Abstract
Purpose
Whether there are differences in the pregnancy outcomes of blastocysts cryopreserved during different developmental stages remains under debate because the results among studies are inconsistent. We analyzed blastocyst quality and pregnancy outcomes by considering blastocyst euploidy and investigated the differences in the development potential between blastocysts of different developmental stages (frozen-thawed day 5 [D5] and day 6 [D6] cycles) and their relationship with clinical pregnancy outcomes.
Methods
In total, 1374 D5 and 255 D6 frozen-thawed blastocyst transfer cycles were retrospectively analyzed. Additionally, the chromosome euploidy and clinical pregnancy rates of 237 blastocysts from 50 pre-implantation genetic diagnosis (PGS) cycles were statistically analyzed. The corresponding euploidy rate and pregnancy outcomes of the D5 and D6 blastocyst transfers were also compared.
Results
The clinical pregnancy rate (47.2 vs 40.0 %; P = 0.04) and implantation rate (34.2 vs 28.8 %; P = 0.03) of the D5 blastocysts were higher than were those of the D6 blastocysts. However, the clinical pregnancy rate (52.4 vs 52.6 %; P = 0.97) and implantation rate (38.9 vs 35.6 %; P = 0.39) of the high-quality D5 blastocysts did not significantly differ from those of the high-quality D6 blastocysts. Analysis of blastocyst euploidy in 237 blastocysts examined in 50 PGS cycles showed that the euploidy rates of the D5 and D6 blastocysts were both 48.1 % (P = 0.99). The clinical pregnancy rate of the D5 blastocysts (48.5 vs 17.6 %; P = 0.03) was higher than that of the D6 blastocysts. The euploidy rates (55.2 vs 55.3 %; P = 0.99) and clinical pregnancy rates (60.0 vs 42.9 %; P = 0.77) of the high-quality D5 and D6 blastocysts did not differ. The euploidy rate (55.3 vs 41.5 %, P = 0.03) and clinical pregnancy rate (54.5 vs 25.0 %, P = 0.03) of the high-quality blastocysts were higher than were those of the poor-quality blastocysts.
Conclusions
The euploidy rates between the D5 and D6 blastocysts did not differ. High-quality D6 blastocysts in frozen-thawed cycles had similar developmental potential and pregnancy outcomes compared to those of high-quality D5 blastocysts. The quality of the blastocysts was an important factor that affected the pregnancy outcomes of the frozen-thawed cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continuous improvement of embryo culture systems and culture environments in recent years has resulted in the increased development and application of blastocyst cultures and transfers in the field of assisted reproductive technology. Blastocyst cultures are conducive to screening embryos that have a greater development potential; this approach can cause embryo-endometrium synchrony. These cultures not only maintain better pregnancy outcomes but also reduce risks due to multiple pregnancies; therefore, blastocyst cultures are a better physiological choice [1]. Blastocyst culture and transfer (particularly elective single-embryo transfer (eSET)) are being used by an increasing number of reproductive centers [2, 3]. Current studies have shown that the clinical pregnancy rate and implantation rate of blastocysts that formed in the fresh embryo transfer cycle and that were transferred on day 5 (D5) were higher than the rates on day 6 (D6) [4, 5]. Most studies proposed that this phenomenon might be due to the reduction of endometrial receptivity during D6 implantation [6, 7]. However, the pregnancy outcomes of D5 and D6 blastocyst transfers in different studies using frozen-thawed cycles were contradictory. The studies of Marek et al. [8] and Kovalevsky et al. [9] showed that the clinical pregnancy rate and implantation rate of D5 frozen-thawed blastocyst transfers were both higher than those of D6; however, the studies of Behr et al. [10] and Kenichiro et al. [11] did not observe a significant difference in the implantation and pregnancy rates between D5 and D6 blastocysts. Levens et al. [12] thawed frozen D5 and D6 expanded blastocysts and showed that the implantation rate of the D5 frozen-thawed blastocysts was significantly higher than that of the D6 frozen-thawed blastocysts. However, there was no significant difference in the clinical pregnancy rates between these groups.
Previous relevant studies only performed simple statistical analyses of the clinical outcomes of frozen-thawed cycles. This study is the first to consider blastocyst chromosome euploidy data. The clinical outcomes of frozen-thawed blastocyst cycles were retrospectively analyzed in our center from January 2014 to June 2015. The euploidy rate and pregnancy conditions of 237 blastocysts from 50 pre-implantation genetic diagnosis (PGS) patients during the same period were also investigated. The pregnancy outcome, embryo quality, and blastocyst euploidy analyses were combined to provide a more in-depth investigation into the differences in the development potential of embryos frozen-thawed on different development days (D5 and D6) and their relationship with clinical pregnancy outcomes.
Materials and methods
Study subjects
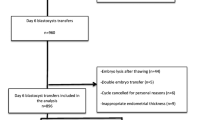
In total, 1732 cycles of frozen-thawed blastocyst transfers were performed in our center from January 2014 to June 2015; of these cycles, 103 cycles of simultaneous D5 and D6 embryo transfers were excluded. A total of 1629 cycles, including blastocyst transfer cycles undergoing 50 PGS cycles, were included in the statistical analyses. All blastocysts were divided into the D5 cryopreservation group (n = 1374) and the D6 cryopreservation group (n = 255) according to the day of embryo development (Fig. 1). The general characteristics of the patients in the D5 and D6 groups (patient age, infertility duration, body mass index (BMI), basal follicle-stimulating hormone (FSH) levels, endometrial thickness, and average implanted embryo number) (P > 0.05; Table 1) did not significantly differ. Patients who met one of the following conditions and signed an informed consent form could receive PGS treatment: (1) age ≥36 years, (2) at least three implantation failures after high-quality embryo transfer, and (3) at least two miscarriages. This study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. All involved patients signed an informed consent form.
Blastocyst culture and scoring
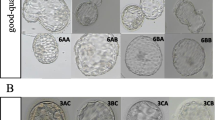
The egg collection day was D0. Eggs were fertilized by conventional in vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI). On D3 of fertilization, two embryos with the best quality were selected for embryo transfer. If the transfer could not be performed due to special conditions, three embryos with the best quality were cryopreserved on D3, and other embryos were transferred into G2 culture medium (Vitrolife, Sweden) and cultured in an incubator under 5 % O2, 6 % CO2, and 89 % N2. The formation and morphology of the blastocysts were observed on D5 and D6, and blastocyst scoring was performed according to the Gardner scoring system [13]. First, staging was performed based on the expansion level of the blastocysts: in stage 1, the blastocoel was present during the early stage, and the blastocoel cavity was less than 50 % of the total volume of the embryo; in stage 2, the blastocoel cavity was more than 50 % of the total volume of the embryo; in stage 3, the blastocyst and cavity completely filled the entire embryo; in stage 4, the blastocyst expanded and the zona pellucida thinned; in stage 5, the blastocyst was being hatched; and in stage 6, the blastocyst was completely hatched. The blastocysts at stages 3–6 were further evaluated for inner cell mass (ICM)/trophectoderm (TE) quality. (1) ICM scoring was categorized as follows: A (many tightly packed, distinguishable ICM cells), B (several loosely grouped ICM cells), and C (very few small ICM cells). (2) TE scoring was categorized as follows: A (many TE cells forming an epithelium with a dense structure), B (a few cells forming a loose epithelium), and C (very few and loose cells forming an epithelium). D5 or D6 blastocysts were scored; blastocysts at stage 3 or above were cryopreserved. If the ICM and TE scores were both above grade B (3BB), the blastocyst was defined as a high-quality blastocyst; otherwise, it was considered a poor-quality blastocyst (Supplementary Fig. 1).
Blastocyst cryopreservation and thawing procedures
Before cryopreservation, the blastocysts were treated with a laser to induce artificial shrinkage [14]. A 2.0-ms laser (Octax Laser Shot™ System, Germany) was used to generate a hole in the TE cell junction away from the ICM location to induce blastocoel shrinkage. Cryopreservation of the blastocysts was achieved using the Cryotop method established by Kuwayama et al. [15]. The vitrification solutions for the frozen-thawed cycles and the carriers were all purchased from Kitazato (Japan). The specific methods were as follows. After shrinkage, the blastocysts were transferred into equilibration solution (ES) and allowed to equilibrate at room temperature for 5–10 min. Then, the blastocysts were washed in vitrification solution (VS) for 1 min, placed in the Cryotop, and directly immersed in liquid nitrogen. After the casing was installed, the blastocysts were cryopreserved. While the blastocysts thawed, the Cryotop was removed and directly immersed in No. 1 thawing solution (TS) prewarmed to 37 °C for less than 1 min. The embryos were immediately transferred to No. 2 TS (dilution solution (DS)) and incubated at room temperature for 3 min. After the embryos were incubated in No. 3 solution (No. 1 washing solution (WS1)) and No. 4 solution (No. 2 washing solution (WS2)) for 5 min each, they were transferred to culture dishes with G2 prewarmed to 37 °C. After the embryos were cultured in an incubator for 24 h, blastocyst expansion was observed. Partial or complete expansion of blastocoels was considered survival, and these cultures could be used for transfer.
Blastocyst biopsy and euploidy diagnosis
Blastocyst culturing was performed after ICSI fertilization for all patients who received PGS. No fresh transfers were performed in any PGS cycle. All embryos were used for blastocyst culture. Biopsy was performed after the blastocysts fully expanded. Because D7 blastocyst culture was not performed, all D6 blastocysts above grade III were used for biopsy. The biopsy process followed the method previously proposed by McArthur et al. Eggs were cultured to D3 after fertilization by ICSI. An 8–10-μm small opening was made in the zona pellucida at the distal gap junctions connecting the blastomeres of the embryo using a laser (Octax Laser Shot™ System, Germany) for assisted hatching. After the blastocysts developed to D5/D6, when the blastocyst expanded and some trophoblasts were hatched, blastocysts were placed in G-MOPS drops in Petri dishes. Blastocysts were fixed using holding pipettes. Hatched trophoblasts were aspirated using a biopsy needle, and three to five trophoblasts were collected using the laser cutting method combined with aspiration biopsy. Biopsy of unhatched blastocysts was performed using a biopsy needle to aspirate some trophoblasts from the opening in the zona pellucida under negative pressure. All the biopsy procedures were performed on the heated stage of a Nikon IX-70 microscope equipped with micromanipulation tools. The ectodermal trophoblasts were collected and placed in 5 μl of 0.2 N KOH for cell lysis; then, whole-genome amplification (WGA) was performed. Chromosome aneuploidy in the blastocysts was detected using a Human CytoSNP-12DNA array (Illumina, San Diego, CA, USA) and an Illumina HiScanSQ BeadArray Reader. The specific steps of this method were described previously [16]. The euploidy rate was defined as the ratio of the number of blastocysts with normal chromosomes and the total number of blastocysts subjected to SNP array detection.
Endometrial programming and observational indicators
All frozen-thawed cycles of endometrium preparation and blastocyst transfer were divided into the natural cycle (NC) group and the artificial cycle (hormone replacement therapy (HRT)) group based on the implantation programs. NC was applicable for patients with a regular menstrual cycle. Follicular development was monitored using B ultrasound on days 8–10 of menstruation. The follicular and endometrial development conditions were assessed and combined with the estradiol (E2) and luteinizing hormone (LH) levels to confirm the ovulation time. Embryo transfer was performed on D5 of ovulation. HRT was applicable for patients with an irregular menstrual cycle, ovulation disorder, or poor endometrial and follicular development in NC. Starting from days 2–3 of menstruation, 2–4 mg/day of estradiol valerate (Progynova, Bayer, Germany) was administered, and the endometrial thickness and serum E2 levels were monitored using B ultrasound. When the endometrial thickness was at least 8 mm, progesterone was additionally administered. Embryo transfer was performed on day 6 of progesterone injection. Observation of the gestational sac by B ultrasound at 35 days after implantation was diagnosed as clinical pregnancy. The implantation rate was defined as the ratio between the number of gestational sacs observed under B ultrasound and the number of transferred blastocysts. First, trimester abortion was defined as the loss of pregnancy without human intervention before 12 weeks of pregnancy.
Statistical analyses
Statistical analyses were performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation (\( \overline{\mathrm{x}}\pm \mathrm{s} \)). The mean values of two groups were compared using the independent samples t test. Percentages were compared using the χ 2 test. P < 0.05 was defined as statistical significance.
Results
Basic patient information
The clinical pregnancy rate and transfer rate in the D5 group were significantly higher than were those in the D6 group (P = 0.04 and 0.03, respectively). The ectopic pregnancy rate in the D5 group was lower than that in the D6 group (P = 0.03). The thawed blastocyst survival rate and early miscarriage rate did not significantly differ between these two groups (Table 1).
The clinical outcomes of the high-quality blastocysts from the D5 and D6 groups did not significantly differ.
The clinical outcome of the poor-quality blastocysts in the D5 group was higher than that in the D6 group. In total, 677 cycles of cryopreserved transfers in the D5 group were high-quality blastocysts, whereas 114 cycles in the D6 group were high-quality blastocysts. The pregnancy outcomes are shown in Fig. 2a. In the high-quality embryo transfer group, the clinical pregnancy rate, implantation rate, first-trimester abortion rate, and ectopic pregnancy rate of high-quality embryos did not exhibit significant differences between the D5 and D6 groups (P = 0.97, 0.39, 0.93, and 0.06, respectively). For the poor-quality blastocysts, the clinical pregnancy rate and implantation rate in the D5 group were significantly higher than those in the D6 group (P = 0.01 and 0.04, respectively), whereas the early abortion rate and the ectopic pregnancy rate did not significantly differ (P = 0.21 and 0.83, respectively) (Fig. 1b).Statistical analysis based on blastocyst quality in the thawing cycles showed that the clinical pregnancy rate and implantation rate in the high-quality blastocyst group were significantly higher than those in the poor-quality blastocyst group (P < 0.01), whereas the first-trimester abortion rate and the ectopic pregnancy rate did not significantly differ between these groups (P = 0.14 and 0.73, respectively) (Fig. 2c).
The relationship between blastocyst development day, quality, and clinical pregnancy outcome. Only statistically significant P values are reported: *P < 0.05, ^P < 0.01. a Pregnancy outcome of high-quality blastocysts in D5 and D6. b Pregnancy outcome of poor-quality blastocysts in D5 and D6. c Pregnancy outcome of high- and poor-quality blastocysts. CPR clinical pregnancy rate, IR implantation rate, FTAR first-trimester abortion rate, EPR ectopic pregnancy rate
Significant differences in the euploidy rates of the 237 blastocysts in 50 PGS cycles were not detected between the D5 and D6 groups.
Sixty-two blastocysts of 50 PGS transfer cycles were thawed, and all blastocysts survived; thus, the thawed survival rate was 100 %.The euploidy rate and the clinical pregnancy rate of the high-quality blastocysts were both significantly higher than were those of the poor-quality blastocysts. The euploidy rates in the D5 and D6 groups were both 48.1 % and thus did not exhibit a difference. However, the clinical pregnancy rate in the D5 group was significantly higher than that in the D6 group (48.5 vs 17.6 %; P = 0.03) (Fig. 3a).The euploidy rate and clinical pregnancy rate of the high-quality blastocysts in the D5 and D6 groups did not significantly differ (Fig. 3b). The euploidy rate and clinical pregnancy rate of the poor-quality blastocysts in the D5 group were both higher than were those in the D6 group; however, the differences were not significant (P > 0.05; Fig. 3c). The statistical analysis based on blastocyst quality showed that the euploidy rate (55.3 vs 41.5 %) and the clinical pregnancy rate (54.5 vs 25.0 %) of the high-quality blastocysts were both higher than those of the poor-quality blastocysts (P = 0.03 in both analyses; Fig. 3d).
Chromosome euploidy rate and clinical pregnancy rate of 237 blastocysts in 50 cases of PGS cycles. Only statistically significant P values are reported: *P < 0.05. a Euploidy rate and clinical pregnancy rate of D5 and D6. b High-quality blastocysts of D5 and D6. c Poor-quality blastocysts of D5 and D6. d Comparison of high- and poor-quality blastocysts. ER euploidy rate, CPR clinical pregnancy rate
Discussion
Assisted reproductive technology has developed rapidly in recent years. Thus, increasing attention has been given to pregnancy quality. With the stable increase in the laboratory blastocyst culture system of embryos and frozen-thawed embryo technology, blastocyst culture and transfer have become the preferred methods of an increasing number of reproductive centers. Recently, vitrification technology and methods have matured, and the thawed blastocyst survival rate has achieved satisfactory improvement [17]. The thawed blastocyst survival rate in this study reached 94.5 % and was not correlated with the development day (D5 and D6) or quality (high quality and poor quality) of the blastocyst, similar to the results of Liebermann et al. [18].
Many studies have examined the pregnancy outcomes of frozen-thawed blastocyst cycles in recent years. The comparison of pregnancy outcomes between D5 and D6 blastocysts remains controversial. Some reports have shown that the clinical pregnancy rate and implantation rate of D5 frozen-thawed blastocyst transfer cycles were higher than those of D6 blastocyst transfers [8, 19]; however, other reports have shown that the clinical outcomes between D5 and D6 cryopreserved blastocyst transfers did not significantly differ [20]. The pregnancy rate and implantation rate of D5 frozen-thawed cycles in this study were both higher than were those of D6 (47.2 vs 40.0 % and 34.2 vs 28.8 %, respectively; P = 0.03 for both analyses). However, the comparison of pregnancy outcomes of high-quality blastocysts between these groups showed that the pregnancy rates and implantation rates were not significantly different (52.4 vs 52.6 % and 38.9 vs 35.6 %; P = 0.97 and 0.39, respectively). Moreover, the pregnancy rate and implantation rate of the poor-quality D6 blastocysts were significantly lower than were those of the poor-quality D5 blastocysts (42.0 vs 29.8 % and 29.7 vs 23.1 %; P = 0.01 and 0.04, respectively). These overall results showed that the differences in pregnancy outcomes between D5 and D6 might be caused by the differences in poor-quality blastocysts. The conclusions in this study were similar to those in a recent meta-analysis by Sunkara et al. [21]. The study by Sunkara et al. included 15 articles on frozen-thawed blastocyst transfers containing 2512 frozen-thawed blastocyst transfer cycles. The statistical analysis showed that the clinical pregnancy rate, ongoing pregnancy rate, and live birth rate after frozen-thawed blastocyst transfer in the D5 group were significantly higher than were those in the D6 group. However, precise comparisons of frozen-thawed blastocyst transfers with D5 and D6 blastocysts that developed into the same stage revealed that the outcomes did not achieve statistical significance.
In addition to the differences caused by the commonly mentioned blastocyst culture systems and freeze-thaw methods, many other factors affect the clinical outcomes of frozen-thawed cycles. Different blastocyst culture strategies and methods in different centers are also important reasons for the differences in study results. Some reproductive centers perform both blastocyst culture and blastocyst transfer (i.e., all embryos receive blastocyst cultures, and high-quality blastocysts are selected for transfer and cryopreservation). Conversely, some centers primarily perform D3 embryo transfers, and patients with grade III embryos or a greater number of embryos receive blastocyst cultures. Our center selects high-quality D3 embryos in the fresh cycles for implantation; the other embryos are used for blastocyst culture, resulting in a low formation rate of blastocysts and a low-percentage of high-quality blastocysts. Additionally, blastocyst scoring has stronger subjectivity, which is also an important reason for the differences in studies from different centers.
We analyzed data from 237 blastocysts from 50 patients undergoing PGS during the same period. The results showed that the euploidy rates of D5 and D6 blastocysts were 48.1 % (75/156) and 48.1 % (39/81), respectively (P = 0.99). The percentages of normal chromosomes in the D5 and D6 blastocysts were identical, suggesting that they had very similar development potentials. However, the euploidy rate of the high-quality blastocysts was 55.3 % (63/114), which was significantly higher than the 41.5 % (51/123) observed for the poor-quality blastocysts (P = 0.03). Therefore, we speculated that the low euploidy rate in the poor-quality blastocysts was an important reason for the low development potential and thus affected the clinical pregnancy and implantation rates. Capalbo et al. analyzed 956 blastocysts from 223 PGS cycles and showed that the euploidy rate of the blastocysts with high-quality morphology was significantly higher than that of the blastocysts with poor-quality morphology. The development and implantation potentials of the blastocysts were closely associated with euploidy but were not significantly associated with the quality and the developmental day [22]. Our study and the study of Capalbo et al. obtained consistent conclusions. In this study, the results of the PGS data analysis were consistent with the results obtained for the entire frozen-thawed cycles; thus, we speculated that the outcomes of blastocyst pregnancy in the frozen-thawed cycles were closely associated with blastocyst quality but not the blastocyst development day (D5 and D6). These results suggested that embryos that did not develop into the blastocyst stage on D5 or that developed into early-stage blastocysts could be cultured for one more day to D6 in the clinic because the high-quality blastocysts that formed on D6 and the high-quality blastocysts that formed on D5 had similar pregnancy outcomes.
Multifactorial logistic regression analysis was performed using the clinical outcome as a dependent variable (pregnancy = 1 and nonpregnancy = 2) and factors that might influence the clinical pregnancy rate, such as age, infertility duration, BMI, basal FSH, endometrial thickness, implanted embryo number, embryonic development day (D5/D6), and embryo quality (high quality/poor quality), as independent variables. The results showed that endometrial thickness (P = 0.01), implanted embryo number (P < 0.01), and embryo quality (P < 0.01) significantly correlated with the pregnancy outcome, while other observational indexes did not correlate with the clinical outcome (P > 0.05). In total, 1110 cycles in this study were implanted with two blastocysts and accounted for 68.1 % of total transplantation cycles. The results of logistic regression analysis also suggested that an increase in the number of implanted embryos could increase the clinical pregnancy rate (P < 0.01). However, the increase in the number of implanted embryos would definitely introduce many risk factors, such as an increase in the multiple pregnancy rate. Therefore, increasing the number of implanted embryos in an attempt to increase the pregnancy rate is not the best approach. The results of logistic regression analysis were consistent with the results of this study, which showed that the clinical pregnancy rate was not associated with the blastocyst development day (P = 0.50) but closely associated with the quality of blastocysts (P < 0.01).
Because of the work strategy, D7 blastocysts were not included in this study. However, a study by Capalbo et al. showed that D7 blastocysts had similar euploidy rates and better ongoing implantation rates compared with D5 and D6 blastocysts. A study by Kovalevsky et al. also showed that D7 blastocysts had higher utilization values. Notably, D6 and D5 blastocysts in this study had similar euploidy rates. Although the clinical pregnancy rate of D6 blastocysts was lower than that of D5 blastocysts, this result did not suggest disposing of the frozen blastocysts formed on D6 or D7 because slow-growing frozen-thawed blastocysts also had relatively high clinical pregnancy rates. In particular, these slow-growing euploidy blastocysts are an economic choice for increasing embryo utilization rates in patients with fewer embryos in clinical practice. The thawing of D5 or D6 high-quality blastocysts is favored in frozen-thawed blastocyst transfers in clinical practice. The preferential selection of blastocysts with excellent development potential for implantation could further increase the implantation rate and pregnancy rate, allow patients to achieve pregnancy as soon as possible, and decrease the psychological and economic burdens of patients.
As a retrospective analysis, this study definitely produced biases, some of which resulted from the culture and implantation strategies of our center. In addition, this study used the data of 50 PGS cycles to validate the frozen-thawed cycle results with a larger sample size. The smaller sample size of the PGS cycles and insufficient effectiveness of validation were also limitations of this study. This study is the first to combine the detection of chromosome aneuploidy in PGS blastocysts and a genetic perspective to analyze the differences in euploidy in blastocysts on different development days and with varied quality. Therefore, the factors that affected the blastocyst pregnancy outcomes in the frozen-thawed cycles were objectively investigated based on the embryonic factor of blastocyst development potential. Overall, the euploidy rate of the D6 blastocysts did not significantly differ from the rate of the D5 blastocysts. Moreover, blastocyst quality was an important factor that affected the pregnancy outcome in the frozen-thawed cycles. The euploidy rate in the high-quality blastocysts was higher than that in the poor-quality blastocysts; the greater development potential might be an important reason for the higher pregnancy rate obtained with the high-quality blastocysts than with the poor-quality blastocysts. In the clinic, high-quality D6 blastocysts in frozen-thawed cycles had developmental potential and pregnancy outcomes similar to those of high-quality D5 blastocysts.
References
Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118.
Sullivan EA, Wang YA, Hayward I, Chambers GM, Illingworth P, McBain J, et al. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum Reprod. 2012;27:3609–15.
Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update. 2011;17(1):107–20.
Barrenetxea G, Lopez de Larruzea A, Ganzabal T, Jimenez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83:49–53.
Utsunomiya T, Ito H, Nagaki M, Sato J. A prospective, randomized study: day 3 versus hatching blastocyst stage. Hum Reprod. 2004;19:1598–603.
Shapiro B, Daneshmand S, Garner F, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. 2008;89:20–6.
Van Voorhis BJ, Dokras A. Delayed blastocyst transfer: is the window shutting? Fertil Steril. 2008;89(1):31–2.
Marek DM, Langley MT, McKean C, et al. Frozen embryo transfer (FET) of day 5 blastocyst embryos compared to transfer of day 6 blastocyst embryos. Fertil Steril. 2000;74(1):S52–3.
Kovalevsky G, Carney SM, Morrison LS, et al. Should embryos developing to blastocysts on day 7 be cryopreserved and transferred: an analysis of pregnancy and implantation rates. Fertil Steril. 2013;100:1008–12.
Behr B, Gebhardt J, Lyon J, Milki A. Factors relating to a successful cryopreserved blastocyst transfer program. Fertil Steril. 2002;77:697–9.
Hiraoka K, Hiraoka K, Miyazaki M, Fukunaga E, Horiuchi T. Perinatal outcomes following transfer of human blastocysts vitrified at day 5, 6 and 7. J Exp Clin Assist Reprod. 2009;6:4.
Levens E, Whitcomb B, Hennessy S, James A, Yauger B, Larsen F. Blastocyst development rate impacts outcome in cryopreserved blastocyst transfer cycles. Fertil Steril. 2008;90:2138–43.
Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Mukaida T, Goto OC, et al. Artificial shrinkage of blastocoels using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Repord. 2006;21(12):3246–52.
Kuwayama M, Vajta G, Kato O, et al. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11(3):300–8.
Li G, He N, Jin H, Liu Y, et al. The influence of single nucleotide polymorphism microarray-based molecular karyotype on preimplantation embryonic development potential. PLoS One. 2015;10(9):e0138234.
Stanger J, Wong J, Conceicao J, Yovich J. Vitrification of human embryos previously cryostored by either slow freezing or vitrification results in high pregnancy rates. Reprod Biomed Online. 2012;24(3):314–20.
Liebermann J. Vitrification of human blastocysts: an update. Reprod Biomed Online. 2009;19 Suppl 4:4328.
Liebermann J, Tucker M. Comparison of vitrification and conventional cryopreservation of day 5 and 6 blastocysts during clinical application. Fertil Steril. 2006;86:20–6.
El-Toukhy T, Wharf E, Walavalkar R, Singh A, Bolton V, Khalaf Y, et al. Delayed blastocyst development does not influence the outcome of frozen thawed transfer cycles. BJOG. 2011;118:1551–6.
Sunkara S, Siozos A, Bolton V, Khalaf Y, Braude P, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. 2010;25(8):1906–15.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The euploidy rates between the D5 and D6 blastocysts did not differ. High-quality D5 and D6 blastocyst transfers had similar pregnancy outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Different grades of blastocysts. A-O represent blastocysts of 3AA,3AB, 3BB,4AA,4AB,4BB,5BB,6BB,3 AC,3BC,4 AC,4BC,4CB, 4CC and 5BC, respectively. Bar = 50 μm. (DOCX 1118 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Yang, Q., Dai, S. et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet 33, 865–872 (2016). https://doi.org/10.1007/s10815-016-0712-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0712-6