Abstract
The present research endeavour was executed with 59 rye introgressed wheat lines including 52 triticale × wheat stable lines and 7 wheat × rye (Lahaul Local) introgressed doubled haploids. The detection of new recombinants through molecular cytogenetic tools viz. genomic in situ hybridization and fluorescence in situ hybridization revealed 1BL.1RS translocation in 33 lines, 1BL.1RS translocation along with substitution in 5 lines, 1BL.1RS translocation along with addition in 8 lines, substitution of single pair of rye chromosomes in 5 lines and addition of rye chromatin in 1 line, while 7 lines were found with no rye chromatin. To validate the effect of rye introgression on drought tolerance and yellow rust resistance, all 59 recombinants were evaluated under well-watered and water stressed conditions at in vivo. Analysis of variance revealed sufficient genetic variability among genotypes for different traits at both in vivo level. Under field conditions, direct influence of rye introgression was observed on plant height, grain yield, biological yield and harvest index. Overall, 1BL.1RS translocations and substitutions were found to exhibit significant influence on most of the drought parameters. The screening for yellow rust resistance revealed differential disease reaction, where 52 recombinants were observed to be resistant. The lines exhibiting superiority for the traits can be used as a parental source in wheat improvement endeavours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread wheat is cultivated as a staple crop in various agro-climatic regions of north-west Himalayas encompassing the hill states viz. Himachal Pradesh, Jammu & Kashmir and Uttarakhand. In these hill states of the country, most of the area is under rainfed cultivation and hence the bread wheat varieties grown there suffer from certain crucial abiotic and biotic stresses. Ultimately, wheat productivity level remains very low (approx. 17–18q ha−1) in comparison with national average (28q ha−1). Among the various abiotic stresses, drought is considered as one of the major stress in such areas. Besides this, these hilly regions provide amiable conditions to various disease pathogens like rusts that may lead to epidemics. In addition, rust pathogens in the hills may pose high risk to the wheat production in plains like Punjab, Haryana and Uttar Pradesh through movement of the inoculum. Hence, the new widely adaptable cultivars are to be developed with broad genetic base carrying resistance genes for biotic, as well as abiotic stresses.
Among the various potential resistance sources against biotic and abiotic stresses, rye (Secale cereale) genome is one of the important sources that have extensively been explored by the breeders for bread wheat improvement programme. Wheat-rye substitutions and translocations have been and are frequently used in resistance breeding (Rabinovich 1998) leading to 1BL.1RS translocation in high yielding cultivars currently grown in many parts of the world (Heslop-Harrison et al. 1990). Nowadays, triticale is used as the bridging species for such introgressions due to the low crossability between wheat and rye (Badiyal et al. 2014; Jamwal et al. 2016).
In an introgression breeding, monitoring of the alien chromatin is a critical step. At present, molecular cytogenetics has smoothened the progress of this approach by identifying new introgressed genes from alien species in wheat. Various versions of molecular cytogenetic approach viz. genomic in situ hybridization (GISH), fluorescence in situ hybridization (FISH), multicolour FISH and extended DNA Fiber mapping emerged recently have excellent applications in various crop improvement programmes. Since first application to visualize DNA sequences on plant chromosomes (Rayburn and Gill 1985), FISH is now the technique of choice to physically visualize genome and chromosomes and the order of chromosome segments, genes and DNA sequences. Simultaneous detection of multiple targets in crop species like wheat (Mukai et al. 1993a, b) has become quite easy through multicolor FISH.
In the light of foregoing, it is clear that drought and yellow rust are the major constraints in harvesting targeted productivity in bread wheat in rainfed areas of the hill state. As rye (Secale cereale) is an excellent gene pool resource for both these traits, there is a dire need to introgress the targeted chromatin/genes through modern plant breeding and biotechnological approaches and also to screen the desired recombinants utilizing the novel tools like GISH and FISH of molecular cytogenetic approach. The targeted rye chromatin/gene(s) introgressed wheat recombinants with minimum linkage drag, expected in the present investigation can be utilized, as such, potential genotypes to be recommended as improved varieties or as potential source for future wheat genetic upgradation endeavours.
Materials and methods
Plant materials
Seeds of 59 recombinant wheat lines including 52 triticale × wheat stable lines (TW lines from 4 series; TW-1, TW-2, TW-4 and TW-6) and 7 wheat × rye (Lahaul Local) introgressed doubled haploids (WRDH) were used for molecular cytogenetic analysis to detect and characterize the introgressed rye chromatin following GISH and FISH approaches (Yamamoto and Mukai 1989; Mukai et al. 1993a, b; Chaudhary et al. 2013) (Table 1). The parentage of all triticale × wheat stable lines and WRDH is given in Table 1. WRDH were produced by crossing elite wheat genotypes with lahaul local rye following Imperata cylindrica- mediated chromosome elimination approach of doubled haploidy breeding (Chaudhary et al. 2005) (Table 1). The experiment of molecular cytogenetic analysis was carried out at The High-tech Molecular Cytogenetics & Tissue culture Lab in the Department of Crop Improvement, CSKHPKV, Palampur during year 2017–2018.
Preparation of Chromosomes spreads
Seeds of selected recombinant wheat lines were kept for germination in petri plates containing germination sheets. After 2–3 days, roots (2–3 cm) were excised from germinated seeds and were kept in vial that were further stored in ice box and cold treatment was given by keeping the ice box at 4 °C for 18–20 h. Then, the fixation of pre-treated roots and slide preparation was done as per Chahota et al. (2015). The prepared slides were kept in the slides box, sealed and stored in 4 °C for further use in FISH experiments.
Labelling of DNA probes
Molecular probes viz. genomic probe of rye, pSc119 (120 bp tandemly repeated DNA sequence isolated from Secale cereale; McIntyre et al. 1990), pTa71 (a 9 kb EcoRI fragment of the repeat unit of 25S-5.8S-18S rDNA isolated from T. aestivum; Bedbrook et al. 1980) and pAs1 (tandem repeat with a monomeric length of 340 bp isolated from T. aestivum; Nagaki et al. 1998) were used to detect and characterize the alien introgressions. The CTAB (cetyltrimethylammonium bromide) method of Murray and Thompson (1980) was used to isolate the genomic DNA of wheat and Himalayan rye. All the probes were labelled by nick translation method that was performed with Dig-Nick Translation and Biotin-Nick Translation kits (Roche Diagnostics) following the manufacturer’s instructions. Detection of the labelled sites was executed by the fluorophores viz. fluorescein-conjugated streptavidin and rhodamine-conjugated anti-digoxigenin.
In situ hybridization
The permanent slides with high metaphase index were selected and treated with pepsin solution with concentration of 50 µg/mL and covered under plastic coverslip and incubated for 5 min at 37 °C. After that, the coverslips were removed and slides were washed in 2 × SSC twice for 5 min. The slides were then re-fixed in a freshly prepared 4% (w/v) paraformaldehyde at room temperature for 10 min and then washed with 2 × SSC twice for 5 min. For in situ hybridization, first of all, probe mixture was prepared in 1.5 mL microtubes as per protocol given by Schwarzacher and Heslop-Harrison (2000). The probe mix was then denatured at 85 °C for 8 min 30 s and cooled on ice for 10 min. 20–25 μL of the denatured probe mixture was applied to each slide and covered with glass coverslip. The probe and chromosomes preparation were then denatured together at 75 °C for 6 min, and slides were transferred quickly to moisture chamber and incubated at 37 °C overnight (about 16–20 h). After overnight hybridization, the slides were washed in 2 × SSC for 4- × 5 min at 37 °C to remove the unbound probe and any remaining hybridization mixture.
For detection of hybridization sites, 50 μL/slide of florescence detection mixture is required. It includes 1 μL antibody (0.5 μL each of strepavidin -FITC (fluorescein isothiocyanate, 0.1 mg/mL) and anti-dig rhodamine conjugated, 0.2 mg/mL) in 100 μL TNB (1 M Tris HCl, 3 M NaCl and 5% blocking reagent). The fluorescence mixture was mixed properly and 50 μL was used for each slide. The 24 × 32 mm size parafilm was cut and placed on the fluorescence detection mixture containing slide. The slides were then incubated for 30–45 min at 37 °C followed by washing in 2 × SSC twice, each for 5 min at room temperature. Subsequently, slides were counterstained with solution that was prepared by mixing 25 μL DABCO (1,4-Diazabicyclo (2,2,2) octane) and 1μL DAPI (per slide) and poured on the slide. The coverslips of size 24 × 32 mm were placed on the slides. The slides were then stored in dark for 30 min or overnight. After the completion of whole procedure, hybridization signals were detected in fluorescence microscope with Olympus CCD camera and analysed using ASI software.
In vivo screening for drought tolerance and yellow rust
The in vivo (field) evaluation of the material was accomplished by raising these genotypes in irrigated and rainfed environments in a randomized block design with three replications during winter season 2017–2018. Under rainfed conditions, no proper rainfall was observed that resulted in water stress conditions during the different stages of growth (Table 2). However, in the irrigated environment, proper irrigation was provided during crown root initiation, late tillering, late jointing, flowering and grain-filling stages. The data were recorded on five randomly selected competitive plants per genotype from each replication on the various morpho-physiological traits: plant height, tillers per plant, spike length, grain yield per plant, biological yield per plant, 1000-grain weight and harvest index.
Statistical analyses were conducted using student’s t test to test the difference in the various drought tolerance-related traits between the translocation lines and non-introgressed lines. To evaluate the response of recombinants for yellow rust, the experimental genotypes were evaluated under natural conditions at HAREC, Kukumseri, Lahaul & Spiti along with two susceptible checks, namely Sonalika and HS 240. The seed material was sown at HAREC during June, 2018 and data pertaining to rust evaluation were recorded in August, 2018. Rust severity (percentage of rust infection on the plant) and field response (type of disease reaction) were assessed by taking consecutive observations according to Modified Cobb scale method (Peterson et al. 1948).
Results
Molecular cytogenetic analysis
The total of 59 lines involving homozygous recombinants (triticale × wheat derived stable lines) and doubled haploids were analysed using sophisticated techniques of molecular cytogenetics, namely GISH and FISH for detecting the rye chromatin introgressions with minimum linkage drag. For aforesaid purpose, different probes, viz. rye genomic probe, repetitive DNA sequence probes like pSc119, pAs1 and pTa71 (rDNA) labelled with fluorescein iso-thio-cyanate (FITC) and rhodamine were utilized. Rye probe was used to detect the rye chromatin labelled, whereas pSc119, pAs1 and pTa71 were utilized to detect the B & R genome chromosomes, D genome chromosomes and NOR regions (1B, 6B and 1R), respectively.
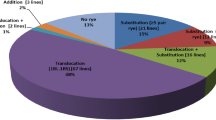
In the present investigation, nearly 56 per cent of recombinants, i.e. out of 59 lines 33 recombinant lines, viz. TW-1-44, TW-159, TW-1-75, TW-1-134, TW-1-201, TW-1-252, TW-2-39, TW-2-50, TW-2-87, TW-2-94, TW-2-95, TW-2-120, TW-2-135, TW-2-158, TW-2-169, TW-2-174, TW-2-187, TW-2-200, TW-2-250, TW-2-318, TW-4-25, TW-4-71, TW-4-126, TW-6-18, TW-6-60, TW-6-62, TW-6-63, TW-6-210, WRDH-2, WRDH-4, WRDH-5, WRDH-6 and WRDH-8 were found to carry 1BL.1RS translocation (Fig. 1a). Out of 5 lines carrying 1BL.1RS translocations along with substitution, 3 lines namely TW-1-212, TW-4-19 and TW-4-138 were found to carry 1BL.1RS translocation along with 1R chromosome pair substitution, whereas, one line viz. TW-2-67 was found with 1BL.1RS translocation along with 4R chromosome pair substitution and one line, TW-4-52 was observed to carry 1BL.1RS translocation and one pair of rye chromosomes substitution (Figs. 1b and 1c). Eight lines (13.56% of total material) namely TW-1-21, TW-1-149, TW-2-54, TW-2-84, TW-2-116, TW-2-291, TW-6-158-3 and TW-6-176 were found to carry an extra chromosome along with 1BL.1RS translocations. Out of these lines, 2 lines TW-1-21 and TW-2-291 were observed to carry 1RL and 1RS, respectively, as additional chromosomes along with 1BL.1RS translocation. While 1R was found to be an additional rye chromosome along with 1BL.1RS translocation in other six recombinant lines (Fig. 1d). The results of molecular cytogenetic analysis revealed that 3 lines namely TW-2-71, TW-2-106 and TW-4-17 were found to carry 1R chromosome pair substitution (Fig. 1e). Whereas in WRDH-1, substitution of 2R chromosome pair (chromosome number 2 of rye) and in WRDH-3, substitution of one pair of rye chromosomes found. In the present investigation only one line namely TW-2-1 was found to carry 1R chromosome in addition to wheat chromosomes. While 7 lines out of 59 lines were observed to be devoid of rye chromatin. These included TW-2-31-4, TW-2-88, TW-2-172, TW-2-173, TW-2-183, TW-4-100 and TW-6-204.
(a) Detection of 1BL.1RS translocation in triticale × wheat stable line (TW-1-59) by using probes: Bio- pSc119 (green) and Dig- rye genomic DNA (red). (b) Detection of 1BL.1RS translocation along with 1R substitution in triticale × wheat (TW-1-212) derived recombinant by using probes: Bio- pSc119 (green) and Dig- rye genomic DNA (red). (c) Detection of 1BL.1RS translocation along with 4R substitution in triticale × wheat (TW-2-67) derived recombinant by using probes: Bio- pSc119 (green) and Dig- rye genomic DNA (red). (d) Detection of 1BL.1RS translocation along with 1R addition in triticale x wheat (TW-2-116) derived recombinant by using probes: Bio- pSc119 (green) and Dig- rye genomic DNA (red). (e) Detection of 1R substitution in triticale x wheat (TW-4-17) derived recombinant by using probes: Bio- pSc119 (green) and Dig- rye genomic DNA (red)
In vivo screening for drought tolerance
To strengthen the results obtained from molecular cytogenetic analysis, performance of rye introgressed bread wheat recombinants was further evaluated at in vivo level for biotic (yellow rust resistance) and abiotic (drought tolerance) stresses. For the aforesaid purpose, all 40 recombinants were screened for the yield and its related traits viz. plant height (cm), tillers per plant, spike length (cm), 1000 grain weight (g), grain yield per plant (g), biological yield per plant (g) and harvest index (%) were screened under irrigated (E1) and rainfed (E2) environments. Under field conditions, rainfall was not evenly distributed throughout the cropping season, which resulted in drought stress conditions (Table 2). The analysis of variance for all the aforesaid traits was worked out under both the environments. The mean sum of squares due to genotypes was found to be significant for all the traits under both the environments (Table 3). According to the level of introgression in various triticale x wheat and wheat x rye recombinants, trait-wise results under two environments are discussed as follows:
Plant height
In the present study, plant height among 59 recombinants ranged from 63.8 cm (TW-6-176) to 107.8 cm (TW-2-172) with an average of 89.4 cm in irrigated (E1) environment. Whereas in rainfed environment (E2), reduction in height was observed where plants were 51.5 cm (TW- 4-71) to 90.7 cm (TW-1-134) tall with an average of 69.3 cm (Table S1). As per the analysis of results following student’s t test, under irrigated conditions (E1), no category of rye introgressed lines was found with significantly reduced height, while non-introgressed lines were found with significantly longer height (Table 4). In the rainfed environment which resulted in stress conditions, effect of rye introgression was observed on plant height and substitution lines (carrying substitution of one pair of rye) were found with significantly decreased height (Table 4).
Tillers per plant
For trait tillers per plant, range among 59 lines varied from 1.4 (TW-4-138) to 5.8 (TW-1-75) with an average of 3.4 in irrigated environment. While under rainfed conditions, tillers per plant varied from 1.0 (TW-4-138 and TW-2-172) to 5.5 (TW-4-19) with mean of 1.9 (Table S1). However, analysis of results following student’s t test revealed that among all the lines, translocation plus addition lines were found to be significantly superior under irrigated conditions (E1) (Table 4). Whereas under water stress conditions of rainfed environment (E2), translocation cum substitution lines were found to exhibit significantly positive response on tillers per plant (Table 4).
Spike length
In the present investigation, field evaluation of 59 lines revealed an average of 10.2 cm and 8.9 cm for spike length under irrigated and rainfed conditions, respectively. In irrigated environment (E1), range of spike length was found to be 6.9 cm (TW-4-19) to 13.6 cm (TW-1-201), whereas in rainfed environment (E2), range was from 6.3 cm (TW-4-19 and TW-4-71) to 13.0 cm (TW-1-134) (Table S1). Under well-watered conditions in E1, rye introgressed lines carrying overall translocation plus addition were found to exhibit significantly positive effect on spike length (Table 4). Under drought stress conditions in rainfed conditions, translocation plus addition lines and addition line were found to exhibit significantly positive response on spike length revealing the positive effect of rye introgression on spike length (Table 4).
1000 grain weight
In irrigated environment, the present study revealed variation from 29.45 g (TW-2-250) to 45.87 g (TW-2-1) in 1000 grain weight with the mean performance of 38.14 g, whereas the mean performance was found to be decreased to 34.44 g with the range from 25.10 g (TW-4-71) to 45.00 g (TW-2-1) under rainfed conditions (Table S1). In the current study, under well-watered conditions in irrigated environment, addition lines were found to be significantly positive for 1000 grain weight on overall mean (Table 4). Likewise under drought stress conditions in rainfed environment, addition lines showed significant positive response.
Grain yield/plant
In the present investigation, among 59 lines, the range of grain yield varied from 3.10 g (TW-4-100) to 8.62 g (TW-4-19) with an average grain yield of 6.15 g under irrigated conditions. Under rainfed conditions, range of grain yield per plant was found to be 1.90 g (TW-2-173) to 6.20 g (TW-2-95) with a mean of 3.78 g (Table S1). The results of current study revealed the positive effect of rye introgression on grain yield per plant under both well-watered conditions and drought stress. In the present investigation, under irrigated conditions and under drought stress conditions in rainfed environment, substitution lines were found to be significantly better for grain yield per plant (Table 5).
Biological yield
Biological yield refers to the total dry matter accumulation of a plant system. In the present investigation, range of biological yield per plant among 59 lines varied from 15.11 g (TW-2-172) to 34.09 g (TW-1-75) with an average of 24.54 g in irrigated environment. While under rainfed conditions, it varied from 13.43 g (TW-2-173) to 26.90 g (TW-2-95) with mean of 19.24 g (Table S1). Among the different categories of rye introgressed lines, under irrigated and rainfed conditions, positive effect of rye introgression on biological yield has been observed, where significant highest biological yield was observed in single pair rye chromosome substitution lines (Table 5).
Harvest index
In irrigated environment, the present study revealed variation from 15.28 per cent (TW-4-100) to 33.32 per cent (TW-4-19) in harvest index with the mean performance of 24.94 per cent, whereas the mean performance was found to be decreased to 19.35 per cent with the range from 14.18 per cent (TW-2-173) to 24.83 per cent (TW-2-54) under rainfed conditions (Table S1). In the present investigation, under both well-watered conditions in irrigated environment and drought stress in rainfed environment, the effect of rye chromatin was evident on harvest index. Under irrigated conditions, addition lines were found to depict significantly positive effect on harvest index (Table 5). On the other side, under drought stress conditions in rainfed environment, substitution lines were found to be significantly superior for harvest index among all the lines (Table 5). The present results for harvest index put substitution lines on the better front than other lines under drought conditions.
Overall, under field conditions, among different significant genotypes for the respective characters, the recombinants which were found significantly superior for most of the traits (five or more than five traits) were TW-2-71, TW-2-106, TW-2-135, TW-2-200, TW-4-19, TW-4-25, TW-4-126, WRDH-3 and WRDH-2. Among these genotypes, TW-2-135, TW-2-200, TW-4-25, TW-4-126 and WRDH-2 were found to carry 1BL.1RS translocation, whereas TW-2-71, TW-2-106 and WRDH-3 were the recombinants that were found to carry substitution of 1R rye chromosome in first two and one rye pair substitution in WRDH-3. And in TW-4-19, 1BL.1RS translocation cum substitution of 1R rye was found. So overall, based on individual performance of recombinants under drought stress in rainfed conditions, present investigation suggested the better response of translocation and substitution recombinants than others.
In vivo screening for yellow rust resistance
In present investigation, all 59 triticale × wheat and wheat × rye recombinants were scored for resistance against yellow rust (Puccinia striiformis f.sp. tritici) according to modified Cobb Scale (Peterson et al. 1948). In current study, field response of 52 lines was observed to be resistant while 7 lines were found to be moderately resistant with different levels of severity in comparison to check varieties viz., Sonalika and HS 240 which were highly susceptible to yellow rust. According to the level of introgression in various triticale × wheat and wheat × rye recombinants, results of yellow rust screening are discussed as follows:
Among the 33 lines carrying 1BL.1RS translocation, variable field response against stripe rust has been recorded where 30 lines were observed to be to be resistant viz. TW-4-25 with trace severity TW-1-44, TW-1-59, TW-1-75, TW-1-134, TW-1-201, TW-2-50, TW-2-87, TW-2-95, TW-2-158, TW-2-169, TW-2-174, TW-2-187, TW-2-200, TW-2-250, TW-4-71, TW-4-126, TW-6-18, TW-6-60, TW-6-62, TW-6-63, TW-6-210, WRDH-2, WRDH-4, WRDH-5, WRDH-8 with 5% severity and TW-2-39, TW-2-94, TW-2-318, WRDH-6 with 10% severity, whereas field response of 2 lines viz. TW-2-120 and TW-2-135 was observed to be moderately resistant with 20% disease severity. Only one line, TW-1-252, was found with moderately resistant field response and 30% severity (Table S2). Among 5 lines carrying 1BL.1RS translocation along with substitution of rye chromosome pair, field response of 2 lines (TW-2-67 and TW-4-19) was found to be resistant with trace severity and field response of 3 lines (TW-1-212, TW-4-52 and TW-4-138) was observed to be resistant with 10% severity (Table S2). Among the 33 lines carrying 1BL.1RS translocation variable field response against stripe rust has been recorded where 30 lines viz., TW-4-25 with trace severity, TW-1-44, TW-1-59, TW-1-75, TW-1-134, TW-1-201, TW-2-50, TW-2-87, TW-2-95, TW-2-158, TW-2-169, TW-2-174, TW-2-187, TW-2-200, TW-2-250, TW-4-71, TW-4-126, TW-6-18, TW-6-60, TW-6-62, TW-6-63, TW-6-210, WRDH-2, WRDH-4, WRDH-5, WRDH-8 each with 5% severity and TW-2-39, TW-2-94, TW-2-318, WRDH-6 each with 10% severity were observed to be resistant whereas field response of 2 lines viz., TW-2-120 and TW-2-135 was observed to be moderately resistant with 20% disease severity (Table S2).
In present investigation, all the 5 lines carrying substitution (TW-2-71, TW-2-106, TW-4-17, WRDH-1 and WRDH-3) were found with resistant field response and 5% disease severity (Table S2). In case of +monosomic addition line, TW-2-1, resistant field response was found with 5% severity. In the current study, 7 lines devoid of rye chromatin were also screened for stripe rust and the results revealed that field response of four lines viz. TW-2-172, TW-2-173, TW-4-100 and TW-6-204 was observed to be resistant with 5% severity. Further, 2 lines (TW-2-31-4 and TW-2-183) were found to reveal 10% severity with resistant field response, whereas one line (TW-2-88) was observed with moderately resistant field response and 20% severity (Table S2).
Discussion
The present research endeavour was undertaken to accelerate the wheat improvement programme through detection and characterization of rye introgressed bread wheat recombinants at in vivo level for drought tolerance and yellow rust resistance. In the present investigation, molecular cytogenetic analysis of different wheat-rye derivatives revealed the wide availability of 1BL.1RS translocation. Present results substantiate the reports provided by Angelova and Georgiev (2006), Ren et al. (2009), Chahota et al. (2015) and Li et al. (2016) who reported wide availability of 1BL.1RS translocation in independently raised intergeneric hybrids. The other translocations, substitutions and additions presented in the present study have also been reported by Chahota et al. (2015) and Fu et al. (2014). The present study revealed the presence of 1R chromosome of rye in lines carrying substitution, addition, as well as in translocation cum substitution and addition. These findings are in concordance with previous studies of Merker (1984) and Devos et al. (1993) where they had shown the most frequent recovery of 1R chromosome followed by 2R and 3R in BC1F3 and F4 generations. It proves higher tendency of 1R to sustain in the wheat background.
The study also showed some lines with translocation plus addition and possible reason for abnormal mitotic behaviour of wheat and 1R chromosomes in the present study might be chromosome–spindle interaction and spindle abnormalities which lead to chromosomal addition as suggested by Bennett et al. 1976. Though addition lines cannot be considered as introgressions, they are stable enough to be maintained and used as initiative for the production of substitution and translocation lines because wheat chromosome pairing hampers in the monosomic addition lines due to the presence of a single rye chromosome. Hence, high frequency of breakage and fusion between rye and wheat chromosomes occur during meiosis (Ren et al. 2012). However, some lines were found with no rye and the reason behind such elimination of the rye chromatin lies in the incompatibility among spindle fibre of wheat origin with that of chromosome of rye. This results in the deviation of rye chromosome from equatorial plate leading to their successive or instant loss. Another possible reason might be backcrossing among the recombinants with respective parental wheat genotypes that facilitated complete loss of rye chromatin.
The lines were further evaluated under irrigated and rainfed conditions to know the effect of rye introgressions on drought-related traits. As per the analysis of results, under well-watered conditions (E1), non-introgressed lines were found with significantly longer height. Our results are in agreement with the findings of Ehdaie et al. (2003), Qi et al. (2016) and Li et al. (2016) where they reported higher plant height among non-carrier lines compared to introgressed lines, i.e. translocation and substitution. From the breeder’s point of view, low plant height is of great importance that plays an important role in lodging resistance. In the present investigation, rye introgressed lines, i.e. substitution lines were observed with less height under rainfed environment that revealed better performance of these lines than that of non-introgressed lines. For tillers per plant, where translocation plus addition lines were found to be significantly superior under irrigated conditions (E1), results are in agreement with the findings of Schneider et al. (2016) who reported increased tillering capacity in 1R addition line than non-introgressed lines. In the present study, for 1000 grain weight, only monosomic rye addition lines performed better than the non-introgressed lines under well-watered conditions in irrigated environment and drought stress in rainfed environment. On contrary to the present findings, Schneider et al. (2016) and An et al. (2015) reported lower 1000 grain weight in 1R and 6R addition lines, respectively, than non-introgressed lines. As per results, for grain yield per plant, substitution lines were found to be significantly better under both the environments. On contrary, Li et al. (2016) and Kumlay et al. (2003) reported the lower grain yield/plant in substitution line (1R) than that of non-introgressed lines. Our findings under water stress conditions are in agreement with earlier reports of Ehdaie et al. (2003) who reported less grain yield in 1BL.1RS translocations under dry field conditions. Singh et al. (1998) also reported negative effect of 1BL.1RS translocation in spring bread on grain yield under droughted and well-watered conditions. The results of current study revealed the positive effect of rye introgression on grain yield per plant under both well-watered conditions and drought stress. Higher grain yield per plant indicated the more accumulation of photosynthates on grain than other plant parts by rye introgressed lines, when exposed to water stressed conditions. The present findings revealed the better performance of substitution lines under both well-watered conditions, as well as drought stress for biological yield per plant. This suggests that substitution lines consistently partitioned the photosynthates to grain production as biological yield indicates total dry matter accumulation in the plant which ultimately contributed to grain yield, by translocating the photosynthates to spikes. On contrary, Li et al. (2016) reported lower biological yield in substitution lines 1R(1B) compared to translocation and non-introgression lines. However, contradictory results for some of characters with previous studies might be attributed to the difference in genotypes used in studies. Looking at the in vivo screening of wheat × rye and triticale × wheat recombinants as a whole, rye introgressed lines were found to perform better for different yield-related traits as compared to non-introgressed lines under drought stress conditions, as well as irrigated conditions. Rye introgressed lines showed high physiological capacity to mobilize photosynthates and translocate them into organs having economic yield under both the environments. These results suggest the presence of drought tolerance genes in the introgressed rye chromatin.
The in vivo screening of 59 lines for resistance against yellow rust revealed differential disease reaction, that is, resistant to moderately resistant with different severity levels. Present results are in agreement with the findings of Ren et al. (2009) and Fu et al. (2010) who observed variable resistance against stripe rust in 1BL.1RS translocation lines. Similarly, Li et al. (2016) observed different response pattern of resistance in the progeny carrying 1BL.1RS translocation developed from same rye parent. It might be due to the interaction of rye chromatin with the wheat germplasm, as well as cytoplasm which affects the pattern of disease resistance. The results of 5 lines carrying substitution are in accordance with the findings of Li et al. (2016) who reported high resistance to some of Pst pathotypes and isolates in 1R(1B) substitution line. The present findings of resistant field response with 5% severity in monosomic addition line, TW-2-1, are contradictory with the study of Schneider et al. (2016) where they reported susceptibility in 1R and 4R disomic addition lines, while 6R addition line was observed to be highly resistant or nearly immune against yellow rust. The current results of non-introgressed lines are in concordance with the findings of Ren et al. (2009) and Yang et al. (2009) who reported resistance in the non-introgressed lines against stripe rust. They proposed that cryptic translocation might be the probable reason for resistance in non-carrier lines. In the present investigation, maximum lines of each group (translocation, translocation cum substitution, translocation plus addition, substitution, addition and no rye) were found under category of resistant. These results are supported by findings of Yang et al. (2009) who studied the response of different rye introgressed lines to P. striiformis f. sp. tritici and reported high level resistance in three lines viz. 1R substitution line (G17), 1BL.1RS translocation line (L9-15) and non-carrier line (L2-10). The potential lines identified in present study can be used as parental source for drought tolerance and yellow rust resistance that opens new vistas in wheat improvement programme.
References
An D, Zheng Q, Luo Q, Ma P, Zhang H, Li L (2015) Molecular cytogenetic identification of a new wheat-rye 6R chromosome disomic addition line with powdery mildew resistance. PLoS ONE 10:e0134534
Angelova Z, Georgiev S (2006) Visualization of Secale cereale DNA in wheat germplasm by genomic in situ hybridization. Biotechnol Biotechnol Equip 20:26–29
Badiyal A, Chaudhary HK, Jamwal NS, Hussain W, Mahato A, Bhatt AK (2014) Interactive genotypic influence of triticale and wheat on their crossability and haploid induction under varied agroclimatic regimes. Cereal Res Commun 42:700–709
Bedbrook J, Jones J, O’Dell M, Thompson RD, Flavell RB (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:525–560
Bennett MD, Finch RA, Barclay IP (1976) The time rate and mechanism of chromosome elimination in Hordeum hybrids. Chromosoma 54:175–200
Chahota RK, Mukai Y, Sharma TR, Chaudhary HK, Rani S (2015) Cytological and morphological characterization of rye wheat derivatives of important agronomic traits. Nucleus 58:2011–2016
Chaudhary HK, Sethi GS, Singh S, Pratap A, Sharma S (2005) Efficient haploid induction in wheat by using pollen of Imperata cylindrica. Plant Breed 124:96–98
Chaudhary HK, Tayeng T, Kaila V, Rather SA (2013) Enhancing the efficiency of wide hybridization mediated chromosome engineering for high precision crop improvement with special reference to wheat × Imperata cylindrica system. Nucleus 56:07–14
Devos KM, Atkinson MD, Chinoy CN, Francis HA, Harcourt RL, Koebner RMD, Liu CJ, Masojc P, Xie DX, Gale MD (1993) Chromosomal rearrangements in the rye genome relative to that of wheat. Theor Appl Genet 85:673–680
Ehdaie B, Whitkus RW, Waines JG (2003) Root biomass, water-use efficiency, and performance of wheat-rye translocations of chromosome 1 and 2 in spring bread wheat “Pavon.” Crop Sci 43:710–717
Fu S, Tang Z, Ren Z, Zhang H (2010) Transfer to wheat (Triticum aestivum) of small chromosome segments from rye (Secale cereale) carrying disease resistance genes. J Appl Genet 51:115–121
Fu SL, Yang MY, Ren ZL, Yan BJ, Tang ZX (2014) Abnormal mitosis induced by wheat–rye 1R monosomic addition lines. Genome 57:21–28
Heslop-Harrison JS, Leitch AR, Schwanzacher T, Anamthawat-Jonsson K (1990) Detection and characterization of 1BL/1R translocation in hexaploid wheat. Heredity 65:385–392
Jamwal NS, Chaudhary HK, Badiyal A, Hussain W (2016) Factors influencing crossability among triticale and wheat and its subsequent effect along with hybrid necrosis on haploid induction. Acta Agric Scand Sect B Plant Soil Sci 66:282–289
Kumlay AM, Baenziger PS, Gill KS, Shelton DR, Graybosch RA, Lukaszewski AJ, Wesenberg DM (2003) Understanding the effect of rye chromatin in bread wheat. Crop Sci 43:1643–1651
Li Z, Ren Z, Tan F, Tang Z, Fu S, Yan B, Ren T (2016) Molecular cytogenetic characterization of new wheat-rye 1R(1B) substitution and translocation lines from a Chinese secale cereale L. Aigan with resistance to stripe rust. PLoS ONE 11:e0163642. https://doi.org/10.1371/journal.pone.0163642
McIntyre CL, Pereira S, Moran LB, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:635–640
Merker A (1984) The rye genome in wheat breeding. Hereditas 100:183–191
Mukai Y, Friebe B, Hatchett JH, Yamamoto M, Gill BS (1993a) Molecular cytogenetic analysis of radiation- induced wheat- rye terminal and intercalary chromosomal translocation and the detection of rye chromatin specifying resistance to Hessian fly. Chromosoma 102:88–95
Mukai Y, Nakahara Y, Yamamoto M (1993b) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nagaki K, Tsujimoto H, Sasakuma T (1998) Dynamics of tandem repetitive Afa-family sequences in Triticeae, wheat-related species. J Mol Evol 47:183–189
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stem of cereals. Can J Res 26:496–500
Qi W, Tang Y, Zhu W, Li D, Diao C, Xu L, Zeng J, Wang Y, Fan X, Sha L, Zhang H, Zheng Y, Zhou Y, Kang H (2016) Molecular cytogenetic characterization of a new wheat-rye 1BL.1RS translocation line expressing superior stripe rust resistance and enhanced grain yield. Planta 244:405–416
Rabinovich SV (1998) Importance of wheat–rye translocations for breeding modern cultivars of Triticum aestivum L. Euphytica 100:323–340
Rayburn AL, Gill BS (1985) Use of biotin-labeled probes to map specific DNA sequences on wheat chromosomes. J Hered 76:78–81
Ren TH, Yang ZJ, Yan BJ, Zhang HQ, Fu SL, Ren ZL (2009) Development and characterization of a new 1BL.1RS translocation line with resistance to stripe rust and powdery mildew of wheat. Euphytica 169:207–213
Ren TH, Chen F, Yan BJ, Zhang HQ, Ren ZL (2012) Genetic diversity of wheat–rye 1BL. 1RS translocation lines derived from different wheat and rye sources. Euphytica 183:133–146
Schneider A, Rakszegi M, Molnar-Lang M, Szakacs E (2016) Production and cytomolecular identification of new wheat-perennial rye (Secale cereanum) disomic addition lines with yellow rust resistance (6R) and increased arabinoxylan and protein content (1R, 4R, 6R). Theor Appl Genet 129:1045–1059
Schwarzacher T, Heslop-Harrison P (2000) Practical in situ hybridization. BIOS Scientific Publishers Ltd, Milton Park, p 203
Singh RP, Huerta-Espino J, Rajaram S, Crossa J (1998) Agronomic effects from chromosome translocations 7DL.7Ag and 1BL.1RS in spring wheat. Crop Sci 38:27–33
Yamamoto M, Mukai Y (1989) Application of fluorescence in situ hybridization to molecular cytogenetics of wheat. Wheat Inf Serv 69:30–32
Yang ZJ, Li GR, Jia JQ, Zeng X, Lei MP, Zeng ZX, Zhang T, Ren ZL (2009) Molecular cytogenetic characterization of wheat: Secale africanum amphiploids and derived introgression lines with stripe rust resistance. Euphytica 167:197–202
Acknowledgements
All the authors highly acknowledge the Department of Science & Technology for providing financial assistance through INSPIRE programme and CSK HP Agricultural University, Palampur for providing experimental material and fields.
Funding
The authors are thankful to DST-INSPIRE (Grant No. IF 150889) for providing financial support for executing the present research work.
Author information
Authors and Affiliations
Contributions
All the authors have contributed towards planning, execution and presentation of the work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Consent to participate
All the listed authors have provided their consent.
Consent for publication
All the listed authors have provided their consent.
Ethics approval
No animals were used in the study.
Additional information
Communicated by M. Molnár-Láng.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, P., Chaudhary, H.K., Kapoor, C. et al. Molecular cytogenetic analysis of novel wheat-rye translocation lines and their characterization for drought tolerance and yellow rust resistance. CEREAL RESEARCH COMMUNICATIONS 50, 655–665 (2022). https://doi.org/10.1007/s42976-021-00212-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-021-00212-7