Abstract
To determine whether embryo developmental stage or morphological grading can predict live birth rate (LBR) from a single blastocyst in nonbiopsied and biopsied frozen embryo transfer (FET) cycles. This retrospective study included 1336 nonbiopsied and 360 euploid FET cycles. Blastocysts were divided according to developmental stage (day 5 [D5] and day 6 [D6]) and morphology (good quality and low quality). Nonbiopsied cycles in which D5 blastocysts were transferred were associated with a significantly higher LBR than those in the D6 group (48.5 vs. 24.3%; p < 0.001), as well as in good-quality embryo transfer cycles than that in low-quality embryo cycles (52.6 vs. 25.3%; p < 0.001). Embryos reaching good-quality blastocysts on D5 yielded significantly higher LBR than those similar quality blastocysts on D6. The same trend was seen in low-quality embryos. Concerning only D5 or D6 blastocyst transfer, the LBRs of good-quality embryos were still superior to those of low-quality embryos. In the case of euploid embryo transfers, the LBR (48.9 vs. 44.9%, p = 0.444) of D5 blastocysts did not significantly differ from that of D6 blastocysts. Good-quality embryos showed a higher LBR than low-quality embryos (51.6 vs. 40.0%, p = 0.030); the adjusted odds ratio remained insignificant after controlling for confounders (aOR 1.56; 95% CI 0.99–2.45; p = 0.056). The LBRs in the same developmental stage or morphology subgroups were not statistically significant. Embryo developmental stage and morphological grade are useful predictors of LBR in nonbiopsied FET cycles. However, no association was found in euploid transfer cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the focus of in vitro fertilization (IVF) has shifted from pursuing higher pregnancy rates to achieving a single, safe, and comfortable pregnancy. Among all the factors that predict the success rate of IVF, the embryo developmental stage is one major factor [1]. Studies have shown that transferring blastocysts can increase the pregnancy rate and live birth rate (LBR) over what is achieved with cleavage-stage embryos [2]. Multifetal pregnancy results in a much higher risk of pregnancy complications and neonatal morbidity than a singleton pregnancy, and is the most significant adverse event of IVF treatment [3]. To date, the single blastocyst transfer (SBT) strategy is the most effective method for avoiding the complications of multiple pregnancies and for producing favorable clinical outcomes [4].
Usually, embryos are cultured to day 5 (D5) after fertilization, but embryos that grow slowly can develop to day 6 (D6) blastocysts or even later. Numerous studies have aimed to determine whether transferring D5 or D6 blastocysts is better in terms of pregnancy outcomes. There is no consensus about the priority of transferring D5 blastocysts to D6 blastocysts in fresh transfer cycles to provide better synchrony between embryos and endometrium [5,6,7]. Additionally, one meta-analysis published in 2010 concluded that transferring blastocysts frozen on day 5 and day 6 was associated with comparable results when embryos were at the same developmental stage [8]. However, with the wide use of vitrification protocols instead of slow-freezing techniques, a large number of studies have shown that D5 blastocysts are superior to D6 blastocysts in frozen-thawed embryo transfer (FET) cycles [9,10,11,12,13,14]. A recent meta-analysis by Bourdon et al. concluded that D5 blastocyst transfers resulted in a higher clinical pregnancy rate (CPR) and LBR than D6 embryos in FET treatment [15].
In addition to the developmental stage of the embryo playing a key role in IVF treatment, embryo quality also determines clinical outcomes. Currently, the most commonly used protocol for assessing blastocyst quality is the analysis of embryo morphology with inner cell mass (ICM) and trophectoderm (TE) grading. One study found no difference in clinical results after transferring good-quality embryos in the D5 and D6 groups [11]. However, this conclusion is still under debate, as some studies indicated better pregnancy outcomes following the use of blastocysts vitrified on day 5 than on day 6 when comparing only good-quality embryos [10, 12].
On the other hand, due to morphological grading being unable to determine the ploidy status of an embryo, preimplantation genetic testing for aneuploidy (PGT-A) has become a common procedure to assess embryo quality. After excluding aneuploid embryos, PGT-A was shown to potentially increase LBRs and decrease early pregnancy failure rates [16]. Conflicting results persist regarding the embryo developmental stage and chromosomal status on transfer outcomes. The implantation rate (IR) and CPR were significantly higher following euploid D5 embryo transfer than they were following euploid D6 transfer in some representative studies [11, 17, 18]. However, some studies challenged that conclusion with data showing no difference in pregnancy results between the two groups [19, 20].
Therefore, we conducted this retrospective study to determine the complete range of clinical outcomes based on embryo developmental stage, embryo quality by morphological grading, and ploidy status to provide more in-depth data for clinicians and patients when facing FET.
Materials and Methods
Patients
This retrospective study was conducted from January 2017 to May 2019 at the reproductive center of the hospital in accordance with the Declaration of Helsinki and with the approval of the Ethics Committee of Nanjing Maternity and Child Health Care Hospital (NJFY-2020KY-051).
Inclusion criteria for participants undergoing nonbiopsied cycles are as follows: (a) patients younger than 42 years old, (b) D5 or D6 single autologous blastocyst transfer in frozen cycles, and (c) previous failed FET cycles ≤ 2.
Patients with normal karyotypes underwent PGT-A treatment following one of the following indications: recurrent pregnancy loss (RPL, ≥ 2 pregnancy losses before 12-week gestation), repeated implantation failure (RIF, ≥ 3 transfers or ≥ 5 optimal quality embryos failing to implant), advanced maternal age (AMA, ≥ 38 years), or severe male factor infertility.
The exclusion criteria for both groups were as follows: (a) endometrial thickness < 7 mm upon the initiation of progesterone exposure, (b) presence of uterus malformation or fallopian hydrosalpinx, (c) cycles of oocyte donation or vitrified oocyte, and (d) missed cycle data and follow-up.
Stimulation and Embryo Culture Protocols
All participants underwent a flexible gonadotropin-releasing hormone (GnRH) antagonist protocol, which started from the third day of the menstrual cycle with the application of recombinant follicular stimulating hormone (rFSH, Gonal-F, Merck Serono, Italy) varying from 150 to 225 IU per day. We monitored follicular growth by ultrasound scan and sex hormone levels (FSH, luteinizing hormone, estradiol, and progesterone) and adjusted the dose of gonadotropin (Gn). When at least two follicles are larger than 18 mm, a 10,000 IU human chorionic gonadotrophin (hCG, Lizhu, China) injection was given to achieve final oocyte maturation, and oocyte retrieval was scheduled 36 h later. Based on sperm quality, conventional IVF or ICSI was performed. For couples who underwent the PGT-A procedure, only insemination via ICSI was conducted. The oocytes and embryos were cultured according to our previously published article [21].
Embryo Biopsy and Euploidy Analysis
Embryos with grades 4 or above and with at least 1 score B for either ICM or TE (AA, AB, BA, BB, BC, and CB) on day 5 or day 6 were considered eligible for biopsy. The blastocyst was positioned on the holding pipette with ICM away from the biopsy pipette. A laser (Hamilton Thorne Inc., Beverly, USA) was used to assist the opening of a 10–20-μm hole in the zona pellucida to make sure the aperture was wide enough to allow the passage of several TE cells. The biopsy pipette was then carefully pressed against the zona and gently aspirated 4–5 cells into the biopsy pipette. Once the biopsy pipette was totally outside the zona pellucida, 2–3 laser pulses were directed at the junctions between cells. After releasing the holding pipette, the target TE cells were separated from the blastocyst by a quick flicking movement from the biopsy pipette against the holding pipette. The aspirated TE cells were released into the biopsy drop and were washed and prepared before processing for next genetic sequencing (NGS). The whole-genome amplification (WGA) procedure involved cell lysis, and fragmentation and amplification procedures on biopsy and control samples using a SurePlex DNA Amplification System (Illumina, San Diego, CA, USA). Later, the amplified samples were analyzed with a VeriSeq PGT Kit (Illumina, San Diego, CA, USA) along with NGS on a MiSeq according to the manufacturer’s recommendations. Euploidy results were then confirmed, with euploid embryos to be transferred and all aneuploid or mosaic blastocysts to be excluded from transfer [22].
Vitrification and Warming Procedures
D5 or D6 blastocysts were scored according to the cavity expansion level, along with ICM and TE grade [23]. Blastocyst expansion was assigned one of the following descriptions: the blastocoel filling < 50% of the nonexpanded embryo (Gardner grade 1), the blastocoel filling > 50% of the embryo (Gardner grade 2), the blastocoel filling ~ 100% of the full blastocyst (Gardner grade 3), an expanded blastocyst with a thin zona pellucida (Gardner grade 4), a hatching blastocyst (Gardner grade 5), and a blastocyst that has completely hatched out of the zona pellucida (Gardner grade 6). The ICM was assigned one of the following grades: numerous tightly packed cells (A), several and loosely packed cells (B), and very few cells (C). Similarly, TE grading was as follows: many cells organized in the epithelium (A), several cells organized in the loose epithelium (B), and few large cells (C). For unbiopsied embryos, D5 or D6 blastocysts at stage 3 or above with at least 1 score B for either ICM or TE were selected for vitrification. In biopsied cycles, embryos reaching grades 4 or above with the same standard of ICM or TE on day 5 or day 6 were vitrified after biopsy. If the ICM and TE scores were both above grade B (≥ 3 BB), the blastocyst was defined as a good-quality blastocyst; otherwise, it was considered a low-quality blastocyst (grades 3–6 AC/BC/CA/CB).
Embryos were vitrified and warmed based on the protocols previously described [24]. Vitrification and thawing solutions, as well as Cryotop, were purchased from Kitazato Corporation (Kitazato BioPharma Co., Shizuoka, Japan). The frozen blastocysts were firstly equilibrated at room temperature and then soaked in vitrification solution for 1 min. For vitrification, the embryos were moved to the top of Cryotop and immediately put into liquid nitrogen. Fully expanded blastocysts were artificially collapsed with a laser-assisted incubation system (200 ms laser). For thawing, the cryotubes were transferred into the thawing solution at 37 °C for 1 min. After processing the embryos in diluent solutions and different washing solutions, the embryos were placed into an equilibrated and warmed culture medium. Before the warmed blastocysts expanded (within 5 min after the warming procedure was completed), assisted hatching was used according to the previous protocols [25]. After surviving the warming procedure, those post-thaw embryos without biopsied were cultured at 37 °C in a 6% CO2, 5% O2, and 89% N2 incubator for another 16 h before transfer. For those biopsied embryos, an additional 2–4-incubation was performed before transfer due to our work schedule. Both good-quality and low-quality embryos classified at vitrified stage were transferred.
Endometrial Preparation and Luteal Phase Support
The choice of endometrial preparation was based on clinical discretion and patient preferences. Participants with regular menstruation treated under a natural cycle (NC) were monitored from day 10 to 12 of their menstrual cycles. We monitored follicular growth, endometrial thickness, and type using serial transvaginal ultrasonography. When the leading follicle was larger than 18 mm, the progesterone level ≤ 1.5 ng/ml, and the endometrial thickness ≥ 7 mm (as determined by tri-laminar lining), 10,000 IU hCG was injected. We then monitor the follicle the next day and 1 day after that if no sign of ovulation was found. It took about 24–48 h after hCG administration to confirm the ovulation by ultrasound. All patients took 30 mg of oral dydrogesterone (Abbott Biologicals B.V., Netherlands) in three divided doses on the day after ovulation confirmation (P + 0) and continued taking it until 14 days after FET.
In artificial cycles (AC), oral estrogen (estradiol valerate, progynova, Bayer, France) was started at 4–6 mg per day on the second day of the menstrual cycle for 1 week and adjusted to 8–10 mg based on the endometrial thickness and serum estradiol (E2) level. This group was further divided according to the addition of GnRH agonist (triptorelin acetate, Diphereline, IPSEN, France), which was administered at the early part of the follicular phase (days 2–4) during the menstrual cycle. After downregulation was confirmed with baseline hormonal and transvaginal ultrasound assessment (i.e., E2 level > 30 pg/ml, luteinizing hormone and FSH levels < 5 IU/L, and endometrial thickness < 5 mm), daily administration of 4–6 mg of oral estrogen was commenced. When the endometrial thickness reached 7 mm and serum E2 level peaked at 200 pg/ml, 90 mg of vaginal progesterone (Crinone, Merck Serono, UK) once a day and 10 mg of dydrogesterone three times daily were administered (P + 0).
All embryo transfer treatments were performed on the 6th day of progesterone exposure (P + 5). To conclude, it took 6–7 days (144–168 h) for embryo transferring after hCG trigger in NC and 5 days (120 h) after progesterone supplementation in AC.
Clinical Outcome
The β-hCG value was examined at 2 weeks after FET. If the pregnancy test was positive, luteal phase support lasted until gestational weeks 8–10. Clinical pregnancy was confirmed by gestational sac identification through ultrasound after 6 weeks of pregnancy. Early miscarriage was termed fetal loss before gestational week 12. Live birth was considered when a living fetus was born after 28 weeks of pregnancy. The primary outcome was LBR. The secondary outcomes included CPR and early miscarriage rate (EMR).
Statistical Analysis
All data were analyzed with SPSS 24.0 (IBM, NY, USA). Comparisons between groups were analyzed by the independent samples t test and Mann-Whitney U test (if data were not normally distributed). Pearson’s chi-square tests were used for categorical data. Multivariate analysis was conducted to confirm whether embryo developmental stage and morphological grading were independently related to LBR after adjusting for confounding factors. The included variables in the multiple regression model were those with a significance on univariate analysis at p < 0.05 or more and those that could potentially affect live birth outcome. Results are expressed as adjusted odds ratio (aOR) and 95% confidence intervals (95% CI). p < 0.05 was considered statistically significant. We performed a post hoc sample size calculation at 80% power and α = 0.05 (two-sided). A sample size of 110 can detect a 25% difference in LBR (baseline rate 50%) in nonbiopsied cycles. In biopsied cycles, an approximate sample sizes of 270 per group was needed to detect a LBR difference between 52 and 40% and of 1550 per group can detect a difference between 50 and 45%.
Results
Study Population
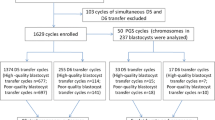
As shown in Fig. 1, we retrospectively enrolled patients undergoing SBT cycles with all the embryos surviving from the vitrified and warmed procedure between January 2017 and May 2019. In the initial recruitment of 2226 SBTs performed in our reproductive center, 519 FETs were excluded for the following reasons: age ≥ 42 years (n = 188), previous failed FET cycles ≥ 3 (n = 202), endometrial thickness at the start of progesterone administration < 7 mm (n = 92), uterus malformation or fallopian hydrosalpinx (n = 29), and cycles of oocyte donation or vitrified oocyte (n = 8). Additionally, 11 FETs were excluded due to missing data or loss of follow-up. A total of 1336 nonbiopsied and 360 biopsied cycles were analyzed in this study. Based on the embryo developmental stage (D5 and D6) and morphological grading (good quality and low quality), we analyzed the CPRs, EMRs, and LBRs in both nonbiopsied and biopsied cycles and each subgroup.
Nonbiopsied Transfer Cycles
The baseline characteristics of the cycles are presented in Table 1. The mean age, body mass index (BMI), basal FSH, luteinizing hormone (LH) and E2 levels, and endometrial thickness on the first day of progesterone administration were comparable between the two groups (D5 and D6; p > 0.05). In addition, the proportions of the type and cause of infertility, as well as endometrium preparation protocol, showed no difference within groups (p > 0.05). We observed a significant difference in the proportion of embryo stage subdivided according to the duration of infertility (p = 0.002). A higher percentage of good-quality embryos was seen in the D5 group (71.6 vs. 28.0%, p < 0.001). In regard to different morphological grading, good-quality embryo groups had statistically younger maternal age (30.8 ± 3.9 vs. 31.4 ± 4.2, p = 0.007) and higher proportion of D5 blastocysts (85.7 vs. 48.0, p < 0.001).
When analyzing clinical outcomes shown in Table 2, we noticed both a significant increase in CPR (overall, 58.2 vs. 32.0%, p < 0.001) and LBR (overall, 48.5 vs. 24.3%, p < 0.001) in the D5 group compared to the D6 group. However, no difference was observed between the two groups in terms of the EMR (overall, 13.6 vs. 17.2%, p = 0.293). In addition, good-quality embryo transfer cycles yielded statistically higher CPR (overall, 61.8 vs. 34.3%, p < 0.001) and LBR (overall, 52.6 vs. 25.3%, p < 0.001) and a statistical lower EMR (overall, 12.0 vs. 20.0%, p = 0.008) than low-quality embryo cycles. The significance still existed after the multiple logistic regression analysis (D5 vs. D6 blastocyst: aOR = 2.01; 95% CI: [1.51–2.68]; p < 0.001 and good-quality vs. low-quality embryo: aOR = 2.51; 95% CI: [1.94–3.24]; p < 0.001) (Table 3). The including confounders were age, BMI, duration of infertility (≤ 2 vs. 3–5 vs. > 5), basal FSH level, endometrial thickness, developmental stage (D5 vs. D6), and morphological grading (good-quality vs. low-quality).
Figure 2 shows LBRs according to morphological grading and developmental stage in nonbiopsied groups. In subgroups where embryos of the same grade were compared between blastocysts cryopreserved on day 5 and day 6, the LBRs were still higher in the D5 group both in good-quality embryo transfer cycles (55.8 vs. 33.0%, p < 0.001) and low-quality embryo transfer cycles (30.1 vs. 20.8%, p = 0.012). A multivariate analysis was performed to adjust for confounding factors (Table 3). The model included patient age, BMI, duration of infertility (≤ 2 vs. 3–5 vs. > 5), basal FSH level, endometrial thickness, and developmental stage (D5 vs. D6). Blastocysts frozen on day 5 were independently associated with a significant increase in the LBRs compared to D6 blastocysts in good-quality embryo transfer cycles (aOR = 2.54; 95% CI: [1.66–3.90]; p < 0.001) and in low-quality embryo transfer cycles (aOR = 1.63; 95% CI: [1.10–2.40]; p = 0.015).
Comparison of live birth rate after single blastocyst transfer according to embryo developmental stage (D5 and D6) and morphological grading (good quality and low quality) in nonbiopsied transfer cycles. A good-quality embryo was defined as a grade 3–6 embryo ≥ BB (AA, AB, BA, BB) before vitrification. A low-quality embryo was classified as a grade 3–6 embryo < BB (AC, CA, BC, CB). (a) Live birth rates of D5 and D6 blastocysts of same morphological grading. (b) Live birth rates of good-quality and low-quality blastocysts in embryos vitrified on the same developmental stage
If we focused on LBR only in the D5 group, good-quality embryos still yielded a significantly higher LBR than low-quality embryos (55.8 vs. 30.1%, p < 0.001). The same trend was also found in the D6 group; the LBR was much lower in low-quality embryo transfer cycles than in good-quality embryo transfer cycles (20.8 vs. 33.0%, p = 0.011). Multivariate regression analysis demonstrated that good-quality embryos were also associated with a statistically higher LBR than low-quality embryos in the D5 group (aOR = 2.81; 95% CI: [2.06–3.82]; p < 0.001) and in the D6 group (aOR = 1.87; 95% CI: [1.14–3.07]; p = 0.013) (Table 3). Confounding factors included in the model were patient age, BMI, duration of infertility (≤ 2 vs. 3–5 vs. > 5), basal FSH level, endometrial thickness, and morphological grading (good-quality vs. low-quality).
Biopsied Transfer Cycles
Table 4 shows patient demographic characteristics in biopsied transfer cycles. Overall, the euploidy rates were 56.5% of usable D5 blastocysts, 44.1% of usable D6 blastocysts, 58.1% of usable good-quality embryos, and 38.6% of usable low-quality embryos (D5 vs. D6: p < 0.001; good-quality vs. low-quality: p < 0.001). Women who underwent embryo transfers from the PGT-A procedure manifested no obvious difference between the D5 and D6 groups and the good-quality versus low-quality groups in terms of general characteristics. There was still a higher proportion of good-quality embryo transfer cycles in the D5 group than in the D6 group (74.5 vs. 44.3%, p < 0.001) and a higher proportion of D5 embryos in the good-quality embryo group than in the low-quality group (63.7 vs. 32.4%, p < 0.001).
Meanwhile, EMR was similar between the D5 and D6 groups (overall, 9.5 vs. 11.1%, respectively, p = 0.716). In contrast to nonbiopsied cycles, CPR in the two groups showed no obvious difference (overall, 57.1 vs. 51.1%, respectively, p = 0.259), and LBR did not either (overall, 48.9 vs. 44.9%, respectively, p = 0.444) (Table 5). There was a slight but not significant difference in favor of D5 blastocyst transfers over D6 transfers (aOR = 1.04; 95% CI: [0.67–1.62]; p = 0.862) (Table 6). In cases of the good-quality and low-quality embryo groups, CPR and LBR were significantly higher in good-quality group than those in low-quality group (58.6 vs. 47.6%, p = 0.040 for CPR; 51.6 vs. 40.0%, p = 0.030 for LBR). EMR depicted no statistical difference between the two groups (overall, 8.7 vs. 13.0%, respectively, p = 0.342) (Table 5). After controlling for all covariates (age, BMI, duration of infertility (≤ 2 vs. 3–5 vs. > 5), basal FSH level, endometrial thickness, developmental stage (D5 vs. D6), and morphological grading (good-quality vs. low-quality)), the difference in LBR between the groups reached no significance (aOR = 1.56; 95% CI: [0.99–2.45]; p = 0.056) (Table 6).
Figure 3 shows LBRs based on morphological grading and developmental stage in the subgroups. No significant differences of LBR were seen between the D5 and D6 groups both in good-quality embryo transfer cycles (51.8 vs. 51.3%, p = 0.939) and low-quality embryo transfer cycles (40.4 vs. 39.8%, p = 0.942). LBRs were higher in the good-quality embryo subgroup compared with that in low-quality subgroups for D5 blastocysts (51.8 vs. 40.4%, p = 0.177) and for D6 blastocysts (51.3 vs. 39.8%, p = 0.128), but the differences were not statistically significant after controlling for the variates (Table 6).
Comparison of live birth rate after single euploid blastocyst transfer according to embryo developmental stage (D5 and D6) and morphological grading (good quality and low quality). A good-quality embryo was defined as a grade 3–6 embryo ≥ BB (AA, AB, BA, BB) before vitrification. A low-quality embryo was classified as a grade 3–6 embryo < BB (AC, CA, BC, CB). (a) Live birth rates of D5 and D6 blastocysts of same morphological grading. (b) Live birth rates of good-quality and low-quality blastocysts in embryos vitrified on the same developmental stage
Discussion
The present study aimed to compare the SBT clinical outcomes in FET cycles according to different blastocyst developmental stages and morphological grading for patients who underwent PGT-A and non-PGT-A treatment. Our results showed that nonbiopsied blastocysts vitrified on day 5 had a significantly higher CPR (58.2 vs. 32.0%, p < 0.001) and LBR (48.5 vs. 24.3%, p < 0.001) than slower growing D6 blastocysts. When analyzing embryos with the same morphology, D5 blastocysts also exhibited significantly higher LBRs than D6 embryos. Moreover, LBRs were higher after transferring good-quality embryos than they were after transferring low-quality embryos in both D5 and D6 groups. In contrast to the nonbiopsied embryos, there were no differences in CPRs and LBRs in euploid embryo transfers after comparing different embryo stages and morphological grades.
More recently, numerous studies have prioritized transferring blastocysts cryopreserved on day 5 rather than day 6 in FET cycles [9,10,11,12,13,14]. Our data are consistent with previously reported results; when combined with both good- and low-quality embryos, blastocysts cryopreserved on day 5 yielded better FET results [11,12,13, 15]. This could be explained in part by the poorer embryo quality [10,11,12], increased abnormal spindle incidence [26], and higher aneuploidy rate of D6 blastocysts in comparison with D5 blastocysts [17, 19, 20, 27].
Although the overall pregnancy results were found to be better after transferring D5 blastocysts, there is a paucity of data comparing pregnancy outcomes of D5 and D6 blastocysts following the same quality embryo transfers. One study found that high-quality blastocysts from D5 and D6 exhibited a comparable CPR (52.4 vs. 52.6%, p = 0.97). For the poor-quality blastocysts, the CPR and IR in the D5 group were significantly higher than those in the D6 group [11]. The average number of embryos transferred in that study by Yang et al. was approximately 1.7 in the D5 group and 1.6 in the D6 group, which may have led to analysis errors. As our present study enrolled only single autologous blastocyst transfers, both good-quality and low-quality D5 embryos exhibited higher LBRs than the D6 blastocysts (55.8 vs. 33.0% and 30.1 vs. 20.8%; p < 0.001 and 0.012, respectively). Our results were also consistent with an article published by Ferreux et al. [12], which showed that good-quality D5 blastocysts had the best clinical outcomes when compared with good-quality D6 and low-quality D5 blastocysts. In addition, when comparing vitrified D5 blastocysts with only good-quality embryos (≥ 3 BB) vitrified on day 6, one study found that blastocysts of higher quality vitrified on day 6 still resulted in a significantly lower CPR than D5 blastocysts (34.4 vs. 44.7%, p = 0.007) [10]. It is therefore important to note that simply performing morphological assessment on D6 alone may be insufficient to indicate embryo potential. In fact, low-quality embryos have higher aneuploidy rates [11, 20, 28]. Capalbo et al. [28] showed that the euploidy rate of blastocysts was 56.4, 39.1, 42.8, and 25.5% in the excellent, good, average, and poor blastocyst morphology groups, respectively (p = 0.01). Another study found that the euploidy rate with high-quality morphology was significantly higher than that with poor-quality morphology (55.3 vs. 41.5%, p = 0.03) [11]. The suggested higher implantation potential of good-quality versus low-quality blastocysts could be explained in part by the 15% lower aneuploidy rate among good-quality embryos.
In a study of Haas et al. [10], D5 blastocysts were subjected to a warming procedure on day 5 of progesterone administration and then were transferred 20–24 h later, while the D6 embryos were thawed on day 6 of progesterone treatment and then were transferred after 2–4 h. Ferreux et al. [12] thawed the blastocysts in an incubator for 2–4 h before transferring to the uterus. The D5 and D6 embryos frozen in our research were both thawed on the 5th day after progesterone administration and then were transferred after an additional 16 h of incubation. However, the pregnancy results were similar with these two reports, which may further elucidate that both the embryo stage and morphology have obvious influences on pregnancy outcomes rather than transfer strategy. To conclude, regardless of embryo ploidy status, both embryo developmental stage (D5 and D6) and morphological grade (good quality and low quality) played prominent roles in FET outcomes.
For patients in favor of obtaining at least one euploid embryo, PGT-A has become a routine tool for ensuring healthy pregnancy outcomes. In China, the cost of PGT-A is totally covered by patients themselves, which is a huge financial burden for most of them. As a result, only patients with medical indications, such as RPL, RIF, AMA and severe male factor infertility, will choose PGT-A treatment. A total of 360 FET cycles were included in the present study for couples with the medical history described above. The LBR was found to be similar after euploid SBT transfer of D5 and D6 embryos (overall, 48.9% and 44.9%, p = 0.444). Meanwhile, CPR and EMR were not found to differ by day of blastocyst vitrification. It is also crucial to note that the results were totally different from those after transferring nonbiopsied embryos. An alternative and plausible explanation was that after eliminating the imbalanced aneuploidy rate between embryos vitrified on day 5 and day 6, D6 blastocysts could achieve comparable pregnancy outcomes with D5 embryos when only euploid embryo FETs were conducted. Moreover, our results were in accordance with those of several previous articles, indicating similar IR after euploidy transfers [19, 20, 27]. Yet, there is still a degree of controversy in the literature in this regard. Yang et al. [11] found that the CPR of D5 blastocyst transfers was much higher than that of D6 blastocysts (48.5 vs. 17.6%; p = 0.03), with a nearly 30% reduction in the D6 group. That study only included 50 PGT-A cycles, and the relatively small sample size might have amplified the analysis error. Additionally, a larger analysis (n = 701) also showed a higher LBR after transferring euploid D5 embryos than it did after transferring euploid D6 blastocysts [18]. We noticed a difference in patient cohorts between their study and ours, as PGT-A was routinely used in all infertile women in their study.
As our data showed no statistically significant difference in the LBRs was observed between the D5 and D6 groups. Nevertheless, the post hoc power analysis calculated a sample size of about 3100 will provide 80% power (α = 0.05) to detect a 5% absolute difference in LBR. Considering the limited sample size, one should be cautious before jumping to any conclusion. In the case of good-quality and low-quality blastocyst transfer, CPR (58.6 vs. 47.6%; p = 0.040) and LBR (51.6 vs. 40.0%; p = 0.030) were significantly higher in good-quality embryo group than those in low-quality embryo group. However, the statistical difference of LBR was not found after the multiple regression analysis (aOR 1.56; 95% CI 0.99–2.45; p = 0.056), possibly owing to the small sample size. There was a higher LBR trend toward good-quality embryos than low-quality embryos in both the D5 (51.8% (71/137) vs. 40.4% (19/47); p = 0.177) and D6 (51.3% (40/78) vs. 39.8% (39/98); p = 0.128) subgroups. Since all the embryos transferred were euploid blastocysts, theoretically, there should be no superior implantation potential between different morphologies within each group. We did see a tendency of favoring good-quality blastocysts. In fact, several articles confirmed that not all euploid blastocysts implant equally; blastocysts with a high morphology score exhibited a significantly higher LBR than those with low morphology score [18, 29]. It has been theorized that some other metabolic or epigenetic differences might play certain roles in that contribution [18]. Because an approximate sample size of 270 per group was needed to detect a LBR difference between 52 and 40%, there is also a necessity to enlarge the sample to confirm the obvious difference between blastocysts with different morphologies.
Though our study was not aimed to investigate the efficacy of PGT-A, it is also interesting to note that LBR was 41.2% (551/1336) in nonbiopsied group and was 46.9% (169/360) in biopsied group (p = 0.052), with no significant increase after PGT-A (Supplemental Table 1). We further noticed a 4% reduction of LBR following euploid D5 transfer compared to good-quality nonbiopsied D5 embryos (51.8 vs. 55.8%). The major underlying reason is the prominent difference of patient characteristics. In the PGT-A group, approximately 35% of patients had RPL, and 40% had RIF. Some authors found that patients with RPL less than 35 years old have a significantly higher rate of chromosomal abnormalities in blastocysts compared with patients with no or sporadic clinical miscarriage [30]. Although euploid embryos were transferred after PGT-A, young patients with RPL still had a worse clinical outcome [30]. Indeed, the cause of first-trimester pregnancy loss is largely due to aneuploidy, and the literature has not suggested an improved LBR when using PGT-A in patients with RPL [31]. Meanwhile, embryo quality and endometrial receptivity are two critical factors in implantation. For patients with RIF, endometrial receptivity played a much more important role than morphology grade and ploidy status. While PGT-A does help select the best quality embryo for transfer, the data currently demonstrate that PGT-A has no significant outcomes on CPRs and LBRs in patients with recurrent IVF failure [32]. Chromosomal abnormalities are not the only cause of RPL and RIF, given that many different etiological factors can contribute. Transferring euploid embryos can provide the maximum increase in IR and reduce the miscarriage rate due to aneuploidy.
Except for the different study populations, another possible explanation for the failure to achieve a more significant beneficial effect of PGT-A might be embryo damage after TE biopsy and misdiagnosis of NGS. A study by Zhang et al. [33] indicated that the biopsied TE cell number for blastocysts with poor TE morphological score negatively affects the embryo implantation potential. In addition, the biopsy timing is crucial for embryo viability. One research elucidated a detrimental impact on clinical outcomes if embryos were not expanded and hatching at the time of biopsy [34]. There is persistent discussion about the efficacy of PGT-A for different prognosis patients [35,36,37]. The mean patient age of the two populations in our study is about 30 years. A recent publication included 220 patients less than 35 years with at least two D5 blastocysts (≥ 2 BB) [38]. The participants were randomized to either the PGT-A group (n = 109) or morphology group (n = 111). There was no statistical difference in LBR between the euploid subgroup and morphology group (56.3% vs. 58.6%, aOR 0.91 (95% CI 0.51–1.63), p = 0.750). Their findings demonstrated that PGT-A does not increase LBR for young patients, suggesting that the effectivity of PGT-A may be offset by the biopsy impairment. In conclusion, there exists negative effects of biopsy damage on embryo implantation potential that is outweighed by the benefit of PGT-A. Other possible reasons for the decreased pregnancy rates in PGT-A patients still need to be disclosed.
Notwithstanding our best efforts to avoid biases in the current study, there are still some limitations and shortcomings in the research. First, the retrospective nature of the study increases the possibility of selection bias. Another limitation of our study is the relatively inadequate sample size of biopsied embryo transfer cycles (n = 360), which may compromise the sufficient effectiveness of validation. Furthermore, the morphology score was based on a combination of ICM and TE grade instead of each separate value or the cavity expansion level. Most of the transferred embryos in this study reached stages 4, 5, and 6, and there is a lack of data on embryos transferred at stage 3 of expansion. Therefore, researching pregnancy results on ICM morphology, TE morphology, or cavity expansion level needs further exploration.
To the best of our knowledge, the present study is the largest clinical study of autologous SBT treatment containing blastocysts vitrified on day 5 and day 6 where embryo quality is based on morphological grading and ploidy status. One strength of our study is that we demonstrated that both developmental stages, morphological criteria, and ploidy status are paramount factors indicating blastocyst quality and influencing FET outcomes. In clinical daily practice, when encountering the question of which blastocyst to transfer, the first choice for patients who did not undergo PGT-A is D5 embryos with a good morphological grade. For patients who have PGT-A indications, regardless of embryo stage and morphology, any euploid embryo can hold a promising clinical result and should be considered for transfer.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Reljic M, Knez J, Kovac V, Kovacic B. Endometrial injury, the quality of embryos, and blastocyst transfer are the most important prognostic factors for in vitro fertilization success after previous repeated unsuccessful attempts. J Assist Reprod Genet. 2017;34(6):775–9. https://doi.org/10.1007/s10815-017-0916-4.
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118. https://doi.org/10.1002/14651858.CD002118.pub5.
American College of O, Gynecologists, Society for Maternal-Fetal M. ACOG Practice Bulletin No. 144: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123(5):1118–32. https://doi.org/10.1097/01.AOG.0000446856.51061.3e.
Practice Committee of Society for Assisted Reproductive T, Practice Committee of American Society for Reproductive M. Elective single-embryo transfer. Fertil Steril. 2012;97(4):835–42. https://doi.org/10.1016/j.fertnstert.2011.11.050.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. https://doi.org/10.1016/j.fertnstert.2014.04.018.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907 e3. https://doi.org/10.1016/j.fertnstert.2015.06.031.
Franasiak JM, Forman EJ, Patounakis G, Hong KH, Werner MD, Upham KM, et al. Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open. 2018;2018(4):hoy022. https://doi.org/10.1093/hropen/hoy022.
Sunkara SK, Siozos A, Bolton VN, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. 2010;25(8):1906–15. https://doi.org/10.1093/humrep/deq143.
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106(6):1370–8. https://doi.org/10.1016/j.fertnstert.2016.07.1095.
Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33(12):1553–7. https://doi.org/10.1007/s10815-016-0818-x.
Yang H, Yang Q, Dai S, Li G, Jin H, Yao G, et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet. 2016;33(7):865–72. https://doi.org/10.1007/s10815-016-0712-6.
Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33(3):390–8. https://doi.org/10.1093/humrep/dey004.
Tubbing A, Shaw-Jackson C, Ameye L, Colin J, Rozenberg S, Autin C. Increased live births after day 5 versus day 6 transfers of vitrified-warmed blastocysts. J Assist Reprod Genet. 2018;35(3):417–24. https://doi.org/10.1007/s10815-017-1097-x.
Sciorio R, Thong KJ, Pickering SJ. Increased pregnancy outcome after day 5 versus day 6 transfers of human vitrified-warmed blastocysts. Zygote. 2019;27(5):279–84. https://doi.org/10.1017/S0967199419000273.
Bourdon M, Pocate-Cheriet K, Finet de Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, et al. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34(10):1948–64. https://doi.org/10.1093/humrep/dez163.
Preimplantation Genetic Testing: ACOG Committee Opinion, Number 799. Obstet Gynecol. 2020;135(3):e133–e7. https://doi.org/10.1097/AOG.0000000000003714.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34(9):1697–706. https://doi.org/10.1093/humrep/dez129.
Irani M, O’Neill C, Palermo GD, Xu K, Zhang C, Qin X, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95–102 e1. https://doi.org/10.1016/j.fertnstert.2018.03.032.
Tiegs AW, Sun L, Patounakis G, Scott RT. Worth the wait? Day 7 blastocysts have lower euploidy rates but similar sustained implantation rates as day 5 and day 6 blastocysts. Hum Reprod. 2019;34(9):1632–9. https://doi.org/10.1093/humrep/dez138.
Whitney JB, Balloch K, Anderson RE, Nugent N, Schiewe MC. Day 7 blastocyst euploidy supports routine implementation for cycles using preimplantation genetic testing. JBRA Assist Reprod. 2019;23(1):45–50. https://doi.org/10.5935/1518-0557.20180089.
Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31(11):1475–81. https://doi.org/10.1007/s10815-014-0317-x.
Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014;29(12):2802–13. https://doi.org/10.1093/humrep/deu277.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11. https://doi.org/10.1097/00001703-199906000-00013.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. https://doi.org/10.1016/j.theriogenology.2006.09.014.
Makrakis E, Angeli I, Agapitou K, Pappas K, Dafereras A, Pantos K. Laser versus mechanical assisted hatching: a prospective study of clinical outcomes. Fertil Steril. 2006;86(6):1596–600. https://doi.org/10.1016/j.fertnstert.2006.05.031.
Hashimoto S, Amo A, Hama S, Ito K, Nakaoka Y, Morimoto Y. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. 2013;28(6):1528–35. https://doi.org/10.1093/humrep/det059.
Majumdar G, Majumdar A, Verma IC, Upadhyaya KC. Relationship between morphology, euploidy and implantation potential of cleavage and blastocyst stage embryos. J Hum Reprod Sci. 2017;10(1):49–57. https://doi.org/10.4103/0974-1208.204013.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81. https://doi.org/10.1093/humrep/deu033.
Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70. https://doi.org/10.1016/j.fertnstert.2016.11.012.
Liu XY, Fan Q, Wang J, Li R, Xu Y, Guo J, et al. Higher chromosomal abnormality rate in blastocysts from young patients with idiopathic recurrent pregnancy loss. Fertil Steril. 2020;113(4):853–64. https://doi.org/10.1016/j.fertnstert.2019.11.016.
Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive Technology. Electronic address Aao, Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive T. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–36. https://doi.org/10.1016/j.fertnstert.2018.01.002.
Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):121. https://doi.org/10.1186/s12958-018-0414-2.
Zhang S, Luo K, Cheng D, Tan Y, Lu C, He H, et al. Number of biopsied trophectoderm cells is likely to affect the implantation potential of blastocysts with poor trophectoderm quality. Fertil Steril. 2016;105(5):1222–7 e4. https://doi.org/10.1016/j.fertnstert.2016.01.011.
Singh S, Hobeika E, Knochenhauer ES, Traub ML. Pregnancy rates after pre-implantation genetic screening for aneuploidy are only superior when trophectoderm biopsy is performed on hatching embryos. J Assist Reprod Genet. 2019;36(4):621–8. https://doi.org/10.1007/s10815-019-01400-5.
Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602. https://doi.org/10.1016/j.fertnstert.2016.04.027.
Kushnir VA, Darmon SK, Albertini DF, Barad DH, Gleicher N. Effectiveness of in vitro fertilization with preimplantation genetic screening: a reanalysis of United States assisted reproductive technology data 2011-2012. Fertil Steril. 2016;106(1):75–9. https://doi.org/10.1016/j.fertnstert.2016.02.026.
Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. https://doi.org/10.1016/j.fertnstert.2013.07.2002.
Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36(4):629–36. https://doi.org/10.1007/s10815-018-01399-1.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number 81871210, 81971386, 81771536 and 81471457) and Natural Science Foundation of Jiangsu Province (BK20171126).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Nanjing Maternity and Child Health Care Hospital (NJFY-2020KY-051).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb).
Rights and permissions
About this article
Cite this article
Ji, H., Zhou, Y., Cao, S. et al. Effect of Embryo Developmental Stage, Morphological Grading, and Ploidy Status on Live Birth Rate in Frozen Cycles of Single Blastocyst Transfer. Reprod. Sci. 28, 1079–1091 (2021). https://doi.org/10.1007/s43032-020-00381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00381-6