Abstract
Neuroinflammation is characterized by reactive microglia and astrocytes (collectively called gliosis) in the central nervous system and is considered as one of the main pathological hallmarks in different neurodegenerative diseases such as Alzheimer’s disease, age-related dementia, and multiple sclerosis. Upon activation, glia undergoes structural and morphological changes such as the microglial cells swell in size and astrocytes become bushy, which play both beneficial and detrimental roles. Hence, they are unable to perform the normal physiological role in brain immunity. Curcumin, a cytokine suppressive anti-inflammatory drug, has a high proven pre-clinical potency and efficacy to reverse chronic neuroinflammation by attenuating the activation and morphological changes that occur in the microglia and astrocytes. This review will highlight the recent findings on the tree structure changes of microglia and astrocytes in neuroinflammation and the effects of curcumin against the activation and morphology of glial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aging, Neuroinflammation, and Neurodegeneration in the CNS
Aging is a complex biological process that involves chronic oxidative and inflammatory stress which disrupts the communication and balance between the brain and the immune system [1, 2]. Aging of the brain, in particular, leads to neuroinflammation and increases the risk of age-related cognitively degenerative diseases such as dementia, including Alzheimer’s disease (AD) [1]. Microarray studies revealed an increase in overall pro-inflammatory and pro-oxidant transcriptional profile with a reduction in growth and anti-oxidant gene profile in older rodent brains compared to adults [3, 4]. Moreover, several studies also reported increased levels of pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α, in the brains of aged rodents and humans [5,6,7,8,9], while a reduction in key regulatory molecules and anti-inflammatory cytokines such as IL-10 and IL-4 was noted [9, 10]. Overall, these studies support glial priming or re-activation and, in turn, increased neuroinflammation, excessive cytokine production, and cognitive deficits in the aged brain [8, 11].
Neuroinflammation, an inflammatory response within the central nervous system (CNS), is initiated in response to a variety of endogenous and exogenous factors including foreign pathogens, neuronal injury, and toxins. Inflammatory episodes are characterized by mainly microglial and astrocytic reactivation (gliosis), the release of pro-inflammatory molecules [cytokines, chemokines, prostaglandins, nitric oxide (NO), and reactive oxygen species (ROS)], increased blood–brain barrier permeability, and recruitment of peripheral immune cells into the CNS [12]. Neuroinflammation leads to neuronal and axonal injury in the CNS which is a common underlying pathoetiology of neurodegenerative conditions such as AD, Parkinson’s disease, and multiple sclerosis [13, 14].
Microglia are the resident immune cells of the CNS. Their function extends beyond the role of immune sentinels and effectors to synaptic pruning and the modulation of higher cognitive functions (learning and memory), macrophage-like activities, and the maintenance of CNS homeostasis [12, 15,16,17]. In normal physiological conditions, physiological ramified microglia, present a round small cell body (large nuclei with thin cytoplasm) and are highly branched, with long thin processes, which continuously scan the entire brain for invading pathogens termed “surveilling” microglia [16, 18, 19]. According to a few investigations, ramified microglial cells have small somas, and long and thin processes, which are the typical characteristics of physiological microglial cells necessary for active surveillance of the brain [20]. Surveillant microglia are highly ramified and bushy, whereas they adopt amoeboid shape when undergoing morphological transformations and de-ramification in response to neuroinflammation with a range of intermediate activation states in between (e.g., ‘intermediately activated’, ‘bipolar’, ‘rod-like’, ‘hypertrophied’, ‘bushy’) [21, 19]. Ramified microglial cells have a key role in surveying the neuronal environment to maintain homeostasis, but under pathological conditions the changes in microglial cell size can influence the entire morphology [22, 23]. Microglia can acquire a primed or pro-inflammatory mRNA, protein, and morphological profile with aging and neurodegenerative disease. Damaged associated molecular patterns (DAMPs) in the microglial environment initiate a rapid morphological transformation in microglia which allows them to migrate to the site of injury/damage, alter their transcriptional profile and produce pro-inflammatory cytokines and chemokines. Reactivated microglia rapidly undergo cytoskeletal rearrangements and change their surface molecular expression thus increase their phagocytic efficiency [24]. Depending on the context, when key regulatory systems are impaired, microglia activation may become maladaptive leading to prolonged neuroinflammation and neurodegeneration. Ionized calcium-binding adaptor molecule 1 (Iba-1) is an actin-binding protein restricted to microglia/macrophages, and its expression is upregulated in reactivated microglia following brain injury and diseases [25, 26]. Thus, their expression increases when microglia morphology changes from quiescent ramified to activated amoeboid microglia [25]. Furthermore, ramified and reactivated microglia display different Iba-1 immunoreactivity with aging, where Iba-1 immunoreactivity increased at all stages of microglia reactivity compared to ramified phenotype [27]. Primed microglia phenotype displays a similar ramification pattern to the physiological microglia, but has a larger oval-shaped cell body [28, 29]. The reactive and amoeboid microglia display large amoeboid-shaped cell bodies often loaded with debris [30]. Moreover, while the reactive phenotype might display less extensive, short, thick processes, the amoeboid microglia seem to have no processes or may display a few within the length of the cell body [28, 30]. It is also important to note that microglia morphology does not necessarily reflect function, dysfunction, or RNA expression phenotype. Rather, it demonstrates how the cells respond to altered homeostasis [31]. Hence, the ramified (physiological) microglia of the healthy CNS can either be hypertrophic (reactive phenotype) due to acute injury or can undergo deterioration (dystrophic) due to age-related processes (predominantly in the neurodegeneration perspective) [31]. The morphological structure of microglia cells is highly variable and rather than categorized into polarized stages, should be considered as a continuum. The morphology can be quantified by measuring the number of processes (branches), sub-branches, and nodes that form a tree structure and a central soma that envelops the cell nucleus. Microglial architecture is more diffuse and complex with multiple primary and secondary branches not confined to a generalizable [32, 33].
Astrocytes contribute to being the most abundant cells in the CNS. Under normal physiological conditions, astrocytes modulate synaptic activity and provide major support for neuronal survival [34]. They regulate the function of microglia, oligodendrocytes, cells of the adaptive immune system and control infiltration of peripheral proinflammatory leukocytes into the CNS during neuroinflammation [35, 36]. Astrocytes are a hallmark of many neuropathologies, activated in response to neurotrauma, stroke, and many neurodegenerative diseases [37]. Reactive astrogliosis involves several processes that vary with the nature of the insult [38]. During neuroinflammation and nerve injury, astrocytes undergo a series of phenotypic and functional changes, also called reactive astrogliosis [39], and release cytokines such as IL-6, nitric oxide (NO), and other potentially cytotoxic molecules [40, 41]. During this process, naïve astrocytes undergo several changes including morphological changes (hypertrophy), proliferation, and functional changes, and differentiate into different subsets, including reactive astrocytes and scar-forming astrocytes. Reactive astrocytes can be divided into toxic A1 astrocytes, which have a toxic role and induce death of neurons, and neuroprotective A2 astrocytes, which enhance neuronal survival and tissue repair [42].
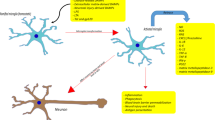
Curcumin, the active compound extracted from the dried rhizomes of Curcuma longa (turmeric), a member of the Zingiberaceae family, is one of the most studied natural compounds within the context of complementary medicine. Turmeric has been used traditionally as a medicinal herb in South East Asia and India for thousands of years for various illnesses including biliary disorders, anorexia, cough, hepatitis, rheumatic arthritis, and a variety of other chronic inflammatory diseases, as well as for its anti-tumorigenic potential [43,44,45]. Curcumin is a known anti-inflammatory agent against various inflammatory conditions. It could cross the blood–brain barrier in its native form and is active against sustained neuroinflammation without any serious adverse effects [46]. It has been reported that curcumin potentially reduced iNOS induction, thus protecting microglial cells against oxidative stress [47. Furthermore, curcumin is attributed to the inhibition of astrocyte hypertrophy in the spinal dorsal horn and phosphorylation of the ERK signaling pathway in a rat model of neuropathic pain [48]. The changes in microglia and astrocytes branched structure and the effects of curcumin to downregulate these alterations are summarized in Fig. 1.
Schematic overview of morphologic and functional changes in glia (microglia and astrocytes) upon re-activation in aging and neuroinflammation, in the rodent brain. Under protective physiological (left) conditions, resident microglia and astrocytes participate in maintaining homeostasis and healthy neuronal activity. Microglia display surveillant motile, fine processes, small cell soma, and distal arborization (light red), while astrocytes (light blue) display complex bushy morphology with fine processes, under normal physiological conditions. Under detrimental/pathological (right) conditions, microglia changes to a de-ramified, re-active state with retracted thick processes, enlarged cell soma, and decreased arborization (dark red). While astrocytes become hypertrophied with elongated, and thicker processes (dark blue). Moreover, continuous upregulation of proinflammatory markers such as interleukin-6 (IL-6) in astrocytes further exacerbates the transition of microglia to reactive de-ramified states leading to neurodegeneration. Damaged or dying neurons release danger-associated molecular patterns (DAMPs), which further contribute to pro-inflammatory glial activation. The vicious cycle of continuous glial activation can be reversed by the anti-inflammatory agent, curcumin. Created with BioRender.com
De-ramification of Microglia During Aging and Neuroinflammation
The physiological microglia continuously scans the brain in ramified morphology with small somas and long dynamic processes [19]. However, the ramified morphology and process activity vary across brain regions [20]. The microglial morphology is highly impacted in their reactive state whereas, there is very limited information concerning the morphological characteristics of microglia in the non-diseased brain. The majority of morphological characterization of microglia has occurred in situations of extensive CNS insult (e.g. traumatic brain injury, AD) [12]. During inflammatory conditions, the microglial cells have been observed to go into an re-activated state, where they become amoeboid-like in their morphology, becoming de-ramified microglia, which are characterized by a swollen cell body and thick processes [49].
Several studies have indicated the morphological variations of microglia across the different regions of the healthy brain [23]. Some of the studies have identified the typical morphology of microglia in rodent brains based on regions. One study has reported that the microglia in each region differs in area and perimeter [20]. The study described clear regional differences in microglial morphology, describing the occurrence of compact microglia with small round soma and short processes found exclusively in sites lacking a blood–brain barrier; longitudinally branched microglia having long primary processes; radially branched microglia with complex processes and spine structure found in the fibre tracts and white matter [20]. A study which examined the morphology of microglia across the mammalian cerebellum has reported variation of microglial morphology according to the extracellular environment of the cells. They further suggested that the microglial structure is based on the synaptic activity within a given region, while highly branched microglia extended in all directions within the cerebellar nuclei, the flattened microglia had processes that extended parallel to the axon projections in the white matter of the cerebellum [50]. Yamada and Jinno, for the first time have classified microglia based on quantitative measurements [51]. They also grouped microglia based on their discrete morphological measurements, revealing that neural tissue contains microglia that progress from highly ramified to compact and thickened processes [51]. Torres-Platas et al. for the first time studied the detailed quantitative neuroanatomical examination of microglia in the human prefrontal cortex [28]. They also identified four classes of microglia: classically ramified microglia; primed microglia (wider cell body with standard ramified processes); reactive microglia (wider cell body, few ramified processes), and amoeboid microglia [28].
Increased numbers of activated microglia in aging brains are the most likely indication of neuroinflammation, which may underlie age-related alterations in the brain’s response to insult and recovery from insult. This is most likely due to the phenotypic alterations of microglia in the aging brain that have reduced arborisation patterns and branch density which results in decreased area of surveillance contributing to the impairment of homeostatic functions [52, 53]. The evidence suggests that the microglia even in the non-pathological aged brain are in a hyperactivated state compared to those in the young healthy brain, in both rodents [54] and humans [55]. A study on microglial morphology in the human cerebral cortex of two nondemented subjects, using high-resolution LN-3 immunohistochemistry, also observed an approximately ten-fold increase in dystrophic microglia in the 68-year-old brain compared with the brain of a 38-year-old subject [56]. Overall, these studies are proposing that microglial senescence and resultant functional changes may provision the development of neurodegenerative diseases like AD and thus exacerbate the risk of cognitive changes associated with normal aging [52, 56, 57]. Microglia may develop an altered profile that resembles an increased inflammatory state, with aging. As reviewed by Nordan et al. [2], this ‘primed’ profile is defined by (1) excessive baseline expression of inflammatory mediators and markers, (2) a lower activation threshold to ‘switch’ to a pro-inflammatory state, and (3) an excessive prolonged inflammatory response following immune activation. Primed microglia of the aged brain is characterized by increased mRNA and protein expression of various pro-inflammatory markers and morphological alterations. The senescent or age-related dystrophic microglial cells convert from a highly ramified morphology to a de-ramified (decreased arborized processes) morphology. The de-ramification includes; loss of finely branched cytoplasmic processes; cytoplasmic beading/spheroid formation; in some instances, partial or complete cytoplasmic fragmentation [58]. A study on microglial morphology in the non-demented aged human cerebral cortex reported shorter and less branched dendritic arbors than microglia of young adults [12]. Several studies have detected similar alterations using Iba-1 immunostaining. Iba-1 microglia from the hippocampus of aged gerbils showed increased cell body size, thickened proximal processes, and decreased ramification of distal branches compared to young adults [59]. Moreover, Iba-1 immunoreactive microglia displayed hypertrophied cell bodies, thickened and de-ramified processes in the dentate gyrus of aged dogs [60]. Nonetheless, expression of the antigen-presenting molecule major histocompatibility complex (MHC) II was increased specifically on microglia of the aged brain [6]. It was also confirmed that the de-ramified morphology of microglia in aged rats corresponded with higher levels of MHC II expression [27], a marker of primed/reactive microglia. Further evidence emerged from positron emission tomography (PET) data that evaluated microglial activation in older humans. PET imaging, using The ligand PK [11C] (R)PK11195 which binds to translocator protein (TSPO) receptors expressed in mitochondria of activated microglia, showed heightened levels in several cortical and subcortical areas of older individuals [61]. Overall, these studies suggest microglial priming increases with aging and may shift towards a more activated morphology contributing to the augmented inflammatory status of the aged brain.
Accumulating shreds of evidence suggest that microglia can become chronically activated by either a single stimulus (e.g., traumatic brain injury (TBI), lipopolysaccharide, d-galactose, or any neuronal damage) or multiple stimuli exposures that result in cumulative neuronal loss with time though the exact mechanism is still unknown [62]. Microglia are activated in response to immunological stimuli, neurodegenerative conditions and brain injuries result in dramatic alterations in morphology, changing from resting, ramified microglia into de-ramified state [63]. The microglial cells are activated in traumatic brain injury and changes in microglial cells morphology have been observed in several TBI rodents models. Microglia undergo considerable remodeling by retracting their processes and adopting an amoeboid morphology [64]. The spatiotemporal changes in microglia morphology over 28 days following rat midline fluid percussion injury (mFPI) as a first step in exploiting microglia morphology to reflect altered brain physiology. The morphology of microglia altered and a de-ramification was observed in the somatosensory cortex barrel field (S1BF) following mFPI [65].
In neuroinflammation, a de-ramification of microglia has been observed, in which the microglia enter an activated state characterized by swollen ramified cells with shorter dendrites [66]. One of our recent study conducted in a mouse model of chronic neuroinflammation (GFAP-IL6 mice) has revealed that microglial cells undergo morphological transformations, such as increased soma size and thickening of the processes in response to IL-6 over expression resulting in chronic neuroinflammation [67]. The study investigated and quantified some of the morphological changes between ramified and non-ramified microglia and found that the microglial cells of GFAP-IL6 mice had significantly larger soma areas, small convex areas, and convex perimeter than those of wild type [67]. Similarly, another study conducted by our group in the same mouse model has revealed a de-ramification of microglia characterized by a significant increase in soma area and soma perimeter in both the hippocampus and cerebellum of the GFAP-IL6 mice compared with those of the wild type mice [68].
Evidence suggests the role of microglial function and morphology changes in the CRND8 mice, a mouse model of AD which carries a mutated form of the human amyloid precursor protein gene. The microglia in the proximity of Aβ plaques were less ramified compared to microglia distant from Aβ plaques, as well as compared to microglia from wild-type mice [69]. A most recent study conducted on human subjects has opened a new window for further investigation of microglial morphology. The morphology of microglial cells was assessed in 32 controls, 44 AD cases, and 16 AD cases from patients immunized against Aβ42 (iAD) using 2D and 3D approaches. The results showed that ramified microglia were fewer in AD compared to the controls but increased in iAD compared to AD and controls whereas, 3D reconstructions highlighted larger cell bodies in AD compared to the controls and increased total process length in iAD compared to AD. Altogether, the reactive/amoeboid microglia were the most represented population in the aged human brain. In contrast, Aβ removal by immunotherapy leads to increased ramified microglia [70].
Astrocytes Hypertrophy During Aging and Neuroinflammation
Astrocytes directly communicate with microglia. In aged rats, astrocytes shift from resting state to hypertrophic activated state, affecting microglial regulation [71, 72]. In the aged brains of both humans and rodents, the astrocyte inflammatory markers glial fibrillary acidic protein (GFAP) and vimentin were increased [72]. A study on the effect of normal aging and LPS-induced inflammation on astroglia-neuron interaction demonstrated that astrocytes were smaller with thicker and shorter branches, as well as less numerous, in the CA1 stratum radiatum of aged rats when compared to adult and lipopolysaccharide (LPS)-treated rats [5]. This study indicated active participation of astrocytes and microglia in the hippocampus of aged and LPS-infused rats in the clearance of cellular debris associated with programmed cell death [5].
Astrocytes exhibit a complex bushy or spongiform morphology, and their very fine processes are in close contact with synapses and other components of brain parenchyma [73]. Shreds of evidence have suggested that the morphometric changes occur in astrocytes during CNS insults but the fine neuroanatomy of astrocytes; however, remains to be investigated in these neurological conditions. Astrocytes are thought to undergo cellular hypertrophy and increase the thickness of their main cellular processes during neuroinflammation [37, 67].
There are two main types of astrocytes in rodents, largely based on their fine anatomical structures: protoplasmic and fibrous astrocytes [74]. Protoplasmic astrocytes are bushier with extended processes located in the gray matter. They form the outermost wall of the blood–brain barrier by extending their processes to blood vessels and enwrap them to form the glial limiting membrane. They play a key role in the modulation of synaptic functions and regulation of local blood flow in response to synaptic activities [75,76,77]. On the other hand, fibrous astrocytes possess straight and long processes and are widely distributed in white matter. This type of astrocyte associated with the blood vessels via their processes just like the protoplasmic astrocytes but their function is not clear [78]. The 3D reconstruction of astrocytes revealed that the reactive astrocytes increased the thickness of their main cellular processes but did not extend to occupy a greater volume of tissue than nonreactive astrocytes [37, 79]. Our recent two studies have described new evidence about the astrocytes hypertrophy during neuroinflammation [67, 68]. These studies [67, 68] analyzed the GFAP-IL6 mice, a chronic neuroinflammatory mouse model, using multiple experimental approaches including immunohistochemistry, 3D reconstruction software Neurolucida360 (MBF Bioscience), Sholl, and morphometric analysis. Specifically, the authors demonstrated that a significant increase in cellular hypertrophy was seen in reactive astrocytes in the inflamed mice which lead to a significant increase in the thickness and length of their main cellular processes in hippocampus and cerebellum areas. Furthermore, a significant increase in the overall convex area (cell area) of the reactive astrocytes occurred compared to nonreactive ones [67, 68]. Astrocyte reactivity in response to injury is termed astrogliosis [80], which involves changes in morphology, increased expression of the intermediate filament proteins, GFAP, and vimentin, heightened proliferation and secretion of inflammatory mediators and growth factors [81, 82]. These reactive astrocytes adopt hypertrophic morphology after injury, involving the extension of processes and swelling of cell bodies. A study conducted in a mouse mild-moderate controlled cortical impact (CCI) model reported hypertrophic astrocytes in the lesional and peri‐lesional area 3 days after TBI [83]. Similarly, astrocytes undergo numerous morphological alterations over time including rapid swelling and dendrites extensions [84, 85] after focal ischemic stroke, resulting from the blockage of cerebral blood vessels, which leads to cell death and brain damage [86]. A study conducted on the morphology of reactive astrocytes after ischemic and hemorrhagic stroke in rats revealed the morphological changes in the astrocytes based on GFAP staining in the regions of the sensorimotor cortex and dorsolateral striatum. There was an increase in the number and length of primary processes (ramification) of reactive astrocytes occurred increased compared with the astrocytes in a sham control group [87].
Astrocytes have different morphology in humans compared to rodents. Recent work has revealed the morphological structure and diversity of cortical astrocytes in humans. Human astrocytes were found to be proportionally larger than rodents and their processes were more elaborate [88]. A study conducted on human brain samples investigated the morphological changes that occur in two different types of astrocytes in depression conditions. The study investigated the morphometric structure of protoplasmic and fibrous astrocytes in Golgi-stained postmortem anterior cingulate cortex (ACC) samples from depressed suicides and matched non-psychiatric controls [89]. Based on literature evidence, patients suffering from depression have significantly higher levels of circulating pro-inflammatory cytokines and local inflammation [90]. Therefore, the fibrous astrocytes in the depressed brains reflect local inflammation in the white matter which leads to the hypertrophy of astrocytes [89].
Curcumin: An Anti-neuroinflammatory Compound and Modified Curcumin Preparation
Curcumin, a polyphenolic compound, an active component of turmeric, is a potent cytokine-suppressive anti-inflammatory drug (CSAID) and exerts a broad range of anti-inflammatory effects [91,92,93]. In the nervous system, curcumin and its modified formulations have been studied in the context of neurodegenerative diseases, e.g. neuroinflammation, AD and Parkinson’s disease, chronic pain, and epilepsy [94]. These studies revealed that curcumin may influence several intracellular signaling pathways, yielding neuroprotective and anti-inflammatory microglia-attenuating effects [47]. Curcumin exerts anti-inflammatory effects through several signaling pathways that are associated with inflammation including Toll-like receptor-4 (TLR-4) pathway [95]. TLR4 plays an important role in the recognition of endogenous agonists, such as heat shock protein, products of proteolytic cascades, intracellular components of ruptured cells, and the genes that are activated by inflammation [96, 97]. Activation of TLRs initiates signal transduction cascade leads to the activation of nuclear factor-kappa B (NF-κB) transcription factor, a transcriptional factor required for the expression of many inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [98], and the mitogen-activated protein kinases (MAPKs). It has been reported that curcumin administration attenuates the TLR4/NF-κB inflammatory signaling pathway [99]. Curcumin was also shown to down-regulate the expression of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), TNF-α, IL-1, -2, -6, -8, and -12 [100]. It has been reported that curcumin achieves its anti-inflammatory activity in the brain by inhibiting of janus kinase (JAK)-STAT signaling pathway [101]. It modulates the activity of several transcription factors (e.g., STAT, nuclear factor-κB, AP-1) and their pro-inflammatory molecular signaling pathways. It inhibits the expression of many pro-inflammatory cytokines which further interferes with the first signaling steps downstream of the IL-6 receptor in microglial activation [102] and astrocyte hypertrophy [103].
During neuroinflammation, the morphology of microglia and astrocytes is dramatically affected, leading to altering their normal physiological functions. Curcumin has known and prominent therapeutic potential to reverse these morphological changes that occur in these cells and can bring them to the normal structure [67, 68]. Curcumin, a CSAID, is frequently used as a drug of choice against inflammatory conditions due to its low toxicity and high preclinical efficacy and has the potential ability to reverse the structural topology of the glial cells [104]. It is a pleiotropic molecule that inhibits microglia transformation to an activated state and subsequent neurodegenerative diseases [105].
Despite its low toxicity profile and wide range of therapeutic applications, curcumin exhibits extremely low bioavailability, mainly due to its poor aqueous solubility, poor stability in solution, and rapid intestinal first-pass and hepatic metabolism [106]. Curcumin is insoluble at room temperature in water at both acidic and neutral pH. While it is soluble in an oil-soluble compound, practically alkali, it is very susceptible to auto-degradation. Therefore, various formulations have been developed in order to get enhanced bioavailability and consequent bio-efficacy [107, 108]. These modified curcumin preparation include liposomes, micelles, emulsions, microemulsions, nano-emulsions, phospholipid complexes, solid lipid nanoparticles, nanostructured lipid carriers, biopolymer nanoparticles, and microgels which enhance the efficacy, absorption, bioavailability, and permeation in the small intestine of curcumin formulation, summarized in Table 1 [109].
Impact of Curcumin on Re-ramified Microglia
It is reported through several pre-clinical trials that neuroinflammation is characterized by the activation of microglia and astrocytes [122], which lead to structural and functional neurological impairments that typify various neurodegenerative diseases [123]. Some of the studies have reported the effects of curcumin in reducing the de-ramification of microglial cells in the brain and bringing microglia back to normal size. A recent study conducted in 5xFAD mice, a mouse model for AD has highlighted the role of curcumin to reduce the de-ramification of microglial cells in the 5xFAD mice. They reported that ip. injection of curcumin for 5 days has significantly reduced the aggregation and de-ramification of microglial cells [124]. Our recent two studies have highlighted the specific effects of curcumin on microglial tree structure in neuroinflammation [67, 68]. One of our studies has reported that the phytosomal curcumin preparation (Meriva®) significantly reduced the soma area and soma perimeter in both hippocampus and cerebellum and reduced the de-ramification of microglia after 4 weeks of treatment via chow (874 ppm) [68]. Similarly, another study conducted on the solid lipid nanoparticle curcumin formulation (Longvida®) has reported the effects of curcumin on the activated and de-ramified microglial cells. Briefly, the study investigated the effect of Longvida® curcumin (LC) in a mouse model of chronic neuroinflammation. The LC diet mixed in chow was fed to 2 months old mice orally in a 500 ppm dose for the period of 6 months. The study confirmed that curcumin has significantly downregulated the de-ramification of microglial cells by reducing the soma size and soma perimeter of activated microglia [67] (Fig. 2).
Effect of Longvida® curcumin (LC) on microglial tree structure. Feeding the LC for 6 months to both WT and GFAP-IL6 mice with high neuroinflammation has significantly reduced the de-ramification of microglia (A–D). The representative images of microglia immunostained for Iba-1 showing that the Iba-1+ microglial cells of the GFAP-IL6 LC fed mice had a significantly reduced soma area (D), soma perimeter, and a higher number of nodes than that of the GFAP-IL6 mice on regular diet. Scale bar 10 µm [67]
The Role of Curcumin in Astrocyte Hypertrophy
Curcumin has a demonstrated role against astrocyte hypertrophy in different CNS conditions. A study conducted on 5xFAD mice, a mouse model of familiar AD, has observed and reported a decrease in activation and hypertrophy of astrocytes after being treated with solid lipid curcumin particles [124]. Emerging studies conducted in rats have reported the effects of curcumin against the hypertrophy of astrocytes in the CNS. One such study has reported that a lipid curcumin formulation was able to decrease astrocyte activation and the number of branches of the astrocytes in the rat hippocampus [5]. Similarly, another study conducted in a Sprague–Dawley rat model of chronic constriction injury model has provided evidence that solid lipid curcumin curcumin attenuates the activation of astrocytes. The study reported a decrease in hypertrophy of astrocytes in the injured rats after being treated with curcumin [48, 124]. We have recently conducted studies in the GFAP-IL6 mouse model of chronic neuroinflammation that have outlined more detailed structural and morphological changes that occurred in astrocytes during chronic neuroinflammation and the effect of modified curcumin preparations [67, 68]. These studies have revealed that astrocytes in the hippocampus and cerebellum regions in the GFAP-IL6 mice on normal-diet mice have a significantly larger dendritic length, the number of processes, convex area, convex perimeter, and the number of nodes compared to the wild types. Consuming curcumin diets, have significantly decreased the hypertrophy of astrocytes by decreasing the dendritic length, the number of processes, convex area, convex perimeter, and the number of nodes of astrocytes [67, 68] briefly; the study investigated the effects of LC in GFAP-IL6 mice by feeding to 2 months old mice for the period of 6 months. The study confirmed that the LC diet has potentially decreased the hypertrophy of astrocytes (Fig. 3).
Effect of Longvida curcumin (LC) on astrocyte hypertrophy showing a significant decrease in overall astrocytes size in LC-fed GFAP-IL6 mice. LC diet reversed the activation of astrocytes resulting in a decrease in the hypertrophy of astrocytes (A–D). 3D reconstruction of astrocytes in wild type and GFAP-IL6 in both showing that the LC diet treated and non-treated mice significantly reduced the astrocytes hypertrophy by decreasing the dendritic length, number of processes, convex area, convex perimeter, and number of nodes than that of GFAP-IL6 normal-fed mice. Scale bar 10 µm [67]
Future Directions
The structural morphology of microglia and astrocytes has an important role in understanding the pathophysiology of neurodegenerative diseases. Research on microglia and astrocytes and their structural and functional changes regarding neuroinflammation, injury, and neuronal disorders is gaining a lot of prominence. Thus, it is important to investigate the glial connections to neuronal networks both during healthy and pathological aging to better understand the etiology of diseases like AD and their impact on memory. It is also still inconclusive whether inflammation is a cause or a result of the observed neurodegeneration in these diseases. Thus, studies focusing on both neuroinflammation and neurodegeneration will help to design target-specific therapeutic interventions when treating related cognitive impairments. There are no therapies purposely designed against microglia- and astrocyte-specific targets in clinical practice. Furthermore, there are limited studies that elaborate on the effects of curcumin against glial cell activation. The presented evidence and findings will hopefully boost more coordinated and better-focused efforts to improve and therapeutically exploit the role of curcumin against reactive microglial cells and astrocytes in chronic neuroinflammation and brain injuries.
Taken together, this review discussed the tree structure of microglial cells and the astrocytes hypertrophy upon activation, in response to neuroinflammation, brain injury, and other kinds of CNS assaults. The study has highlighted the effects of curcumin against the glial cell activation and its potential to reverse the morphological changes that occur upon activation. This work showed that curcumin has a key role to reverse the activation of glial cells and, decrease the de-ramification of microglia and hypertrophy of astrocytes. This study could be the possible clue to cope with the neuroinflammation characterized by activation of microglia and astrocytes and alteration in their structural morphology.
References
Gamage R et al (2020) Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front Cell Neurosci. https://doi.org/10.3389/fncel.2020.577912
Norden DM, Muccigrosso MM, Godbout JP (2015) Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96(Pt A):29–41
Godbout JP et al (2005) Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 19(10):1329–1331
Lee EG et al (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289(5488):2350–2354
Cerbai F et al (2012) The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE 7(9):e45250
Henry CJ et al (2009) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23(3):309–317
Gavilán MP et al (2007) Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem 103(3):984–996
Sparkman NL et al (2006) Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci 26(42):10709–10716
Ye SM, Johnson RW (2001) An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 9(4):183–192
Maher FO, Nolan Y, Lynch MA (2005) Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging 26(5):717–728
Chen JL, Penhune VB, Zatorre RJ (2008) Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex 18(12):2844–2854
Streit WJ, Mrak RE, Griffin WST (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm 1(1):14
Sen MK et al (2020) Revisiting the pathoetiology of multiple sclerosis: has the tail been wagging the mouse? Front Immunol 11:572186
Xie A et al (2014) Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. Biomed Res Int 2014:648740
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77(1):10–18
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468
Wolf SA, Boddeke H, Kettenmann H (2017) Microglia in physiology and disease. Annu Rev Physiol 79:619–643
Kozai TD et al (2012) In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J Neural Eng 9(6):066001
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Lawson LJ et al (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39(1):151–170
Gomez-Nicola D, Perry VH (2015) Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist 21(2):169–184
Vinet J et al (2012) Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflamm 9:27
Hinwood M et al (2013) Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 23(8):1784–1797
Heindl S et al (2018) Automated morphological analysis of microglia after stroke. Front Cell Neurosci 12:106
Hovens IB et al (2014) Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun 38:202–210
Ohsawa K et al (2004) Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem 88(4):844–856
VanGuilder HD et al (2011) Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflamm 8:138
Torres-Platas SG et al (2014) Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflamm 11:12
Heneka MT et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Karperien A, Ahammer H, Jelinek HF (2013) Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci 7:3
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9(1):62–66
Abdolhoseini M et al (2019) Segmentation, tracing, and quantification of microglial cells from 3D image stacks. Sci Rep 9(1):8557
Clarke LE, Barres BA (2013) Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 14(5):311–321
Voskuhl RR et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29(37):11511–11522
Mayo L, Quintana FJ, Weiner HL (2012) The innate immune system in demyelinating disease. Immunol Rev 248(1):170–187
Wilhelmsson U et al (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA 103(46):17513–17518
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647
Hara M et al (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 23(7):818–828
Mrak RE, Griffin WS (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 26(3):349–354
Ahmad MH, Fatima M, Mondal AC (2019) Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: rational insights for the therapeutic approaches. J Clin Neurosci 59:6–11
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487
Chandran B, Goel A (2012) A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 26(11):1719–1725
Sanmukhani J et al (2014) Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res 28(4):579–585
Rao CV, Simi B, Reddy BS (1993) Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 14(11):2219–2225
Sundaram JR et al (2017) Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s disease. J Alzheimer’s Dis 60(4):1429–1442
Parada E et al (2015) Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol Nutr Food Res 59(9):1690–1700
Ji FT et al (2013) Curcumin exerts antinociceptive effects by inhibiting the activation of astrocytes in spinal dorsal horn and the intracellular extracellular signal-regulated kinase signaling pathway in rat model of chronic constriction injury. Chin Med J (Engl) 126(6):1125–1131
Ralay Ranaivo H et al (2006) Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci 26(2):662–670
Vela JM et al (1995) Morphology and distribution of microglial cells in the young and adult mouse cerebellum. J Comp Neurol 361(4):602–616
Yamada J, Jinno S (2013) Novel objective classification of reactive microglia following hypoglossal axotomy using hierarchical cluster analysis. J Comp Neurol 521(5):1184–1201
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17(3):157–172
Sparkman NL, Johnson RW (2008) Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 15(4–6):323–330
Perry VH, Matyszak MK, Fearn S (1993) Altered antigen expression of microglia in the aged rodent CNS. Glia 7(1):60–67
Sheng JG, Mrak RE, Griffin WS (1998) Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol 95(3):229–234
Streit WJ et al (2004) Dystrophic microglia in the aging human brain. Glia 45(2):208–212
Perry VH, Newman TA, Cunningham C (2003) The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci 4(2):103–112
Streit WJ et al (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118(4):475–485
Choi JH et al (2007) Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci 69(11):1131–1136
Hwang IK et al (2008) Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochem Res 33(7):1309–1315
Schuitemaker A et al (2012) Microglial activation in healthy aging. Neurobiol Aging 33(6):1067–1072
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Bedi SS et al (2018) Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J Neuroinflamm 15(1):84
Morrison H et al (2017) Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep 7(1):13211
Davis EJ, Foster TD, Thomas WE (1994) Cellular forms and functions of brain microglia. Brain Res Bull 34(1):73–78
Ullah F et al (2020) Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model. Sci Rep 10(1):2365
Ullah F et al (2020) Evaluation of phytosomal curcumin as an anti-inflammatory agent for chronic glial activation in the GFAP-IL6 mouse model. Front Neurosci. https://doi.org/10.3389/fnins.2020.00170
Plescher M et al (2018) Plaque-dependent morphological and electrophysiological heterogeneity of microglia in an Alzheimer’s disease mouse model. Glia 66(7):1464–1480
Franco-Bocanegra DK et al (2021) Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci Rep 11(1):15955
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54(10):8071–8089
Kabba JA et al (2018) Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol 38(1):53–71
Bushong EA, Martone ME, Ellisman MH (2004) Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 22(2):73–86
Miller RH, Raff MC (1984) Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4(2):585–592
Henneberger C et al (2010) Long-term potentiation depends on release of d-serine from astrocytes. Nature 463(7278):232–236
Uwechue NM et al (2012) Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J Physiol 590(10):2317–2331
Takano T et al (2006) Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9(2):260–267
Marín-Padilla M (1995) Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol 357(4):554–572
Stone DJ et al (1998) Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. J Neurosci 18(9):3180–3185
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Gorina R et al (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59(2):242–255
Paintlia AS et al (2013) Modulation of Rho-Rock signaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-α-dependent mechanism. Glia 61(9):1500–1517
Villapol S, Byrnes KR, Symes AJ (2014) Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol 5:82
Liu Z et al (2014) Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 62(12):2022–2033
Li H et al (2014) Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci 15:58
Stapf C et al (2002) Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry 73(3):294–298
Mestriner RG et al (2015) Astrocyte morphology after ischemic and hemorrhagic experimental stroke has no influence on the different recovery patterns. Behav Brain Res 278:257–261
Oberheim NA et al (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10):3276–3287
Torres-Platas SG et al (2011) Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 36(13):2650–2658
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9):732–741
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1/A):363–398
Ullah F et al (2017) High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch Toxicol 91(4):1623–1634
Amalraj A et al (2017) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med 7(2):205–233
Farkhondeh T et al (2019) The impact of curcumin and its modified formulations on Alzheimer’s disease. J Cell Physiol 234(10):16953–16965
Panaro MA et al (2020) The emerging role of curcumin in the modulation of TLR-4 signaling pathway: focus on neuroprotective and anti-rheumatic properties. Int J Mol Sci 21(7):2299
Johnson GB, Brunn GJ, Platt JL (2003) Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol 23(1–2):15–44
Kigerl KA et al (2007) Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem 102(1):37–50
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16(1):3–9
Ni H et al (2015) Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med 38(2):199–206
Bharti AC, Donato N, Aggarwal BB (2003) Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol 171(7):3863–3871
Kim HY et al (2003) Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol 171(11):6072–6079
Ray B, Lahiri DK (2009) Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol 9(4):434–444
Kodali M et al (2018) Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immun 69:499–514
Burgos-Morón E, López-Lázaro M et al (2010) The dark side of curcumin. Int J Cancer 126:177
Ghasemi F et al (2019) Effects of curcumin on microglial cells. Neurotox Res 36(1):12–26
Chin D et al (2013) Neuroprotective properties of curcumin in Alzheimer’s disease—merits and limitations. Curr Med Chem 20(32):3955–3985
Jamwal R (2018) Bioavailable curcumin formulations: a review of pharmacokinetic studies in healthy volunteers. J Integr Med 16(6):367–374
Antony B et al (2008) A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci 70(4):445–449
Stohs SJ et al (2020) Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review. Molecules 25(6):1397
Cuomo J et al (2011) Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 74(4):664–669
Gota VS et al (2010) Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 58(4):2095–2099
Jäger R et al (2014) Comparative absorption of curcumin formulations. Nutr J 13:11
Klickovic U et al (2014) Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. Biomed Res Int 2014:458592
Shoba G et al (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Purpura M et al (2018) Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur J Nutr 57(3):929–938
Gopi S et al (2017) Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: an open-label parallel-arm study. Phytother Res 31(12):1883–1891
Kumar D et al (2016) Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following the oral administration of a food-grade formulation with fenugreek dietary fibre: a randomised double-blind crossover study. J Funct Foods 22:578–587
Sasaki H et al (2011) Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 34(5):660–665
Kanai M et al (2012) Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol 69(1):65–70
Madhavi D, Kagan D (2014) Bioavailability of a sustained release formulation of curcumin. Integr Med (Encinitas) 13(3):24–30
Briskey D et al (2019) Increased bioavailability of curcumin using a novel dispersion technology system (LipiSperse®). Eur J Nutr 58(5):2087–2097
Gyengesi E et al (2018) Investigation into the effects of tenilsetam on markers of neuroinflammation in GFAP-IL6 mice. Pharm Res 35(1):22
Campbell IL et al (1993) Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA 90(21):10061–10065
Maiti P, Paladugu L, Dunbar GL (2018) Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci 19(1):7
Acknowledgements
Not applicable.
Funding
No special funding was received for this work.
Author information
Authors and Affiliations
Contributions
FU designed, drafted, and wrote the manuscript. RG wrote sections, edited and reviewed the manuscript and prepared Fig 1. MKS reviewed and edited the manuscript. EG supervised, advised, edited and reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ullah, F., Gamage, R., Sen, M.K. et al. The Effects of Modified Curcumin Preparations on Glial Morphology in Aging and Neuroinflammation. Neurochem Res 47, 813–824 (2022). https://doi.org/10.1007/s11064-021-03499-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03499-4