Abstract
The study of placental lipid metabolism in uncomplicated pregnancies has not been developed in the literature to date. Its importance lies in expanding the knowledge of placental function to enable comparison with pathological pregnancies in future research. The aim of the present study was to compare the lipid metabolic activity and storage of the maternal and fetal sides of the placenta in healthy pregnancies. Moreover, we compare singleton vs. twin pregnancies to determine if placental metabolic needs differ. We analyzed placental explants from uncomplicated pregnancies, 20 from singleton and 8 from bichorial-biamniotic twin pregnancies (n = 28). Six cotyledon fragments were collected from each placenta at different distances from the umbilical cord, three close to the chorionic plate (hereinafter, we will refer to them as “fetal side”) and another three close to the anchoring villi into the decidua basalis (referred to as “maternal side”). The samples were analyzed for quantitative assay placental fatty acid oxidation (FAO) and esterification (FAE) activities and triglyceride levels. The location of lipid storage in the chorionic villi was assessed by Oil red-O staining. Placental fatty acid oxidation did not show differences when comparing the maternal and fetal sides of the placenta or between single and twin pregnancies. When comparing placental sides, FAE was increased twofold in the maternal side compared to the fetal side of the placenta (P = 0.013). The tendency for lipogenesis in the placenta was exemplified by the FAE/FAO ratio, which was a 37.1% higher on the maternal side (P = 0.019). Despite this, triglyceride levels were five times higher in the fetal side than in the maternal one (P = 0.024). When analyzing singleton vs. twins, FAE was superior in the fetal side in multiple pregnancies (× 2.6, P = 0.007) and the FAE/FAO ratio was significantly higher in twins than in singleton pregnancies, on both sides of the placenta. Despite this finding, triglyceride levels were similar in twin and singleton pregnancies. Comparing the placentas of twins in the same pregnancy, there were no differences in lipid metabolism (FAO or FAE) or placental triglyceride levels between the two co-twins. Using Oil red-O staining, lipid storage in chorionic villi was found to be located on the syncytiotrophoblast cells and not in the connecting axis. The maternal side of the placenta is more active in the esterification of fatty acids, while the storage of neutral lipids concentrates on the fetal side. Moreover, multiple gestations have increased esterification without changes in the concentration of placental triglycerides, probably due to a higher transfer to the fetal circulation in response to the greater energy demand from twin fetuses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The placenta and placental transfer are vital for fetal growth and development, providing all the nutrients and oxygen that the fetus requires. The placenta is very metabolically active and regulates the energy requirements of the fetus and of itself; any injury to it may have a significant impact on fetal development. A better understanding of the placental function and its relationship with possible pathologies begins with the study of its function in healthy pregnant women. So far, limited research on fatty acid metabolism and storage in the placenta has been developed, and most of it focuses on its involvement with pathologic function.
The knowledge of placental energy metabolism becomes more relevant in cases of twin gestations. The maternal energy supply must provide for an increased placental mass with sufficient output for its subsistence and distribution to both fetuses for growth and development. It must be taken into account that twin pregnancies are related to an increase in pathologies in which alterations of energy metabolism could be involved such as intrauterine growth restriction (IUGR), gestational diabetes mellitus (GDM), preeclampsia, or preterm delivery [1, 2]. Therefore, this gap in the scientific literature makes it essential to expand the study of placental lipid metabolism in uncomplicated singleton and twin pregnancies.
Fatty acids are considered essential in fetal and placental development as they take part in multiple cellular and metabolic processes. Their functions include being energy substrates, components of cell membranes, precursors of the synthesis of oxylipins, and participating in the modulation of inter and intracellular hormonal signaling [3]. The study of lipids at the placental level is constantly evolving. It was not until 2002 that it was observed that enzymes of placental beta-oxidation were active [3], which led to the conclusion that the placenta, apart from having a function in lipid transport, could also metabolize fatty acids as a source of energy [4]. Until a few years ago, it was assumed that the absorption of nutrients and transport of fatty acids took place through the total surface of the cells of the syncytiotrophoblast of the placental villi. However, recent research has shown different metabolic activity, location, and composition of lipid droplets in the two trophoblast layers of the term human placenta [5,6,7,8]. On the other hand, to our knowledge, the analysis of placental lipid metabolism has not been described in twin gestations. The few articles that study lipids in twin gestations focus on fatty acids levels, such as Okita et al. (1983) who found differences in essential fatty acid (EFA) levels in chorion laeve in singleton and bichorial-biamniotic twin pregnancies, even in different regions of the chorion [9].
The major nutrient needs for fetal growth vary throughout pregnancy and the placenta adapts to this supply; therefore, any defect produced in the development and maturation of the placenta can lead to damaging effects on the fetus. Currently, placental lesions are classified according to the placental side in which the injury is found [10], considering that maternal and fetal sides have different pathologies and implications, especially in cases of malperfusion. Clinical usefulness of several well-established histological placental lesions has been confirmed such as fetal growth restriction, acute fetal distress, uterine hypoxia, severe ascending infection, placental abruption, excessive extravillous trophoblasts, placental hydrops, fetal thrombotic vasculopathy, and stem obliterative endarteritis [11]. According to this, those pathologies in which physiopathogenesis influences lipid and glycid metabolism could have different relevance depending on whether the lesion was found on the maternal or fetal side of the placenta. Nevertheless, despite its importance, the functional differences between both sides of the placenta, especially in metabolic terms, are an unexplored topic.
The aim of the present study was to expand the knowledge about the processes of metabolism and storage of lipids in healthy pregnant women. The main metabolic tendency in the placenta towards lipolysis or lipogenesis was analyzed through fatty acid oxidation (FAO) and fatty acid esterification (FAE). The level of neutral lipid storage was measured as well as the location of such accumulation (trophoblast vs. connective tissue). We compared these metabolic parameters between singleton and twin pregnancies, and between the maternal and fetal sides of the placenta.
Materials and Methods
Study Design
Participants were enrolled in this prospective observational study at La Paz University Hospital (Department of Obstetrics and Gynecology) between May 2014 and May 2016. This study was approved by the Local Ethical Committee and all the participants signed informed consent forms.
Twenty-four women were included, 20 with singleton pregnancies and 4 with bichorial-biamniotic twin pregnancies (eight placentas). All deliveries were performed by cesarean section for clinical reasons not affecting placental metabolism or perfusion, as shown in Table 1. The reason to include only patients with cesarean deliveries in the study was to avoid the potential effects of labor contractions on placental metabolism.
The inclusion criteria were as follows: normal blood pressure through all pregnancy, pregnancy at term (37–41 weeks), no medical history of chronic metabolic diseases or any pathology that could involve lipid or carbohydrate metabolism disorders, and no pregnancy complications. Exclusion criteria included major fetal anomalies, women with a history of long-chain 3-hydroxyacyl-coA dehydrogenase (LCHAD) deficiency or mitochondrial trifunctional protein (TFP) deficiency, acute fatty liver of pregnancy (AFLP), history of chronic hypertension or other metabolic diseases, and women with an obstetric history of hypertensive disorders induced by pregnancy gestational such as hypertension, preeclampsia, eclampsia, or HELLP syndrome (hemolysis, elevated liver enzymes, low platelets).
Sample Collection
All samples were collected on the delivery day, including a fasting peripheral blood extraction prior to surgery. All the blood analysis results, as well as the clinical, obstetric, and perinatal data recorded, are shown in Table 1.
Immediately after birth (less than 2 h after delivery), the fresh placenta was transported on dry ice to the laboratory. Wedges were taken from the placenta and dissected by removing the membranes and calcium deposits. Cotyledon fragments were extracted as explants for each assay. They were collected from regions located central, intermediate, and peripheral to the umbilical cord insertion. Three 100-mg explants were obtained close to the chorionic plate (hereinafter referred to as “fetal side”) and another three 100-mg explants close to the anchoring villi into the decidua basalis (referred to as “maternal side”). Global results of each assay were obtained calculating the mean of the maternal and fetal sides of the placenta.
Lipid Analysis
Placental Fatty Acid Oxidation
Measurements of FAO assays in placental explants were performed according to the method previously described by our group [12,13,14]. FAO was quantified by nanomole of [3H]-palmitate per gram of tissue per hour (nmol/g/h).
Placental Fatty Acid Esterification
After thawing the explants, they were washed and homogenized in 500 μL of cold phosphate-buffered saline (PBS). An aliquot of 100 μL was used to extract the lipid content following the method previously described [15, 16]. FAE was also quantified by nanomole of [3H]-palmitate per gram of tissue per hour (nmol/g/h).
Quantification of Placental Triglyceride Concentrations
As previously described [17], thawed placental fragments of 20 mg were obtained and homogenized in 400 μL of HPLC-grade acetone. The placental lipid content was calculated as milligrams of triglyceride per milligrams of total placental proteins (TG/Prot ratio) to eliminate heterogeneity biases in the composition of the placenta and to accurately establish the placental lipid content.
Oil Red-O Histological Staining
Staining with Oil red-O solution was performed following the manufacturer’s instructions [18] to evaluate the histological place of lipid storage in chorionic villi, both on the fetal side and the maternal side of the placenta. The staining with Oil red-O was visually compared with hematoxylin-eosin staining, and both were observed under a × 4 to × 40 microscope.
Statistical Analysis
All data were analyzed with SPSS 20.0. Distributions were checked with the Saphiro-Wilk test. When a variable was distributed normally, data were presented as mean ± standard deviation and comparisons were carried out using the Student’s t test. In cases of non-normal distribution, data were shown as median and interquartile range and the tests used for the analysis were either the Wilcoxon test (for paired samples) or Mann-Whitney’s U test (for independent samples). The qualitative variables were analyzed by Chi-square test. We used a paired sample t test to compare placental metabolic data from twins of the same pregnancy. Pearson’s correlation coefficient (r) and stepwise backward linear regression (R2) were used to evaluate the association between numerical variables and to adjust for confounding variables. Differences were considered significant at P value < 0.05.
Results
Maternal and Obstetric Characteristics
Maternal characteristics and obstetric-perinatal outcomes are shown in Table 1. Pregestational maternal body mass index was normal in 87.5% (21/24); the remaining 12.5% (3/24) were overweight but none were obese. The fasting serum values of glucose and lipid profiles were normal in all pregnant women. Insulin resistance was observed in 16.7% (4/24) of the patients, defined as a homeostatic model assessment for insulin resistance (HOMA-IR) ≥ 2.6 in pregnant women in the third trimester [19]. There were no cases of IUGR or small-for-gestational-age (SGA) fetuses.
The study groups (singleton vs. twin pregnancies) were similar, although some differences were found such as higher low-density lipoprotein (LDL) and triglyceride maternal values together with an expected higher weight gain in twin pregnancies. In obstetric data, the difference in the mode of conception stands out (P = 0.005), being in vitro fertilization (IVF) in all of the twins compared to 20.8% in singleton pregnancies. Regarding the indication for cesarean section, there were differences in their indication (P = 0.034); half of the surgeries performed on twin pregnancies were indicated for twinning associated with an unfavorable Bishop test, according to the labor induction protocol of our center. On perinatal results, the gestational age at delivery, the neonatal, and placental weight were lower in twins than in singleton newborns.
Analysis of Placental Explants
Comparison Between the Maternal and Fetal Placental Sides
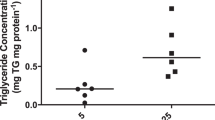
The main results of FAO, FAE assays, and triglyceride content comparing the maternal and fetal sides of the placental explants are shown in Table 2 and Fig. 1. Data are presented pooled and separately considering singleton and twin pregnancies. When investigating fatty acid beta-oxidation of the placentas, no statistically significant differences were observed. Nonetheless, differences were found in fatty acid esterification, which was significantly higher in the maternal side compared to the fetal side when analyzing both the pooled samples and the cases of singleton pregnancies (× 2.1, P = 0.013 and × 1.8, P = 0.012, respectively). However, in the twin cases, esterification was similar in both sides of the placenta. The ratio between esterification and oxidation of placental fatty acids (FAE/FAO ratio) was calculated to explore the main metabolic tendency in the placenta towards lipogenesis or lipolysis. The esterification/oxidation ratio was positive, being 37.1% higher in the maternal placenta than in the fetal one on the pooled sample (P = 0.019), with an even greater difference (64.5%) in singleton gestations when analyzing them separately (P = 0.021). Again, in the cases of twin pregnancies, no statistically significant differences were observed in the esterification/oxidation ratio between the maternal and fetal sides. Regarding the evaluation of placental triglyceride content, the triglyceride concentration was found to be × 4.9 times higher in the fetal side than in the maternal side on the pooled sample (P = 0.024). Although this result remained stable when analyzing separately singleton and twin pregnancies, differences did not reach statistical significance probably due to the wide dispersion of the results. Furthermore, the lipid content of placental triglycerides was adjusted for the amount of protein in the placenta using the TG/Prot ratio. Similarly to triglyceride concentration, the TG/Prot ratio was × 4.8 times higher in the fetal side than in the maternal side of the placenta (P = 0.017); however, in the subgroups, singleton vs. twins analysis differences remained but they were not significant.
Comparison between the maternal and fetal placental sides in the pooled sample. Quantitative assay of placental fatty acid oxidation (FAO) and esterification (FAE) activities and placental triglyceride levels were analyzed in 28 explants of healthy pregnant women. a FAE was higher in the maternal side of the placenta compared to the fetal one (P = 0.013). b The tendency towards lipogenesis was proven by the FAE/FAO ratio, which was higher in the maternal placenta than in the fetal one (P = 0.019). c Triglyceride levels were evaluated with the protein-adjusted triglyceride ratio (TG/Prot). It was higher in the fetal side than in the maternal side of the placenta (P = 0.017). This reflects a tendency towards lipogenesis in the maternal side of the placenta, while in the fetal side, there is a greater accumulation of lipids in the form of triglycerides adjusted for the amount of placental protein. FAO fatty acid oxidation, FAE fatty acid esterification, TG/Prot triglyceride/protein. Horizontal lines represent mean ± standard deviation
Considering the distribution of the TG/protein data (Fig. 1), especially in the fetal side of the placenta, we performed a correlation analysis between this variable and maternal parameters. We found significant correlations of higher free fatty acids with increased TG/Prot ratio on the maternal side (r = 0.56; P = 0.006) and on the mean (r = 0.43, P = 0.04). C-peptide had a positive association with TG/Prot ratio on the fetal side of the placenta (r = 0.42, P = 0.04) and on the mean (r = 0.41, P = 0.04). Correlation of TG/Prot ratio with birthweight centile was of borderline significance when studying the fetal side of the placenta (r = − 0.35, P = 0.06). We studied the distribution of these three variables and we could not find any correlation with the distribution of the TG level in the placenta.
With Oil red-O staining, triglyceride deposits were shown to accumulate in the syncytiotrophoblast and not in connective tissue (Fig. 2). Moreover, greater lipid accumulation can be verified on the fetal side than on the maternal side by quantitative analysis.
Lipid droplets in trophoblast tissue. These histological samples correspond to placental explants from a singleton pregnancy. The maternal side of the placenta is shown on a image, and fetal side on the b image (magnification × 40). The lipid content stained with Oil red-O is found in the trophoblast and not in the connective axis of the chorionic villi. There is a greater accumulation of lipid droplets on the fetal side than on the maternal side of the placenta
Comparison Between Singleton and Twin Gestations
Differences in metabolism and lipid content between singleton and twin gestations were analyzed. The results are shown in Table 3 and Fig. 3, with the analysis on the maternal, fetal and, mean of both placental sides separated. No statistically significant differences were found in beta-oxidation between singleton and twin pregnancies, neither in the maternal side of the placenta, the fetal side, nor the mean. Esterification was significantly higher, around twofold, in twin pregnancies in comparison to singleton pregnancies in both the fetal side (× 2.6, P = 0.015) and the mean (× 2, P = 0.007), but not in the maternal side. Likewise, in twin pregnancies, a greater esterification/oxidation ratio was obtained in the maternal side of the placenta, the fetal side, and the mean compared to singleton pregnancies (P = 0.041, 0.003 and 0.002, respectively). However, the analysis of the total triglyceride content, as well as the adjusted value with the TG/Prot ratio, did not differ significantly in singleton pregnancies and twins in the maternal and fetal sides of the placenta and the mean.
Comparison between singleton vs. twin pregnancies, analysis of the maternal and fetal sides of the placenta. a Fatty acid esterification (FAE) was higher in the fetal side in twin pregnancies with respect to singleton pregnancies (P = 0.015), but not in the maternal side. In singletons, there was greater esterification on the maternal side compared to the fetal side (P = 0.012); however, there were no differences between both sides in twins. b The esterification/oxidation ratio (FAE/FAO) was higher in twins, both on the maternal and fetal sides (P = 0.041 and P = 0.003, respectively). In singleton pregnancies, there was a more marked tendency in the placenta towards lipogenesis on the maternal side compared to the fetal side (P = 0.021). c No differences in the content of adjusted placental triglycerides (TG/Prot) were found among singleton and twin pregnancies, nor between maternal and fetal sides on each group. FAO fatty acid oxidation, FAE fatty acid esterification, TG/Prot triglyceride/protein. Horizontal lines represent mean ± standard deviation
We found statistically significant positive correlations between FAE on the fetal side of the placenta with the presence of twin pregnancy (r = 0.45, P = 0.01), mode of conception by IVF (r = 0.45, P = 0.01), and inversely with gestational age at study (r = − 0.55, P = 0.005). When adjusting for these variables by using linear regression, all were independent predictors of FAE on the fetal side of the placenta, including twin pregnancy (R2 = 0.21, P = 0.04). Along the same line, the mean FAE was significantly related to the presence of twins (r = 0.51, P = 0.007), IVF (r = 0.45, P = 0.01), higher total cholesterol (r = 0.62, P = 0.001), and increased LDL (r = 0.61, P = 0.001). All of these variables, including twins, were independent predictors of the mean placental esterification (R2 = 0.23, P = 0.03) when adjusting by linear regression.
Comparison Between Twins Pregnancies
Lipid metabolism was analyzed within twin pairs to investigate differences between the same-pregnancy placentas. No significant differences were found in any of the metabolic processes studied (FAO and FAE) or in the protein-adjusted triglyceride level when comparing the larger and the smaller twins, both on the maternal and the fetal side of the placenta.
Discussion
Principal Findings
The present study is first to demonstrate that lipid metabolism and storage in the maternal and fetal side of the placenta are different and that these processes change in twin pregnancies. Furthermore, this paper is novel at being carried out in healthy patients, setting a basis for the study of placental function.
Interpretation of Results
Our results support the importance of lipid metabolomic knowledge of pregnancy as an advance in the investigation of potential obstetric pathology related to the placenta.
Due to the predominant maternal catabolic state in the third trimester, beta-oxidation is increased in the final stages of gestation [3, 20, 21]. Maternal fat accumulated in the adipose tissue during early pregnancy becomes available for placental transfer during the last trimester of pregnancy, satisfying the exacerbated fetal demand for fatty acids. Beta-oxidation degrades fatty acids by various enzymatic chain reactions that are mediated by enzymes such as LCHAD or medium-chain acyl-coenzyme A dehydrogenase (MCAD). LCHAD gene expression has been proved to be higher in the placenta than in other maternal tissues, while MCAD gene expression was five times higher in fetal than maternal blood [22]. This could give us an idea about the metabolic importance of this organ at the end of gestation and the efficient use of energy from different fatty acids as required in maternal or fetal tissue. A mathematical model developed for lipid metabolism described that in the maternal-facing microvillous plasma membrane of the syncytiotrophoblast, the consumption of fatty acids predominates with respect to the fetal-facing basal membrane, also suggesting greater permeability [23]. In contrast, according to our results, placental fatty acid consumption as an energy substrate is homogeneous in all parts of the placenta and is similar in singleton and twin pregnancies.
However, big differences were found in lipid esterification and storage in the placenta. It is well established that most fatty acids are found in serum as esterified forms linked to triglycerides, phospholipids, or cholesterol esters [24]. Ferchaud-Roucher et al. [6] demonstrated that a similar process could occur in in vitro trophoblast cells. We observed on the pooled sample that the maternal side of the placenta has a clear tendency towards the synthesis of fatty acids esters, which may be consistent with changes in the maternal serum and lipid metabolism during pregnancy. On the other hand, lipolysis tends to increase in maternal adipocytes leading to serum hyperlipidemia in the third trimester mainly due to hypertriglyceridemia [21, 23]. On the same topic, it has been previously described that pregnant women with gestational diabetes show an inverse relationship between fatty acid oxidation and esterification [12]. However, despite the increase in lipid esterification on the maternal side of the placenta, the triglyceride level is higher on the fetal side, suggesting intraplacental transport. Computational modeling of compartments in the placenta already suggested the existence of an intracellular lipid metabolic pool (probably in the syncytiotrophoblast cells), which modulates the transport of fatty acids to the fetus. These fatty acids available for transport would be a very small percentage of the total, which could be stored in a free or esterified form [24]. In the 1970s, a study in rats described that the fatty acids transported from the mother to the fetus did so through a placental compartment that only contained 5% of the total of fatty acids [25]. Similarly, Rebholz et al. [26] found that in rats, the amount of lipids the fetus receives is independent of the maternal intake, supporting the theory that the placenta also behaves as energy storage and can regulate lipid transfer. They also observed a greater accumulation of lipids in the placenta with respect to other tissues such as adipose or striated muscle. Moreover, fat deposits in placentas from malnourished pregnant women who received parenteral nutrition with daily lipid emulsions were found in Hofbauer cells and vacuolated syncytial cells in the chorionic villi [27]. Along the same line, we demonstrate with Oil red-O staining that lipid storage occurred not in the connective tissue but syncytiotrophoblast cells. Consequently, any alteration in the latter could influence the contribution of fatty acids to the fetus.
Given these findings, we conclude that although the placenta seems to be more active in the processes of esterification of fatty acids in the maternal side, once the triglycerides are esterified, these might be transported through still unknown mechanisms to the fetal surface. There, they could be stored waiting to fulfill the fetal energy demand. As the previously mentioned publications suggest, the placenta could modulate lipid transfer according to fetal needs [26, 28]. This hypothesis would explain why in twins there is a greater synthesis of esters in the fetal side of the placenta without increasing the content of placental triglycerides, probably due to a greater transfer of lipids to the fetal circulation secondary to higher energy demand from multiple fetuses. Monochorionic twins are considered an ideal model to study the regulation of the placenta given their identical genotypes and shared maternal environment. Research involving lipid metabolism in multiplets has focused on comparing IUGR-twins as well as epigenetic changes (such as DNA methylation) that inhibit expression of genes involved in fetal growth, such as DCRE1 (which encodes a mitochondrial enzyme of the unsaturated fatty acid (UFA) β-oxidation pathway) or LEPR (which encodes the leptin receptor) [29]. Differences have also been observed in the amount of saturated fatty acids, UFA, and polyunsaturated fatty acid (PUFA) in the placenta and umbilical cord plasma in the IUGR-twin compared with the unaffected co-twin or with singletons [30], finding differences in the level of fatty acids in amniotic fluid pre- and post-fetoscopic laser coagulation in twin-twin transfusion syndrome [31]. Having understood that there are differences in the lipid metabolism and storage at the placental level between twins and singletons, it would be interesting to investigate the variations in lipid transport between both placental sides in future studies.
In this study, we focused on neutral lipids (triglycerides) as energy substrates and storage lipids, although investigations about lipid class fraction show a higher percentage of phospholipids and cholesterol (both membrane lipids) than triglycerides [32, 33]. We believe that the global esterification rate should not change if we considered the latter; we believe that they would likely be increased in the fetal side similar to triglycerides, due to increased fetal requirement for cell membrane formation. However, future studies are necessary to verify this hypothesis. Moreover, previous studies on the composition of crude lipids in the placenta show that long-chain polyunsaturated fatty acids (LCPUFA) are the most prevalent fatty acids in the placenta [32, 33], even though their supplementation through the diet is necessary. This is probably due to a process called biomagnification, in which the transport of these fatty acids undergoes a selection process in favor of the fetal circulation to improve neurodevelopment [7, 33, 34]. In our study, we evaluated the metabolism and storage of palmitic acid (C16:0). We believe that if LCPUFA were analyzed, the amount of stored triglyceride could be found in a lower percentage due to a high transfer to the fetal circulation, as it has already been described [8, 16]. In fact, previous research comparing EFA levels between singleton and multiple pregnancies demonstrates that the EFA levels in maternal and umbilical plasma and in the umbilical cord vessel wall are lower in multiplets than in singletons [35, 36]. For this reason, we believe that it is wise not to extrapolate the conclusions of this study to the placental metabolism of LCPUFA until further studies clarify this issue.
Clinical and Research Implications
The metabolic differences between the maternal and fetal sides of the placenta described in this study offer no clinically relevant findings, but the utility of this research extends beyond the original focus. There is growing evidence in the literature that transport, metabolism, or storage defects of placental fatty acids could be involved in different pathologies such as obesity [37,38,39,40,41], GDM [42, 43], IUGR [44], preeclampsia, HELLP syndrome [12, 45, 46], AFLP, or preterm delivery [4, 13, 23, 33, 39]. Our paper contributes to a better understanding of the lipid metabolism in the placenta of healthy pregnancies, valuable data that can be used for comparison in future research focused on these pathologies; it is so that the knowledge of placental lipid metabolism could have clinical implications in the future. Moreover, the potential increased demand for fetal lipids as an energy substrate in twin pregnancies suggested by our results may have implications for maternal and fetal nutrition or specific twin pathologies.
Strengths of the Study
To our knowledge, this is the first report of placental lipid metabolism and storage compartmental analysis on healthy pregnant women, which compares the metabolism between the maternal and fetal sides of the placenta. The differences found contribute to a greater knowledge of the use of energy resources by the fetus and the placenta. The study is strengthened by the differences obtained between singleton and multiple pregnancies, showing greater energy demand and different lipid storage or consumption. Most of the maternal and obstetric-perinatal characteristics studied in both populations did not show significant differences, and those variables with significant differences between singletons vs. twins group showed a relationship by twinning when adjusting for confounding variables. The process of collecting and analyzing the lipid metabolomics of the placental explants had previously been developed with promising results [12, 13, 22].
Limitations of the Study
We believe that given the greater tendency to catabolism during the late stages of gestation, the results of this study (performed in placentas at term) may not be applicable to early stages of gestation. One obvious limitation of this study is that it is an ex vivo study, with conclusions that may not be extrapolated in vivo. Transport through the placenta is determined by different factors, such as placental structure and vasculature, hormonal influence during pregnancy, the maternal contribution of nutrients, and placental nutrient sensing and growth signals. These conditions are not reproducible in the laboratory and may affect the metabolism and transport through the placenta. On the other hand, the study is limited by a small sample size, especially with respect to the analysis by subgroups between singleton and twin pregnancies, whose results could be influenced by the biological variability between placentas. However, this study provides interesting driving information for future metabolic pathways analysis with a larger sample size.
Conclusions
The maternal side of the placenta is metabolically more active than the fetal side of the placenta, especially regarding fatty acid esterification. However, the latter is the preferential compartment for triglycerides storage, ready to be used by the fetal circulation according to its energy needs. In twin pregnancies, fatty acid esterification is higher on the fetal side of the placenta without increasing the placental triglycerides levels, suggesting a transfer to the fetal circulation in response to the even greater energy demand of the growing twin fetuses.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Santana DS, Surita FG, Cecatti JG. Multiple pregnancy: epidemiology and association with maternal and perinatal morbidity. Rev Bras Ginecol Obstet. 2018;40(9):554–62.
Santana DS, Silveira C, Costa ML, Souza RT, Surita FG, Souza JP, et al. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: evidence from the WHO Multicountry Survey on Maternal and Newborn Health. BMC Pregnancy Childbirth. 2018;18(1):449.
Rakheja D, Bennett MJ, Foster BM, Domiati-Saad R, Rogers BB. Evidence for fatty acid oxidation in human placenta, and the relationship of fatty acid oxidation enzyme activities with gestational age. Placenta. 2002;23(5):447–50.
Shekhawat P, Bennett MJ, Sadovsky Y, Nelson DM, Rakheja D, Strauss AW. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am J Physiol Endocrinol Metab. 2003;284(6):E1098–105.
Kolahi K, Louey S, Varlamov O, Thornburg K. Real-time tracking of BODIPY-C12 long-chain fatty acid in human term placenta reveals unique lipid dynamics in cytotrophoblast cells. PLoS One. 2016;11(4):e0153522.
Ferchaud-Roucher V, Rudolph MC, Jansson T, Powell TL. Fatty acid and lipid profiles in primary human trophoblast over 90h in culture. Prostaglandins Leukot Essent Fat Acids. 2017;121:14–20.
Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407.
Kolahi KS, Valent AM, Thornburg KL. Real-time microscopic assessment of fatty acid uptake kinetics in the human term placenta. Placenta. 2018;72–73:1–9.
Okita JR, Johnston JM, MacDonald PC. Source of prostaglandin precursor in human fetal membranes: arachidonic acid content of amnion and chorion laeve in diamnionic-dichorionic twin placentas. Am J Obstet Gynecol. 1983;147(5):477–82.
Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140(7):698–713.
Stanek J, Biesiada J. Clustering of maternal-fetal clinical conditions and outcomes and placental lesions. Am J Obstet Gynecol. 2012;206(6):493 e1–8.
Bartha JL, Visiedo F, Fernandez-Deudero A, Bugatto F, Perdomo G. Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta. 2012;33(2):132–4.
Visiedo F, Bugatto F, Sanchez V, Cozar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–12.
Perdomo G, Commerford SR, Richard AM, Adams SH, Corkey BE, O’Doherty RM, et al. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem. 2004;279(26):27177–86.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7.
Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metab Clin Exp. 2007;56(11):1500–7.
Perdomo G, Kim DH, Zhang T, Qu S, Thomas EA, Toledo FG, et al. A role of apolipoprotein D in triglyceride metabolism. J Lipid Res. 2010;51(6):1298–311.
Oil red O color solution: instructions for use [Internet]: Germany: Merk KGaA; 2019 [cited 2019 Dec 8] [Available from: http://www.emdmillipore.com/US/en/product/Oil-red-O-color-solution,MDA_CHEM-102419?bd=1.
Reyes-Muñoz EM-HE, Ortega-Gonzalez C, Arce-Sanchez L, Avila-Carrasco A, Zamora-Escudero R. HOMA-IR and QUICKI reference values during pregnancy in Mexican women. Ginecol Obstet Mex. 2017;85(5):306–13.
Lindsay KL, Hellmuth C, Uhl O, Buss C, Wadhwa PD, Koletzko B, et al. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS One. 2015;10(12):e0145794.
Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res. 2006;65(Suppl 3):59–64.
Bartha JL, Bugatto F, Fernandez-Deudero A, Fernandez-Macias R, Perdomo G. Tissue specific expression of human fatty acid oxidation enzyme genes in late pregnancy. Lipids Health Dis. 2016;15(1):200.
Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–54.
Chavan-Gautam P, Rani A, Freeman DJ. Distribution of fatty acids and lipids during pregnancy. Adv Clin Chem. 2018;84:209–39.
Hummel L, Zimmermann T, Schirrmeister W, Wagner H. Synthesis, turnover and compartment analysis of the free fatty acids in the placenta of rats. Acta Biol Med Ger. 1976;35(10):1311–6.
Rebholz SL, Burke KT, Yang Q, Tso P, Woollett LA. Dietary fat impacts fetal growth and metabolism: uptake of chylomicron remnant core lipids by the placenta. Am J Physiol Endocrinol Metab. 2011;301(2):E416–25.
Jasnosz KM, Pickeral JJ, Graner S. Fat deposits in the placenta following maternal total parenteral nutrition with intravenous lipid emulsion. Arch Pathol Lab Med. 1995;119(6):555–7.
Hanebutt FL, Demmelmair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr. 2008;27(5):685–93.
Roifman M, Choufani S, Turinsky AL, Drewlo S, Keating S, Brudno M, et al. Genome-wide placental DNA methylation analysis of severely growth-discordant monochorionic twins reveals novel epigenetic targets for intrauterine growth restriction. Clin Epigenetics. 2016;8:70.
Wang L, Han TL, Luo X, Li S, Young T, Chen C, et al. Metabolic biomarkers of monochorionic twins complicated with selective intrauterine growth restriction in cord plasma and placental tissue. Sci Rep. 2018;8(1):15914.
Dunn WB, Allwood JW, Van Mieghem T, Morris RK, Mackie FL, Fox CE, et al. Carbohydrate and fatty acid perturbations in the amniotic fluid of the recipient twin of pregnancies complicated by twin-twin transfusion syndrome in relation to treatment and fetal cardiovascular risk. Placenta. 2016;44:6–12.
Klingler M, Demmelmair H, Larque E, Koletzko B. Analysis of FA contents in individual lipid fractions from human placental tissue. Lipids. 2003;38(5):561–6.
Brown SH, Eather SR, Freeman DJ, Meyer BJ, Mitchell TW. A lipidomic analysis of placenta in preeclampsia: evidence for lipid storage. PLoS One. 2016;11(9):e0163972.
Watkins OC, Islam MO, Selvam P, Pillai RA, Cazenave-Gassiot A, Bendt AK, et al. Metabolism of 13C-labeled fatty acids in term human placental explants by liquid chromatography-mass spectrometry. Endocrinology. 2019;160(6):1394–408.
Zeijdner EE, van Houwelingen AC, Kester AD, Hornstra G. Essential fatty acid status in plasma phospholipids of mother and neonate after multiple pregnancy. Prostaglandins Leukot Essent Fat Acids. 1997;56(5):395–401.
Foreman-van Drongelen MM, Zeijdner EE, van Houwelingen AC, Kester AD, Al MD, Hasaart TH, et al. Essential fatty acid status measured in umbilical vessel walls of infants born after a multiple pregnancy. Early Hum Dev. 1996;46(3):205–15.
Barbour LA. Metabolic culprits in obese pregnancies and gestational diabetes mellitus: big babies, big twists, big picture : the 2018 Norbert Freinkel Award Lecture. Diabetes Care. 2019;42(5):718–26.
Ferchaud-Roucher V, Barner K, Jansson T, Powell TL. Maternal obesity results in decreased syncytiotrophoblast synthesis of palmitoleic acid, a fatty acid with anti-inflammatory and insulin-sensitizing properties. FASEB J. 2019;33(5):6643–54.
Nam J, Greenwald E, Jack-Roberts C, Ajeeb TT, Malysheva OV, Caudill MA, et al. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J Nutr Biochem. 2017;49:80–8.
Barbour LA, Hernandez TL. Maternal lipids and fetal overgrowth: making fat from fat. Clin Ther. 2018;40(10):1638–47.
Lewis RM, Wadsack C, Desoye G. Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care. 2018;21(2):78–82.
Hulme CH, Nicolaou A, Murphy SA, Heazell AEP, Myers JE, Westwood M. The effect of high glucose on lipid metabolism in the human placenta. Sci Rep. 2019;9(1):14114.
Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2017;36(2):513–21.
Chassen SS, Ferchaud-Roucher V, Gupta MB, Jansson T, Powell TL. Alterations in placental long chain polyunsaturated fatty acid metabolism in human intrauterine growth restriction. Clin Sci (Lond). 2018;132(5):595–607.
Lin YP, Xu CL, Lin KS, Gu HB, Chen L, Wang Y, et al. Study on the correlation between adipocyte fatty-acid binding protein, glucolipid metabolism, and pre-eclampsia. J Obstet Gynaecol Res. 2018;44(4):655–662.
Wojcik-Baszko D, Charkiewicz K, Laudanski P. Role of dyslipidemia in preeclampsia-a review of lipidomic analysis of blood, placenta, syncytiotrophoblast microvesicles and umbilical cord artery from women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018;139:19–23.
Acknowledgments
We thank the staff of the Department of Obstetrics and Gynaecology at the La Paz University Hospital (Madrid, Spain) for their help in patient enrolment and placental tissue collection.
We also thank Lucía Durán Alcalde for the technical assistance and the processing of samples, and Israel John Thuissard Vasallo and David Sanz Rosa, from the Department of Biostatistical at the Infanta Sofía University Hospital (Madrid, Spain), for the statistical assistance.
Funding
This study was funded by Instituto de Salud Carlos III (Madrid, Spain), project PI12/01947.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the intellectual content and have analyzed, reviewed, and revised the manuscript, as well as approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Approval was obtained from the ethics committee of La Paz University Hospital (Madrid, Spain). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data and photographs.
Code Availability
The codes supporting the current study have not been deposited in a public repository but are available from the corresponding author on request.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abascal-Saiz, A., Fuente-Luelmo, E., Haro, M. et al. Placental Compartmentalization of Lipid Metabolism: Implications for Singleton and Twin Pregnancies. Reprod. Sci. 28, 1150–1160 (2021). https://doi.org/10.1007/s43032-020-00385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00385-2