Abstract
A total of 139 triticale × wheat progenies (including recombinant inbred lines and doubled haploids) were developed indigenously. Screening at cytogenetic level using genomic in situ hybridization and fluorescence in situ hybridization revealed maximum progenies (48%) carrying 1RS.1BL translocation. D-R genome substitution ranging from 1 to 7 chromosomes of rye showed significantly more biological yield (9.29 g), 1000 grain weight (48.26 g), number of tillers per plant (6.67) and plant height (106.06 cm) than other progenies depicting the contribution of rye chromatin towards plant yield. Thirteen per cent of lines were found without rye chromatin. Screening of all progenies against yellow rust pathogen revealed about 85% lines to be resistant with varying levels of resistance. In vitro testing for drought tolerance showed non-significant difference among progenies carrying variable rye introgressions. The progenies developed in the present study may present novel germplasm and utilized in the future wheat improvement programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rye, Secale cereale (2n = 2x = 14), a diploid species originated from the Near East (Salamini et al. 2002) has been established as a potential source of resistance genes to wheat since the development of first amphiploid triticale (Triticosecale x Wittmack) by Rimpau in 1888. Rye stands out from other cereals in terms of its nutritional value being rich in iron (Fe) and zinc (Zn) (Johansson et al. 2020, 2014) as well as allelopathic potential (Bertholdsson et al. 2012), both qualities are indispensable for wheat breeders. Most notable rye chromatin transfer to wheat is that of 1RS chromosome segment, in different forms like 1AL.1RS, 1BL.1RS, and 1DL.1RS translocations (Mago et al. 2015) contributing several resistance genes for powdery mildew, leaf, stripe and stem rusts which has inevitably led to Green Revolution. But continuously evolving pathogen races like Ug99 have started breaking down the resistance posed by rye chromatin (Crespo-Herrera et al. 2017). Hence, to maintain sustainable resistance in elite wheat cultivars, newer genes have to be introgressed from variable rye sources. Recently, some novel genes like Sr59, Yr83 and Pm56 have been transferred from rye to wheat (Rahmatov et al. 2016; Hao et al. 2018; Li et al. 2020), which may be utilized as durable sources against fungal diseases. For sustainable improvement of elite wheat cultivars by integrating rye chromatin from variable sources, successful hybridization among them is a pre-requisite. But incompatibility among the wheat and rye genomes hampers successful transfer of genes from rye to wheat hence triticale is being used as a bridging species for the introgressions.
Taking into consideration the above information, the work was planned to transfer rye chromatin into wheat through indigenously developed triticale lines. These triticale-wheat progenies (including doubled haploids and RILs) were evaluated at cytogenetic as well as agro-morphological levels to assess the effect of rye chromatin on yield-related traits, yellow rust resistance and drought tolerance.
Materials and methods
Molecular cytogenetic analysis
A total 139 stable triticale x wheat progenies (including RILs through SSD and doubled haploids through Imperata cylindrica-mediated chromosome elimination approach of doubled haploidy breeding) were developed. The parentage of all the progenies is given in Supplementary Table 1.
These lines were subjected to molecular cytogenetic analysis using genomic in situ hybridization (GISH) and fluorescence in situ hybridization (FISH) (Schwarzacher and Heslop-Harrison 2000) at Molecular Biology Lab, Department of Genetics, University of Leicester, UK. Slides with good metaphasic chromosome spreads were prepared according to Yamamoto and Mukai (1989). These were subjected to FISH using genomic rye DNA and repetitive DNA sequence as probes, viz. pSc119.2 (a 120 bp tandem repeated DNA sequence from Secale cereale), pTa71 (a repeat unit of 25S–5.8S–18S rDNA from T. aestivum), pAs1 (a tandem repeat with a monomeric length of 340 bp from T. aestivum). Probes were labelled with biotin-16-dUTP or digoxigenin-16-dUTP (Roche diagnostics) by random priming kit (Bioprime DNA labelling system, Invitrogen).
Agro-morphological evaluation
All 139 wheat-rye progenies (RILs as well as doubled haploids) were evaluated in the experimental fields of Department of crop Improvement, CSK HPKV, Palampur, for agronomically important yield-related traits and resistance against yellow rust, which was scored on 0–100 scale prescribed by Paterson et al. (1948). For evaluating the drought tolerance, in vitro screening of the genotypes (10 plants each) was done by subjecting them to low (− 0.2 MPa) and moderate (− 0.4 MPa) moisture stress induced by polyethylene glycol (PEG-6000) (Michel and Kaufmann 1973) with slight modifications. The seeds were disinfected in 1% sodium hypochlorite solution for 5 min followed by washing (three times) with distilled water. Petri dishes along with Whatman paper were sterilized in autoclave. Ten seeds of each variety were transferred into each sterilized glass Petri dish with a diameter of 9 cm in which the filter papers were placed. Five millilitre of distilled water was added to each Petri dish. Then, after 24 h 10 ml PEG solution of respective concentration for each treatment was added to the Petri dish. The germinated seeds were counted till full germination. The seeds whose root length was 2 mm or more were considered as the germinated ones. On the 8th day, the germinated seeds were taken out of the Petri dishes and the stem and root were separated to assess the morphological parameters.
Results
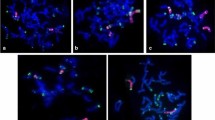
Molecular cytogenetic analysis of 139 triticale x wheat progenies including conventionally developed lines and doubled haploids (DHs) revealed different levels of rye chromatin introgression , i.e., translocation, substitution, addition and their combinations (Fig. 1). Out of 139 lines, 67 (48%) were observed to carry 1RS.1BL translocation (Fig. 2a).
Molecular cytogenetic analysis of rye introgressions in wheat a 1RS.1BL translocation in TW-1–49 pSc119 (red) rye DNA (green) b 1RS.1BL translocation along with 3R(3D) substitution in TW-1–26 pSc119 (red), rye DNA (green). c 1RS.1BL and 6BS.6RL translocations along with 2R(2D) substitution in TW 10 pSc119 (red), rye DNA (green) d 1RS.1BL translocation along with seven pair rye substitution in WR12 rye DNA (red), pTa71 (green). e Six pair rye chromosome substitution in TW 24 pAs1 (red), rye DNA (green) f) 1R and 1RL addition in TW-2–55 pAs1 (red), rye DNA (green)
There were 33 (24 per cent) lines in which substitution has been observed, 12 lines have shown single pair rye substitution and rest with substitution of five and more pairs rye chromosomes (Fig. 2e). These lines recorded significantly more yield per plant (9.29 g), 1000 grain weight (48.26 g), number of tillers per plant (6.67) and plant height (106.06 cm) than other groups included in the study (Table 1). Besides, maximum resistance to yellow rust was observed in these lines depicting 33.33 per cent lines to be immune, 28.57 per cent to be highly resistant and 38.10 per cent to be resistant (Table 2). Fourteen lines showed 1RS.1BL translocation along with substitution of 3D with 3R (Fig. 2b). During the triticale wheat hybridization programmes, 18 progenies (12.95 per cent) revealed the absence of rye chromosomes showing reversion towards wheat parentage. During the present investigation, 18 progenies (12.95 per cent) revealed the absence of rye chromosomes showing reversion towards wheat parentage.
Discussion
Present investigation has been envisioned towards exploring novel combinations of wheat, triticale and rye in the form of translocations, substitutions and additions after hybridizing diverse genotypes. Among wheat rye progenies 48% lines were observed to carry 1RS.1BL translocation (Fig. 2a) advocating the prevalence of this translocation in majority of cultivated wheat cultivars. Wide availability of this translocation has also been reported by Kumar et al. (2003), Angelova and Georgiev (2006), Ren et al. (2009), Chahota et al. (2015), Li et al. (2016) and Sharma et al. (2022) in independently raised intergeneric hybrids.
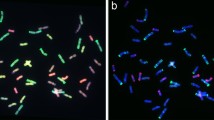
Substitution in twenty-four per cent progenies may be attributed to wheat–rye genome interactions leading to preferred elimination of wheat D-genome chromosomes (Li et al. 2015). Wheat-rye progenies carrying five or more rye chromosomes genetically revert to triticale type, showing improved agronomic performance in the progenies carrying seven rye chromosomes followed by six and five chromosome substitutions, respectively. This might be due to agronomically important genes present on rye chromosomes (2R, 4R, 5R and 7R) (Falke et al. 2009; Jeberson et al. 2021). Among the substitution lines developed in the study, no such agronomic superiority was observed which contradicts the reports by Seerja and Reddy (2013) who observed significantly high yield among five pair rye chromosomes followed by seven and six pair rye chromosome carrying progenies. However, under drought stress, these lines revealed better germination, root length and weight owing for wide adaptability (Fig. 3).
In the present study, 3R substitution along with 1Bl.1RS translocation was observed in fourteen lines. The 3R chromosome is known to be associated with plant height, spike length, number of spikelets per spike, number of kernels per spike and kernel weight (Myśków et al. 2014). But in the present study, improvement in grain yield and harvest index was observed rather than plant height and grain weight. However, these lines revealed highest germination percentage in control (97.25 per cent) as well as at mild stress of − 0.2 MPa (89.75 per cent). The same progenies recorded maximum root length (5.04 cm) and seedling vigour index (1166.18) in the control which may be due to genotype and environment interactions.
Addition of alien chromosomes in wheat genome is desirable to study the dosage effect of genes. The current study revealed 5 lines (3.60 per cent) with monosomic addition of rye chromosomes among which, three were carrying translocation also. These results are in accordance with the findings of Fu et al. (2014) where one line with 1RS.1BL translocation along with addition of 1R chromosome was observed. Though addition lines cannot be considered as introgressions, these are stable enough to be maintained and used as initiative for the production of substitution and translocation lines. The agronomic performance of such lines in the present work was poor for most of the traits which contradicts by the results of Schneider et al. (2016) who reported the significant influence of 1R on the grain yield when compared to 4R and 6R addition. Screening for drought tolerance of these lines reported improvement in fresh and dry weight of shoot and root. This improvement might be due to more copy of genes present on 1R because according to Waines and Ehdaie (2007), 1RS promote root biomass growth.
The absence of rye chromosomes in the progenies showed reversion towards wheat parentage. Similar findings have been reported by Chahota et al. (2015) where six lines of triticale x wheat derivatives were lacking in rye chromatin. The reason behind such elimination of the rye chromatin lies in the incompatibility among spindle fibre of wheat origin with that of rye chromosomes. This results in the deviation of rye chromosome from equatorial plate leading to their successive or instant loss. Present work revealed these lines to be non-significantly different from those with rye chromatin introgression for yield-related traits (Table 1). Agronomic performance of these lines revealed poor grain yield, tiller number and harvest index whereas higher 1000 grain weight and biological yield. The findings by Ehdaie et al. (2003) and An et al. (2015) also support present observations as they observed higher plant height and lower grain yield respectively, among non-introgressed lines as compared to introgressed lines , i.e. translocation and substitution. Although under drought stress, the rye introgressed lines performed better than non-introgressed progenies (Fig. 3).
In conclusion, the research work presents novel genetically stable wheat rye progenies in the form of translocations, substitutions and additions. Furthermore, the screening of the progenies for yield and related traits, resistance towards yellow rust and tolerance for drought stress (− 0.2 and − 0.4 MPa) revealed positive effect of rye introgression. Since triticale and wheat lie in different gene pools, the present study generated new germplasm which can be used further in wheat improvement programmes.
Availability of data and material
Supplementary data have been provided.
References
An D, Zheng Q, Luo Q, Ma P, Zhang H, Li L (2015) Molecular cytogenetic identification of a new wheat-rye 6R chromosome disomic addition line with powdery mildew resistance. PLoS ONE 10:e0134534
Angelova Z, Georgiev S (2006) Visualization of Secale cereale DNA in wheat germplasm by genomic in situ hybridization. Biotechnology 20:26–29
Bertholdsson NO, Andersson SC, Merker A (2012) Allelopathic potential of Triticum spp., Secale spp. and Triticosecale spp. and use of chromosome substitutions and translocations to improve weed suppression ability in winter wheat. Plant Breed 131:75–80
Chahota RK, Mukai Y, Sharma TR, Chaudhary HK, Rani S (2015) Cytological and morphological characterization of rye wheat derivatives of important agronomic traits. Nucleus 58:2011–2016
Crespo-Herrera LA, Garkava-Gustavsson L and Åhman I (2017) A systematic review of rye (Secale cereale L.) as a source of resistance to pathogens and pests in wheat (Triticum aestivum L.). Hereditas 154:14.
Ehdaie B, Whitkus RW, Waines JG (2003) Root biomass, water-use efficiency, and performance of wheat-rye translocations of chromosome 1 and 2 in spring bread wheat “Pavon.” Crop Sci 43:710–717
Falke KC, Wilde P, Wortmann H, Geiger HH, Meidaner T (2009) Identification of genomic regions carrying QTL for agronomic and quality traits in rye (Secale cereale) introgression libraries. Plant Breed 128:615–623
Fu SL, Yang MY, Ren ZL, Yan BJ, Tang ZX (2014) Abnormal mitosis induced by wheat–rye 1R monosomic addition lines. Genome 57:21–28
Hao M, Liu M, Luo J, Fan C, Yi Y, Zhang L (2018) Introgression of powdery mildew resistance gene Pm56 on rye chromosome arm 6RS into wheat. Front Plant Sci 9:1040
Jeberson MS, Chaudhary HK, Chahota RK, Wani SH (2021) Doubled haploid production in advanced back cross generations and molecular cytogenetic characterization of rye chromatin in triticale× wheat derived doubled haploid lines. Biocell 45(6):1651
Johansson E, Henriksson T, Prieto-Linde ML, Andersson S, Ashraf R, Rahmatov M (2020) Diverse wheat-alien introgression lines as a basis for durable resistance and quality characteristics in bread wheat. Front Plant Sci 11:1067
Johansson E, Hussain A, Kuktaite R, Andersson SC, Olsson ME (2014) Contribution of organically grown crops to human health. Int J Environ Res Public Health 11:3870–3893
Kumar S, Kumar N, Balyan HS, Gupta PK (2003) 1BL.1RS translocation in some Indian bread wheat genotypes and strategies for its use in future wheat breeding. Caryologia 56:23–30
Li J, Dundas I, Dong C, Li G, Trethowan R, Yang Z (2020) Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor Appl Genet 133:1095–1107
Li Z, Ren T, Yan B, Tan F, Yang M and Ren Z (2016) A mutant with expression deletion of gene Sec-1 in a 1RS.1BL line and its effect on production quality of wheat. PLoS ONE 11: e0146943
Li H, Guo X, Wang C, Ji W (2015) Spontaneous and divergent hexaploid Triticales derived from common wheat x rye by complete elimination of D-genome chromosomes. PLoS ONE 10:e0120421
Mago R, Zhang P, Vautrin S, Šimková H, Bansal U, Luo MC (2015) The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nature Plants 1:15186
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Myśków B, Hanek M, Banek-Tabor A, Maciorowski R, Stojałowski S (2014) The application of high-density genetic maps of rye for the detection of QTLs controlling morphological traits. J Appl Genet 55(1):15–26
Paterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stem of cereals. Can J Res 26:496–500
Rahmatov M, Rouse MN, Nirmala J, Danilova T, Friebe B, Steffenson BJ (2016) A new 2DS·2RL Robertsonian translocation transfers stem rust resistance gene Sr59 into wheat. Theor Appl Genet 129:1383–1392
Ren TH, Yang ZJ, Yan BJ, Zhang HQ, Fu SL, Ren ZL (2009) Development and characterization of a new 1BL.1RS translocation line with resistance to stripe rust and powdery mildew of wheat. Euphytica 169:207–213
Salamini F, Özkan H, Brandolini A, Schäfer-Pregl R, Martin W (2002) Genetics and geography of wild cereal domestication in the Near East. Nature Reviews on Genetics 3:429–441
Schneider A, Rakszegi M, Molnar-Lang M, Szakacs E (2016) Production and cytomolecular identification of new wheat-perennial rye (Secale cereanum) disomic addition lines with yellow rust resistance (6R) and increased arabino-xylan and protein content (1R, 4R, 6R). Theor Appl Genet 129:1045–1059
Schwarzacher T, Heslop-Harrison P (2000) Practical in situ hybridization. BIOS Scientific Publishers Ltd., England, p 203.
Seerja PS, Reddy VRK (2013) Identification of rye chromosome substitutions in Triticale and its relation with kernel characters and seed setting through Giemsa C-banding technique. Asian Pacific J Reprod 2:289–296
Sharma P, Chaudhary HK, Kapoor C, Manoj NV, Singh K, Sood VK (2022) Molecular cytogenetic analysis of novel wheat-rye translocation lines and their characterization for drought tolerance and yellow rust resistance. Cereal Res Commun 50(4):655–665
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Ann Bot 100:991–998
Yamamoto M, Mukai Y (1989) Application of fluorescence in situ hybridization to molecular cytogenetics of wheat. Wheat Inform Service 69:30–32
Acknowledgements
The authors are highly thankful to Monsanto’s for providing financial support through prestigious Beachell Borlaug International Fellowship-2012.
Funding
The authors are thankful to Monsanto’s Beachell Borlaug International Fellowship-2012 for providing financial support for executing the present research work.
Author information
Authors and Affiliations
Contributions
All the authors have contributed towards planning, execution and presentation of the work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethics approval
No animals were used in the study.
Consent to participate
All the listed authors have provided their consent.
Consent for publication
All the listed authors have provided their consent.
Ethics of publication
The authors declare that the work is original and complete. The work has not been submitted to any other journal for publication. The results presented in the manuscript are original.
Additional information
Communicated by Márta Molnár-Láng.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jamwal, N.S., Badiyal, A., Chaudhary, H.K. et al. Molecular cytogenetic analysis of newly developed progenies from triticale × wheat crosses for yield and stress tolerance. CEREAL RESEARCH COMMUNICATIONS 52, 859–865 (2024). https://doi.org/10.1007/s42976-023-00410-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-023-00410-5