concentration-dependent, with stimulation of cell growth at lower concentrations between 10 and 1.0 nM but at higher doses between 1 and 10 μM, and above inhibition is observed in both ER-positive and ER-negative cells. At lower concentrations, genistein competes with estradiol for binding to the ER and thus mimic as an estrogen agonist [21]. The antiproliferative effects are clearly non-ER mediated. It induces apoptosis in both ER-positive MCF7 cell lines and ER-negative MDA-MB-468 cancer cell lines. It inhibits the growth of cancer cells by intervening in cell cycle progression (affecting CDKs), which ultimately results in cessation of cell proliferation. PTKs (protein tyrosine kinases) that are involved in tumorigenesis are inhibited by genistein action, and this efficacy has led many laboratories to investigate its therapeutic potential against breast and prostate cancer [22]. In addition to PTKs, other mechanisms by which genistein exhibits anticarcinogenic activity are inhibition of angiogenesis (inhibits TGF-β signaling and the suppression of growth factor receptor VEGF-mediated signaling pathway), BRCA1 modulation, induction of apoptosis through inhibitory effect on NF-κB signaling and Akt pathway, inhibition of aromatase enzyme, and inhibiting DNA replication enzymes DNA topoisomerases I and II.
Androgens are crucial in prostate cancer genesis and act via activation of androgen receptor (AR). AR-responsive gene, prostate-specific antigen (PSA) acts as major key factor in prognosis and progression in prostate cancer patients. Genistein, at high concentrations, inhibits the growth of androgen-dependent and independent cells in human prostate cancer cell lines by inhibiting PSA synthesis in prostate cancer cells [23]. It displays anti-metastatic efficacy in prostrate tissue in phase I and phase II clinical trial patients [24]. Genistein inhibit cancer progression in colon cells by inducing apoptosis or inhibiting proliferation, upregulating LDL receptors [25], and affecting epidermal growth factors [26].
Genistein in combination with other chemotherapeutic agents such as centchroman, cisplatin, gemcitabine, docetaxel, and 5-fluorouracil potentiates their antitumor activity [27,28,29,30]. Several other combination therapies such as curcumin-genistein [31], terazosin-genistein [32], and genistein-cetuximab [33] have proved to be effective in various cancer cells. A derivative of genistein, phenoxodiol ((3-(4-hydroxyphenyl)-2H-chromen-7-ol, Fig. 15.4), belonging to the family of drugs called signal transduction inhibitors can arrest several cancer cell lines in the G1 stage of the cell cycle [34]. It stimulates apoptosis via multiple pathways. It is a molecule with broad antitumor activity and high specificity for tumor cells. Its biochemical tendencies are predominantly well-matched to reversal of chemoresistance. It is being developed as a chemo-sensitizer of standard chemotherapeutics in solid cancers [35].
2.1.1.1.2 Osteoporosis
Osteoporosis is a bone disease causing brittleness in bone due to a reduction in bone tissue. Osteoclasts responsible for bone destruction are dependent on tyrosine kinase enzyme. Estrogen deficiency causes an abundance of the osteoclast. Genistein is a well-known tyrosine kinase inhibitor and can be used as the antiosteoporotic agent. Genistein along with other phytoestrogens play a favorable effect in maintaining a balance between adipogenesis and osteogenesis. It inhibits the expansion of osteoclasts while stimulates the growth of osteoblasts [36, 37]. Antigenotoxic effects of genistein in postmenopausal women leading to positive effects on bone suggest the fact that genistein can be used for the prevention of bone loss in postmenopausal women without getting significant adverse effects [38]. However, later studies suggested that treatment with genistein to ovariectomized rats resulted in improved biomechanical results and enhancement of morphologic parameters but risk involved with the uterus and mammary glands was unclear [39].
2.1.1.1.3 Antidiabetic Effects
Diabetes is a metabolic disorder either caused by defective insulin secretion or insulin action or sometimes both play a role. Genistein preserves pancreatic β-cells, ameliorates glucose and lipid metabolisms, and elevates insulin levels [40]. It decreases insulin-induced lipid synthesis from glucose in isolated adipocytes and also inhibits insulin-stimulated glucose oxidation [41]. The antidiabetic potential is mediated by counteracting reactive oxygen species and chelating metals [42]. Since oxidative stress inflammation also plays a role in promoting metabolic disorders, genistein in the dose of 1 mg/kg/day in 30% dimethyl sulfoxide for 45 days ameliorates the inflammatory state to endorse its antidiabetic efficacy [43].
2.1.1.1.4 Neuroprotective Effects
Neurodegenerative disorders mainly comprise of Alzheimer’s disease (AD) and Parkinson’s disease (PD) where oxidative stress-induced neuron cellular apoptosis is believed to be involved in the development of both these diseases. Parkinson’s disease (PD) is characterized by the progressive loss of dopaminergic neurons. PD primary pathological events leading to dopaminergic neurons degeneration is supposed to occur via oxidative stress. Increased lipid peroxidation and DNA damage are also the result of overproduction of reactive oxygen species (ROS). So, genistein being a potent antioxidant attenuates the neuronal damage and loss through counteracting oxidative stress. Genistein has the potential to suppress PTK expression, which is interpreted to be neuroprotective [44]. It has neuroprotective effects on dopaminergic neurons in the nigrostriatal system, and this effect may be accredited to enhancing Bcl-2 gene expression [45]. Activation of the IGF-I receptor signaling pathway is another neuroprotective effects associated with genistein [46].
Alzheimer’s disease (AD) is manifested by different cellular and molecular events and primarily involves deposition of the β-amyloid peptide into senile plaques which initiate oxidative stress and accelerates the degeneration of neurons [47].
Genistein has antioxidant and neuroprotective effects on Alzheimer’s disease (AD). It mediates protective effect against Aβ-induced toxicity via estrogen receptors. This action stimulates MAP kinases, activates the NF-ĸB signaling pathway, and thereby induces overexpression of antioxidant manganese superoxide dismutase (MnSOD) [48]. Acute genistein treatment has been suggested to be useful in improving memory deficits in ovariectomized rats [49]. Genistein (10mg/kg) improved the memory of Aβ-injected rats due to the estrogen-like activity instead of antioxidant effect [50]. It has been found that genistein increased PPARg (peroxisome proliferator-activated receptor gamma) levels, which in turn will make overexpressing apolipoprotein E (apoE) thus mediates the degradation of amyloid β (Aβ). Therefore, a clinical trial is undergoing, which studies the role of genistein on treatment of AD via activation of PPARg levels [51]. Some of the genistein-polyamine conjugates possessed momentous cholinesterases (ChEs) inhibitory activity with an IC50 value of 2.75 μmol/L which was better than that of rivastigmine (5.60 μmol/L) [52].
2.1.1.2 Daidzein
Daidzein (7,4’-dihydroxyisoflavone) (Fig. 15.5) is commonly found in Pueraria mirifica and Kudzu or Pueraria lobata . It commonly exists as inactive glucoside daidzein. It is also obtained from precursor formononetin, which on breakdown by intestinal glucosidases is converted to daidzein. Daidzein is further partially metabolized to equol and O-desmethylangiolensin (O-DMA).
Daidzein is a comprehensively studied phytoestrogen with respect to its skeletal effects. It can promote various therapeutic effects such as prevention of cancer, cardiovascular diseases, etc. Daidzein inhibits the growth of human prostate, colon, breast, and ovarian carcinomas [53, 54]. It causes cell cycle arrest at the G1 and G2/M phases in human breast cancer cells [40], induces apoptosis [55], and affects angiogenesis/metastasis [56]. Carboxymethyl derivatives of daidzein act as a carrier for daunomycin (anticancer drug used for ovarian cancer) enhancing anticancer potential in human model of ovarian cancer cell lines [57].
Antiosteoporotic functions of daidzein include stimulation of osteoblast and inhibition of osteoclast through ERs and stimulation of protein synthesis in osteoblasts [58] that are further enhanced in synthetic analogs [59].
Ipriflavone, a synthetic isoflavone (prenyl isoflavone derivative) derived from daidzein, has been developed as an oral treatment for acute leukemias [60]. It showed best results for prevention of osteoporosis suggesting that it is a useful and safe alternative to ERT in treating osteoporosis in postmenopausal women [61].
Daidzein displays good antioxidant activity by upregulating the antioxidant enzyme catalase and is thereby linked to chemopreventive potential in many human diseases, including cancer and heart disease [62]. Daidzein along with genistein is among one of the most potent modulators of immune functions [63], but daidzein is also involved in immunosuppressive effects [64]. Daidzein along with genistein can protect against oxidative stress-induced cell apoptosis and cell proliferation inhibition in the diabetic endothelial cell model. This combination therapy may thereby prove to be beneficial for diabetes and chronic lung diseases [65]. Moreover, daidzein anti-oxidative potential imparts neuroprotective effects against Alzheimer’s disease [66]. Other beneficial effects of daidzein include decreased LDL oxidation and lipoprotein disorders [67], anti-obesity, and metabolic disorders [68].
2.1.1.3 Glycitein
Glycitein is commonly found in Glycine max (L.) and Pueraria montana and accounts for 5–10% of the total isoflavones in soy food products. Glycitein has a structural similarity to those of genistein and daidzein. It has weak estrogenic activity, comparable to that of the other soy isoflavones but much lower than that of diethylstilbestrol (DES) and 17β-estradiol [69]. Glycitein prominently has therapeutic potential for prevention of neurodegenerative disorders [70]. It helps in lowering cholesterol and LDL contents [71] and has preventive measures in cardiovascular diseases [72].
2.1.1.4 Formononetin
Formononetin is an isoflavonoid found abundantly in Astragalus mongholicus Bunge and Trifolium pratense L. (red clover). The extracts of these herbs have been used clinically to treat different diseases including cardiovascular diseases since long time. In addition, it has been reported to exert anti-oxidative and estrogenic effects [73] along with hypolipidemic properties. Formononetin has also been found to be beneficial in cardiovascular disorders as it shows vasorelaxation effects [74].
2.1.2 Flavones
Flavonoidal chemical entity comprises of 2-phenyl-benzo[α]pyrane or flavane nucleus, which consists of two benzene rings linked through a heterocyclic pyran ring. Flavonoids exert a wide spectrum of biological activities in a multitude of disease states such as cancer, cardiovascular disease, and neurodegenerative disorders. There are reports about their beneficial effects as anti-allergic [75], anti-inflammatory [76], antiviral [77], antioxidant [78], and antitumor activities [79]. Apart from this, they are known to possess hypercholesterolemic, anti-hepatotoxic, and antifertility activities [80] including anti-inflammatory potential [81, 82]. Some of them are also thought to act as natural fungicides and UV protectants. Apigenin and naringenin are some of the extensively studied flavonoids.
2.1.2.1 Apigenin
Apigenin (Fig. 15.6) is commonly found in Medicago sativa, Allium sativum, and Acinos suaveolens. Apigenin is a weak mutagen showing both anticarcinogenic activity [83] and antigenotoxic effect [84].
Apigenin acts as antiproliferative by inducing cell cycle arrest and apoptosis [85]. It enhances the efficacy of standard chemotherapeutic drug gemcitabine by increasing its antitumor effects [86]. It also possesses antioxidant potential [87] and neuroprotective effects [88]. It enhances the effect of chemotherapeutic drug cisplatin in prostate cancer cell lines [89]. 8-Prenylapigenin displays anti-inflammatory and vascular protective properties [90].
2.1.2.1.1 8-Prenylnaringenin
8-Prenylnaringenin (8-PN) contained in the female flower of hops (Humulus lupulus L.). It is one of the strongest plant-derived estrogen receptor ligands and regarded as one of the most potent phytoestrogens [91, 92]. It strongly binds to both estrogen receptor (ER) α and β with a relative binding affinity of about 0.01 (estradiol = 1), ten times higher than that of genistein [93]. 8-PN anticancer effects include induction of apoptosis in hormone-dependent breast cancer cell lines in a dose-dependent manner [94] and intervening in cell signaling pathways [95]. Later studies suggested proliferative effects of 8-PN [96]. Platelet aggregation is involved in a number of vascular diseases such as atherosclerosis, myocardial infarction, coronary artery disease, and thrombosis. 8-PN anti-aggregatory and anti-adhesive potency on human platelets leads to prevention of these diseases [97].
2.1.2.2 Kaempferol
Kaempferol is one of the most commonly found dietary flavonoids. It is mainly isolated from grapefruit, tea, and broccoli. It exerts its biological effect not only through estrogen receptors but also through some estrogen-related receptors [98]. Its preventive role in postmenopausal conditions, osteoporosis, and cardiovascular diseases has been established [99]. Anticancer effects are observed in colorectal, lung, and prostate cancers. It can induce apoptosis through inhibiting DNA synthesis, inhibiting kinase activities, inducing nuclear DNA degradation, cell cycle arrest, and telomerase inhibition [100,101,102,103]. Recent reports suggest its antiviral potential against influenza virus-induced severe lung damage [104].
2.1.2.3 Icariin
Icariin is the main active flavonoid glucoside isolated from Epimedium pubescens. It is used as antirheumatics (anti-inflammation) and in tonics (for health promotion) [105]. Epimedium flavonoids extract containing prominently icariin have a therapeutic effect on osteoporosis [106, 107], and it also enhances bone healing and thus reduces osteoporosis [108]. Recent studies explored its cardioprotective potential [109], antiangiogenesis against liver fibrosis [110], and neuroprotective efficacies [111].
2.1.2.4 Silymarin
Silymarin is a purified extract from milk thistle (Silybum marianum) comprising of a mixture of four isomeric flavonolignans: silibinin (its main, active component), isosilibinin, silydianin, and silychristin [112]. It has been used for centuries as a natural remedy for liver diseases and claimed for clinical applications in the treatment of viral hepatitis [113]. Additionally, silymarin has also been identified for its anti-inflammatory and cytoprotective effects [114], exhibiting antiosteoporotic [115], immunomodulatory [116], and antitumor activities [117] and antiviral potencies [118]. Silymarin is also effective against neurodegeneration and aging-related disorders [119] and fungal infections [120].
2.1.2.5 Baicalein
Baicalein is a flavonoid extracted from Scutellaria baicalensis. It has been reported to have multiple pharmacological activities including anti-inflammatory, anti-allergic, antiviral, and anti-oxidative effects [121,122,123]. Antitumor effects of baicalein have been widely explored on various human cancer cell lines [124,125,126,127]. Silymarin and baicalein in combination have synergistic anticancer effect on human hepatoma HepG2 cells [128]. It also has beneficial effects in treating memory loss and may have potential as therapeutic lead in combating Parkinson’s disease [129]. It is also effective in the prevention of noise-induced hearing loss [130].
2.1.3 Isoflavan
The isoflavans are a subclass of flavonoids, composed of C6-C3-C6 skeleton with the central heterocyclic pyran ring. Most common example of this class is equol. Other examples include isoflavans glabridin and hispaglabridins A and B.
2.1.3.1 Equol
Equol (Fig. 15.7) is a bioactive metabolite of daidzein that is produced by intestinal bacteria and has estrogenic activity exceeding that of daidzein [131].
It is considered to have therapeutic potential similar to that of the isoflavones genistein and daidzein. Equol being a chiral molecule is profoundly found in nature as enantiomer S-equol. This stereoisomer displays a 13-fold higher relative binding affinity (RBA) for ERβ than ERα which is greater than its parent compound daidzein [132]. Contrary, the R-enantiomer has a stronger RBA for ERα. Equol is known to possess the best antioxidant activity among all isoflavones. It also has anticancerous effects and induces apoptosis in human breast cancer cells [133]. Equol possesses substantial vasodilator [134] and protects cells from H2O2-induced cell death to prevent vascular atherosclerosis [135]. To date, few clinical trials have considered equol as a molecule having potential to enhance bone density among postmenopausal women [136, 137]. Studies revealed the fact that ingestion of equol and resveratrol as dietary supplements may improve postmenopausal health problems [138].
2.1.4 Flavonol
Any class of flavonoid compounds that are hydroxy derivatives of flavone can be classified as flavonol. One of the most common examples of flavonol is quercetin.
Quercetin (Fig. 15.8) is found in the peel of apples and red onions. Naturally it occurs in form of two glycosides, quercetrin when linked to rhamnose or rutin when linked to rutinose.
Quercetin is presumed to have beneficial health effects such as prevention of cancer and cardiovascular disease [139, 140] and is also a strong antioxidant [141]. Its therapeutic efficacy has been thoroughly investigated for its abilities to express antiproliferative effects by in both prostate cancer and human endometrial adenocarcinoma cell lines [142]. It induces apoptosis in colorectal cancers [143] and in ER-α negative cancer cell lines [144]. Various quercetin derivatives are reported to have DNA topoisomerase activities [145]. Quercetin-amino acid conjugates as safe modulators for multidrug resistance (MDR) [146]. It has protective anti-inflammatory effect against rheumatoid arthritis [147].
2.1.5 Coumestans
Coumestans (Fig. 15.9) are plant phenols having estrogenic activity most pronounced among all the phytoestrogens.
2.1.6 Coumestrol
Coumestrol (Fig. 15.10) is isolated from ladino clover (Trifolium repens L.), strawberry clover (Trifolium fragiferum), and alfalfa or lucerne (Medicago sativa L.). This has a similar structural scaffold to a steroid and thus interacts with estrogen receptors (ERs).
It is reported to have higher binding affinities to ERs than the other phytoestrogen compounds apart from genistein [148], showing similar biphasic effects as genistein on cell growth [149]. Being a phytoestrogen, it displays a wide scale of biological effects on the breast, uterus, and bone. It imparts cytotoxic effects on breast cancer [150] and antitumor effects on prostate cancer [151]. It is a unique molecule as it inhibits resorption of the bone and, at the same time, it stimulates bone mineralization [152]. Since it also has less estrogenic activity than 17β-estradiol, it may prove to be a more potent drug against bone diseases including osteoporosis. Considering the fact, coumestrol and its analogs, coumestrol diacetate and coumestrol dimethyl ether have been evaluated for these effects in vivo with favorable [153]. Psoralidin, a prenylated coumestan and coumestrol, exerts bone-protective effects via osteoblast proliferation and differentiation, thus enhancing bone formation [154]. Coumestrol also displays anticarcinogenic activity [155] and neurobehavioral actions [156] and has neuroprotective effects [157].
2.2 Non-flavonoids
2.2.1 Lignans
Lignans (Fig. 15.11) are defined as dimeric phenylpropanoid (C6-C3) compounds containing a dibenzylbutane skeleton and are widely distributed in nature as minor constituents of some plant species.
Lignans occur in high concentration in flaxseed and in lesser concentration in whole grain cereals, vegetables, and fruits. Lignans as they occur in plants are not active estrogens. Such activity is achieved only after the gut flora metabolism of these precursors to the so-called mammalian lignans. They have low molecular weight and with phenolic groups at the meta position of the aromatic rings.
As with the isoflavone phytoestrogens, lignans have health-promoting effects particularly in the areas of hormone-dependent cancers and cardiovascular disease [158, 159] and possess estrogenic as well as antiestrogenic activity [160]. Lignans intervene in estrogen metabolism by affecting sex hormone binding globulin (SHBG) [161]. Secoisolariciresinol and matairesinol are the most well-known phytoestrogenic lignans. They are not estrogenic by themselves but on conversion to enterodiol and enterolactone acquire estrogenic action. Enterodiol is further metabolized to enterolactone.
Secoisolariciresinol (Fig. 15.12) is isolated from Taxus baccata and Urtica dioica. It is effective in lowering serum cholesterol and in preventing the development of diabetes mellitus and has also proved to be good antioxidant along with enterodiol and enterolactone [ 162 ]. Enterolactone has a strong protective effect on the breast cancer [163]. It inhibits estradiol proliferative effect on MCF-7 breast cancer cells in culture [164]. Secoisolariciresinol diglucoside (SDG) increases bone mass in offspring in rats when taken during lactation [165]. SDG exerts antidepressant-like effect in mice [166] and is antidiabetic [167].
2.2.2 Stilbenes
Stilbenes (Fig. 15.13) are 1,2-diarylethenes. Stilbenoids, hydroxylated derivative of stilbenes, are of pharmaceutical interest. They have been explored for their chemopreventive effects, lipid-lowering and vascular activities, and antidiabetic potential. Trans-resveratrol is one the most pronounced and major active compounds of all the monomeric stilbenes.
Resveratrol (Fig. 15.14) is a secondary plant metabolite and exists as cis and trans isomers. Anticancer properties exhibited by this molecule have drawn considerable attention, since it has a remarkable inhibitory potential in various stages of tumor development.
It may block tumor development at various stages by targeting kinases [168], steroid hormone receptors [169], reactive oxygen species [170], ribonucleotide reductase [171], and DNA polymerases [172]. Resveratrol being structurally similar to the synthetic estrogen diethylstilbestrol (DES: 4,4’-dihydroxy-trans-a,b-diethylstilbene) behaves as a strong estrogen receptor agonist. Unlike other phytoestrogens, it can bind with equal affinity to both the estrogen receptors ERα and ERβ. Among both the enantiomeric forms, the trans isomer exerts higher activity in ER-positive cancer cell lines. At lower concentrations (10–25 nM), it displays proliferative effects on MCF-7 cell lines, whereas at concentrations of 0.1 and 1 μM, it had no effect. At concentrations of 10 μM, resveratrol competes with the endogenous estrogen 17β estradiol and inhibits its binding to ER. This phenomenon can be explained by the fact that in the presence of estrogen resveratrol shows agonist as well as antagonistic effect in ER-positive breast cancer cells, while lack of estradiol leads to induction of ER-dependent transcriptional events by this molecule [173]. Resveratrol anticancer effects include cell cycle arrest (S/G2 phase transition of the cell cycle) and induction of differentiation and apoptosis in several of human cancer cell lines, such as breast, prostate, leukemia, colon, and esophageal cells [174]. Resveratrol in combination therapy with standard chemotherapeutic drugs increases the efficacy of the therapy and concurrently reduces the undesired side effects. Roscovitine (ROSC), a selective cyclin-dependent kinase (CDK) inhibitor in MCF-7 breast cancer cells in combination with resveratrol, enhanced the ROSC-mediated inhibition of cell proliferation and cell cycle arrest [175]. Resveratrol-based aspirin prodrugs exert improved anticancer properties [176]. Apart from combination therapy, resveratrol analogs with improved bioavailability displayed positive results [177]. It has been reviewed extensively as MDR reversion molecule particularly against breast cancer [178]. Considering the above facts, resveratrol might be the most promising candidate for HRT and chemoprevention of breast cancer.
Other activities of resveratrol include antioxidant and neuroprotective properties showing positive effects on Alzheimer’s disease [179] and Parkinson’s disease [180]. It is in clinical trials phase II to evaluate its neuroprotective potential in patients with AD [181]. Resveratrol also protects the cardiovascular system by a large number of mechanisms [182], possesses anti-inflammatory activity [183] and antimicrobial effects [184], and demonstrates suppression of oxidative DNA damage [185]. Resveratrol also affects in modulation of lipoprotein metabolism by affecting lipogenesis and lipolysis in adipocytes of normal rats [186] and could aid in the treatment of chronic intestinal inflammation [187]. The pure compound is now available in tablets and is recommended as a dietary supplement.
2.2.3 Chalcones
Basic chalcone skeleton (Fig. 15.15) comprises of an α, β double bond. Presence of a hydroxyl group at position 2′ and/or 3′ imparts ER-ligand activities, and thus chalcones possess anticarcinogenic potency and antiproliferative effects for different cancer cells [188]. Some chalcone derivatives show biphasic effect in breast cancer cells in a dose-dependent manner. They show proliferative effects at low concentrations but exhibit an ER-independent inhibitory activity at levels ≥10 μM [189]. Two of the commonly found plant chalcones are isoliquiritigenin and licochalcone A.
2.2.3.1 Isoliquiritigenin
Isoliquiritigenin (Fig. 15.16), a chalcone primarily reported from licorice, has been identified in terms of its anti-oxidative effect [190], anti-inflammatory effect, antiplatelet aggregation [191] and most of all estrogenic properties. Isoliquiritigenin can behave as antitumor agent in several human carcinoma cell lines and induce apoptosis. HL-60 human promyelocytic cell line [192], colon cancer cells [193], human prostate cancer [194], and human hepatoma cells [195] have been found to be inhibited by the action of isoliquiritigenin. Biphasic effects of isoliquiritigenin in MCF-7 breast cancer cells are well-documented. Antitumor mechanism of isoliquiritigenin involves induction of apoptosis [196], inhibition of cell signaling pathways, and inhibition of angiogenesis [197]. Some studies evaluated its antiangiogenetic effects and considered it to be pronounced enough in leukemia cells to be considered as a drug [198]. Not only in cancer, isoliquiritigenin also showed neuroprotection [199], anti-inflammatory potential [200], and bone-protective efficacy [201].
2.2.3.2 Licochalcone A
Licochalcone A is also a molecule of therapeutic interest and possesses various biological activities including antibacterial [202], anti-parasitic [203], estrogenic, and antitumor effects [204]. Its antiproliferative potency has been found to be very pronounced [205]. Like other phytoestrogens, licochalcone induces apoptosis in MCF-7 breast cancer [206] and leukemia cells [207]. It is also effective in prostate cancer [208] and gastric cancer as it can induce apoptosis by causing cell cycle arrest at G2/M transition [209]. Furthermore, licochalcone A is also effective as an anti-obesity molecule [210] and has bone healing effects [211].
3 Phytoestrogens as Therapeutic Agents
The therapeutic potential of phytoestrogens cannot be clearly evaluated as the dose of phytoestrogens administered, their dietary composition, and duration of use come into effect and thus makes them difficult to compare. Data regarding considerable health benefits to cardiovascular, breast, bone, and menopausal symptoms are not strong enough to conclude. Thus the degree to which phytoestrogen consumption confers health benefits excluding side effects still remains unresolved.
3.1 Menopausal Symptoms and Prevention of Osteoporosis
Phytoestrogen consumption came into effect as a natural alternative to hormone replacement therapy. They were found to relieve not only from vasomotor perimenopausal symptoms including hot flushes but also played a crucial role in prevention of osteoporosis. Most of the phytoestrogens from all the category including coumestrol, resveratrol, genistein, daidzein, and others are reported to have bone-protective effect in vivo. Although their therapeutic potential seemed dependent on the dose, duration, and route of administration and on the animal model employed, their osteoprotective potential is dependent on their effects on the uterus and mammary glands and the risks involved in using these molecules as osteoprotective agents [39]. Although mixed results have been obtained, still dietary supplements containing numerous phytoestrogens continue to be popular as a natural alternative to hormone replacement.
3.2 Cardiovascular Health and Prevention of Heart Disease
Postmenopausal health problems also accompany heart diseases as there is decrease in estradiol levels during menopause [212]. In the current scenario of rise in cardiovascular diseases, soy consumption has been correlated with reduced risk of cardiovascular disorders. Isoflavonoids in particular play a role by increasing plasma level of the good cholesterol (HDL) and deteriorate bad cholesterol (LDL) [213]. Owing to this, phytoestrogens received the great attention and simultaneously stimulated consumption and adoption of soy foods in Western countries. However, there are escalating evidences to suspect the impact of soy consumption on LDL levels, and other cardiovascular diseases might be spurious. Regardless, people at risk for heart disease still consider soy intake as part of their daily meals.
3.3 Breast Cancer: Pro or Con?
Role of phytoestrogens in the area of breast cancer as perpetrator or protector has proven to be one of the trickiest questions to be tackled. Although extensive studies regarding this factor have been done, still results have been frustratingly incongruous. The fact that phytoestrogens bind to ERs with relatively high affinity may lead to the idea that high phytoestrogen intake could be the factor behind the increased risk of carcinogenesis. In such a case, breast cancer survivors are put at risk for reoccurrence. For instance, the phytoestrogen genistein enhances multidrug resistance in breast cancer cell [214]. Still, the relatively opposite results with traditionally low cancer rates in Asia stand as evidence of phytoestrogen benefit though this difference could be accounted on the difference in traditional diet of Asian and Western women [215]. On account of these reports, extensive studies have been done taking epidemiological approach to address these concerns, but no concluding results could be obtained due to numerous factors involved. It has been a difficult task to get a clear consensus as to whether or not phytoestrogens are beneficial or harmful particularly in the area of breast cancer. But since the mounting evidences of quantifiable benefits on bone and postmenopausal health, women without grave threat factors for breast cancer or a family history of the same are likely to integrate soy into their diet without momentous concern.
3.4 Other Therapeutic Applications
The therapeutic effects of PEs on neurodegenerative disorders have been clearly stated and have extensive effect on CNS. Although recent studies have also suggested negative outcome in case of cognitive functions [216]. Phytoestrogens have benefecial effects in hepatic alterations and have antidiabetic potential as seen with many of the molecules. They display antiaging effect on the skin via estrogen receptors [217]. Their antiviral properties toward a range of exogenous viruses have been clearly established [11]. Phytoestrogens have noteworthy antioxidant potential and are also good anti-inflammatory agents. They display a plethora of therapeutic properties such as antibacterial, antifungal, antidepressant, and anti-obesity to name a few.
3.5 Cons: The Endocrine Disrupting Properties of Phytoestrogens
Although phytoestrogens have been established as source of mediating a possible protective role in the areas of certain diseases such as cardiovascular diseases and some hormone-dependent cancers, conversely they also exert a detrimental effect on normal cells. Mutagenic effects, genotoxicity, and cancer cell-promoting properties are some of the perpetrator effect held by them. Isoflavones fit into the category of being an endocrine disruptor, which can be defined as compounds having ability to alter the function(s)/structure of the endocrine system and causing adverse effects. There have been reports implicating that phytoestrogens consumption during critical timings of development may hamper with the organizational role of estrogen and simultaneously causing myriad of adverse health outcome. This has been related to occurrence of malformations in the reproductive system, cancers, and reduced fertility. This also leads to early puberty and disrupted brain organization [218]. Paradoxically, phytoestrogens being effective antioxidants may also cause oxidative DNA damage and may lead to immunosuppressive effects. Phytoestrogens can influence human endogenous retrovirus expression (HERV) causing injurious effects of HERVs on cancer causing genes.
4 Conclusions
Phytoestrogens are intriguing, as although their beneficial effects have dominated the research areas, the potentially adverse effects of these compounds have been overlooked. Clearly some of the phytoestrogens have paradoxical effects, and many key factors could be attributed to it. To begin with the ligand-binding affinity of phytoestrogens, both ER could play a role. Also since they act within ER-mediated and ER-independent pathways, this may lead to additive, synergistic, or possible antagonistic interactions. In in vitro and in vivo, studies deliver varying outcome due to contribution of various factors such as involvement of multiple pathways, dosage, and bioavailability. Phytoestrogenic actions are dependent on concentration, varying with the target tissue and upon number and type of ER behaving as selective estrogen receptor modulators (SERM) which makes them difficult to predict the effect. Other key factors are also involved in the discrepancy obtained in the results. Ethnicity of the patient and bioavailability and genetic polymorphism of the PE metabolizing enzyme vary considerably with ethnicity and region of the population involved. Phytoestrogenic content in the supplements and extracts varies reasonably, and the difference in bioavailability of the compound must also be taken into account [219]. The presence or absence of endogenous estrogens also influences the effect of phytoestrogens [220]. All these factors can be attributed for the clinical efficacy of phytoestrogens which is also the necessity of the present scenario in the field of chemotherapy, but the undesired effects cannot be neglected unless critically observed and evaluated. As with many other compounds found in nature, there are many pros and cons associated with phytoestrogens, but overall, phytoestrogen supplements have a safe side-effect profile. To conclude, phytoestrogens are worthy of further investigations to achieve maximum beneficial output with a corresponding minimization of their undesired effects.
References
Rietjens IM, Sotoca AM, Vervoort J, Louisse J (2013) Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol Nutr Food Res 57:100–113
Knight DC, Eden JA (1996) A review of the clinical effects of phytoestrogens. Obstet Gynecol 87:897–904
Yildiz F (2005) Phytoestrogens in Functional Foods. Taylor & Francis Ltd, Boca Raton, pp 3–5:210–211
Paterni I., Granchi C., Katzenellenbogen J.A, Minutolo F. (2014) Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids 90 13–29 S0039-128X(14: 00151–00152
Böttner M, Thelen P, Jarry H (2013) Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J Steroid Biochem Mol Biol 139:245–251 pii: S0960-0760(13)00052-6
Anderson JJB, Anthony M, Messina M, Garner SC (1999) Effects of phyto-oestrogens on tissues. Nutr Res Rev 12:75–116
Messina MJ, Loprinzi CL (2001) Soy for breast cancer survivors: a critical review of the literature. J Nutr 131:3095S–3108S
Adlercreutz H (1998b) Evolution, nutrition, intestinal microflora, and prevention of cancer: a hypothesis. Proc Soc Exp Biol Med 217:241–246
Kurzer MS, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17:353–381
Carlson S, Peng N, Prasain JK, Wyss JM (2008) Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gen Med 5:S76–S90
Martin JHJ, Crotty S, Warren P, Nelson PN (2007) Does an apple a day keep the doctor away because a phytoestrogen a day keeps the virus at bay? A review of antiviral properties of phytoestrogen. Phytochemistry 68:266–274
Januario AH, Lourenco MV, Domezio LA, Pietro R, Castilho MS, Tomazela DM, da Silva M, Vieira PC, Fernandes JB, Franca SD (2005) Isolation and structure determination of bioactive isoflavones from callus culture of Dipteryx odorata. Chem Pharm Bull 53:740
Hu J-Y, Aizawa T (2003) Quantitative structure–activity relationships for estrogen receptor binding affinity of phenolic chemicals. Water Res 37:1213–1222
Brzozowski AM, Pike ACW, Dauter Z et al (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758
Vaya J, Tamir S (2004) The relation between the chemical structure of flavonoids and their estrogen-like activities. Curr Med Chem 11:1333–1343
Cassidy A, Bingham S, Setchell K (1995) Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr 74:587–601
Fatima A, Singh R (2016) The chemistry and pharmacology of genistein. Nat Prod J 6:3–12
Makiewicz L, Garey J, Adlercreutz H, Gurpide E (1993) In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol 45:399–405
Shutt DA, Cox RI (1972) Steroid and phyto-estrogen binding to sheep Uterine receptors in vitro. J Endocrinol 52:291–310
Kuiper GGJM, Lemmen JG, Carlsson B et al (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen. Endocrinology 139:4252–4263
Wang TT, Sathymoorthy N, Phang JM (1996) Molecular effects genistein on estrogen receptor mediated pathways. Carcinogenesis 17:271–275
Agarwal R (2000) Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol 60:1051–1059
Lakshman M, Li X, Vijayalakshmi A, Joshua C, Takimoto Chris H, Irene H, Pelling Jill C, Bergan Raymond C (2008) Dietary Genistein inhibits metastasis of human prostate cancer in mice. Cancer Res 68:20–24
Pavese JM, Krishn SN, Bergan RC (2014) Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am J Clin Nutr 100:431S–436S
Caruso MG, Notarnicola M, Cavallini A, Guerra V, Misciagna G, Di Leo A (1993) Demonstration of low density lipoprotein receptor in human colonic carcinoma and in surrounding mucosa by immunoenzymatic assay. Ital J Gastroenterol 25:361
Qi W, Weber CR, Wasland K, Savkovic SD (2011) Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 11:219
Kaushik S, Shyam H, Sharma R, Balapure AK (2016) Genistein synergizes centchroman action in human breast cancer cells. Indian J Pharm 48:637–642
Sarkar FH, Li Y (2006) Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res 66:3347–3350
Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH (2006) Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer 106:1260–1268
Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ (2005) Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 338:694–699
Aditya NP, Shim M, Yang H, Lee YJ, Ko S (2014) Antiangiogenic effect of combined treatment with curcumin and genistein on human prostate cancer cell line. J Funct Foods 8:204–213
Chang KL, Cheng HL, Huang LW, Hsieh BS, Hu YC, Chi TT, Shyu HW, Su SJ (2009) Combined effects of terazosin and genistein on a metastatic, hormone-independent human prostate cancer cell line. Cancer Lett 276:14–20
Park HJ, Della-Fera MA, Hausman DB, Rayalam S, Ambatia S, Baile CA (2009) Genistein inhibits differentiation of primary human adipocytes. J Nutr Biochem 20:140–148
Aguero MF, Fachinetti MM, sheleg Z, Senderowicz AM (2005) phenoxodiol: a novel isoflavone, induces G1 arrest by specific loss in cyclin dependent kinase 2 activity by p- 53 independent induction of p21WAF1/CIP1. Cancer Res 65:3364–3373
Choueiri TK, Mekhail T, Hutson TE, Ganapathi R, Kelly GE, Bukowsky RM (2006) Phase I trial of phenoxodiol delivered by continuous intravenous infusion in patients with solid cancer. Ann Oncol 17:860–865
Rickard DJ, Monroe DG, Ruesink TJ, Khosla S, Riggs BL, Spelsberg TC (2003) Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors alpha and beta. J Cell Biochem 89:633–646
Yamagishi T, Otsuka E, Hagiwara H (2001) Reciprocal control of expression of mRNAs for osteoclast differentiation factor and OPG in osteogenic stromal cells by genistein: evidence for the involvement of topoisomerase II in osteoclas- togenesis. Endocrinology 142:3632–3637
Atteritano M, Pernice F, Mazzaferro S, Mantuano S, Frisina A, D'Anna R, Cannata ML, Bitto A, Squadrito F, Frisina N, Buemi M (2008) Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur J Pharmacol 589:22–26
Sehmisch S, Uffenorde J, Maehlmeyer S, Tezval M, Jarry H, Stuermer KM, Stuermer EK (2010) Evaluation of bone quality and quantity in osteoporotic mice–The effects of genistein and equol. Phytomedicine 17:424–430
Choi EJ, Kim G (2008) Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 15:683–690
Bhathena SJ, Velasquez MT (2002) Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 76:1191–1201
Hamideh S, Marcus BM, Antonio A, Marcelo HL (2011) The antioxidant effect of genistein on the in vitro metal -mediated formation of free radicals. Clin Biochem 44:S226–S226
Palanisamy N, Kannappan S, Anuradha CV (2011) Genistein modulates NF-kB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur J Pharmacol 667:355–364
Gelinas S, Martinoli MG (2002) Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J Neurosci Res 70:90–96
Liu LX, Chen W, Xie J, Wong M (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res 60:156–161
Gao Q, Xie J, Wong M, Chen W (2012) IGF-I receptor signaling pathway is involved in the neuroprotective effect of genistein in the neuroblastoma SK-N-SH cells. Eur J Pharmacol 677:39–46
Jakob-Roetne R, Jacobsen H (2009) Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Eng 48:3030–3059
Bagheri M, Roghanid M, Joghataeib M, Mohsenia S (2012) Genistein inhibits aggregation of exogenous amyloid-beta1-40 and alleviates astrogliosis is in the hippocampus of rats. Brain Res 1429:145–154
Alonso A, Gonzalez-Pardo H, Garrido P et al (2010) Acute effects of 17 beta-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age (Dordr) 32(4):421–434
Bagheri M, Joghataei M-T, Mohsen S, Roghani M (2011) Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer’s disease. Neurobiol Learn Mem 95:270–276
ClinicalTrials.gov identifier: NCT01982578l Genistein as a Possible Treatment for Alzheimer's Disease. (GENISTEÍNA_2)l juy 2015).
Zhang X, Wang J, Hong C, Luon W, Wang C (2015) Design, synthesis and evaluation of genistein-polyamine conjugates as multi-functional anti-Alzheimer agents. Acta Pharm Sin B 5:67–73
Sathyamoorthy N, Wang TT (1997) Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur J Cancer 33:2384–2389
Guo JM, Kang GZ, Xiao BX, Liu DH, Zhang S (2004) Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int J Gynecol Cancer 14:882–888
Lee JS, Son KH, Sung MK, Kim YK, Yu R, Kim JS (2003) Anticarcinogenic properties of a daidzein-rich fraction isolated from soybean. J Med Food 6:175–181
Rabiau N, Kossaï M, Braud M, Chalabi N, Satih S, Bignon YJ, Bernard-Gallon DJ (2010) Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol 34:200–206
Somjen D, Katzburg S, Nevo N, Gayera B, Hodged RP, Reneveyd MD, Kalchenko V, Meshorere A, Sternc N, Kohena F (2008) A daidzein–daunomycin conjugate improves the therapeutic response in an animal model of ovarian carcinoma. J Steroid Biochem Mol Biol 110:144–149
Yamaguchi M, Sugimoto E (2000) Stimulatory effect of genistein and daidzein on protein synthesis in osteoblastic MC3T3-E1 cells: activation of aminoacyl-tRNA synthetase. Mol Cell Biochem 214:97
Yadav DK, Gautam AK, Kureel J, Srivastava K, Sahai M, Singh D, Chattopadhyay N, Maurya R (2011) Synthetic analogs of daidzein, having more potent osteoblast stimulating effect. Bioorg Med Chem Lett 21:677–681
Pagano L, Teofili L, Mele L, Piantelli M, Ranelletti FO et al (1999) Oral ipriflavone (7-isopropoxy-isoflavone) treatment for elderly patients with resistant acute leukemias. Ann Oncol 10:124–125
Petilli M, Fiorolli G, Benvenuti S, Frediani U, Gorif BMLIBI, estrogen r (1995) Calcif Tissue Int 56:160–165
Kampkötter A, Chovolou A, Kulawik A, Röhrdanz E, Weber N, Proksch P, Wätjen W (2008) Isoflavone daidzein possesses no antioxidant activities in cell-free assays but induces the antioxidant enzyme catalase. Nutr Res 28:620–628
Gredel S, Grad C, Rechkemmer G, Watzl B (2008) Phytoestrogens and phytoestrogen metabolites differentially modulate immune parameters in human leukocytes. Food Chem Toxicol 46:3691–3696
Yum MK, Jung MY, Cho D, Kim TS (2011) Suppression of dendritic cells’ maturation and functions by daidzein, a phytoestrogen. Toxicol Appl Pharmacol 257:174–181
Xu SZ, Zhong W, Ghavideldarestani M, Saurabh S, Lindow SW, Atkin SL (2009) Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radic Biol Med 47:167–175
Hsieh H, Wua W, Hu M (2009) Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol 47:625–632
Borradaile NM, Dedreu LE, Wilcox LJ (2002) Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J 366:531–539
He Y, Niu W, Xia C, Cao B (2016) Daidzein reduces the proliferation and adiposeness of 3T3-L1 preadipocytes via regulating adipogenic gene expression. J Funct Foods 22:446–453
Song TT, Hendrich S, Murphy PA (1999) Estrogenic activity of glycitein, a soy isoflavone. J Agric Food Chem 47:1607–1610
Gutierrez-Zepeda A, Santell R, Wu Z, Brown M, Wu Y, Khan I, Link CD, Zhao B, Luo Y (2005) Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci 6:54
Chen ZY, Ma KY, Liang Y, Peng C, Zuo Y (2011) Role and classification of cholesterol-lowing functional foods. J Funct Foods 3:61–69
Lin CC, Chen TS, Lin YM, Yeh YL, Li YH, Kuo WW, Tsai FJ, Tsai CH, Yen SK, Huang CY (2013) The p38 and NFjB signaling protein activation involved in glycitein protective effects on isoproterenol-treated H9c2 cardiomyoblast cells. J Funct Foods 5:460–465
Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW (2008) Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine 16:314–319
Wu J, Li Q, Wu M, Guo D, Chen H, Chen S, Seto S, Alice LSA, Poon CCW, Leung GPH, Lee SMY, Kwan Y, Chan S (2010) Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J Nutr Biochem 21:613–620
Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, Ogata A, Kawase I, Tanaka T (2007) Flavonoids and related compounds as anti-allergic substances. Allergol Int 56:113–123
Theoharides TC, Alexandrakis M, Kempuraj D, Lytinas M (2001) Anti-inflammatory actions of flavonoids and structural requirements for new design. Int J Immunopathol Pharmacol 14:119–127
Arao T, Udayama M, Kinjo J, Nohara T, Funakoshi T, Kojima S (1997) Preventive effects of saponins from Puerariae radix (the root of Pueraria lobata Ohwi) on in vitro immunological injury of rat primary hepatocyte cultures. Biol Pharm Bull 20:988–991
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614
Joshi KS, Rathos MJ, Joshi RD, Sivakumar M, Mascarenhas M, Kamble S, Lal B, Sharma S (2007) In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor. P276-00. Mol Cancer Ther 6:918–925
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation. Heart disease and cancer. Pharmacol Rev 52:673–751
Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ (2006) Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem 54:9798–9804
Khan TH, Jahangir T, Prasad L et al (2006) Inhibitory effect of apigenin on benzo(a)pyrene mediated genotoxicity in Swiss albino mice. J Pharm Pharmacol 58:1650–1655
Siddique YH, Afzal M (2009) Antigenotoxic effect of apigenin against mitomycin C induced genotoxic damage in mice bone marrow cells. Food Chem Toxicol 47:536–539
King JC, Lu Q, Li G, Moro A, Takahashi H, Chen M, Go VLW, Reber HA, Eibl G, Hines OJ (2012) Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim Biophys Acta 1823:593–604
Strouch MJ, Milam BM, Melstrom LG, McGill JJ, Salabat MR, Ujiki MB, Ding XZ, Bentrem DJ (2009) The flavonoid apigenin potentiates the growth inhibitory effects of gemcitabine and abrogates gemcitabine resistance in human pancreatic cancer cells. Pancreas 38:409–415
Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK et al (2012) Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol Pharm Bull 35:1440–1446
Jagan K, Priya CS, Kalpana K, Vidhya R, Anuradha CV (2017) Apigenin attenuates hippocampal oxidative events, inflammation and pathological alterations in rats fed high fat, fructose diet. Biomed Pharmacother 89:323–331
Erdogan S, Turkekul K, Serttas R, Erdogan Z (2017) The natural flavonoid apigenin sensitizes human CD44+ prostate cancer stem cells to cisplatin therapy. Biomed Pharmacother 88:210–217
Paoletti T, Fallarini S, Gugliesi F, Minassi A, Appendino G, Lombardi G (2009) Anti-inflammatory and vascular protective properties of 8-prenylapigenin. Eur J Pharmacol 620:120–130
Milligan SR, Kalita JC, Heyerick A, Rong H, Cooman L, Keukeleire D (1999) Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab 83:2249–2252
Milligan SR, Kalita JC, Pocock V, Van De Kauter V, Stevens JF, Deinzer ML, Rong H, De Keukeleire D (2000) The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab 85:4912–4915
Matsumura A, Ghosh A, Pope GS, Darbre PD (2005) Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 94:431–443
Brunelli E, Minassi A, Appendino G, Moro L (2007) 8-Prenylnaringenin, inhibits estrogen receptor-a mediated cell growth and induces apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol 107:140–148
Brunelli E, Pinton G, Chianale F, Graziani A, Appendino G, Moro L (2009) 8-Prenylnaringenin inhibits epidermal growth factor-induced MCF-7 breast cancer cell proliferation by targeting phosphatidylinositol-3-OH kinase activity. J Steroid Biochem Mol Biol 113:163–170
Helle J, Kräker K, Bader MI, Keiler AM, Zierau O, Vollmer G, Welsh JE, Kretzschmar G (2014) Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol Cell Endocrinol 392:125–135
Vito CD, Bertoni A, Nalin M, Sampietro S, Zanfa M, Sinigaglia F (2012) The phytoestrogen 8-prenylnaringenin inhibits agonist-dependent activation of human platelets. Biochimica et Biophysica Acta (BBA) 1820:1724–1733
Wang J, Fang F, Huang Z, Wang Y, Wong C (2009) Kaempferol is an estrogen-related receptor a and c inverse agonist. FEBS Lett 583:643–647
Strauss L, Santti R, Saarinen N, Streng T, Joshi S, Mäkelä S (1998) Dietary phytoestrogens and their role in hormonally dependent disease. Toxicol Lett 102–103:349–354
Sahu SC, Gray GC (1994) Kaempferol-induced nuclear DNA damage and lipid peroxidation. Cancer Lett 85:159–164
Zhang Y, Chen AY, Li M, Chen C, Yao Q (2008) Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J Surg Res 148:17–23
Kim S-H, Choi K-C (2013) Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res 29:229–234
Kashafi E, Moradzadeh M, Mohamadkhani A, Erfaniand S (2017) Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed Pharmacother 89:573–577
Zhang R, Ai X, Duan Y, Xue M, He W, Wang C, Xu T, Xu M, Liu B, Li C, Wang Z, Zhang R, Wang G, Tian S, Liu H (2017) Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-kB and MAPK signaling pathways. Biomed Pharmacother 89:660–672
Wu J, Du J, Xu C, Le J, Liu B, Xu Y et al (2010) In vivo and in vitro anti-inflammatory effects of a novel derivative of icariin. Immunopharmacol Immunotoxicol 33:49–54
Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM (2005) Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine 12:189–193
Sakai S, Tamura M, Mishima H, Kojima H, Uemura T (2008) Bone regeneration induced by adenoviral vectors carrying til-1/Cbfa1 genes implanted with biodegradable porous materials in animal models of osteonecrosis of the femoral head. J Tissue Eng Regen Med 2:164–167
Hsieh T, Sheu S, Sun J, Chen M, Liu M (2010) Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine 17:414–423
Qian ZQ, Wang YW, Li YL, Li YQ, Zhu L, Yanga DL (2017) Icariin prevents hypertension-induced cardiomyocyte apoptosis through the mitochondrial apoptotic pathway. Biomed Pharmacother 88:823–831
Algandaby MM, Breikaa RM, Eid BG, Neamatallah TA, Abdel-Naim AB, Ashour OM (2017) Icariin protects against Thioacetamide-induced liver fibrosis in rats: implication of anti-angiogenic and anti-autophagic properties. Pharmacol Rep. https://doi.org/10.1016/j.pharep.2017.02.016
Xiong D, Deng Y, Huang B, Yin C, Liu B, Shi J et al (2016) Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol 30:157–162
Wu JW, Lin LC, Tsai TH (2009) Drug–drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol 121:185–193
Wellington K, Jarvis B (2001) Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs 15:465–489
Sonnenbichler J, Zetl I, Cody V, Middleton E, Karbone JB, Berck A (eds) (1988) Specific binding of a flavonolignane derivative to an estradiol receptor. Prog Clin Biol Res 280:369–374
El-Shitany NA, Hegazy S, El-desoky K (2010) Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine 17:116–125
Gharagozloo M, Velardi E, Bruscoli E, Agostini M, Di Sante M, Donato V, Amirghofran Z, Riccard C (2010) Silymarin suppress CD4+ T cell activation and proliferation: effects on NF-κB activity and IL-2 production. Pharmacol Res 61:405–409
Wu T, Liu W, Guo W, Zhu X (2016) Silymarin suppressed lung cancer growth in mice via inhibiting myeloid-derived suppressor cells. Biomed Pharmacother 81:460–467
Song JH, Choi HJ (2011) Silymarin efficacy against influenza A virus replication. Phytomedicine 18:832–835
Srivastava S, Sammi SR, Laxman TS, Pant A, Nagar A, Trivedi S, Bhatta RS, Tandon S, Pandey R (2017) Silymarin promotes longevity and alleviates Parkinson’s associated pathologies in Caenorhabditis elegans. J Funct Foods 31:32–43
Yun DG, Lee DG (2017) Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochim Biophys Acta 1859:467–474
Kimata M, Inagaki N, Nagai H (2000) Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med 66:25–29
Hong T, Jin GB, Cho S, Cyong JC (2002) Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med 68:268–271
Piao YS, Du YC, Oshima H, Jin JC, Nomura M, Yoshimoto T, Oshima M (2008) Platelet-type 12-lipoxygenase accelerates tumor promotion of mouse epidermal cells through enhancement of cloning efficiency. Carcinogenesis 29:440–447
Kuntz S, Wenzel U, Daniel H (1999) Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 38:133–142
Tong WG, Ding XZ, Adrian TE (2002a) The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res Commun 296:942–948
Tong WG, Ding XZ, Witt RC, Adrian TE (2002b) Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther 1:929–935
Chung H, Choi HS, Seo EK, Kang DK, Oh ES (2015) Baicalin and baicalein inhibit transforming growth factor-b1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem Biophys Res Commun 458:707–713
Chen C, Huang T, Wong C, Hong C, Tsai Y, Liang C, Lu F, Chang W (2009) Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol 47:638–644
Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL (2004) The flavonoid Baicalein inhibits fibrillation of Synuclein and disaggregates existing fibrils. J Biol Chem 279:26846–26857
Kang TH, Hong BN, Park C, Kim SY, Park R (2010) Effect of baicalein from Scutellaria baicalensis on prevention of noise-induced hearing loss. Neurosci Lett 469:298–302
Setchell KD, Brown NM, Lydeking-Olsen E (2002) The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr 132:3577
Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA (2004) Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med Chem 12:1559–1567
Choi EJ, Ahn WS, Bae SM (2009) Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem Biol Interact 177:7–11
Jackman A, Woodman OL, Chrissobolis S, Sobey CG (2007) Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res 1141:99–107
Chung J, Kim SY, Jo HH, Hwang SJ, Chae B, Kwon DJ, Lew YO, Lim Y, Kim JH, Kim EJ, Kim J, Kim M (2008) Antioxidant effects of equol on bovine aortic endothelial cells. Biochem Biophys Res Commun 375:420–424
Setchell KD, Lydeking-Olsen E (2003) Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr 78:593S–609S
Lampe JW (2009) Is equol the key to the efficacy of soy foods? Am J Clin Nutr 89:1664S–1667S
Davinelli S, Scapagnini G, Marzatico F, Nobile V, Ferrara N, Corbi G (2017) Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: a randomized, placebo-controlled study. Maturitas 96:77–83
Knekt P, Jarvinen R, Seppanen R, Hellovaara M, Teppo L, Pukkala E, Aroma A (1997) Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol 146:223–230
Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC (2002) Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr 75:880–886
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Agullo G, Gamet-Payrastre L, Fernandez Y, Anciaux N, Demigne C, Remesy C (1996) Comparative effects of flavonoids on the growth, viability and metabolism of a colonic adenocarcinoma cell line (HT29 cells). Cancer Lett 105:61–70
Russo M, Palumbo R, Tedesco I, Mazzarella G, Russo P, Iacomino G, Russo GL (1999) Quercetin and anti-CD95(Fas/ Apo1) enhance apoptosis in HPB-ALL cell line. FEBS Lett 462:322–328
Sotocaa AM, Ratman D, Saag PV, Ström A, Gustafsson JA, Vervoort J, Rietjens IMCM, Murk AJ (2008) Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ER_/ER_ ratio. J Steroid Biochem Mol Biol 112:171–178
Bernad FX, Sable S, Cameron B (1997) glycosylated flavones as selective inhibitors of topoisomerases IV. Antimicrob Agents Chemother 41:992–998
Kim MK, Choo H, Chong Y (2014) Water-soluble and cleavable Quercetin−amino acid conjugates as safe modulators for P-glycoprotein-based multidrug resistance. J Med Chem 57:7216−7233
Haleagrahara N, Miranda-Hernandez S, Alim MA, Hayesa L, Bird G, Ketheesan N (2017) Therapeutic effect of quercetin in collagen-induced arthritis. Biomed Pharmacother 90:38–46
Whitten PL, Naftolin F (1998) Reproductive actions of phytoestrogens. Bailliere Clin Endocrinol Metab 12:667–690
Saarinen NM, Penttinen PE, Smeds AI, Hurmerinta TT, Makela SI (2005) Structural determinants of plant lignans for growth of mammary tumors and hormonal responses in vivo. J Steroid Biochem Mol Biol 93:209–219
Zafar A, Singh S, Naseem I (2016) Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: insights into the molecular mechanism. Food Chem Toxicol. https://doi.org/10.1016/j.fct.2016.11.034
Lim W, Jeong M, Bazer FW, Song G (2017) Coumestrol inhibits proliferation and migration of prostate cancer cells by regulating AKT, ERK1/2, and JNK MAPK cell signaling cascades. J Cell Physiol 232(4):862–871
Tsutsumi N (1995) Effect of coumestrol on bone metabolism in organ culture. Biol Pharm Bull 18:1012–1015
Wang H, Li H, Moore LB, Johnson MDL, Maglich JM, Goodwin B, Ittoop ORR, Wisely B, Creech K, Parks DJ, Collins JL, Willson TM, Kalpana GV, Venkatesh M, Xie W, Cho SY, Roboz J, Redinbo M, Moore JT, Mani S (2008) The phytoestrogen coumestrol is a naturally occurring antagonist of the human Pregnane X receptor. Mol Endocrinol 22:838–857
Zhai Y, Li Y, Wang Y, Cui J, Feng K, Kong X, Chen L (2017) Psoralidin, a prenylated coumestan, as a novel anti-osteoporosis candidate to enhance bone formation of osteoblasts and decrease bone resorption of osteoclasts. Eur J Pharmacol Apr 15(801):62–71
Hong Y, Cho M, Yuan Y, Chen S (2008) Molecular basis for the interaction of four different classes of substrates and inhibitors with human aromatase. Biochem Pharmacol 75:1161–1169
Whitten PL, Patisaul HB, Younga LJ (2002) Neurobehavioral actions of coumestrol and related isoflavonoids in rodents. Neurotoxicol Teratol 24:47–54
Canal Castro C, Pagnussat AS, Orlandi L, Worm P, Moura N, Etgen AM, Alexandre Netto C (2012) Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res 1474:82–90
Dai Q, Franke AA, Jin F, Shu XO, Hebert JR et al (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomark Prev 11:815–821
De Kleijn MJJ, Van der Schouw YT, Wilson PWF, Grobbee DE, Jacques PF (2002) Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. women: the Framingham study. J Nutr 132:276–282
Aehle E, Müller U, Eklund PC, Willför SM, Sippl W, Dräger B (2011) Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry 72:2396–2405
Adlercreutz H, Höckerstedt K, Bannwart C, Bloigu S, Hämäläinen E, Fotsis T, Ollus A (1987) Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). Journal of Steroid Biochemistry 27:1135–1144
Prasad K (2000) Antioxidant activity of secoisolariciresinol diglucoside- derived metabolites secoisolariciresinol. Enterodiol, and enterolactone. Int J Angiol 9:220–225
Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A (2004) Serum enterolactone levels and the risk of breast cancer in women with palpable cysts. Eur J Cancer 40:84–89
Mousavi Y, Adlercreutz H (1992) Enterolactone and estradiol inhibit each other’s proliferative effect on MCF-7 breast cancer cells in culture. J Steroid Biochem Mol Biol 41:615–619
Figueiredo MS, Maia LA, Guarda DS, Lisboa PC, Moura EG (2017) Flaxseed secoisolariciresinol diglucoside (SDG) during lactation improves bone metabolism in offspring at adulthood. J Funct Foods 29:161–171
Ma X, Wang R, Zhao X, Zhang C, Sun J, Li J, Zhang L, Shao T, Ruan L, Chen L, Xu Y, Pan J (2013) Antidepressant-like effect of flaxseed secoisolariciresinol diglycoside in ovariectomized mice subjected to unpredictable chronic stress. Metab Brain Dis 28:77–84
Hu P, Mei QY, Ma L, Cui WG, Zhou WH, Zhou DS, Zhao Q, Xu DY, Zhao X, Lu Q, Hu ZY (2015) Secoisolariciresinol diglycoside, a flaxseed lignan, exerts analgesic effects in a mouse model of type 1 diabetes: engagement of antioxidant mechanism. Eur J Pharmacol 767(15):183–192
Stewart JR, Christman KL, O’Brian CA (2000) Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases: inhibition of protein kinase D but not protein kinase C isozyme autophosphorylation. Biochem Pharmacol 60:1355–1359
Bhat KPL, Lantvit D, Christov K, Mehta RC, Pezzuto JM (2001) Estrogenic and antiestrogenic properties of resveratrol in mammary tumour models. Cancer Res 61:7456–7463
Stojanovic S, Sprinz H, Brede O (2001) Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys 391:79–89
Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O (1998) Resveratrol: a remarkable inhibitor of ribonucleotide reductase. FEBS Lett 421:277–279
Locatelli GA, Savio M, Forti L, Shevelev I, Ramadan K, Stivala LA, Vannini V, Hubscher U, Spadari S, Maga G (2005) Inhibition of mammalian DNA polymerases by resveratrol: mechanism and structural determinants. Biochem J 389:259–268
Bhat KPL, Lantvit D, Christov K, Mehta RC, Pezzuto JM (2001) Estrogenic and antiestrogenic properties of resveratrol in mammary tumour models. Cancer Res 61:7456–7463
Sakamoto T, Horiguchi H, Oguma E, Kayama F (2010) Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem 21(9):856–864
Wesierska-Gadek J, Kramer MP, Maurer M (2008) Resveratrol modulates roscovitine-mediated cell cycle arrest of human MCF-7 breast cancer cells. Food Chem Toxicol 46:1327–1333
Zhu Y, Fu J, Shurlknight KL, Soroka DN, Hu Y, Chen X, Sang S (2015) Novel resveratrol-based aspirin prodrugs: synthesis, metabolism, and anticancer activity. J Med Chem 58(16):6494–6506
Du C, Dong MH, Ren YJ, Jin L, Xu C (2016) Design, synthesis and antibreast cancer MCF-7 cells biological evaluation of heterocyclic analogs of resveratrol. J Asian Nat Prod Res 3:1–13
Alamolhodaei NS, Tsatsakis AM, Ramezani M, Hayes AW, Karimi G (2017) Resveratrol as MDR reversion molecule in breast cancer: an overview. Food Chem Toxicol 103:223–232
Anekonda TS, Reddy PH (2006) Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem 96:305–313
Karlsson J, Emgard M, Brundin P, Burkitt MJ (2000) trans-resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J Neurochem 75:141–150
ClinicalTrials.gov Identifier: NCT01504854 Phase II Study to Evaluate the Impact on Biomarkers of Resveratrol Treatment in Patients With Mild to Moderate Alzheimer’s Disease April 2016
Wallerath T, Deckert G, Ternes T, Anderson H, Li H et al (2002) Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106:1652–1658
Das S, Das DK (2007) Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6:168–173
Stagos D, Portesis N, Spanou C, Mossialos D, Aligiannis N, Chaita E, Panagoulis C, Reri E, Skaltsounis L, Tsatsakis AM (2012b) Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from greek domestic Lamiaceae species. Food Chem Toxicol 50:4115–4124
Mizutani K, Keda K, Kawai Y, Yamori Y (2001) Protective effect of resveratrol on oxidative damage in male and female stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 28:55–59
Szkudelska K, Nogowski L, Szkudelski T (2009) Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol 113:17–24
Sanchez-Fidalgo S, Cardeno A, Villegas I, Talero E, Alarcon de la Lastra C (2010) Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol 633:78–84
De Vincenzo R, Scambia G, Panici PB, Ranelletti FO, Bonanno G, Ercoli A, Delle MF, Ferrari F, Piantelli M, Mancuso S (1995) Effect of synthetic and naturally occurring chalcones on ovarian cancer cell growth: structure–activity relationships. Anticancer Drug Des 10:481–490
Tamir S, Eizemberg M, Somjen D, Izrael S, Vaya J (2001) Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol 78:291–298
Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T (1998) Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg Med Chem 6:339–347
Tawata M, Aida K, Noguchi T, Ozaki Y, Kume S, Sasaki H, Chin M, Onaya T (1992) Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharmacol 212:87–92
Chowdhury SA, Kishino K, Satoh R, Hashimoto K, Kikuchi H, Nishikawa H, Shirataki Y, Sakagami H (2005) Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res 25:2055–2063
Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T (2004) Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci 95:448–453
Jung JI, Chung E, Seon MR, Shin HK, Kim EJ, Lim SS, Chung WY, Park KK, Park JH (2006) Isoliquiritigenin (ISL) inhibits ErbB3 signaling in prostate cancer cells. Biofactors 28:159–168
Hsu YL, Kuo PL, Lin LT, Lin CC (2005) Isoliquiritigenin inhibits cell proliferation and induces apoptosis in human hepatoma cells. Planta Med 71:130–134
Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, Menichini F, Amdoc S (2002) Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol 82:315–322
Kanga S, Choi J, Choi Y, Bae J, Li J, Kim DS, Kim J, Shin S, Lee Y, Kwun I, Kang Y (2010) Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J Nutr Biochem 21:55–65
Li D, Wang Z, Chen H, Wang J, Zheng Q, Shang J, Li J (2009) Isoliquiritigenin induces monocytic differentiation of HL-60 cells. Free Radic Biol Med 46:731–736
Zhan C, Yang J (2006) Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol Res 53:303–309
Chi JH, Seo GS, Cheon JH, Lee SH (2017) Isoliquiritigenin inhibits TNF-α-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur J Pharmacol 796:101–109
Liu S, Zhu L, Zhang J, Yu J, Cheng X, Peng B (2016) Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem Pharmacol 106:82–93
Tsukiyama R, Katsura H, Tokuriki N, Kobayashi M (2002) Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother 46:1226–1230
Chen M, Christensen SB, Blom J, Lemmich E, Nadelmann L, Fich K et al (1993) Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob Agents Chemother 37:2550–2556
Iwata S, Nishino T, Nagata N, Satomi Y, Nishino H, Shibata S (1995) Antitumorigenic activities of chalcones I. Inhibitory effects of chalcone derivatives on 32P-Incorporation into phospholipids of Hela cells promoted by 12-O- tetradecanoyl-phorbol 13-acetate (TPA). Biol Pharm Bull 18:1710–1713
Chen X, Liu Z, Meng R, Shi C, Guo N (2017) Antioxidative and anticancer properties of Licochalcone A from licorice. J Ethnopharmacol 198:331–337
Rafi MM, Rosen RT, Vassil A, Ho CT, Zhang H, Ghai G, Lambert G, Di Paola RS (2000) Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res 20:2653–2658
Rafi M, Vastano BC, Zhu N, Ho CT, Ghai G, Rosen RT, Gallo MA, DiPaola RS (2002) Novel polyphenol molecule isolated from licorice root (Glycyrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J Agric Food Chem 50:677–684
Fu Y, Hsieha T, Guo J, Kunicki J, Lee MYWT, Darzynkiewicz Z, Wu JM (2004) Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun 322:263–270
Xiao X, Hao M, Yang X, Ba Q, Li M, Ni S, Wang L, Du X (2011) Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett 302:69–75
Won S, Kim S, Kim Y, Lee P, Ryu J, Kim J, Rhee H (2007) Licochalcone A: a lipase inhibitor from the roots of Glycyrrhiza uralensis. Food Res Int 40:1046–1050
Shang F, Ming L, Zhou Z, Yu Y, Sun J, Ding Y, Jin Y (2014) The effect of licochalcone A on cell-aggregates ECM secretion and osteogenic differentiation during bone formation in metaphyseal defects in ovariectomized rats. Biomaterials 35:2789–2797
Oliveira PJ, Carvalho RA, Portincasa P, Bonfrate L, Sardao VA Fatty acid oxidation and cardiovascular risk during menopause: a mitochondrial connection? J Lipids 2012, 2012:365798
Jenkins DJ, Mirrahimi A, Srichaikul K et al (2010) Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr 140(12):2302S–2311S
Rigallia JP, Tocchettib GN, Aranab MR, Villanueva SSM, Catania VA, Theile D, Ruiz ML, Weiss J (2016) The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett 376-1:165–172
Henderson BE, Bernstein L (1991) The international variation in breast cancer rates: an epidemiological assessment. Breast Cancer Res Treat 18(1): S11–S117
Soni M, White LR, Kridawati A, Bandelow S, Hogervorst E (2016) Phytoestrogen consumption and risk for cognitive decline and dementia: with consideration of thyroid status and other possible mediators. J Steroid Biochem Mol Biol 160:67–77
Gopaul R, Knaggs HE, Lephart ED (2012) Biochemical investigation and gene analysis of equol: a plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors 38:44–52
Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ Jr (2008) Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90:911–940
This P, Cremoux PD, Leclercq G, Jacquot Y (2011) A critical view of the effects of phytoestrogens on hot flashes and breast cancer risk. Maturitas 70:222–226
Glazier MG, Bowman MA (2001) A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 161:1161–1172
Acknowledgments
AA is supported by a research fellowship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fatima, A., Alam, A., Singh, R. (2018). Therapeutic Potential of Phytoestrogens. In: Rani, V., Yadav, U. (eds) Functional Food and Human Health. Springer, Singapore. https://doi.org/10.1007/978-981-13-1123-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-1123-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1122-2
Online ISBN: 978-981-13-1123-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)