Abstract
Specific features of structural self-organization of C60 fullerene (1 nm size range), antitumor antibiotic doxorubicin (Dox) and their complex in physiological solution (0.9 % NaCl) have been investigated by means of atomic-force microscopy, dynamic light scattering, and small-angle X-ray scattering. Significant ordering of the mixed system, C60 + Dox, was observed, suggesting the complexation between these drugs, and giving insight into the mechanism of enhancement of Dox antitumor effect on simultaneous administration with C60 fullerene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A big challenge in the development of modern biotechnology is a targeted use of biocompatible low-toxic nanomaterials for treatment of various diseases including cancer. In particular, of paramount importance now are such elements of medical treatment as early detection, determination of localization of malignant tumors, targeted delivering of drugs to tumor, development of methods of selective therapy etc.
Within the group of currently known antitumor agents the C60 fullerene attracts special interest of researches (Cataldo and Da Ros 2008; Montellano et al. 2011; Prylutska et al. 2011) due to the relative simplicity of synthesis, high stability, wide range of available possibilities of chemical modification, and high reduction ability (C60 molecule may accept up to six electrons). However the bioavailability of C60 fullerene is limited by its intrinsic hydrophobicity leading to poor solubility in polar solvents (Hirsch et al. 2005). The preparation of highly stable pristine C60 fullerene aqueous solutions (C60FAS) has been reported by different authors (Deguchi et al. 2001; Andrievsky et al. 2002; Fortner et al. 2005; Prylutskyy et al. 2014a), which has made a breakthrough in further utilization of C60FAS in biomedical applications. Theoretical computations as well as microscopy and spectroscopy data (Deguchi et al. 2001; Amer et al. 2005; Avdeev et al. 2004; Brant et al. 2005; Prylutskyy et al. 2013, 2014b) have shown that the C60FAS contains both single hydrated C60 molecules and sphere-like hydrated clusters (aggregates) with diameters up to 200 nm. Recently, a surface hydroxylation of the C60 fullerene nanoparticles was reported by means of FTIR spectroscopy (Prylutskyy et al. 2014a; Labille et al. 2009) which is considered to be the main reason for C60 fullerene stabilization in aqueous solution.
Anthracycline antibiotic doxorubicin (Dox, Fig. 1) is one of the most extensively used drug in chemotherapy of cancer.
However, the most significant drawback in practical utilization of this antibiotic is its high cardiotoxicity and relatively low selectivity of biological action which significantly reduces its overall medical effect (Carvalho et al. 2014). Recently, extended physico-chemical investigation (Evstigneev et al. 2013, 2014) showed that Dox molecules may bind with C60 molecule suggesting that such complexation may affect the biological function of the antibiotic. Indeed, both in vivo (Panchuk et al. 2015; Prylutska et al. 2014) and in vitro (Panchuk et al. 2015; Skamrova et al. 2014) studies have proved this and have shown that immobilization of Dox on C60 fullerene reduces side toxic effects of this drug with respect to normal cells and increases its accumulation in target tumor cells. The proposed biological effect of C60 + Dox complexation is very similar to the long-known effect of Dox–xanthine complexation resulting in alteration of cytotoxic and/or mutagenic consequences of the antibiotic action in vitro (Traganos et al. 1991; Piosik et al. 2002; Evstigneev 2014). It thus follows that C60 fullerene–drug complexation may act as a key step in the process of their synergistic interaction in biological media and the link between C60 fullerene–drug complexation and the observed enhancement of antitumor effect of the drug creates a new perspective in combinational anticancer therapy.
It is important to note that previous physico-chemical investigations of the complexation between C60 fullerene and Dox cited above have been accomplished in non-salted aqueous environment, thus making ambiguous the projection of the proposed mechanism of complexation onto biological system. Further utilization of C60 fullerene in medical nanotechnologies, in particular, evaluation of the in vivo antitumor activity in combination with Dox, requires reliable data on structural state of C60 fullerene, Dox, and their complex in solution close by its properties to physiological solution as a common media suitable for direct administration of these drugs in vivo. Such information is also needed for getting insight into the mechanism of specific biological action of these drugs, viz. membranotropic, immunomodulating, radioprotector etc. So, the aim of the present work was the investigation of structural self-organization of C60 fullerene, Dox, and their complex in physiological solution (0.9 % NaCl) as effective therapeutic agents against cancer.
Experimental
C60FAS in concentration 0.15 mg/ml was prepared according to the method described in (Prylutskyy et al. 2014a). Homogeneity of the media containing C60 fullerene dissolved in physiological solution was achieved by mixing C60FAS and physiological solution in equal volumes (1:1) with further treatment of that mixture in ultrasonic bath (BK-9050, Germany; power 50 W, frequency 40 kHz, mixing time 3 h).
In all experiments the Dox solution (‘Doxorubicin-TEVA’, Pharmachemie B.V., lyophilized powder, 10 mg) was prepared by means of dissolving the powder in physiological solution at initial concentration of 0.15 mg/ml.
Immobilization of Dox on C60 fullerene was made as follows. Initial stocks of C60FAS and Dox were dissolved in physiological solution and then mixed together under volume ratio 1:2, which was considered to be the most optimal for loading of C60 fullerene clusters with Dox (Evstigneev et al. 2013). The resulting mixture was treated in ultrasonic dispergator during 30 min with further mixing within 12 h in magnetic stirrer at room temperature.
Structural state of C60 fullerene, Dox, and their complex was monitored with an aid of atomic-force microscopy (AFM). The droplets of physiological solution were deposited onto a cleaved mica substrate (V-1 Grade, SPI Supplies) and the measurements were carried out in dry layers after complete evaporation of the solvent. The sample visualization was carried out in a semi-contact (tapping) mode using NSG10 AFM probes in the ‘Solver Pro M’ AFM microscope (NT-MDT, Russia).
The size distribution of the particles in physiological solution was estimated by dynamic light scattering (DLS) and small-angle X-ray scattering (SAXS) methods. DLS instrument (Zetasizer Nano ZS90, Malvern, Worcestershire, UK), equipped with a He–Ne laser (max. 5 mW) operating at the wavelength of 633 nm, was used. SAXS experiments were carried out on a Rigaku X-ray instrument with a high-speed Cu rotating anode SMAXS-3000 Point SAXS system (Moscow Institute of Physics and Technology, Dolgoprudniy, Russia) using a standard transmission configuration. An X-ray wavelength of λ = 1.54 Å was used, resulting in a momentum transfer, Q in the range of 0.007–1 Å−1, where \(Q = \left( {4\pi /\lambda } \right)\sin \left( {\theta /2} \right)\) and θ is the scattering angle. The samples studied were placed in borosilicon capillaries having 1.5 mm diameter and 0.01 mm wall thickness (W. Muller, Berlin, Germany).
Results and discussion
Preliminary investigation of the possibility of complexation between Dox and C60 fullerene in physiological solution was carried out by means of UV–Vis spectroscopy. The absorption spectra of native Dox and C60 fullerene with Dox mixture were measured in the range of wavelengths λ = 400–600 nm at room temperature. The pronounced hypochromic effect observed as a result of such mixing (data not shown) indicates the formation of a stable complex between Dox and C60 fullerene similar to that reported before in non-salted aqueous solution (Evstigneev et al. 2013, 2014). This result enabled further detailed investigation of the C60 + Dox solution using probe microscopy and light scattering techniques.
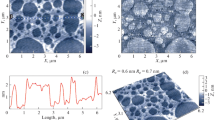
The process of water evaporation from the droplet of physiological solution containing C60 fullerenes without Dox was monitored by means of optical microscopy. The formation of salt crystals spread over the substrate initially covered by the solution was observed on the mica surface. AFM investigation was performed on the smooth regions of the surface not containing the salt crystals. The islands with characteristic lateral dimension ~1 μm were observed in these regions (Fig. 2).
The height of all islands is similar and amounts to 0.8 ± 0.2 nm which well agrees with the diameter of single C60 fullerene. It means that the C60 molecules are grouped on the mica surface into densely packed monolayer clusters. Besides the C60 fullerene islands, the acicular nano-objects having ~10 nm height and lateral length up to ~1 μm were also seen which may be assigned to NaCl crystals, formed after evaporation of water. It is important to note that in previous AFM investigations of the C60FAS structure (Prylutskyy et al. 2013, 2014a), the formation of the monolayer islands was not observed. Hence, it may be concluded that the formation of the densely packed C60 fullerene islands is due to the presence of Na and Cl ions in the studied solution.
A set of papers is currently available in literature dealing with the investigation of C60 fullerene aggregation in electrolytes. In particular, in Deguchi et al. (2001) the shift of the C60 fullerene absorption peak toward red range of UV–Vis spectrum on the addition of sodium chloride was reported. This process was accompanied by the formation of yellow sediment which indicated coagulation of the C60 molecules. Along with that it has long been known that the C60 fullerene clusters are negatively charged in aqueous solution (the typical values of ζ-potential are −20 to −50 mV) (Brant et al. 2005). Thus the electrostatic repulsion between C60 molecules is thought to be one of the reasons responsible for stabilization of the disperse system as a whole. In contrast, diluted solutions of electrolytes (<0.001 M) induce destabilization of the C60 fullerene suspensions (Deguchi et al. 2001). The observed gathering of C60 molecules into islands in the presence of the ions is obviously a consequence of the decreasing of electrostatic repulsion due to shielding effect, also confirmed by the increase in the negative value of ζ-potential on lowering the ionic strength of C60FAS (Chen et al. 2009). As a result the attractive non-electrostatic forces (van der Waals and hydrophobic) facilitate the formation of the densely packed monolayer islands of C60 molecules during the process of solvent evaporation.
The obtained results also suggest that C60 molecules influence the growth of the crystals of salt. In particular, in certain regions of the surface the NaCl crystals, looking like the three-arm ‘stars’, are seen (Fig. 2). The height of such crystals equals approximately to 8–30 nm and the arm lengths reach ~1 μm. This observation points out the changing of the surface free energy of the NaCl crystals in solution under the influence of the closely located C60 molecules. This is confirmed by the fact that the NaCl nanocrystals are either attached to the C60 fullerene islands or fully surrounded by the C60 molecules. It may be assumed that the negatively charged C60 molecules or their clusters in solution are bound or surrounded by Na+ ions. Reassociation of the Na+ and Cl− ions on water evaporation is accompanied by liberation of the C60 fullerenes which can then form islands located in close proximity to the salt crystals.
On water evaporation from the Dox solution and from the mixture of Dox with C60 fullerene containing NaCl, a non-homogeneous distribution of the precipitated material on the mica surface was observed, which is similar to that in the case of investigation of the physiological solution containing C60 molecules. NaCl crystals are localized in the ‘salt’ spot which was clearly seen in optical microscope. The area of the spot occupies ~50 % of the whole surface area initially covered by the solution. It was established that the main fraction of NaCl and the studied compounds, i.e., Dox and C60 fullerene, is localized within the ‘salt’ spot (the region of high concentration).
In the region of high concentration, Dox molecules form ordered long-chain branched nanostructures having the height of 6–20 nm (Fig. 3, region I). In the vicinity of Dox, one can notice the nanocrystals of salt from physiological solution which are seen on the picture as white points (Fig. 3, region II). The height of these nanocrystals is approximately ~35 nm.
Investigations of the layer of the C60 fullerene with Dox mixture showed that in the range of high concentration, its structure is similar to that of the Dox layer (see Figs. 3, 4). It thus may be concluded that the principal influence on the process of formation of the C60 fullerene–Dox-containing system is due to the Dox molecules or salt ions which are always present in all studied solutions.
In the region of the surface away of the ‘salt’ spot (the range of low concentration) the structure of the layer of C60 fullerene–Dox-containing system is seen as an island-like structure (Fig. 5). It qualitatively differs from the Dox layer structure (Fig. 3), as well as quantitatively differs from the C60 fullerene structure (Fig. 2), which were precipitated from physiological solution in separate. The height of the observed islands is more than 1 nm, indicating the presence of molecular complexes of C60 fullerene with Dox in their structure, which well agrees with the available predictions (Evstigneev et al. 2013, 2014; Skamrova et al. 2014).
Once the formation of molecular complexes has been visualized by AFM under condition of solvent evaporation, further confirmation of complex formation directly in physiological solution may be obtained from light scattering techniques.
A typical result of DLS experiment shown in Fig. 6 gives the distribution of the number of light scattering particles according to their mean hydrodynamic diameters in the studied systems. The main fraction of light scattering particles had diameters in the range of 132–33 nm for the mixture of C60 fullerene with Dox and for C60 fullerene without Dox, respectively, in physiological solution. It is important to note that in the previous DLS investigations of the C60FAS structure in non-salted solution the particles having diameters around 100 nm were reported (Prylutskyy et al. 2013, 2014a). Hence the observed decrease of average dimensions of fullerene clusters may be due to the presence of Na and Cl ions. In contrast, the addition of Dox to C60FAS significantly increases the average cluster size (~132 nm) which evidences the C60-Dox complexation. Importantly, this result was obtained in physiological solution and fully agrees with the same conclusion reported before in non-salted media (Evstigneev et al. 2013, 2014). The complexation most likely occurs via π-stacking of the aromatic moieties of fullerene and Dox molecules (Evstigneev et al. 2013) in a manner similar to well-investigated C60 fullerene binding with calixarenes, porphyrins, and other aromatic molecules (Balch et al. 1999; Boyd and Reed 2005).
The AFM and DLS results described above have additionally been tested by means of SAXS technique. The SAXS data (Fig. 7) clearly indicate the existence of particles with a characteristic size <100 nm in the studied systems. A power law decreasing of the X-ray scattering intensity with a power exponent −4 was revealed. It suggests the spherical shape of the nanoparticles (aggregates). From the SAXS curves one can estimate the gyration radius of the particles in C60 fullerene with Dox in physiological solution and C60 fullerene in physiological solution, which appeared to be similar in both systems, viz. R g ≈ 14 nm. The mean diameter of the spherical nanoparticles can be calculated from the gyration radius and its value was found to be about 36 nm. This value is lower than that obtained from DLS which is quite expected because of different sensitivity of these methods to different sizes (i.e., SAXS is more sensitive to smaller sizes whereas DLS better ‘sees’ large aggregates).
Taken together, the results of probe microscopy and light scattering presented above evidence the complexation of C60 fullerene with Dox in physiological solution. It supports the previously published hypothesis (Evstigneev et al. 2013, 2014) based on the experiment carried out in non-salted aqueous media which states that Dox molecule may induce additional C60 fullerene aggregation in physiological stock solution prepared for administration in biosystem. When such mixture is injected into biosystem the C60 fullerene clusters incorporate antibiotic molecules and thereby act as nano-containers protecting the antibiotic molecule from binding to plasma proteins and scavenging molecules in biological fluid, and deliver Dox to target cells. Such mechanism resembles the well-described binding of C60 fullerene to micelles (Dallavalle et al. 2014) and may explain the observed in vivo and in vitro biological synergism (see “Introduction” section) when the Dox and C60 molecules are administered together. On the other hand, it is worth noting that C60 fullerene itself exerts cytotoxicity (Aschberger et al. 2010), membranotropic action (Prylutska et al. 2012), and possesses antioxidant properties (Gharbi et al. 2005; Prylutska et al. 2008) which may also be responsible, in part, for the biological interaction of fullerene and Dox. However, at least two indirect evidences support the hypothesis of complexation as the predominant mechanism:
-
(i)
The biological synergism was shown to be the most pronounced specifically when Dox and C60FAS enter the biosystem as a mixture, and is less pronounced when they are administered sequentially (Panchuk et al. 2015),
-
(ii)
The biological synergism of various aromatic molecules (including Dox), originating from their direct non-covalent interaction in biological fluid, has long been known and termed ‘the interceptor action’ (Traganos et al. 1991; Piosik et al. 2002; Woziwodzka et al. 2013; Evstigneev 2014). The interceptor action suggests that the observed features of drugs’ biological interaction should be more dependent on physico-chemical parameters of their interaction (i.e., affinity constants and concentrations) rather than on the chemical properties of each of the interacting molecules (e.g., the ability to generate/absorb reactive oxygen species). In fact this statement has recently received preliminary confirmation due to the existence of correlation of the observed change in biological effect in vitro with the parameters of intermolecular interaction for various aromatic drug molecules (including Dox) in the presence of three potential interceptor molecules, viz. caffeine, chlorophylline, and C60 fullerene, (Skamrova et al. 2014; Buchelnikov and Evstigneev 2014).
Although understanding of the mechanism of biological interaction of Dox and C60 fullerene requires further investigation, results of the present work, complemented by literature data, point out that this mechanism may be due to their complexation in biological fluid. If so, the complexation of fullerene with other important aromatic antitumor drugs may be expected, e.g., with mitoxantrone, topotecan, actinomycin D etc., and, hence, similar biological interaction may be anticipated. Investigation of this issue is currently under way in our laboratory.
Conclusions
Based on literature data, the complexation of C60 fullerene with antitumor antibiotic Dox is currently considered to be the key step in the mechanism of antitumor effect being developed during simultaneous administration of C60 and Dox in vivo. Previous physico-chemical investigations of C60–Dox complexation have been performed in non-physiological environment, thus making it difficult to project the obtained results onto biological system.
In the present work the structural self-organization of C60 fullerenes, Dox molecules, and their complex has been investigated using probe microscopy and light scattering techniques in physiological solution (0.9 % NaCl). It was found that C60 molecules form densely packed monolayer clusters. Such structure was found to differ considerably from the structure of C60 fullerene layers, precipitated from water solution. The observed difference originates from shielding of the C60 fullerene negative charge by the salt ions. It was also established that within the ‘salt’ spot (the range of high concentration) the structure of the layer of the C60 fullerene–Dox-containing system is similar to the structure of the Dox layer, which consists of ordered long-chain branched nano-objects with inclusions from salt nanocrystals. However, in the region away from the ‘salt’ spot (the range of low concentration) the structure of the layer of the C60 fullerene–Dox-containing system demonstrates an island-like type, which is qualitatively different from the Dox layer structure and is quantitatively different from the structure of C60 fullerene precipitated from physiological solution. These results point out on complex formation between C60 fullerene and Dox molecules occurring when these compounds present together in physiological solution. SAXS data clearly demonstrate the existence of particles with a characteristic size <100 nm in the studied systems. Along with that the main fraction of nanoparticles has diameters in the range of 36 nm that well agrees with DLS data for C60 fullerene in physiological solution.
In general, the obtained data have confirmed the existence of well-pronounced complexation between the fullerene and Dox in solution close by its properties to physiological one, which allows further discussion of the effect of complexation in projection onto biological system. In this context the most important feature of the obtained data is the increase of the average C60 fullerene cluster size on addition of Dox in physiological solution, suggesting that these clusters incorporate antibiotic molecules and, presumably, act as nano-containers protecting the antibiotic molecule from binding to plasma proteins and scavenging molecules in biological fluid. This result is in agreement with literature data on C60 fullerene complexation with Dox performed in non-salted media, and supports the previously suggested mechanism of C60–Dox complexation as the key step in biological synergistic interaction of fullerene and Dox on their simultaneous administration in biosystem. It may therefore act as a starting hypothesis in any evaluation of the in vivo and in vitro biological synergism when aromatic drugs and C60 molecules are administered together.
References
Amer MS, Elliott JA, Maguire JF, Windle AH (2005) Calculations of the Raman spectra of C60 interacting with water molecules. Chem Phys Lett 411:395–398
Andrievsky GV, Klochkov VK, Bordyuh AB, Dovbeshko GI (2002) Comparative analysis of two aqueous-colloidal solutions of C60 fullerene with help of FTIR reflectance and UV-Vis spectroscopy. Chem Phys Lett 364:8–17
Aschberger K, Johnston HJ, Stone V, Aitken RJ, Tran CL, Hankin SM et al (2010) Review of fullerene toxicity and exposure-appraisal of a human health risk assessment, based on open literature. Regul Toxicol Pharmacol 58:455–473
Avdeev MV, Khokhryakov AA, Tropin TV, Andrievsky GV, Klochkov VK, Derevyanchenko LI et al (2004) Structural features of molecular-colloidal solutions of C60 fullerenes in water by small-angle neutron scattering. Langmuir 20:4363–4368
Balch AL, Olmstead MM (1999) Structural chemistry of supramolecular assemblies that place flat molecular surfaces around the curved exteriors of fullerenes. Coord Chem Rev 185–186:601–617
Boyd PDW, Reed CA (2005) Fullerene-porphyrine constructs. Acc Chem Res 38:235–242
Brant J, Lecoanet H, Wiesner MR (2005) Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanopart Res 7:545–553
Buchelnikov AS, Evstigneev MP (2014) Quantitative correlation of the in vitro biological effect with parameters of molecular complexation in mutagen-interceptor systems. J Theor Biol 357:268–271
Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ (2014) Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 34:106–135
Cataldo F, Da Ros T (eds) (2008) Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Carbon materials: chemistry and physics, vol 1. Springer, Dordrecht
Chen KL, Elimelech M (2009) Relating colloidal stability of fullerene (C60) nanoparticles to nanoparticle charge and electrokinetic properties. Environ Sci Technol 43:7270–7276
Dallavalle M, Leonzio M, Calvaresi M, Zerbetto F (2014) Explaining fullerene dispersion by using micellar solutions. ChemPhysChem 15:2998–3005
Deguchi S, Alargova RG, Tsujii K (2001) Stable dispersions of fullerenes, C60 and C70, in water. Preparation and characterization. Langmuir 17:6013–6017
Evstigneev MP (2014) Hetero-association of aromatic molecules in aqueous solution. Int Rev Phys Chem 33:229–273
Evstigneev MP, Buchelnikov AS, Voronin DP, Rubin YuV, Belous LF, Prylutskyy YuI et al (2013) Complexation of C60 fullerene with aromatic drugs. ChemPhysChem 14:568–578
Fortner JD, Lyon DY, Sayes CM, Boyd AM, Falkner JC, Hotze EM et al (2005) C60 in water: nanocrystal formation and microbial response. Environ Sci Technol 39:4307–4316
Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F (2005) [C60] fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 5:2578–2585
Hirsch A, Brettreich M, Wudl F (2005) Fullerenes: chemistry and reactions. Wiley, New York
Labille J, Masion A, Ziarelly F, Rose J, Brant J, Villieras F et al (2009) Hydration and dispersion of C60 in aqueous systems: the nature of water-fullerene interactions. Langmuir 25:11232–11235
Montellano A, Da Ros T, Bianco A, Prato M (2011) Fullerene C60 as multifunctional system for drug and gene delivery. Nanoscale 3:4035–4041
Panchuk RR, Prylutska SV, Chumak VV, Skorokhyd NR, Lehka LV, Evstigneev MP et al (2015) Application of C60 fullerene-doxorubicin complex for tumor cell treatment in vitro and in vivo. J Biomed Nanotechnol 11:1139–1152
Piosik J, Zdunek M, Kapuscinski J (2002) The modulation by xanthines of the DNA-damaging effect of polycyclic aromatic agents, part II. The stacking complexes of caffeine with doxorubicin and mitoxantrone. Biochem Pharmacol 63:635–646
Prylutska SV, Grynyuk II, Matyshevska OP, Prylutskyy YuI, Ritter U, Scharff P (2008) Anti-oxidant properties of C60 fullerenes in vitro. Fuller Nanotub Carbon Nanostruct 16:698–705
Prylutska SV, Burlaka AP, Klymenko PP, Grynyuk II, Prylutskyy YuI, Schuetze Ch et al (2011) Using water-soluble C60 fullerenes in anticancer therapy. Cancer Nanotechnol 2:105–110
Prylutska S, Bilyy R, Overchuk M, Bychko A, Andreichenko K, Stoika R et al (2012) Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J Biomed Nanotechnol 8:522–527
Prylutska S, Grynyuk I, Matyshevska O, Prylutskyy Y, Evstigneev M, Scharff P, Ritter U (2014) C60 fullerene as synergistic agent in tumor-inhibitory doxorubicin treatment. Drugs R D 14:333–340
Prylutskyy YuI, Buchelnikov AS, Voronin DP, Kostjukov VV, Ritter U, Parkinson JA et al (2013) C60 fullerene aggregation in aqueous solution. Phys Chem Chem Phys 15:9351–9360
Prylutskyy YuI, Petrenko VI, Ivankov OI, Kyzyma OA, Bulavin LA, Litsis OO et al (2014a) On the origin of C60 fullerene solubility in aqueous solution. Langmuir 30:3967–3970
Prylutskyy YuI, Evstigneev MP, Pashkova IS, Wyrzykowski D, Woziwodzka A, Gołuński G et al (2014b) Characterization of C60 fullerene complexation with antibiotic doxorubicin. Phys Chem Chem Phys 16:23164–23172
Skamrova GB, Laponogov I, Buchelnikov AS, Shckorbatov YG, Prylutska SV, Ritter U et al (2014) Interceptor effect of C60 fullerene on the in vitro action of aromatic drug molecules. Eur Biophys J 43:265–276
Traganos F, Kapuscinski J, Darzynkiewicz Z (1991) Caffeine modulates the effects of DNA-intercalating drugs in vitro: a flow cytometric and spectrophotometric analysis of caffeine interaction with novantrone, doxorubicin, ellipticine, and the doxorubicin analogue AD198. Cancer Res 51:3682–3689
Woziwodzka A, Gołuński G, Wyrzykowski D, Kaźmierkiewicz R, Piosik J (2013) Caffeine and other methylxanthines as interceptors of food-borne aromatic mutagens: inhibition of Trp-P-1 and Trp-P-2 mutagenic activity. Chem Res Toxicol 26:1660–1673
Acknowledgments
This work was supported, in part, by Russian Science Fund, Project No. 14-14-00328.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prylutskyy, Y.I., Evstigneev, M.P., Cherepanov, V.V. et al. Structural organization of C60 fullerene, doxorubicin, and their complex in physiological solution as promising antitumor agents. J Nanopart Res 17, 45 (2015). https://doi.org/10.1007/s11051-015-2867-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2867-y