Abstract
Nanoparticles (20–50 nm) of Na+, CO32−-containing calcium phosphate (Na: 1.49 wt% and C: 1.53 wt%) with apatite-type structure were prepared by precipitation method from aqueous solution. According to FTIR spectroscopy data, the partial substitution of phosphate by carbonate (B-type) realized in the apatite-type structure. Obtained Na+, CO32−-hydroxyapatite (HAP) was used for the preparation of hybrid biocomposites with Alginate (Alg) with weight ratio HAP: Alg = 1:1 or 2:1 and C60 fullerene (C60; from 0.2 to 4 wt%) and their mechanical properties were determined. It was found, that sample with weight ratio HAP: Alg = 2:1 and containing 4.0 wt% of C60 has the highest Young's modulus 429 MPa comparing with other determined samples. The structure modeling of the investigated system showed that the formation of triple complexes Na+, CO32−-HAP–Alg–C60 is stabilized by solvophobic and stacking interactions. The created biocomposites can be used as an effective implant material for bone restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The design of composites containing hydroxyapatite (HAP) with other materials represents one of the most promising strategies in developing effective biomaterials for bone regeneration. This approach is mainly based on the imitation of the composition and structure of human hard tissues, which are a real typical nanocomposites containing the polymer and ceramic components [1,2,3,4,5,6,7,8,9,10,11,12,13].
Ceramic component such as hydroxyapatite demonstrates adequate osteoconductivity and osteoinductivity as well as bright prospects in drug delivery field attributed to its characteristic pore structure, high surface area and chemical stability, but it has poor mechanical performance and uncontrollable biodegradability [2, 5, 10]. Taken into account, that most of the biological apatites contain several foreign ions, mainly carbonate (CO32−) and traces of Na+, Mg2+, HPO42−, F− [14, 15], the synthetic HA are modified by different ions in order to influence on its properties. Thus, carbonate ions in apatite structure play a vital role in the bone and can influence the structure and morphology of the apatite [16, 17]. It should be noted, that carbonated apatite is bioceramic that has been shown to have high osteoconductivity and can be degraded. Thus, the use of carbonated apatite in bone tissue engineering applications is more appropriate than HAP because it is chemically more similar to bone apatite [18] and has a higher rate of resorption.
The natural and synthetic polymers such as collagen [19], alginate (Alg) [19,20,21,22], gelatin [23], poly(lactic acid), poly(glycolic acid) [24], chitosan [25,26,27,28], etc. can be used as components for hybrid biocomposites. Among all known polymers the sodium Alg is often used because of the low cost, biocompatibility [19, 22,23,24] and biodegradability [19, 22]. It can be made porous [22], non-toxic, and has the ability to modify the surface to generate the appropriate flexibility to suit its function. Previously it was shown that Alg has been commonly used for drug delivery application [20, 22] and encapsulation [20, 21].
Thus, the combination of Alg as polymer component with chemically modified hydroxyapatite as a ceramic component allows to obtain hybrid biocomposite with optimum mechanical properties and tissue interaction. At the same time obtained scaffold must be biocompatible and has compatibility in living tissue, not cause foreign body response, and non-being toxic.
On the other hand, recent progress has increased interest in the biomedical application of the carbon nanostructure—C60 fullerene (C60) that demonstrates unique physicochemical properties. Water-soluble pristine C60 is able to penetrate the cell membrane [29,30,31] and be non-toxic in vitro and in vivo systems [32] to exert specific health effects (e.g., antibacterial and antiviral) [33,34,35]. C60 is a spherical-like molecule, which surface consists of 60 carbon atoms, interconnected with single and double chemical bonds. The last is electron deficient, which determines the electron-acceptor properties of the molecule and its ability to attach reagents containing unpaired electrons (free radicals) easily [36,37,38]. Due to this ability, in biological systems C60 acts as a powerful scavenger of reactive oxygen species (ROS), allowing it to exhibit anti-inflammatory properties [39, 40]. C60 is a highly stable compound does not react with oxygen, is resistant to the action of alkalis and acids. Based on foregoing, C60 can be as an effective nanoplatform for the delivery of anticancer drugs [41, 42].
Thereby, the aim of this work focuses on the preparation of hybrid bioactive scaffolds based on apatite-related Na+, CO32−-containing calcium phosphate, Alginate and C60 fullerene for biomedical application. On the first step, the chemically modified calcium phosphate has been prepared and characterized. Then it was used for fabrication of hybrid biocomposites with different amount of Alg and C60. The mechanical properties of the prepared biocomposites were investigated as well as structural modeling of Na+, CO32−-HAP–Alg–C60 system was done.
2 Experimental
2.1 Synthesis of Na + , CO 3 2−-containing apatite nanoparticles
Apatite-related Na+, CO32−-containing calcium phosphate was synthesized using the chemical precipitation method. As initial reagents, an analytical graded Ca(NO3)2·4H2O, Na2CO3 and NaH2PO4 were used. Na2CO3 and NaH2PO4 solutions were added to a solution of Ca(NO3)2·4H2O under stirring. Interaction was held at fixed molar ratios Ca/P = 1.67 and CO32−/PO43− = 1.5 at temperature 25 °C. The reaction mixture was stirred for 2 h and then resulting slurry was filtrated and washed up several times with water. The obtained precipitate was dried overnight at 100 °C. The prepared phosphate was characterized and then used for the preparation of composites.
2.2 Preparation of pristine C 60 aqueous colloid solution and Na + , CO 3 2− -HAP–Alg–C 60 biocomposites
The pristine C60 aqueous colloid solution (C60FAS) was prepared by C60 transfer from toluene to water using continuous ultrasound sonication as described by Ritter et al. [43]. The obtained C60FAS was characterized by 0.15 mg/ml C60 concentration, 99% purity, stability and homogeneity; the average size of nanoparticles was 80 nm [44].
On the first stage, the Na+, CO32−-HAP–C60 composites were prepared by mixing of Na+, CO32−-HAP powder with C60FAS and with further evaporation of water. Then, the obtained Na+, CO32−-HAP–C60 mixture and the necessary amount of sodium Alg powder was well grinding in an agate mortar. Then 10 ml of water was added. The obtained Na+, CO32−-HAP–Alg–C60/H2O mixture was added dropwise into 0.1 M Ca(NO3)2 solution. The obtained microspheres were washed with distilled water, filtered, dried at 80 °C and analyzed.
2.3 Characterization techniques
The phase and chemical composition of prepared calcium phosphate were determined using X-ray powder diffraction (XRD) and elemental analysis methods, respectively. The diffractogram was obtained using a Shimadzu XRD-6000 diffractometer with Cu–Kα radiation. The range of the diffraction angles (2θ) was from 5 to 90° with step 0.02. The elemental composition of calcium phosphate was defined by atomic absorption spectroscopy (Thermo Electron M-Series instrument) and CHN elemental analysis (Elementar-Analysensysteme GmbH, Donaustraβe 7, D-63452, Germany).
Fourier-transform infrared spectroscopy (FTIR) spectra of prepared Na+, CO32−-HAP and Na+, CO32−-HAP-Alg-C60 biocomposites were measured with PerkinElmer Spectrum BX spectrometer as KBr pellets in the range 400–4000 cm−1 at room temperature. The resolution of the spectrometer is 1 cm−1.
The morphology and crystallite size of the prepared Na+, CO32−–HAP particles were investigated using a scanning electron microscope (SEM; FEI Quanta 400 ESEM instrument). The powder was previously coated with the Au/Pd.
For measuring the strength under the uniaxial compression, the powder of Na+, CO32−-HAP and its hybrid composites with different amount of Alg and C60 were prepared in the form of tablets with diameter 5 mm and thickness 1.7–2.0 mm by cold pressing of granulated powders in molds using the hydraulic press (at ~ 100 MPa). Mechanical properties of prepared samples under loading were investigated using the original automated equipment [45].
2.4 Structural modeling
The spatial structure of the elementary cell of apatite was reported in [46] and was taken by us from the American Mineralogist Crystal Structure Database in the form of AMC-file (code 0002297). Four 2 × 2 cells of Na+, CO32−-HAP crystal structure with substitution of 2 calcium atoms with Na+ and 2 phosphorous groups with CO32− was built using the VESTA program (version 3.4.4) [47].
The structure of C60 was taken from the Protein Data Bank32 (PDB codes Ids C60) [48], built using HyperChem 8.0 software and energy minimized using Gaussian09W on DFT (B3LYP) level of theory with 6-31G* basis set.
The structure of sodium Alginate was built using HyperChem 8.0 software (the Sugar Builder module) by alternating hyaluronic (HYL) and mannuronic (MAN) units, linked by α-1,4 glycosidic bonds. The length of the oligosugar was set in a way to cover the perimeter of the interface between HAP and C60 and comprised 17 units: (HYL–MAN)8-HYL. The hydrogen atoms of sugar carboxyls were substituted by sodium atoms.
The structure of the triple complex Na+, CO32−-HAP–Alg–C60 was built using HyperChem 8.0 software and energy minimized using the molecular mechanics method MM + .
3 Results and discussion
3.1 Synthesis and characterization of chemically modified apatite-related calcium phosphate
On the first step, the chemically modified apatite-related calcium phosphate as a ceramic component of composites was prepared using chemical precipitation method from an aqueous solution according to the scheme:
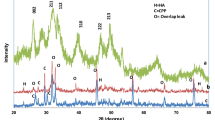
Obtained solid was filtrated, dried at 100 °C and characterized. According to XRD and SEM data, the sample dried at 100 °C is poorly crystalline calcium phosphate with the size of particles in the range of 20–50 nm (Fig. 1a).
The chemical composition of prepared phosphate was determined using both an atomic absorption spectroscopy and CHN elemental analysis. The obtained results are the following: Ca: 26.47wt%, Na: 1.49 wt% and C: 1.53 wt%.
FTIR spectra for obtained powder showed the characteristic peaks of substituted calcium phosphate containing the carbonate anion. The adsorption bands at 879, 1440 and 1490 cm–1 correspond to the vibration of CO32– and indicate the realization of B-type substitution of phosphate-group by carbonate in the apatite-type structure [49, 50]. Modes in the regions of 570–620 cm−1 and 980–1150 cm−1 were assigned to symmetric and asymmetric stretching vibrations (ν4, ν1 and ν3) of phosphate tetrahedron, respectively. The broad peak in the range of (3275–3750) cm–1 appears due to sorbed water and the hydroxyl group stretching in apatite-type structure (Fig. 2, curve 1).
In order to confirm the formation of the apatite-related phase the obtained and dried at 100 °C sample was heated to 600 °C and characterized using the powder XRD method. The formation of apatite-related calcium phosphate that belongs to the hexagonal system was established. The calculated lattice parameters: a = 9.409(6) and c = 6.891(7) Å are close to corresponding values early reported in [49]. The formation of impurities was not detected (Fig. 1b).
Based on the XRD, SEM, FTIR and elemental analysis data the nanoparticles of apatite-type Na+, CO32−–calcium phosphate were prepared.
3.2 Preparation and characterization of Na + , CO 3 2− -HAP–Alg–C 60 hybrid biocomposites
Obtained chemically modified calcium phosphate was used as an inorganic component for the preparation of hybrid biocomposites with Alg and C60. Four samples with different content of Alg and C60 were fabricated (Table 1). Photos of obtained biocomposite microspheres are shown in Fig. 3.
FTIR spectra of obtained Na+, CO32−-HAP–Alg–C60 biocomposites showed the characteristic vibrational bands of phosphate group PO4 in apatite in the ranges 1190–960 cm−1 (ν3) and 610–550 cm−1 (ν4) (Fig. 2, curves 2–5). The main difference of the spectra of initial Na+, CO32−-HAP and obtained composites Na+, CO32−-HAP–Alg–C60 is the presence of mode at 1420 cm−1 that is assigned to the symmetric stretching vibration of the COO− groups of Alg. The peak at 1630 cm−1 corresponding to the asymmetric stretching vibration of carbonyl (C = O) of Alg and intensivity of this band correlate with the amount of Alginate in composites (Fig. 2, curves 2–5). Besides, the evidence of methylene (CH2) at 2850 cm−1 and 2920 cm−1 from the Alginate can be also observed in the spectra, which confirmed the existence of Alginate [51]. According to reported data, the characteristic FTIR vibrational modes for C60 are at 524, 574, 1182 and 1420 cm−1 [52]. In the spectra of prepared Na+, CO32−-HAP–Alg–C60 biocomposites, the peaks of C60 (at 524, 574, 1182 and 1420 cm−1) overlap with vibrational bands of phosphate group PO4 in apatite and vibration of Alginate.
3.3 Compressive strength of prepared samples
Results of the studies of compressive strength (σc) of initial apatite-related Na+, CO32−-containing calcium phosphate and prepared Na+, CO32−-HAP–Alg–C60 biocomposites are represented in Table 2 and Figs. 4, 5.
As it is seen from Fig. 4, only sample N1 (with weight ratio HAP: Alg = 1:1 and 0.2 wt% C60) was destructed at relatively low compressive loading (~ 58 MPa) and maximal relative deformation εdestr is equal to 0.22. Another three samples, namely N2, N3 and N4 (with weight ratio HAP: Alg = 2:1 and different amount of C60 (Table 1)) are flattened, its thickness was decreased on 30–60% (large relative deformation εdestr = 0.36–0.63), while the diameter was increased. After unloading these samples are not restored, i.e. under compression up to loading in 121–241 MPa the large plastic deformations occur, that may lead to ductile failure. Figure 5 presents the “loading–unloading” diagrams σ(ε) of investigated samples during three cycles of uniaxial compression up to 12 MPa. The Youngʼs modulus (E) estimation has shown that maximal value of E = 429 MPa was observed for the sample N4 (with weight ratio HAP: Alg = 2:1 and 4.0 wt% C60), and for other samples, N1, N2 and N3, the value of E lies within (327–394) MPa. This result indicates the influence of C60 addition to Phosphate-Alginate mixture on the mechanical properties of hybrid composites.
Summarizing, prepared biocomposites in system Na+, CO32−-HAP–Alg–C60 can be used as biomaterials with special mechanical properties for bone restoration as well as in delivery systems with controlled drug release [56].
3.4 Structural modeling of Na + , CO 3 2−–HAP–Alg–C 60 system
The possibility of complexation between the components of designed hybrid biocomposite was testified by means of molecular mechanics. The model of 3D structure of the triple Na+, CO32−-HAP–Alg–C60 complex is shown on Fig. 6. Such arrangement of molecules is mainly stabilized by solvophobic/van der Waals interactions between the C60 and Na+, CO32−-HAP surfaces, and is additionally stabilized by the hydrogen bonds between the OH-groups of Alg and oxygen atoms of Na+, CO32−-HAP (≈2 bonds).
Within the framework of the MM + method, we calculated the energies of intra- and intermolecular interactions in the triple complex, viz.
Etotal = 3142.54 kcal/mol, E\(_{\text{C}_{60}}\) = 224.63 kcal/mol, E\(_{\text{Na}^{+},{\text{CO}}_{3}^{2-}}\)-HAP= 1259.86 kcal/mol, EAlg = 1672.92 kcal/mol.
It allowed to estimate the contribution of the intermolecular interactions into the total energy of complex formation: ΔE = Etotal − E\(_{\text{C}_{60}}\) − E\(_{\text{Na}^{+},{\text{CO}}_{3}^{2-}}\)-HAP − EAlg ≈ −14.88 kcal/mol. This value should be increased by the magnitude of complex stabilization from solvophobic interactions. The latter could be estimated from solvent accessible surface areas, A, of each molecule in separate and in complex, viz. Atotal = 5373.72 Å2, A\(_{\text{C}_{60}}\) = 524.82 Å2, A\(_{\text{Na}^{+},{\text{CO}}_{3}^{2-}}\)2−-HAP = 2597.03 Å2, AAlg = 3775.57 Å2. Taking the microscopic surface tension, γ = 0.05 kcal/mol·Å2, for water [57], one can finally estimate the solvophobic contribution as ΔGhyd = γ(Atotal − A\(_{\text{C}_{60}}\) − A\(_{\text{Na}^{+},{\text{CO}}_{3}^{2-}}\)-HAP − AAlg) ≈ −76.19 kcal/mol. It is seen that the energy of solvophobic interactions in absolute value is more than the absolute value of total stabilization energy. It means that some contributions can be positive and destabilize complex, whereas contributions from van der Waals stacking energy and H-bonds are small in absolute value.
In summary, it may be concluded that the physical adsorption of C60 into Na+, CO32−-HAP–Alg system seems possible by means of forming triple complexes stabilized by solvophobic and stacking interactions, whereas H-bonds give relatively small contribution into stabilization.
4 Conclusion
According to the powder XRD, SEM, FTIR and elemental analysis data the nanoparticles (20–50 nm) of apatite-related Na+, CO32−-containing calcium phosphate were prepared. Then, obtained inorganic component was used for the design of hybrid biocomposites Na+, CO32−-HAP–Alg–C60 with different amounts of Alg and C60. Studies of compressive strength of prepared Na+, CO32−-HAP–Alg–C60 biocomposites showed that sample containing 4.0 wt% of C60 (with weight ratio HAP: Alg = 2:1) has the highest Young's modulus 429 MPa. This result may be indicated that the addition of C60 into apatite-Alg composite influence on its mechanical properties. Results of structural modeling of Na+, CO32−-HAP–Alg–C60 system showed that the physical adsorption of C60 into Na+, CO32−-HAP–Alg composite seems possible by means of forming triple complexes stabilized by solvophobic and stacking interactions.
Hereby, the subject and results of the presented investigation have by both methodological meaning as the general approach to study interaction inorganic particles with organic substance and can be used for practical fabrication of effective materials in system Na+, CO32−-HAP–Alg–C60 with special mechanical properties for biomedical applications.
References
R. Zhang, P.X. Ma, J Biomed Mater Res 44, 446 (1999)
S.J. Peter, L. Lu, D.J. Kim, A.G. Mikos, Biomaterials 21, 1207 (2000)
G. Wei, P.X. Ma, Biomaterials 25, 4749 (2004)
S.S. Kim, M.S. Park, S.J. Gwak, C.Y. Choi, B.S. Kim, Tissue Eng 12, 2997 (2006)
S.S. Kim, M.S. Park, O. Jeon, C.Y. Choi, B.S. Kim, Biomaterials 27, 1399 (2006)
K.M. Woo, J. Seo, R. Zhang, P.X. Ma, Biomaterials 28, 2622 (2007)
X.L. Deng, G. Sui, M.L. Zhao, G.Q. Chen, X.P. Yang, J Biomater Sci 8, 117 (2007)
S.W. Kang, H.S. Yang, S.W. Seo, D.K. Han, B.S. Kim, J Biomed Mater Res A 85, 747 (2008)
J. Li, X. Yuan, F. He, A.F. Mak, J Biomed Mater Res 86B, 381 (2008)
K.H. Włodarski, P.K. Włodarski, R. Galus, Ortop Traumatol Rehabil 10(3), 201 (2008)
P. Zhang, Z. Hong, T. Yu, X. Chen, X. Jing, Biomaterials 30, 58 (2009)
J. Venkatesan, I. Bhatnagar, P. Manivasagan, K.-H. Kang, S.-K. Kim, J Biolog Macromol 72, 269 (2015)
K. Jahan, M. Tabrizian, Biomater Sci 4(1), 25 (2016)
R.Z. LeGeros, Nature 206, 403 (1965)
A. Bigi, G. Cojazzi, S. Panzavolta, A. Ripamonti, N. Roveri, M. Romello, J Inorg Biochem 68, 45 (1997)
G. Xu, I.A. Aksay, J.T. Groves, J Am Chem Soc 123, 2196 (2001)
A. Krajewski, M. Mazzocchi, P.L. Buldini, A. Ravaglioli, A. Tinti, P. Taddei, C. Fagnano, J Mol Struct 744–747, 221 (2005)
I.D. Ana, S. Matsuya, K. Ishikawa, Engineering 2, 344 (2010)
G.H. Kim, S.H. Ahn, Y.Y. Kim, Y.S. Cho, W. Chun, J Mater Chem 21, 6165 (2011)
C.K. Kuo, P.X. Ma, Biomaterials 22, 511 (2001)
N. Mohan, P.D. Nair, Trends Biomater Artif Org 18(2), 219 (2005)
J.-W. Lu, Y.-L. Zhu, Z.-X. Guo, P. Hu, J. Yu, Polymer 47, 8026 (2006)
H.-W. Kim, B.-H. Yoon, H.-E. Kim, J Mater Sci 16, 1105 (2005)
T.-J. Lee, S.-W. Kang, S.H. Bhang, J.M. Kang, B.-S. Kim, J Biomater Sci 21, 635 (2010)
K. Tuzlakoglu, R.L. Reis, J Mater Sci Mater Med 18, 1279 (2007)
C.I. Dias, J.F. Mano, N.M. Alves, J Mater Chem 18, 2493 (2008)
A.R. Costa-Pinto, V.M. Correlo, P.C. Sol, M. Bhattacharya, P. Charbord, B. Delorme, R.L. Reis, N.M. Neves, Biomacromol 10, 2067 (2009)
B. Li, Y. Wang, D. Jia, Y. Zhou, W. Cai, Biomed Mater 4, 015011 (2009)
S. Foley, C. Crowley, M. Smaihi, C. Bonfils, B.F. Erlanger, P. Seta, C. Larroque, Biochem Biophys Res Commun 294, 116 (2002)
C. Schuetze, U. Ritter, P. Scharff, A. Bychko, S. Prylutska, V. Rybalchenko, Yu. Prylutskyy, Mater Sci Eng C 31, 1148 (2011)
Yu. Prylutskyy, A. Bychko, V. Sokolova, S. Prylutska, M. Evstigneev, V. Rybalchenko, M. Epple, P. Scharff, Mater Sci Eng C 59, 398 (2016)
S.V. Prylutska, A.G. Grebinyk, O.V. Lynchak, I.V. Byelinska, V.V. Cherepanov, E. Tauscher, O.P. Matyshevska, Yu.I. Prylutskyy, V.K. Rybalchenko, U. Ritter, M. Frohme, Fuller Nanotubes Carbon Nanostruct 27, 715 (2019)
C. Kepley, J Nanomed Nanotechnol 3, 6 (2012)
S. Goodarzi, T. Da Ros, J. Conde, F. Sefat, M. Mozafari, Mater Today 20(8), 460 (2017)
F. Moussa, Nanobiomaterials 25, 113 (2018)
N. Gharbi, M. Pressac, M. Hadchouel, H. Szwarc, S.R. Wilson, F. Moussa, Nano Lett 5(12), 2578 (2005)
C.A. Ferreira, D. Ni, Z.T. Rosenkrans, W. Cai, Nano Res 11(10), 4955 (2018)
S.V. Eswaran, Curr Sci 114(9), 1846 (2018)
V. Dragojevic-Simic, V. Jacevic, S. Dobric, A. Djordjevic, D. Bokonjic, M. Bajcetic, R. Injac, Dig J Nanomater Biostruct 6, 819 (2011)
O.O. Gonchar, A.V. Maznychenko, N.V. Bulgakova, I.V. Vereshchaka, T. Tomiak, U. Ritter, Yu.I. Prylutskyy, I.M. Mankovska, A.I. Kostyukov, Oxidative Med Cell Longev 2018, 17 (2018)
Yu.I. Prylutskyy, M.P. Evstigneev, V.V. Cherepanov, O.A. Kyzyma, L.A. Bulavin, N.A. Davidenko, P. Scharff, J Nanopart Res 17, 45 (2015)
S. Prylutska, R. Panchuk, G. Gołuński, L. Skivka, Yu. Prylutskyy, V. Hurmach, N. Skorokhyd, A. Borowik, A. Woziwodzka, J. Piosik, O. Kyzyma, V. Garamus, L. Bulavin, M. Evstigneev, A. Buchelnikov, R. Stoika, W. Berger, U. Ritter, P. Scharff, Nano Res 10(2), 652 (2017)
U. Ritter, Y.I. Prylutskyy, M.P. Evstigneev, N.A. Davidenko, V.V. Cherepanov, A.I. Senenko, O.A. Marchenko, A.G. Naumovets, Fuller Nanotub Carbon Nanostruct 23, 530 (2015)
Yu.I. Prylutskyy, V.V. Cherepanov, M.P. Evstigneev, O.A. Kyzyma, V.I. Petrenko, V.I. Styopkin, L.A. Bulavin, N.A. Davidenko, D. Wyrzykowski, A. Woziwodzka, J. Piosik, R. Kaźmierkiewicz, U. Ritter, Phys Chem Chem Phys 17, 26084 (2015)
L. Vovchenko, O. Lazarenko, L. Matzui, Yu. Perets, A. Zhuravkov, V. Fedorets, F. Le Normand, Phys Status Solid A 211, 336 (2014)
R.M. Wilson, J.C. Elliot, S.E.P. Dowker, Am Miner 84, 1406 (1999)
K. Momma, F. Izumi, J Appl Crystallogr 44, 1272 (2011)
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne, Nucleic Acids Res 28, 235 (2000)
N. Strutynska, I. Zatovsky, N. Slobodyanik, A. Malyshenko, Y. Prylutskyy, O. Prymak, I. Vorona, S. Ishchenko, N. Baran, A. Byeda, A. Mischanchuk, Eur J Inorg Chem 2015, 622 (2015)
N. Strutynska, N. Slobodyanik, A. Malyshenko, I. Zatovsky, I. Vorona, Y. Prylutskyy, O. Prymak, N. Baran, S. Ishchenko, V. Nosenko, Solid State Phenom 230, 133 (2015)
L.B. Sukhodub, L.F. Sukhodub, O.O. Litsis, Yu.I. Prylutskyy, Mater Chem Phys 217, 228 (2018)
Yu.I. Prylutskyy, V.I. Petrenko, O.I. Ivankov, O.A. Kyzyma, L.A. Bulavin, O.O. Litsis, M.P. Evstigneev, V.V. Cherepanov, A.G. Naumovets, U. Ritter, Langmuir 14, 3967 (2014)
R.Z. LeGeros, J.P. LeGeros, in An introduction to bioceramics. ed. by L.L. Hench, J. Wilson (Word Scientific, London, 1999), p. 139
N. Kanasan, S. Adzila, N.A. Mustaffa, P. Gurubaran, Proc Eng 184, 442 (2017)
W.H. Tham, M.U. Wahit, M. Rafiq, A. Kadir, T.W. Wong, Songklanakarin. J Sci Technol 35(1), 57–61 (2013)
L.B. Sukhodub, L.F. Sukhodub, M.O. Kumeda, S.V. Prylutska, V. Deineka, Yu.I. Prylutskyy, U. Ritter, Carbohydr Polym 223, 115067 (2019)
V.V. Kostjukov, N.M. Khomytova, A.A.H. Santiago, A.M.C. Tavera, J.S. Alvarado, M.P. Evstigneev, J Chem Thermodyn 43(10), 1424 (2011)
Acknowledgements
This work was partially supported by the DFG (N RI 966/18-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strutynska, N., Malyshenko, A., Tverdokhleb, N. et al. Design, characterization and mechanical properties of new Na+, CO32−-apatite/alginate/C60 fullerene hybrid biocomposites. J. Korean Ceram. Soc. 58, 422–429 (2021). https://doi.org/10.1007/s43207-020-00107-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-020-00107-z